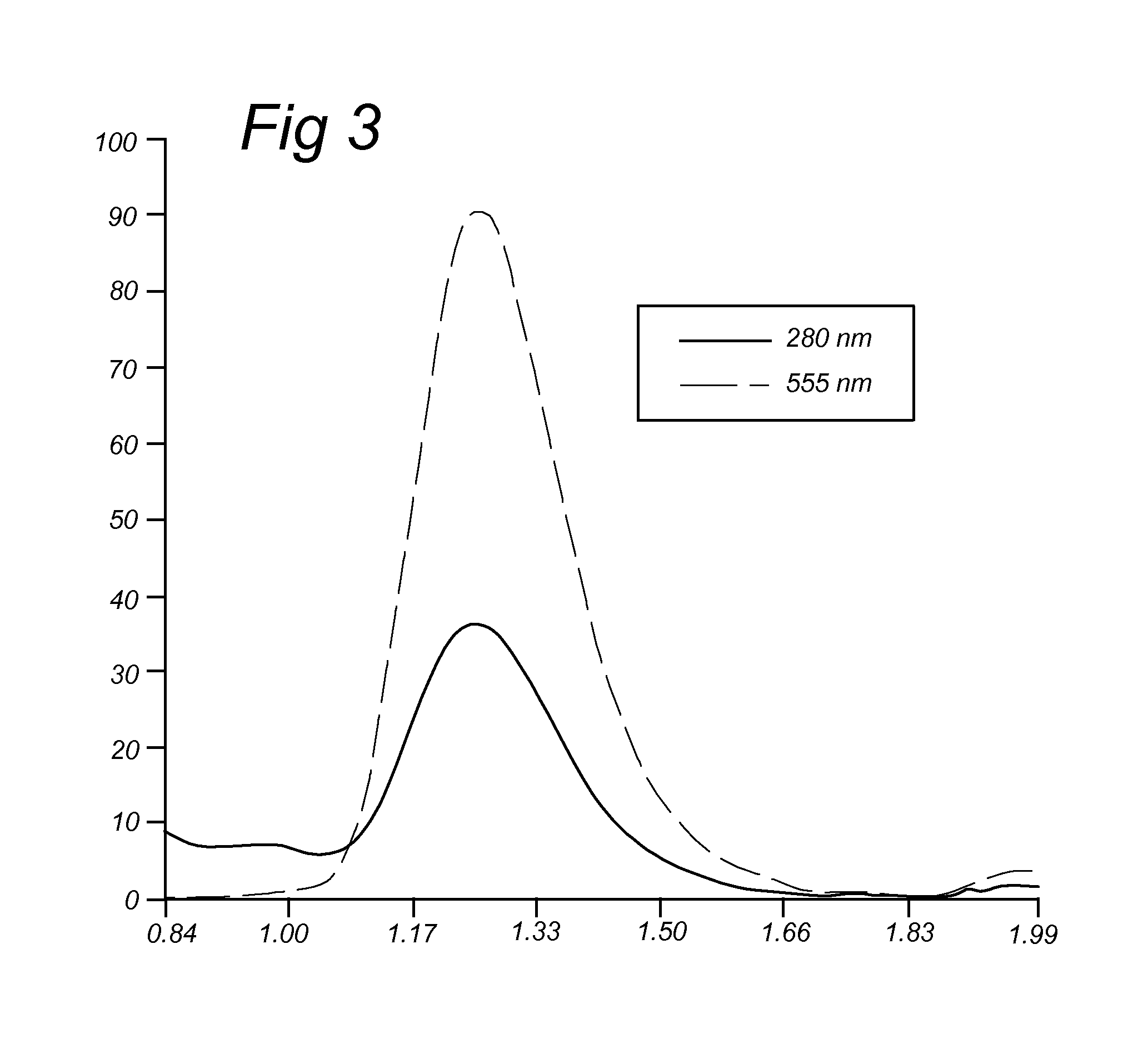
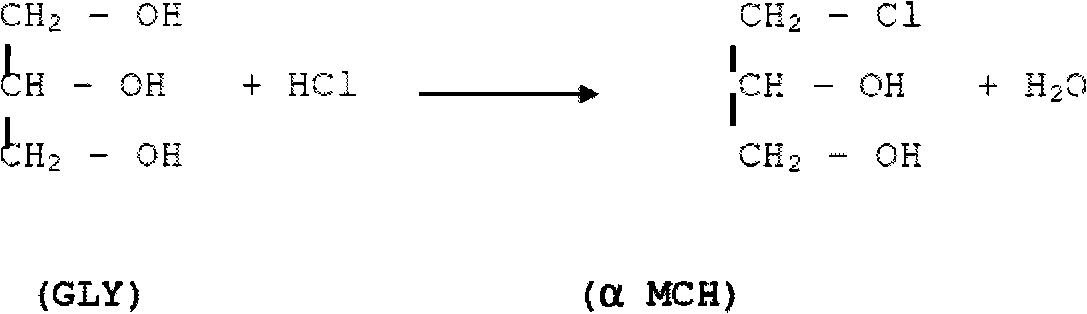
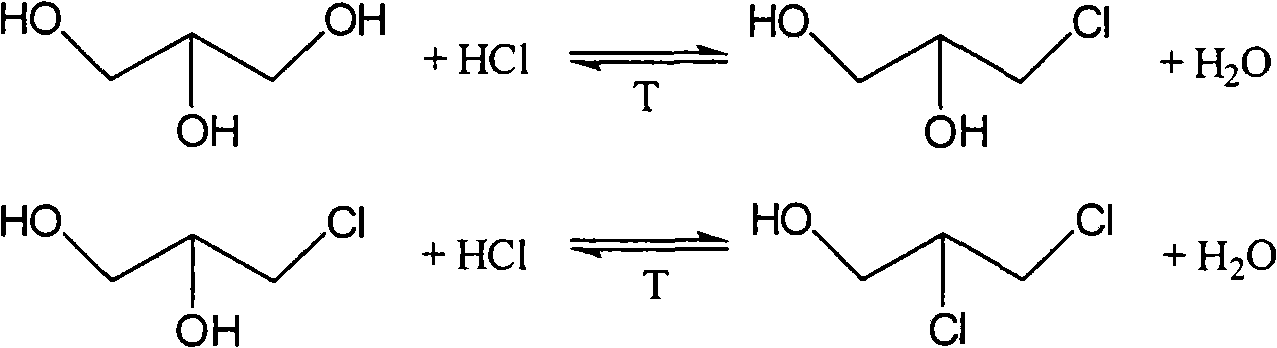
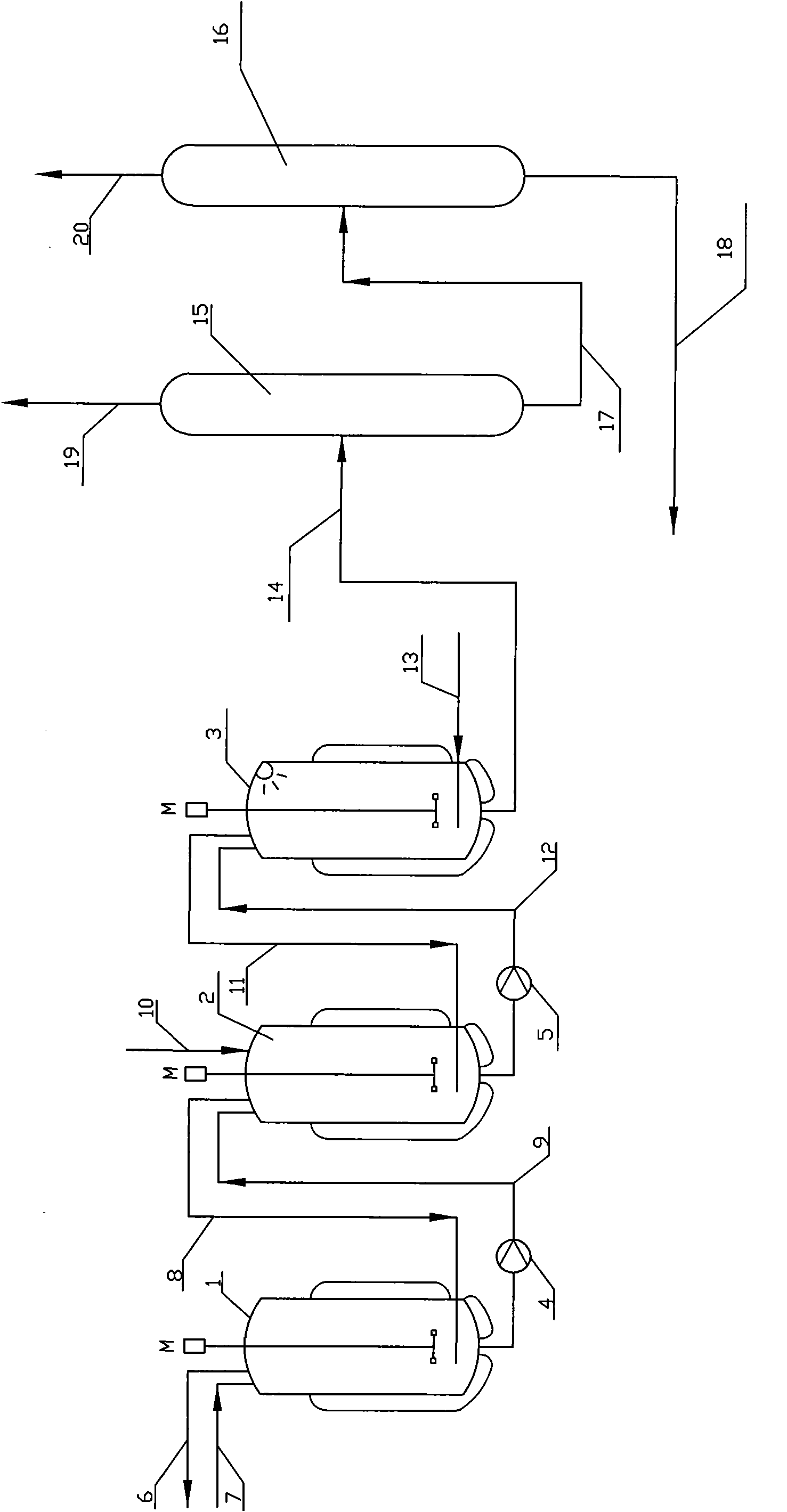
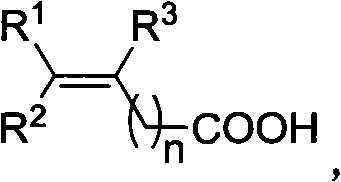
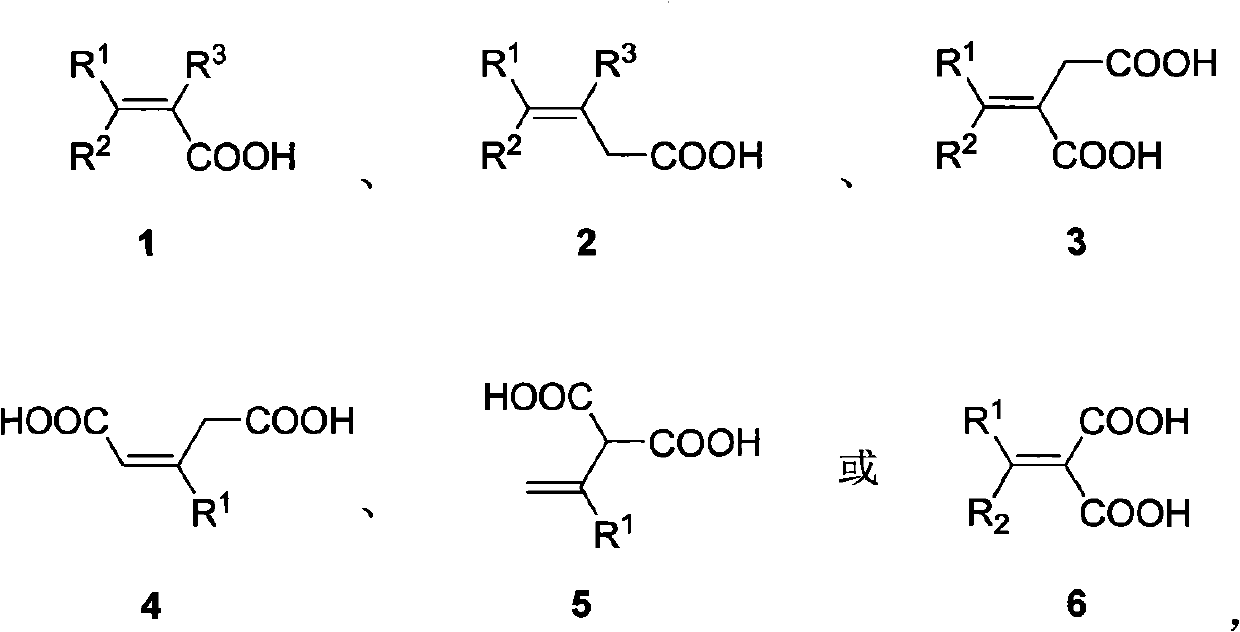
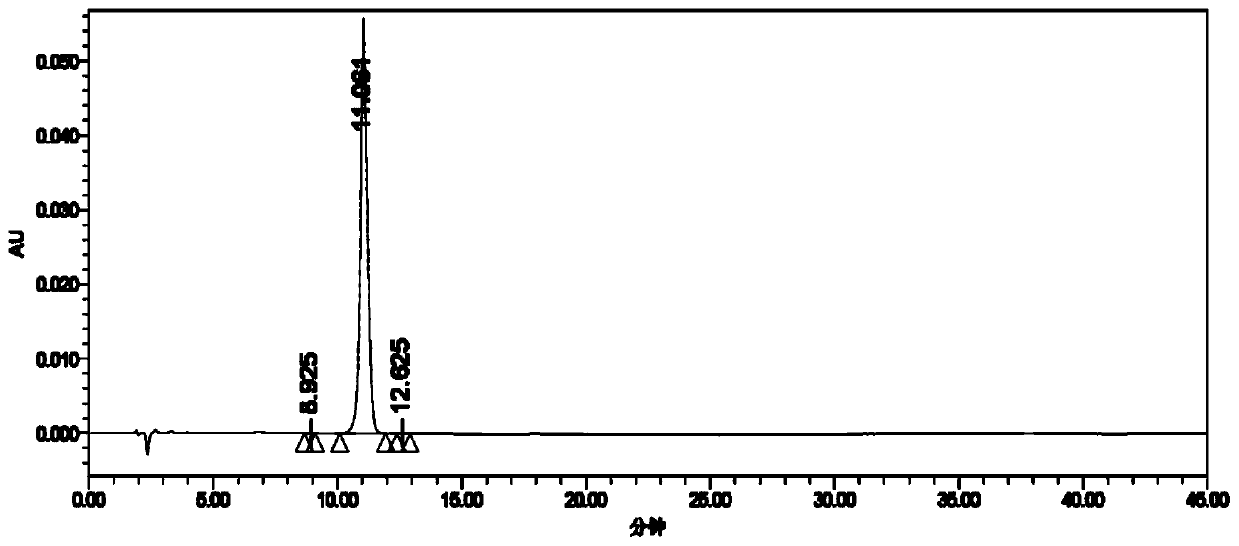
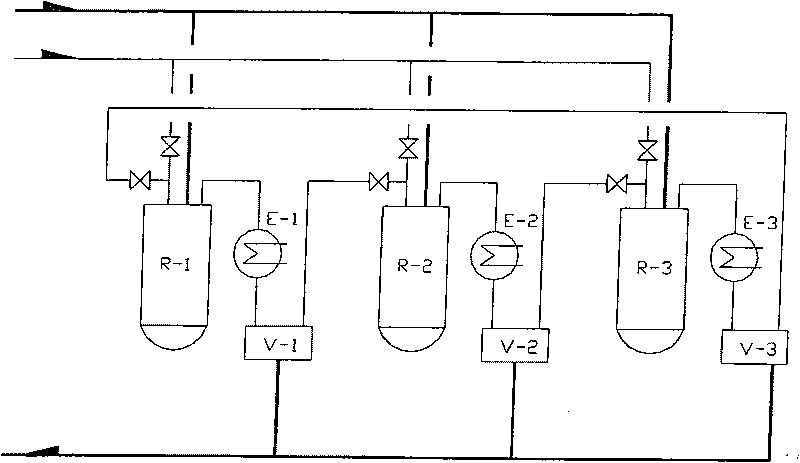
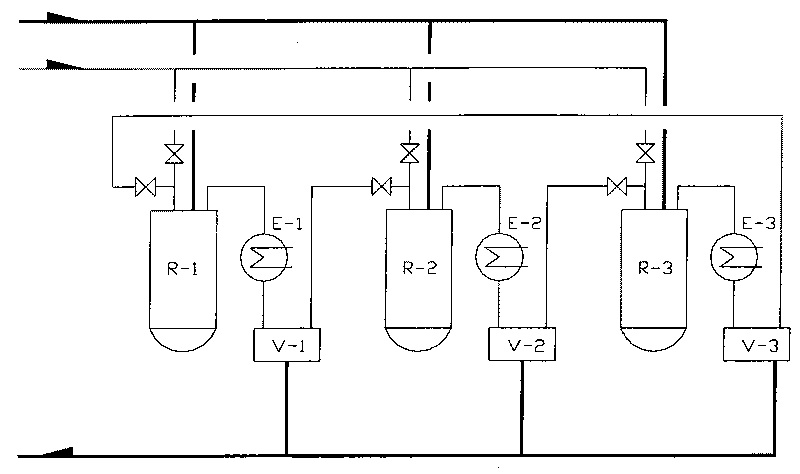
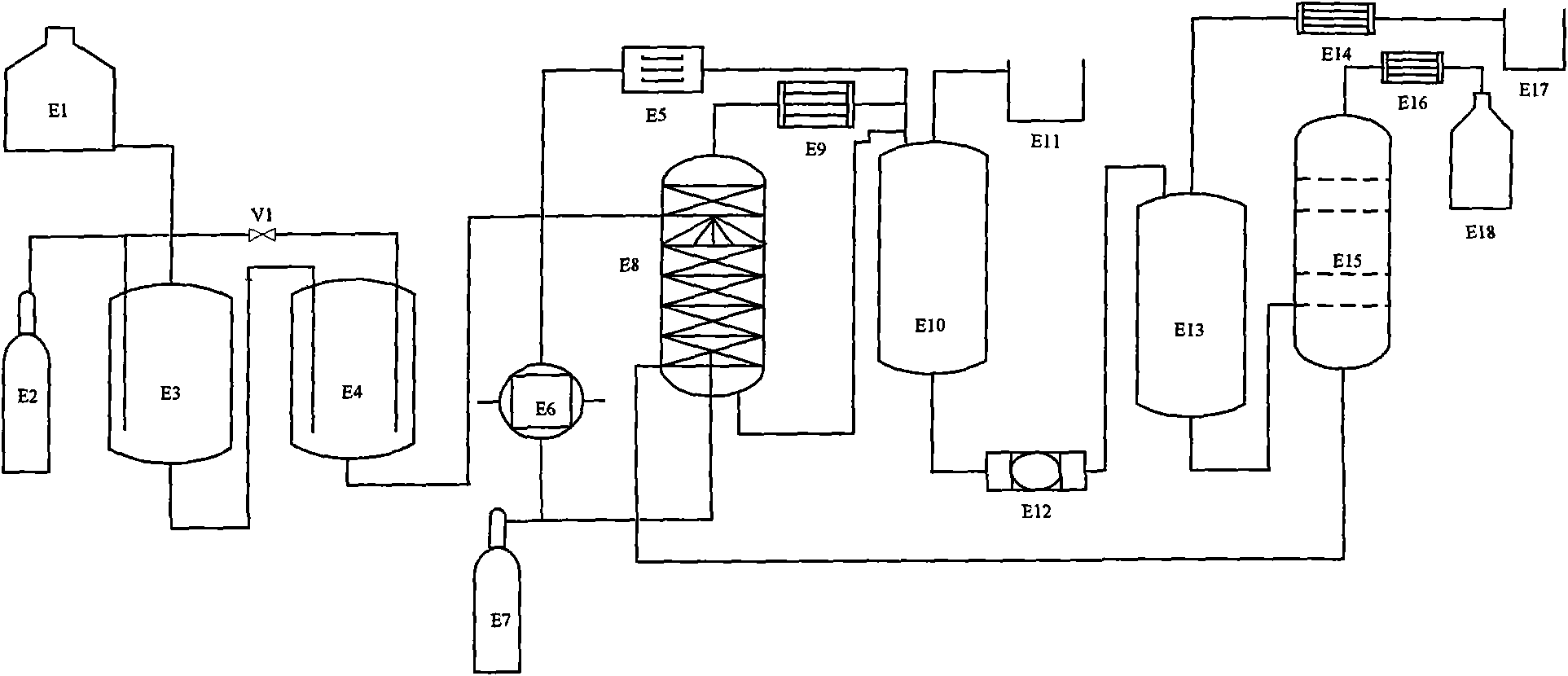
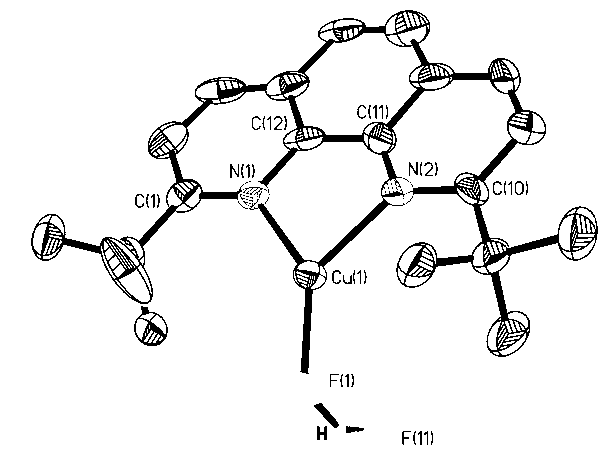
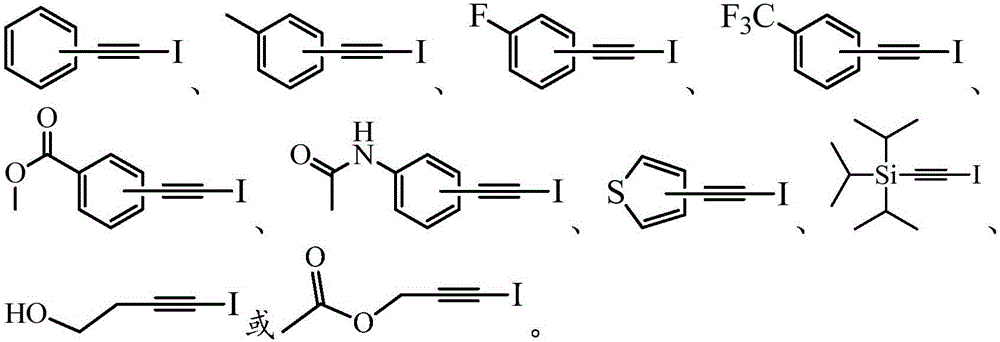
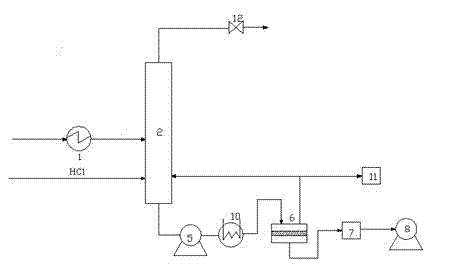
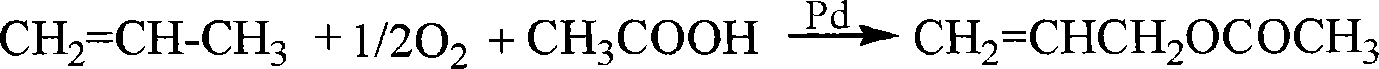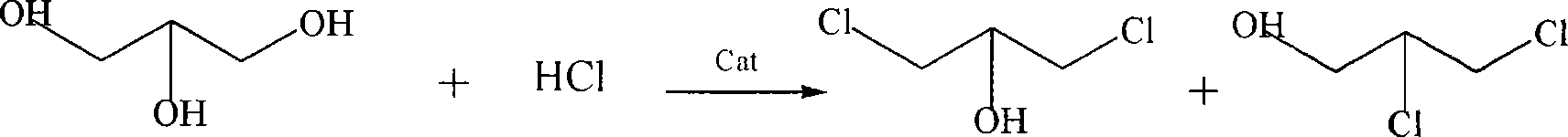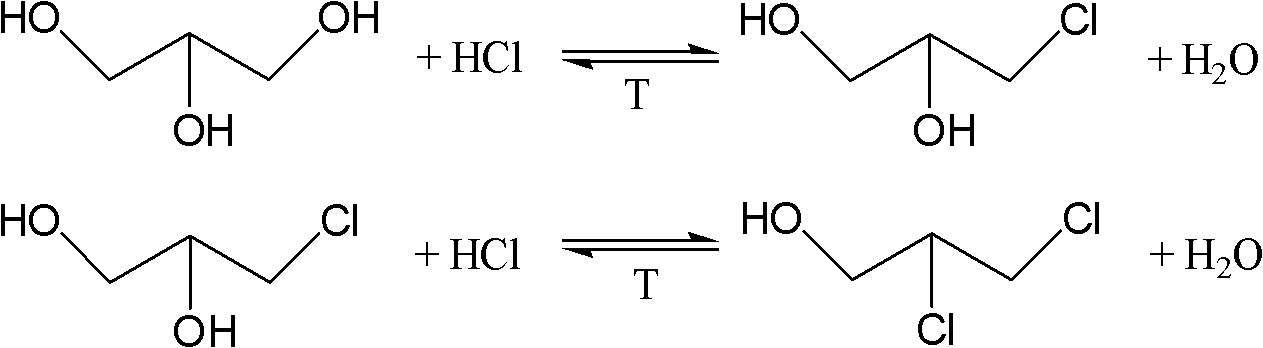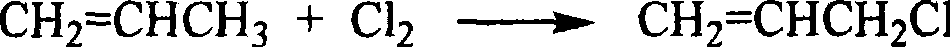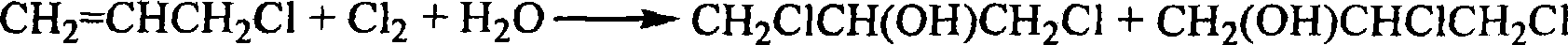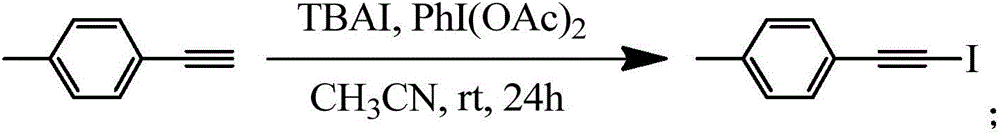Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
340results about "Preparation by halogen introduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Process for the conversion of a crude glycerol, crude mixtures of naturally derived multicomponent aliphatic hydrocarbons or esters thereof to a chlorohydrin
ActiveUS20080015370A1Minimize formationHigh selectivityOrganic compound preparationCarboxylic acid esters preparationSufficient timeAliphatic hydrocarbon
A process for converting a crude glycerol, crude mixtures of naturally derived multihydroxylated-aliphatic hydrocarbons or esters thereof to a chlorohydrin, by contacting the crude glycerol, crude mixtures of naturally derived multihydroxylated-aliphatic hydrocarbons or esters thereof starting material with a source of a superatmospheric partial pressure of hydrogen chloride for a sufficient time and at a sufficient temperature, and wherein such contracting step is carried out without substantial removal of water, to produce the desired chlorohydrin product; wherein the desired product or products can be made in high yield without substantial formation of undesired overchlorinated byproducts; wherein said crude glycerol, said ester of crude glycerol, or mixture thereof is derived from a renewable raw material. Chlorohydrins made by the process of the present invention are useful in preparing epoxides such as epichlorohydrins.
