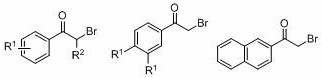
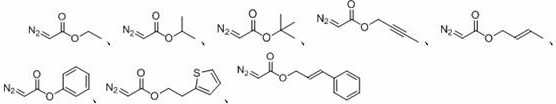
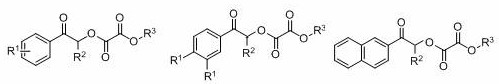
A kind of method for preparing oxalate
A technology of oxalate ester and -br, which is applied in the field of preparation of oxalate ester, can solve the problem that there is no method for synthesizing oxalate ester green, and achieve the effects of easy functional grouping, high atom economy, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] Add compound 1a (0.5 mmol, 101.6 mg), compound 2a (2 mmol, 224 vL), eosin Y (Eosin Y) (0.00 5 mol, 3.6 mg), N,N-dimethylformazol into a 25 mL Schlenk tube Amide (2 mL); then reacted in oxygen for 36 hours under the irradiation of a 12 W green LED lamp; after the reaction, extracted with ethyl acetate (10 mL × 3), dried over anhydrous magnesium sulfate, and removed with a rotary evaporator Solvent, silica gel adsorption, and simple column chromatography to obtain product 3a with a yield of 85%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0036] 1 H NMR (400 MHz, CDCl 3 ) δ 7.94 – 7.89 (m, 2H), 7.63 (t, J =7.4 Hz, 1H),7.50 (t, J =7.7 Hz, 2H), 5.55 (s, 2H), 4.40 (q, J =7.1 Hz, 2H), 1.40 (t, J =7.2Hz, 3H); 13 C NMR (101 MHz, CDCl 3 ) δ 189.79, 156.91, 156.88, 134,10, 133.48,128.84, 127.65, 67.59, 63.35, 13.75; HRMS (ESI...
Embodiment 2
[0038]
[0039] Add compound 1b (0.5 mmol, 114.2 mg), compound 2a (2 mmol, 224 vL), eosin Y (Eosin Y) (0.005 mol, 3.6 mg), N,N-dimethylformazol into a 25 mL Schlenk tube Amide (2 mL). Then the system was reacted in oxygen for 36 hours under the irradiation of 12 W green LED lamp. After the reaction, it was extracted with ethyl acetate (10 mL × 3), dried over anhydrous magnesium sulfate, the solvent was removed by a rotary evaporator, adsorbed on silica gel, and the product 3b was obtained by simple column chromatography with a yield of 73%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0040] 1 H NMR (400 MHz, CDCl 3 ) δ 8.03-7.97 (m, 1H), 7.66-7.59 (m, 1H), 7.33-7.27 (m, 1H), 7.24-7.17 (m, 1H), 5.43 (d, J =3.7 Hz, 2H), 4.42 (q, J =7.1 Hz,2H), 1.42 (t, J =7.2 Hz, 3H); 13 C NMR (101 MHz, CDCl 3 ) δ 188.00, 187.97,163.65, 161.12, 157.04,...
Embodiment 3
[0042]
[0043] Add compound 1c (0.5 mmol, 119.5 mg), compound 2a (2 mmol, 224 vL), eosin Y (Eosin Y) (0.005 mol, 3.6 mg), N,N-dimethylformazol into a 25 mL Schlenk tube Amide (2 mL). Then the system was reacted in oxygen for 36 hours under the irradiation of 12 W green LED lamp. After the reaction, it was extracted with ethyl acetate (10 mL × 3), dried over anhydrous magnesium sulfate, the solvent was removed by a rotary evaporator, adsorbed on silica gel, and the product 3c was obtained by simple column chromatography with a yield of 90%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0044] 1 H NMR (400 MHz, CDCl 3 ) δ 7.50-7.36 (m, 3H), 7.18-7.13 (m, 1H), 5.52(s, 2H), 4.40 (q, J =7.1 Hz, 2H), 3.84 (s, 3H), 1.40 (s, 3H); 13 C NMR (101 MHz, CDCl 3 ) δ 189.64, 159.82, 156.88, 134.69, 129.82, 120.47, 119.98, 111.95, 67.63, 63.29, 55.27,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com