Sialic acid Neu5Ac derivative as well as preparation method and application thereof
A sialic acid and derivative technology, applied in the field of sialic acid Neu5Ac derivatives and their preparation, can solve problems such as in-depth research, achieve a wide range of applications, improve identification and binding, and have obvious application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
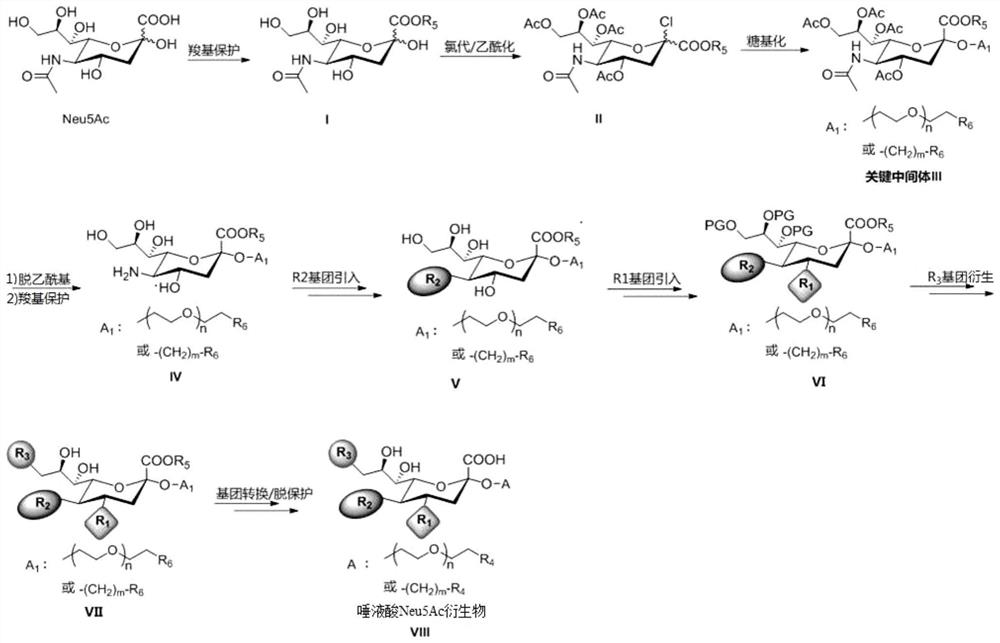
[0088] The preparation method of sialic acid Neu5Ac derivative specifically comprises the following steps:
[0089] Using sialic acid Neu5Ac as the starting material, the carboxyl group was protected to introduce R 5 The compound of formula I is obtained by protecting the group, and the compound of formula I is prepared by chlorination and acetylation at the same time to obtain the fully acetylated 2-chlorosugar of formula II, and the compound of formula II derives a functional side chain at the 2-position of the sugar ring through glycosylation Obtain the compound of formula III; The compound of formula III obtains the compound of formula IV after deacetylation and carboxyl protection to expose the amino and hydroxyl groups on the sugar ring; the compound of formula IV is obtained according to R 2 →R 1 →R 3 The introduction of the group is carried out in the order to obtain the compound of formula VII; the compound of formula VII is converted through side chain group (side ...
Embodiment 1
[0093] Example 1: Synthesis of customized sialic acid ligand functional molecule 1:
[0094]
[0095] Concentrated hydrochloric acid (1 mL) was dropped into methanol (100 mL), natural sialic acid Neu5Ac (10 g, 32.3 mmol) was added in batches, the reaction was stirred until the solution was clear, the reaction solution was neutralized, the solvent was evaporated, and the residue was rotary evaporated three times by adding toluene , drained to obtain the crude intermediate of formula 1-1, which was directly used in the next step reaction;
[0096] The crude product of formula 1-1 obtained in the previous step was dissolved in chloroacetyl (40 mL), stirred at room temperature until the reaction was complete, and the solvent was evaporated to obtain the crude product of formula 1-2, which was directly used in the next reaction;
[0097] The crude product of formula 1-2 obtained in the previous step and HOPEG 4 OBn (13.8g, 48.5mmol) was mixed with dry dichloromethane (60mL), si...
Embodiment 2
[0104] Example 2: Synthesis of customized sialic acid ligand functional molecule 2:
[0105]
[0106] Natural sialic acid Neu5Ac (10g, 32.3mmol) was dissolved in ethanol (100mL), potassium carbonate (5.36g, 38.8mmol) was added, stirred for 15 minutes, benzyl bromide (6.08g, 35.6mmol) was added dropwise, stirred at 50°C After 2 hours, TLC detected that the reaction was complete, and the reaction solution was spin-dried, and the residue was spin-evaporated three times with toluene, and then sucked dry to obtain the crude intermediate of formula 2-1, which was directly used in the next reaction;
[0107] The crude product of formula 2-1 obtained in the previous step was dissolved in chloroacetyl (20 mL), stirred at room temperature until the reaction was complete, and the solvent was evaporated to obtain the crude product of formula 2-2, which was directly used in the next reaction;
[0108] The crude product of formula 2-2 obtained in the previous step and HOPEG 4 OBn (13.79...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com