Owner:BLUE CUBE IP
Fused cyclooctyne compounds and their use in metal-free click reactions
InactiveUS8859629B2Easy to synthesizeAmenable to simple and straightforward modification of the various parts of the moleculeBiocideCarbamic acid derivatives preparationCombinatorial chemistry1,3-dipole
Owner:SYNAFFIX
Fused cyclooctyne compounds and their use in metal-free click reactions
InactiveUS20130137763A1Easy to synthesizeAmenable to simple and straightforward modification of the various parts of the moleculeBiocideCarbamic acid derivatives preparationCombinatorial chemistry1,3-dipole
The invention relates to fused cyclooctyne compounds, and to a method for their preparation. The invention also relates to a conjugate wherein a fused cyclooctyne compound according to the invention is conjugated to a label, and to the use of these conjugates in bioorthogonal labeling, imaging and / or modification, such as for example surface modification, of a target molecule. The invention further relates to a method for the modification of a target molecule, wherein a conjugate according to the invention is reacted with a compound comprising a 1,3-dipole or a 1,3-(hetero)diene.
Owner:SYNAFFIX
Batch, semi-continuous or continuous hydrochlorination of glycerin with reduced volatile chlorinated hydrocarbon by-products and chloracetone levels
ActiveUS20080015369A1High selectivityQuick conversionOrganic compound preparationCarboxylic acid esters preparationSufficient timeChloroacetone
The present invention relates to a process for converting a multihydroxylated-aliphatic hydrocarbon or ester thereof to a chlorohydrin, by contacting the multihydroxylated-aliphatic hydrocarbon or ester thereof starting material with a source of hydrogen chloride at superatmospheric, atmospheric and subatmospheric pressure conditions for a sufficient time and at a sufficient temperature, preferably wherein such contracting step is carried out without substantial removal of water, to produce the desired chlorohydrin product; wherein the desired product or products can be made in high yield without substantial formation of undesired overchlorinated byproducts; said process carried out without a step undertaken to specifically remove volatile chlorinated hydrocarbon by-products or chloroacetone, wherein the combined concentration of volatile chlorinated hydrocarbon by-products and chloroacetone is less than 2000 ppm throughout any stage of the said process.
Owner:BLUE CUBE IP
Conversion of a Multihydroxylated-Aliphatic Hydrocarbon or Ester Thereof to a Chlorohydrin
ActiveUS20080045728A1High selectivityQuick conversionOrganic compound preparationPreparation by halogen introductionSufficient timeAliphatic hydrocarbon
The present invention relates to a process for converting a multihydroxylated-aliphatic hydrocarbon or ester thereof to a chlorohydrin, by contacting the multihydroxylated-aliphatic hydrocarbon or ester thereof starting material with a source of a superatmospheric partial pressure of hydrogen chloride for a sufficient time and at a sufficient temperature, and wherein such contracting step is carried out without substantial removal of water, to produce the desired chlorohydrin product; wherein the desired product or products can be made in high yield without substantial formation of undesired overchlorinated byproducts. In addition, certain catalysts of the present invention may be used in the present process at superatmospheric, atmospheric and subatmospheric pressure conditions with improved results.
Owner:BLUE CUBE IP
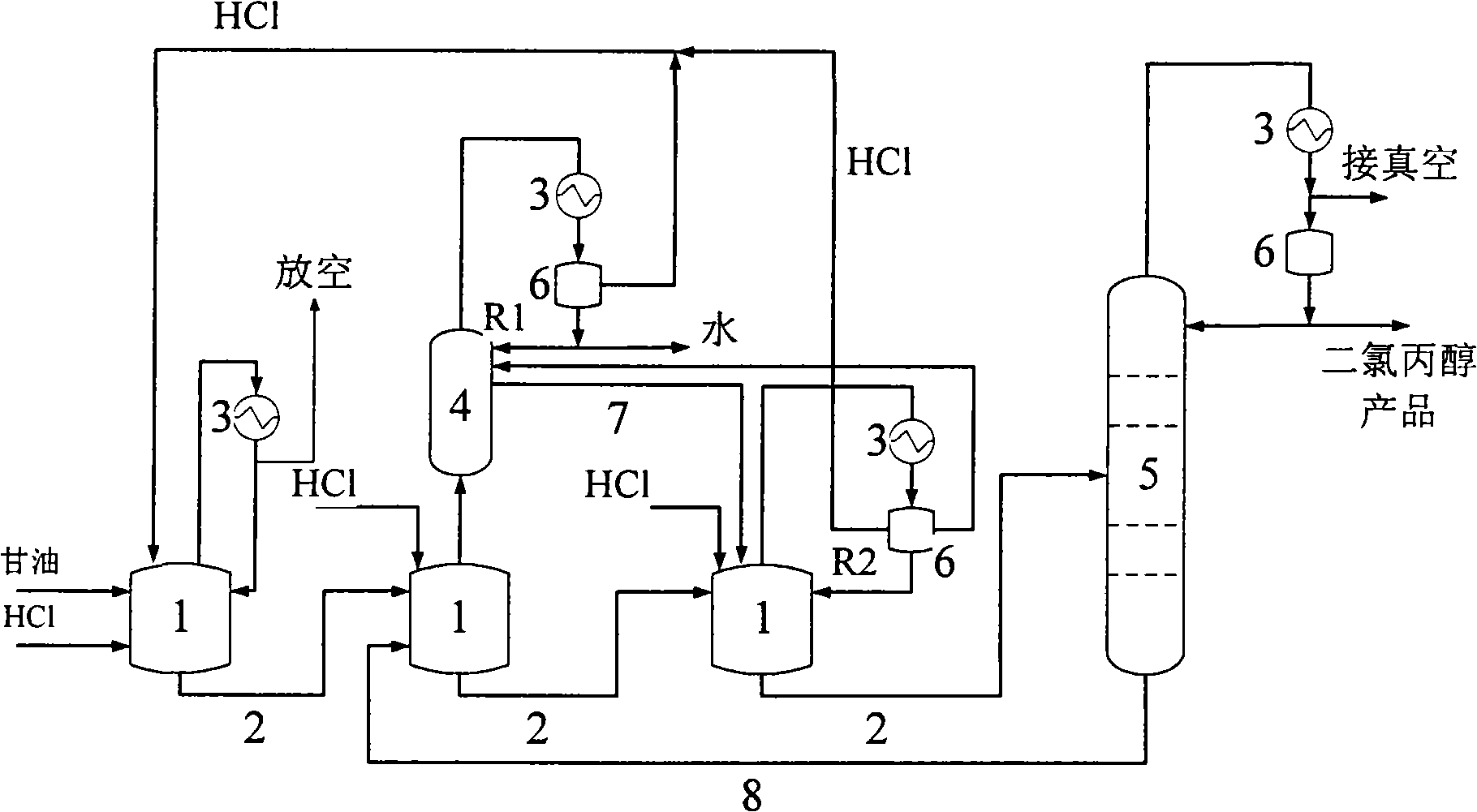
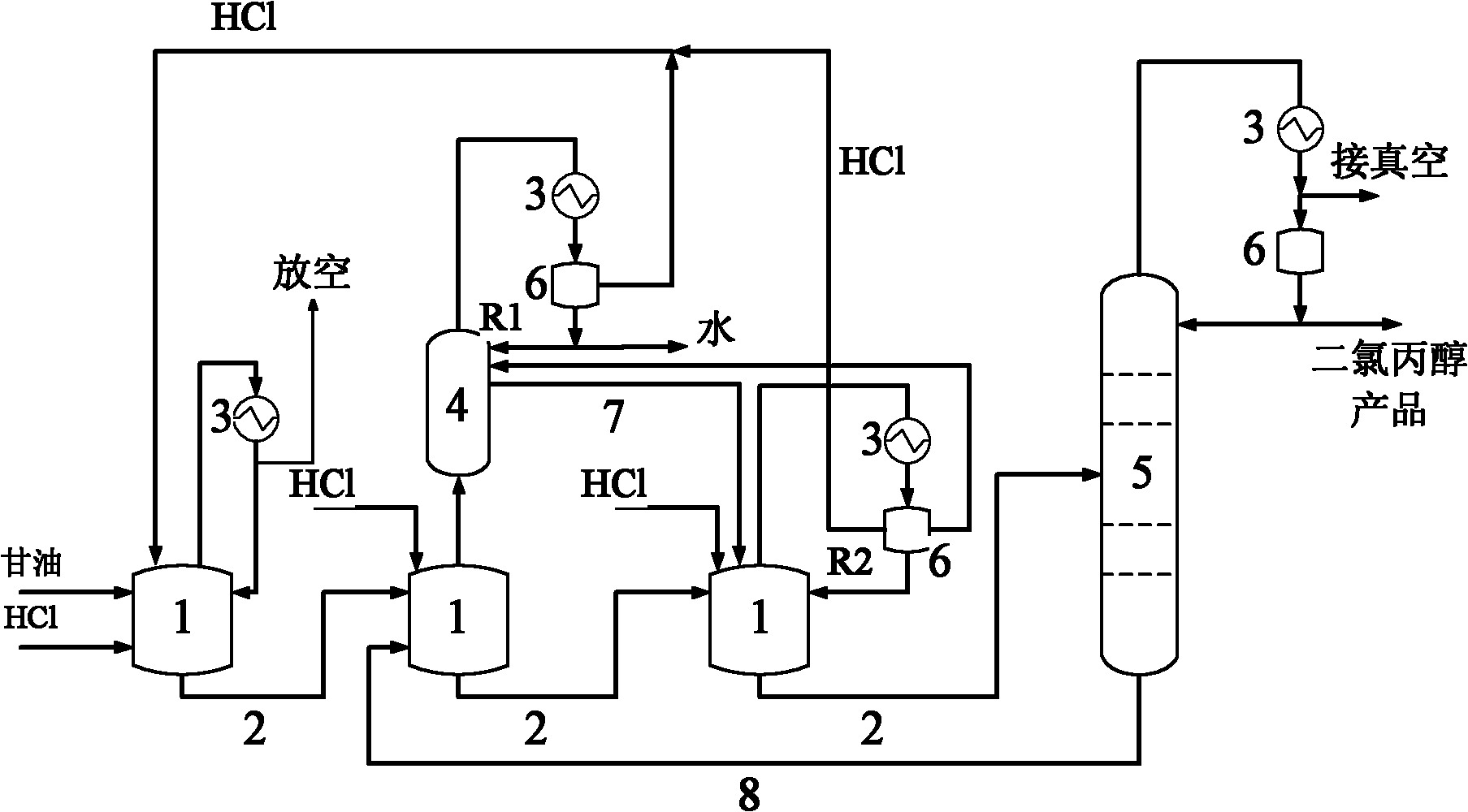
Method for preparing dichloropropanol by glycerol
InactiveCN101182283AImprove conversion rateIncrease reaction ratePreparation by halogen introductionGlycerolCirculating pump
A method of preparing for dichloropropanol from glycerol is that the glycerol and catalyst are added into a spraying type reactor; a liquid circulating pump is used to pump the glycerol and the catalyst our from the spraying type reactor, then the glycerol and the catalyst are sent into the spraying type reactor from the top of the spraying type reactor by the manner of spraying; HCl gas which is absorbed from the lateral part of the spraying type reactor is mixed completely and react with the materials of the glycerol and the catalyst inside the spraying type reactor; at the same time the negative pressure produced by spraying brings a resultant inside the spraying type reactor in the form of an azeotropic matter of water, the dichloropropanol and the HCl; the water and the dichloropropanol in the azeotropic matter are condensed down by a condenser, and the unreacted HCl gas and the complementary HCl gas are sent into the spraying type reactor together for cyclic reaction. The invention has the advantages of the improvement of the reaction velocity and high glycerol transformation rate.
Owner:CHINA RES INST OF DAILY CHEM IND
Conversion of glycerine to dichlorohydrins and epichlorohydrin
ActiveCN101541418AMethod optimizationReduce consumptionPreparation by halogen introductionChemical/physical/physico-chemical stationary reactorsNuclear engineeringGlycerol
The present invention relates to a process for the production of dichlorohydrin by catalyzed hydrochlorination of glycerine where the reaction is performed in at least two subsequent stages operating continuously at different pressures with both vapor and liquid recycle. The first reactor, low pressure (L. P.) reactor, operating at a pressure ranging from 1 to 4 bar and at a temperature from 900C to 1300C converts most of GLY to MHC. The second reactor, medium pressure (M. P. ) reactor, operating at a pressure ranging from 5 to 20 bar and at temperature from 90 DEG c to 1300C converts the effluent from the L. P. reactor to DCH with an adequate degree of conversion. Each reactor is followed by a stripping unit.
Owner:CONSER
Conversion of a multihydroxylated-aliphatic hydrocarbon or ester thereof to a chlorohydrin
InactiveCN1976886ANot significantly removedHigh selectivityPreparation by halogen introductionSufficient timeAliphatic hydrocarbon
The present invention relates to a process for converting a multihydroxylated-aliphatic hydrocarbon or ester thereof to a chlorohydrin, by contacting the multihydroxylated-aliphatic hydrocarbon or ester thereof starting material with a source of a superatmospheric partial pressure of hydrogen chloride for a sufficient time and at a sufficient temperature, and wherein such contracting step is carried out without substantial removal of water, to produce the desired chlorohydrin product; wherein the desired product or products can be made in high yield without substantial formation of undesired overchlorinated byproducts. In addition, certain catalysts of the present invention may be used in the present process at superatmospheric, atmospheric and subatmospheric pressure conditions with improved results.
Owner:DOW GLOBAL TECH LLC
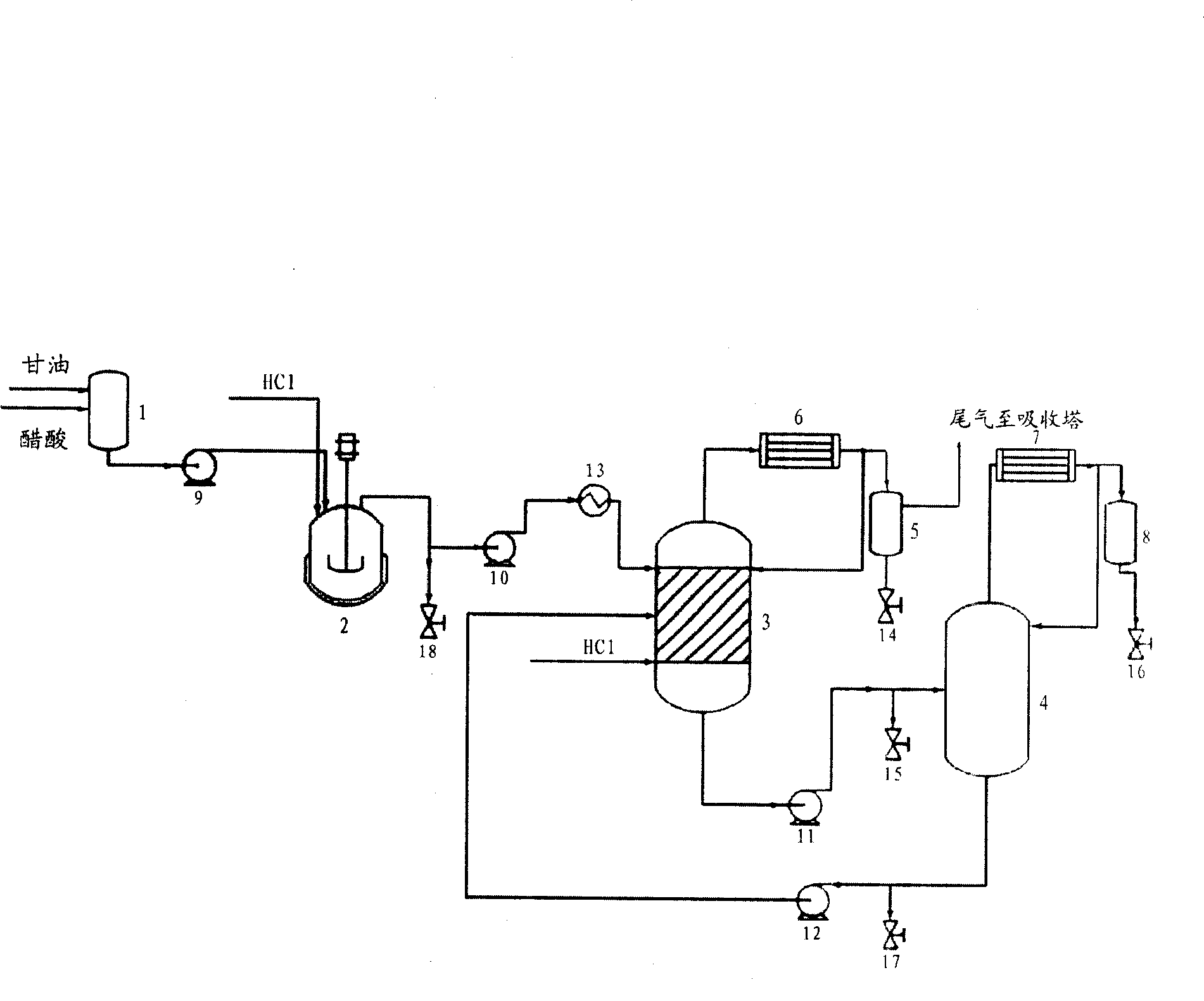
Preparation method of dichloropropanol
ActiveCN103333047AHigh yieldLow costPreparation by halogen introductionSide reactionHydrogen chloride
The invention provides a preparation method of dichloropropanol. Dichloropropanol is obtained by subjecting glycerin and a chloridizing agent (such as hydrogen chloride) to chlorination in a chlorination reaction tower in the presence of a catalyst. A stannum compound salt is used as the catalyst; a one-time investment of the catalyst is needed, and the catalyst is capable of being recycled. Advantages of the tower reactor are that: uniform contact of solid and liquid is ensured, the time of backmixing is reduced, and the tower reactor is suitable for large-scaled industrial production. Advantages of the preparation method of the invention are that: reaction conditions are mild, side reactions are few, and the yield of dichloropropanol is high.
Owner:河北珈奥甘油化工有限公司
Technique and system for preparing dichloropropanol by autocatalysis reaction of glycerine and hydrogen chloride
ActiveCN101357880AReduce usageEliminate formationPreparation by halogen introductionAutocatalysisBoiling point
The invention discloses a technique for preparing dichloropropanol by the autocatalytic reaction of glycerol and hydrogen chloride. Glycerol and hydrogen chloride carry out autocatalytic reaction at the temperature of 105 to 160 DEG C and the pressure of 0.05 to 0.5Mpa, and the water generated in the reaction is distilled and removed. The invention also discloses a system for preparing dichloropropanol by the autocatalytic reaction of glycerol and hydrogen chloride gas. By adopting the technique of autocatalytic reaction of glycerol and hydrogen chloride, the invention avoids the using of catalyst, lowers production cost obviously, eliminates the formation of by-products with high boiling point, improves reaction selectivity and has the advantages of simple technique and low production cost. The system can transform glycerol fully and greatly improves the selectivity of dichloropropanol; the purity of the dichloropropanol obtained finally is more than 99.5 percent.
Owner:NANTONG TIANSHI CHEM
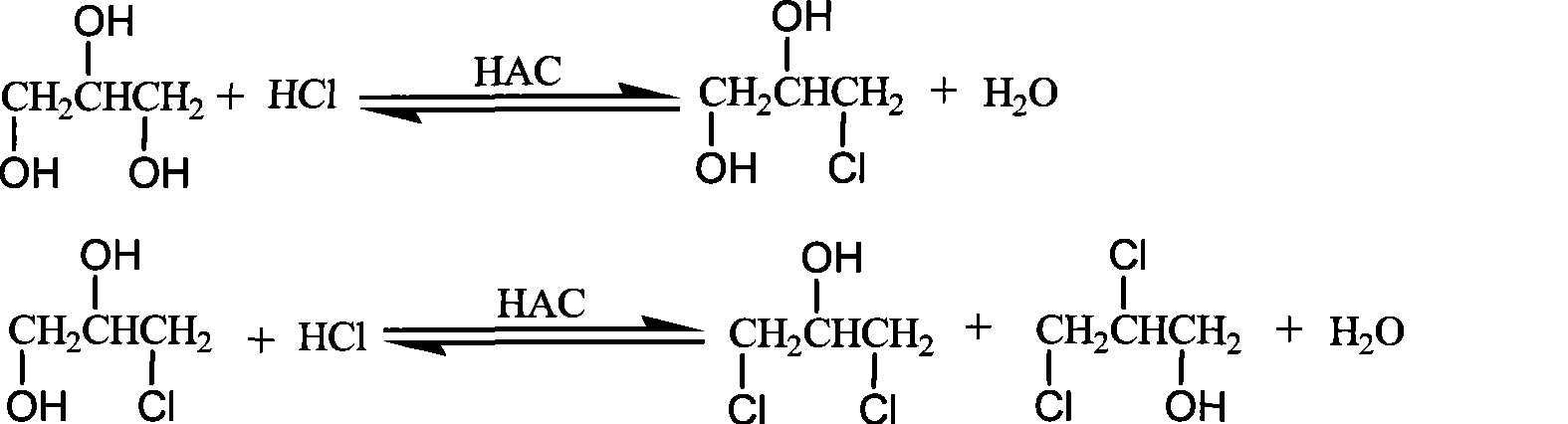
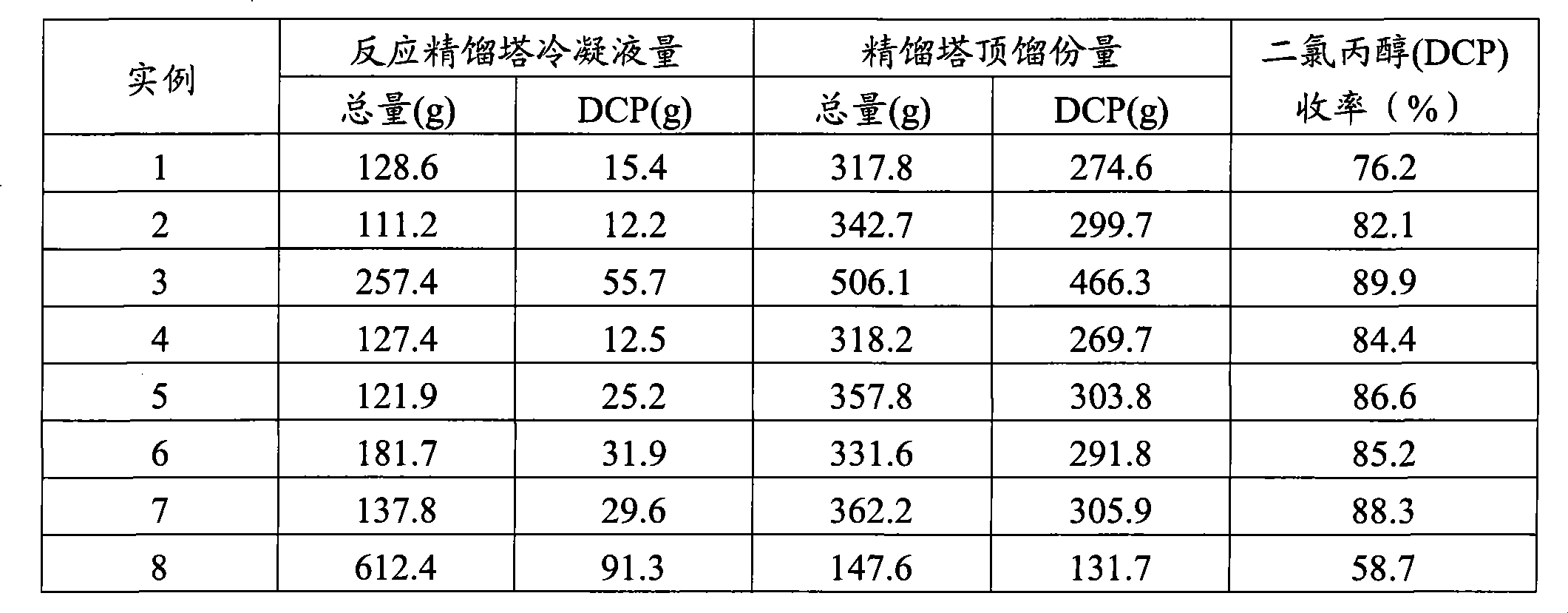
Production method of dichloropropanol
ActiveCN101805243AImprove conversion rateReduce dosagePreparation by halogen introductionBoiling pointDistillation
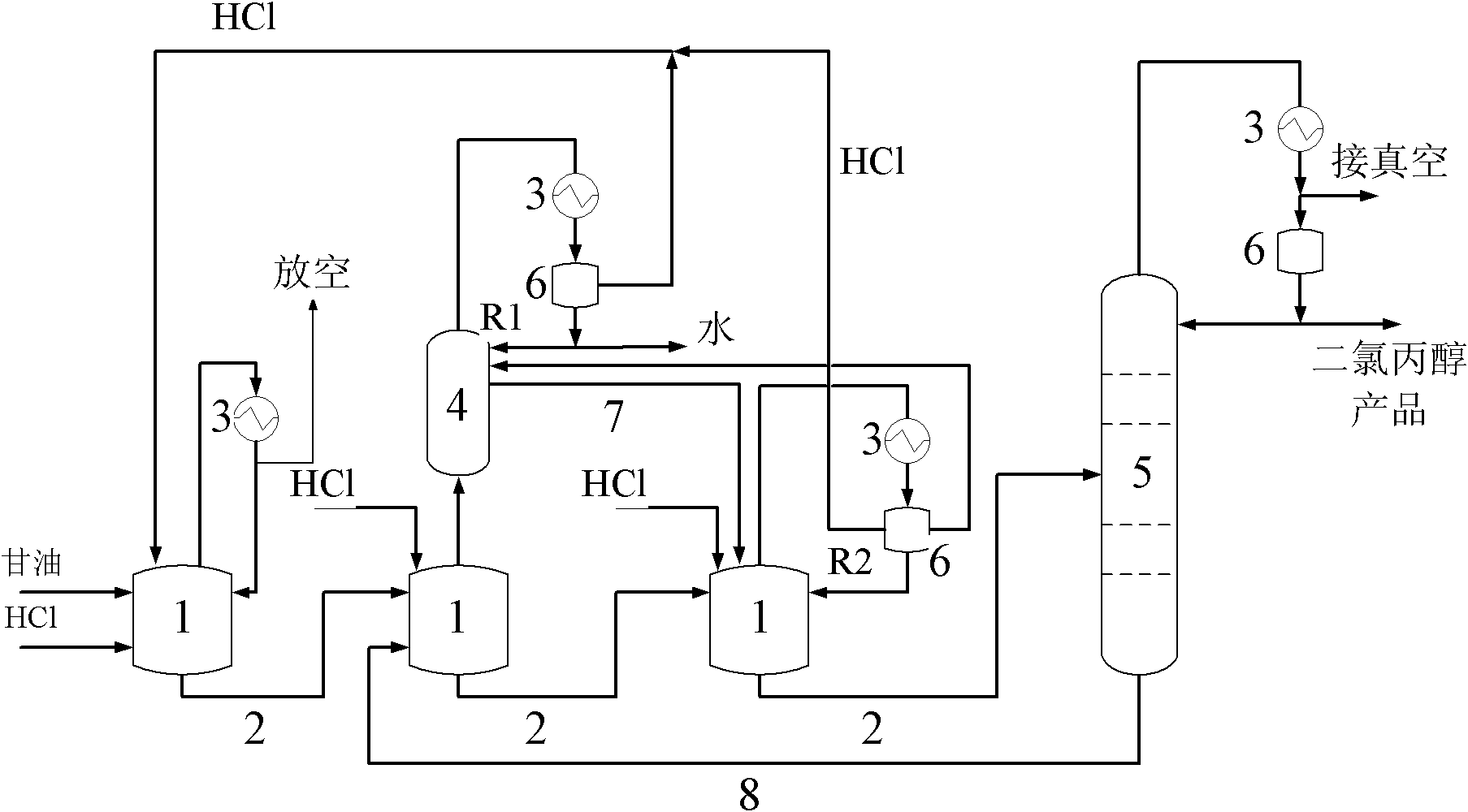
The invention relates to a production method of dichloropropanol, comprising the following reaction steps: glycerol reacts with gaseous HCl in the presence of organic carboxylic acid catalyst, reaction liquid reactant reacts with gaseous HCl in the presence of Lewis acid catalyst and then reacts with gaseous HCl again under the action of microwave and the stirring action; after passing through a low-boiling-point substance distillation tower and a rectifying tower, the mixture is prepared into the product of the invention. Compared with the prior art, HCl conversion rate is improved by about one time. When the combined catalysis technology of the invention is adopted, the dosage of the organic carboxylic acid catalyst is lowered by half, glycerol conversion rate is improved by 2%, and product yield is improved by 5%; and meanwhile, side reaction is reduced so as to avoid the defect that extra potentially toxic organic carboxylic acid side reaction generates a great quantity of waste, thus protecting environment.
Owner:NINGBO HUANYANG CHEM
Fluorination method of vinyl carboxylic acid
ActiveCN102219638AOrganic compound preparationPreparation by halogen introductionCarboxylic acidSolvent
The invention relates to a decarboxylation and fluorination method of vinyl carboxylic acid. In the method, in an aqueous or polar organic solvent or a mixed solvent of aqueous and polar organic solvents, vinyl carboxylic acid, an additive and an electrophilic fluorinating reagent are reacted at room temperature-reflux temperature so as to synthesize monofluoro olefin, allyl fluoride, difluoro alkyl substituted alcohol, mono-difluoro alkyl substituted alcohol, monofluoro alkyl substituted epoxide, beta-monofluoro / difluoro substituted cyclic lactone or beta-monofluoro substituted unsaturated cyclic lactone compounds. The structural formula of vinyl carboxylic acid is shown in the specification. According to the method in the invention, raw materials are available, reaction is simple and convenient, and the method can be used for preparing compounds which are difficult to prepare by using a common method.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
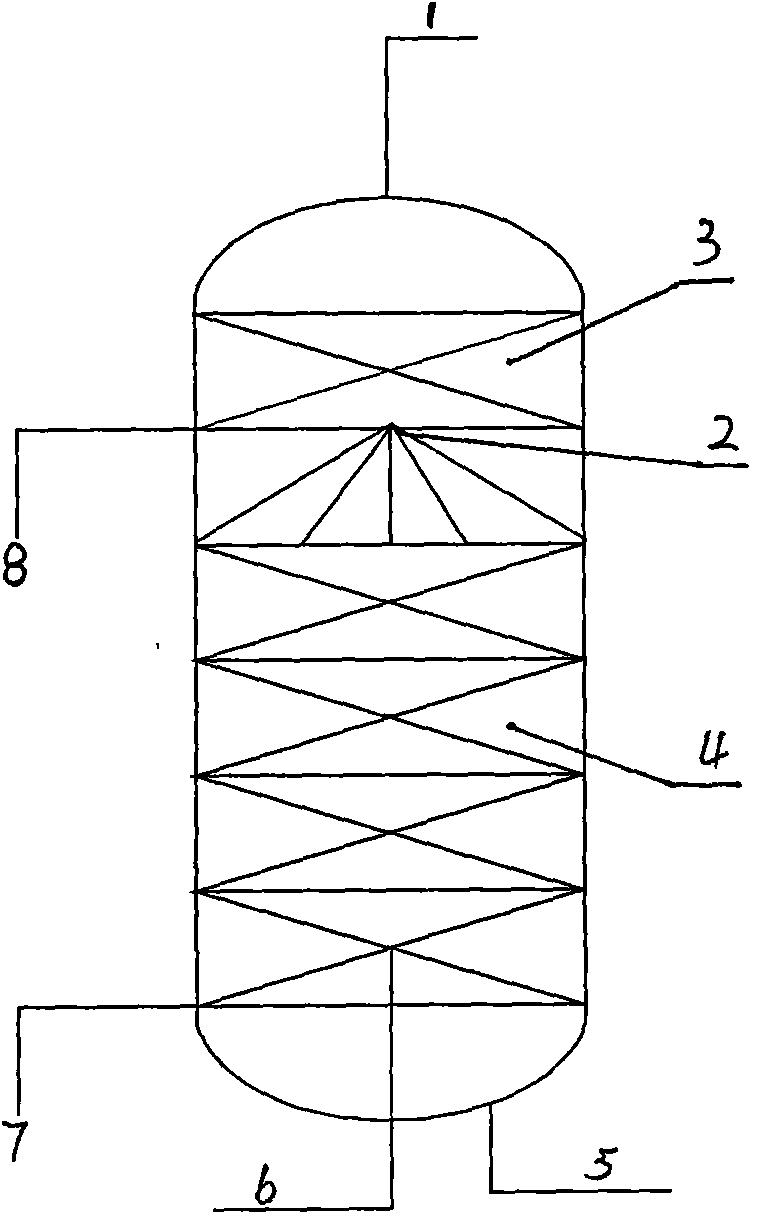
Production process for refining dichlorohydrin by glycerol reaction distillation
InactiveCN101481298ASimple processReaction temperatureChemical industryPreparation by halogen introductionOligomerGlycerol
The invention provides a production process for preparing dichloropropanol by reactive distillation of glycerol. The production process comprises the following steps: the raw material glycerol containing a homogeneous catalyst is sent to a reactive rectification tower from the top after heat exchange, hydrogen chloride gas enters from the lower part of a rectification section of the tubular reactive rectification tower, and the raw material glycerol and countercurrent flow of the hydrogen chloride gas are subject to absorption and chlorination reaction by a packing layer at the temperature of 90-120 DEG C and the temperature of 0.001-0.1MPa (absolute pressure) and are rectified; omonochloropropylene glycol at the bottom of the tubular reactive rectification tower, glycerol and glycerol oligomer are sent to a heavy constituent separation tower for decompressing and recovering the monochloropropylene glycol and the glycerol is returned to the raw material glycerol; and product gas on the top of tubular reactive rectification tower is cooled by a heat exchanger to obtain the dichloropropanol which is sent to a cyclization working procedure, and tail gas is further washed with lye, and is discharged from by a vacuum pump.
Owner:SHANDONG UNIV OF SCI & TECH
A method for continuously preparing dichloropropanol with glycerol and hydrochloric acid
InactiveCN102295529ABroaden the range of sourcesReduce manufacturing costPreparation by halogen introductionAlkaline earth metalPretreatment method
The object of the invention is to provide a method for synthesizing dichloropropanol with glycerin and hydrochloric acid. After pretreated industrial hydrochloric acid or pretreated industrial by-product hydrochloric acid or reagent hydrochloric acid is mixed with glycerin and catalyst in a batching tank, continuous Put it into a reactor equipped with a fractionation device on the top, continue heating under normal pressure for chlorination reaction, the temperature is controlled at 100-200°C, while continuously performing chlorination reaction, continuously distill the material in the reactor, and the product dichloro Propanol and water are continuously taken out from the reaction system after being condensed into liquid by the condenser; the same pretreatment method of industrial hydrochloric acid and industrial by-product hydrochloric acid of the present invention is to add one or more phosphorus-containing and / or sulfur-containing hydrochloric acid to the hydrochloric acid to be treated. Alkali metal and / or alkaline earth metal salts of inorganic acids, added in an amount of 0.1 to 15% of the weight of the hydrochloric acid to be treated, stirred at a temperature of 0 to 100°C for 0.5 to 15 hours; And the by-product hydrochloric acid provides a good outlet, which is conducive to reducing the production cost of dichloropropanol.
Owner:江西省化学工业研究所 +1
Method for preparing tranexamic acid
ActiveCN110156620AEasy to separateRaw materials are cheap and easy to getOrganic compound preparationOrganic chemistry methodsCyclohexanedimethanolAminolysis
The invention relates to a method for preparing tranexamic acid. The method comprises the following steps: reacting 1,4-cyclohexanedimethanol, used as starting material, with an HX acid to generate 4-chloromethylcyclohexylmethanol or 4-iodomethylcyclohexylmethanol, carrying out an oxidation reaction in an atmosphere of a gas containing 21-100% of oxygen in order to produce 4-chloromethylcyclohexylcarboxylic acid or 4-iodomethylcyclohexylcarboxylic acid, carrying out an aminolysis reaction in an autoclave, introducing liquid ammonia or ammonia water having an ammonia content of 15-28% to the aminolysis reaction system, and transforming the obtained reaction product with an alkali after the aminolysis reaction is finished in order to obtain the tranexamic acid, wherein X is Cl or I. Comparedwith the prior art, the preparation method has the advantages of cheap and easily available raw materials and low cost. The method also has the advantages of simplicity in operation, high yield, andsuitableness for industrial production.
Owner:VALIANT CO LTD
Method for synthesizing dichloropropanol by catalyzing glycerol for chlorination under existence of dicarboxylic acid-rare earth chloride
ActiveCN101704722AHigh activityImprove reaction efficiencyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDistillationRare earth
The invention relates to a method for synthesizing dichloropropanol by catalyzing glycerol for chlorination under the existence of dicarboxylic acid-rare earth chloride, which comprises the following steps of: mixing glycerol and a dicarboxylic acid-rare earth chloride composite catalyst in a chloridizing reactor R-1, and then introducing chlorine hydride gas, constantly evaporating out water and partial dichloropropanol generated in the reactor; introducing chlorine hydride as a tail gas into a chloridizing reactor R-2 filled with the glycerol and the catalyst; after the chlorination of the reactor R-1 is ended, evaporating dichloropropanol by decompression and then adding the glycerol for further chlorination; during the distillation of the chloridizing reactor R-1, starting to introduce chlorine hydride into the chloridizing reactor R-2, and the chlorine hydride as the tail gas into a chloridizing reactor R-3, wherein the glycerol and the catalyst added into the chloridizing reactors R-1, R-2 and R-3 have the same dosage; after the chlorination of the chloridizing reactor R-2 is ended, repeating the procedures of distillation, feeding and chlorination in the chloridizing reactor R-1; connecting the chloridizing reactors R-1,R-2 and R-3 from head to tail, and introducing the chlorine hydride as the tail gas into the chloridizing reactor R-1 while introducing the chlorine hydride into the chloridizing reactor R-3; transferring a mixed liquor of the dichloropropanol, water and the chlorine hydride to a distillation still, and adding an organic solvent to remove water by azeotropy. The method has the advantages of high reaction speed, high utilization ratio of the chlorine hydride and high yield.
Owner:SHANDONG TIANCHENG CHEM CO LTD
Method for preparing dichlorohydrin and reaction device
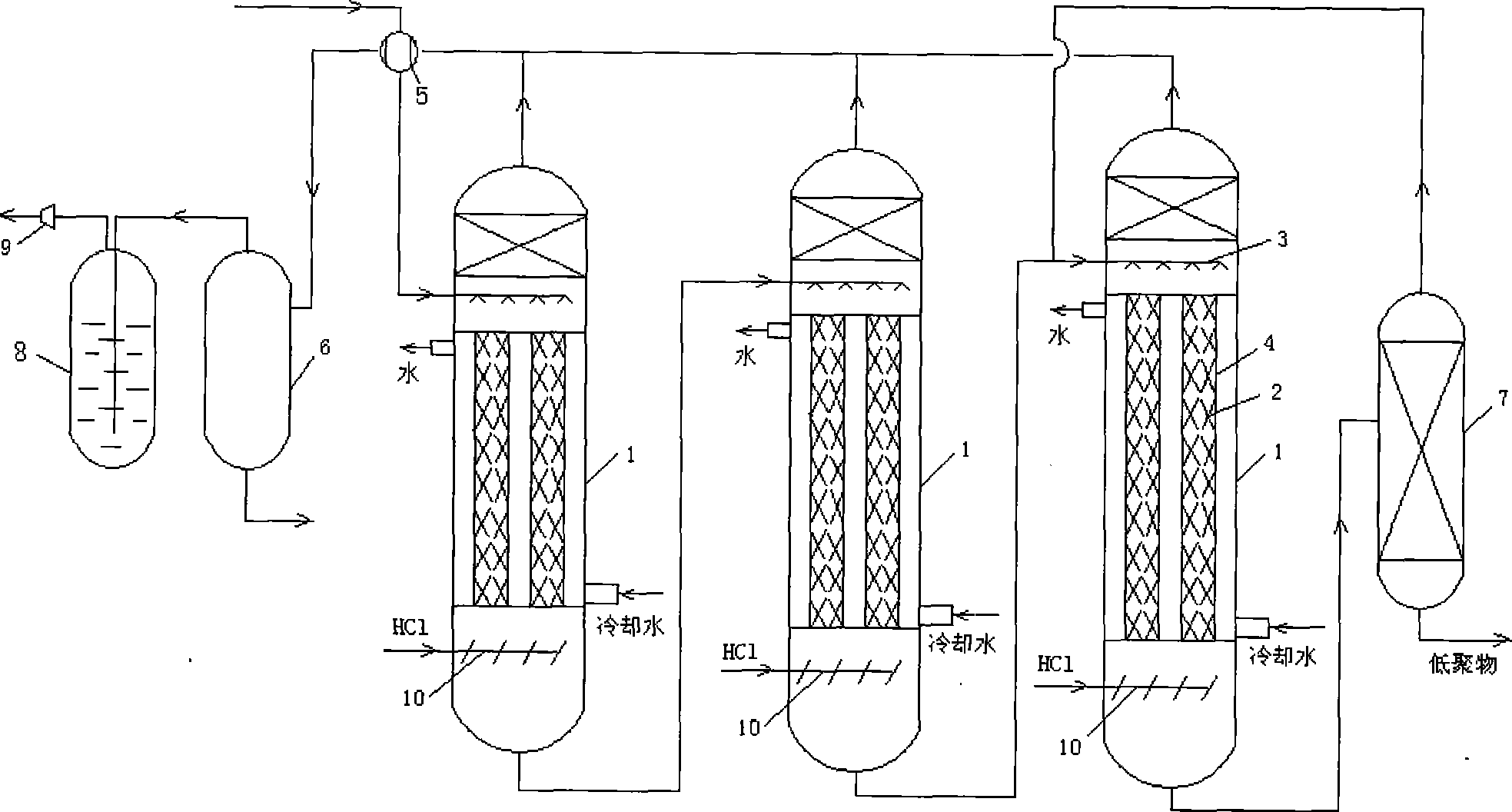
ActiveCN101774886ALow viscosityFully contactedPreparation by halogen introductionLiquid-gas reaction processesStrong acidsTower
The invention relates to improvement on a method for preparing dichlorohydrin by chlorinating glycerin and a reaction device. The chlorinating reaction is the series reaction of a reaction kettle and a sprinkling reaction tower which is filled with filler. The sprinkling reaction tower is sequentially provided with a placing filter zone, a hollow built-in sprinkling device zone and a placing filter zone from top to bottom. After viscosity decrease by kettle type preaction, the sprinkling reaction is carried out, the reaction contact is sufficient, the reaction time is long, the reaction efficiency is greatly enhanced, the conversion rate of the glycerin achieves 100%, and the reaction time is short and only needs 6-8 hours. The dichlorohydrin yield can be enhanced to more than 96%, and the yield can be enhanced to more than 99.0% by adding kreatine and strong acid mixing catalyst. The invention has the advantages of simple process flow, convenient reaction operation and high reaction efficiency.
Owner:江苏德纳化学股份有限公司 +1
Method for producing dichlorohydrin with glycerol
InactiveCN101429099AImprove responseReduce construction costsPreparation by halogen introductionReaction rateGlycerol
The invention relates to a method for preparing dichlorohydrin from glycerin. The method comprises the following steps: the glycerin is mixed with carboxylic acid catalyst, and mixture obtained and HCl are subjected to chlorination reaction in a chlorination reactor; reaction mixture obtained is sent into a reaction rectification tower (3), contacts the HCl and is subjected to chlorination reaction continuously; water, a hydrochloric acid and partial dichlorohydrin generated after reaction are rectified from the top of the reaction rectification tower (3), and products are reclaimed after condensation; and tower bottoms of the reaction rectification tower (3) enter into a purification and rectification tower (4) for purification and separation. The method is simple and convenient to operate, can improve the reaction rate and the HCl utilization rate, and can improve the dichlorohydrin yield to more than 80 percent.
Owner:WANHUA CHEM GRP CO LTD +2
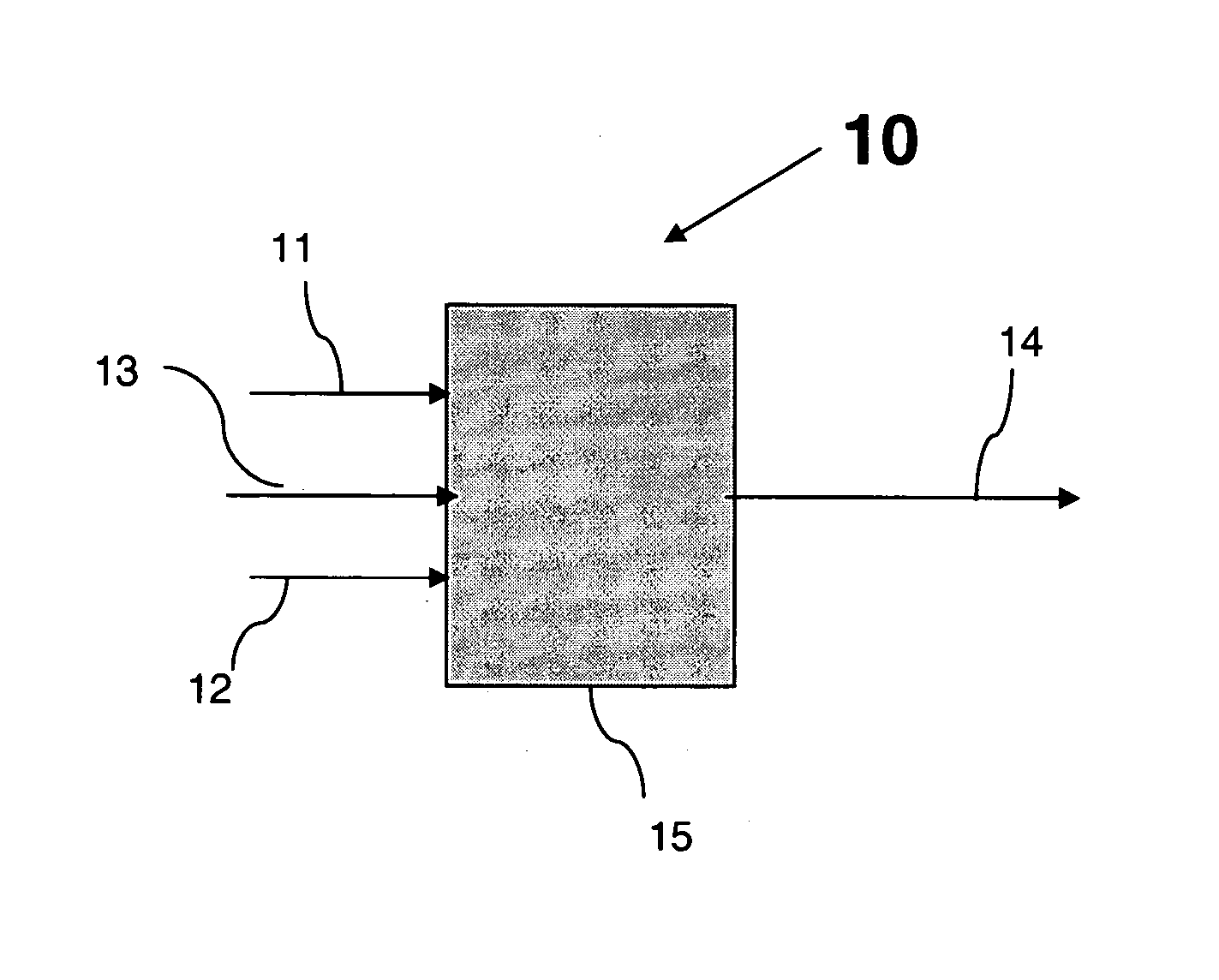
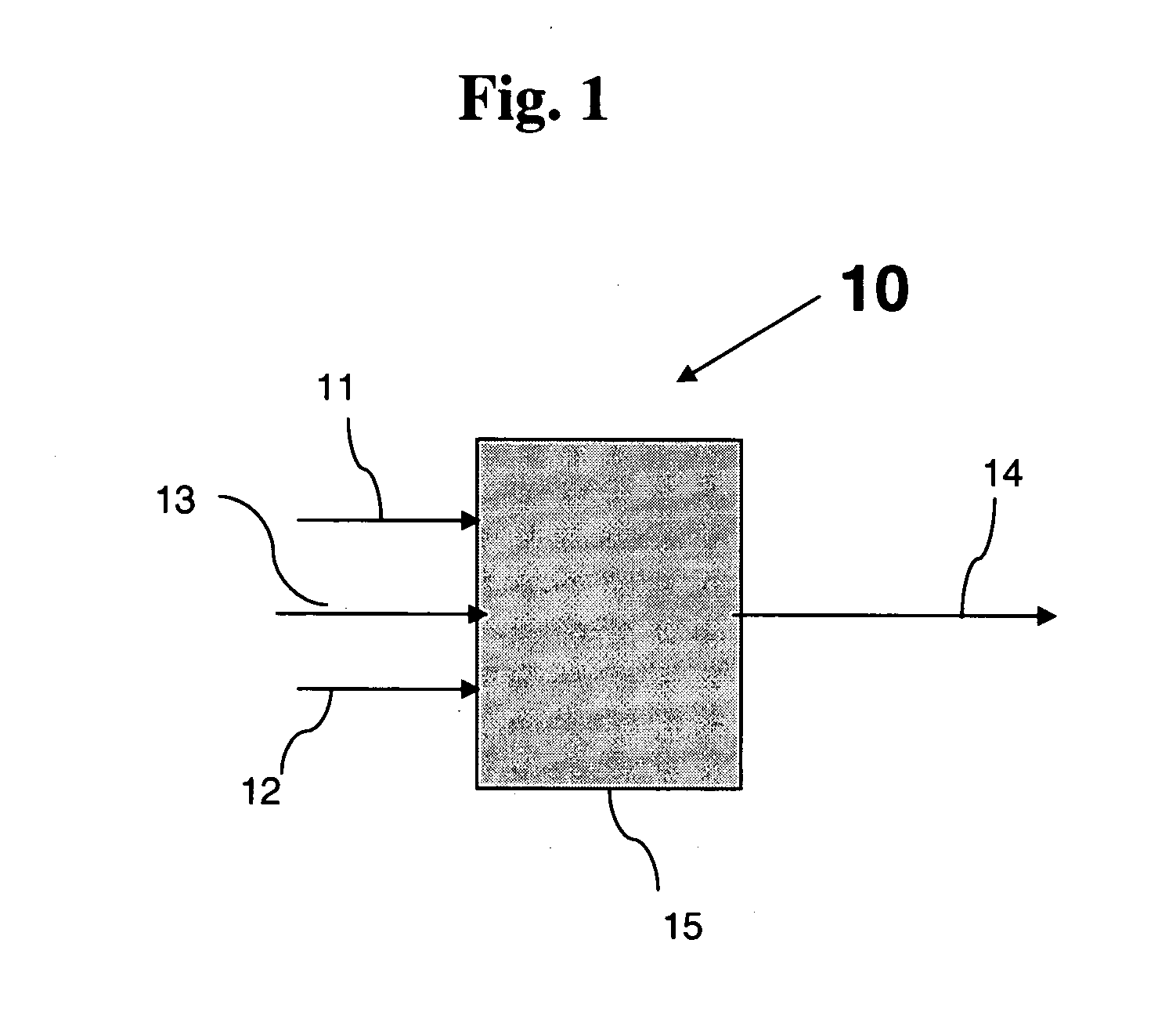
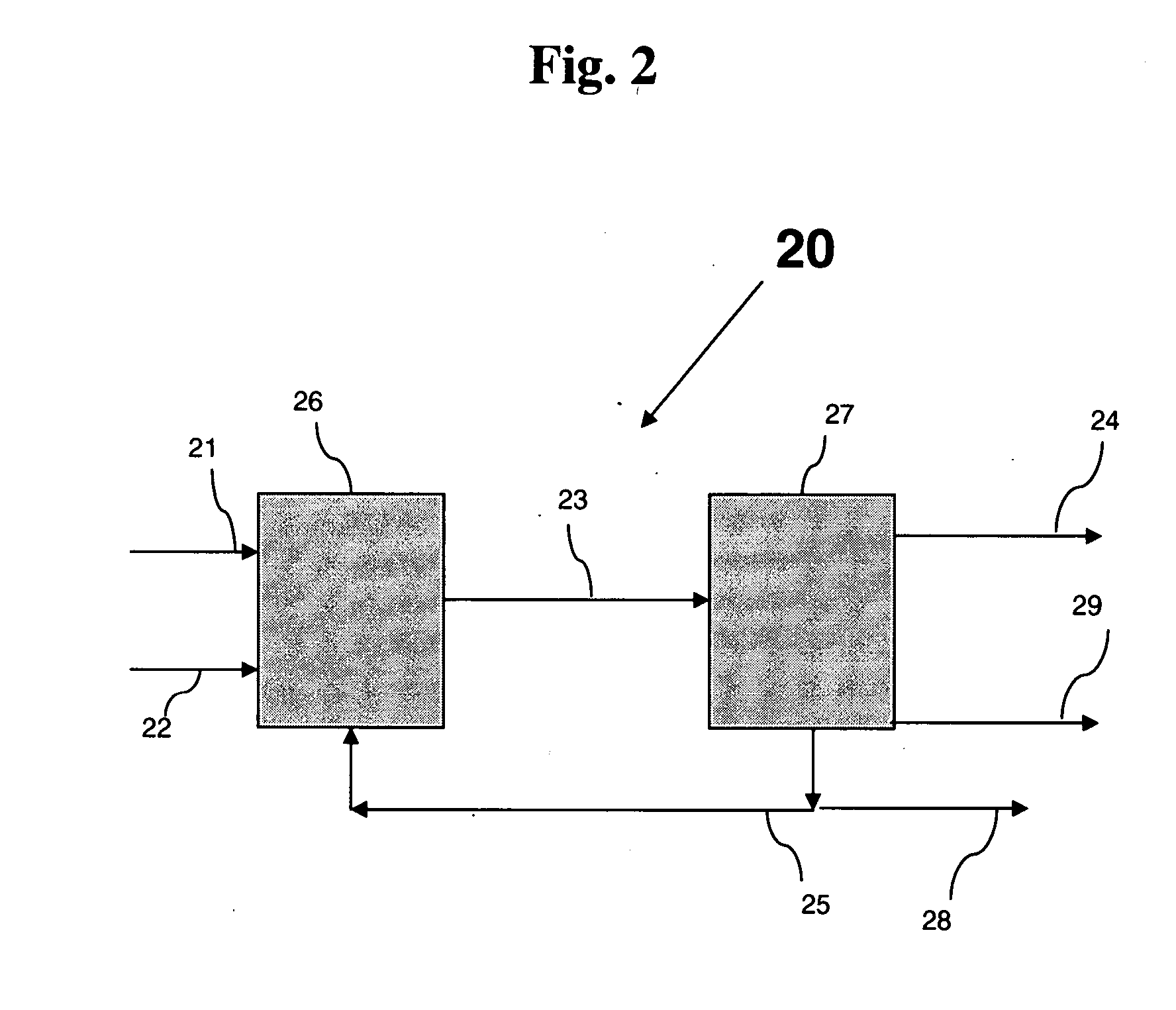
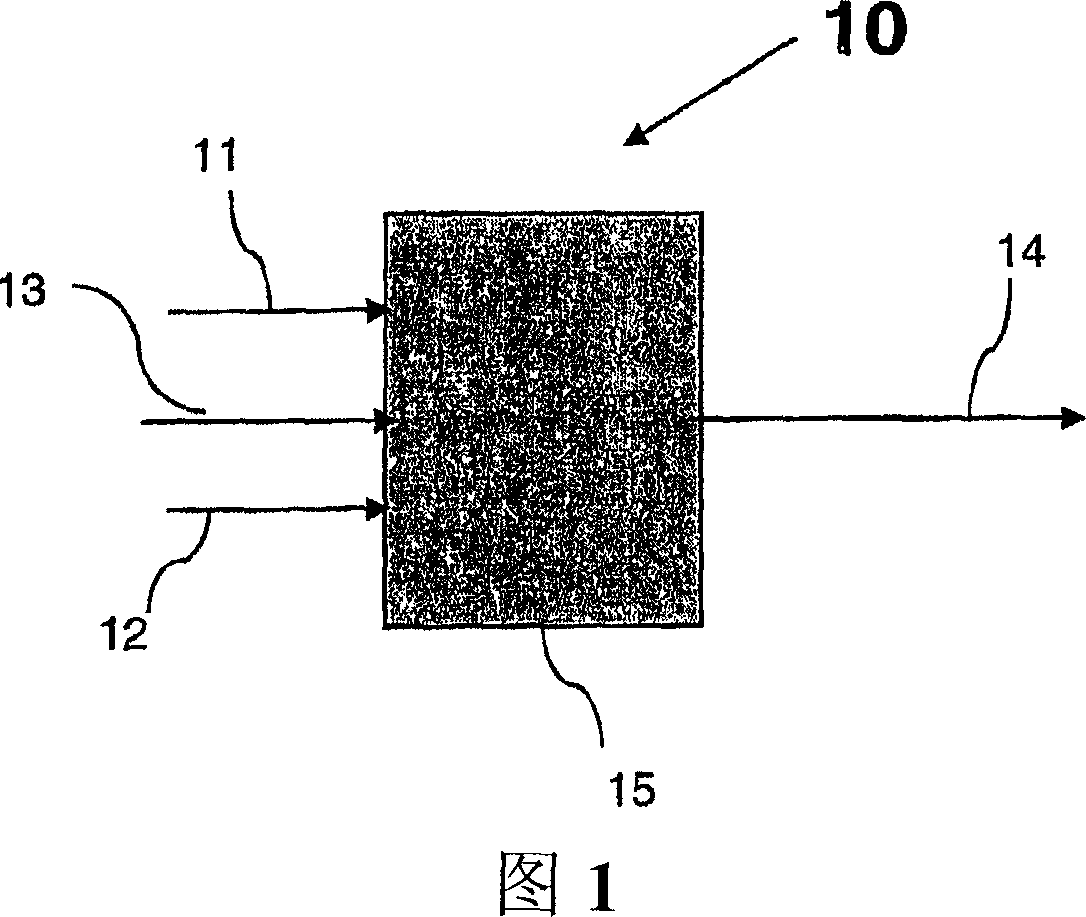
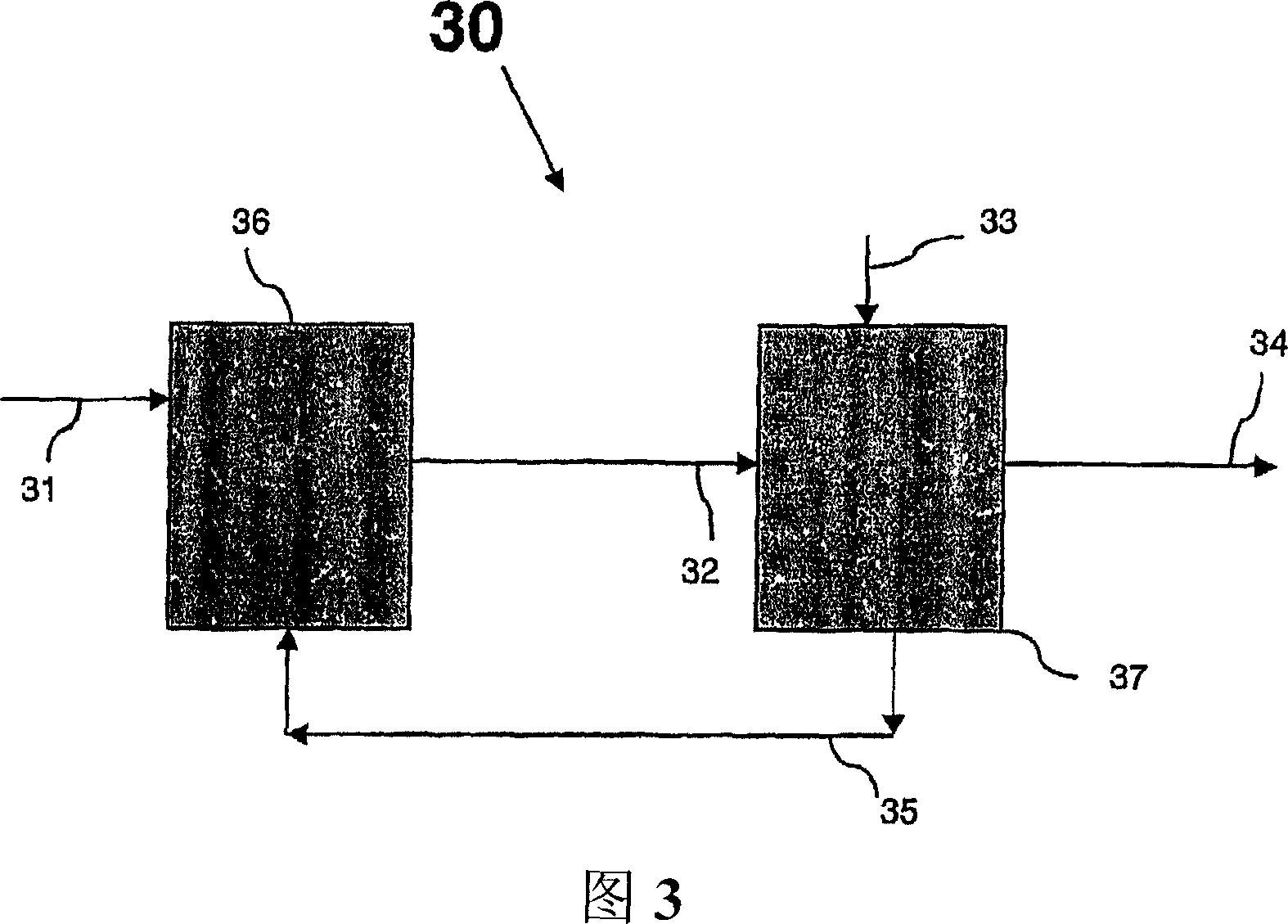
Process and apparatus for vapor phase purification during hydrochlorination of multi-hydroxylated aliphatic hydrocarbon compounds
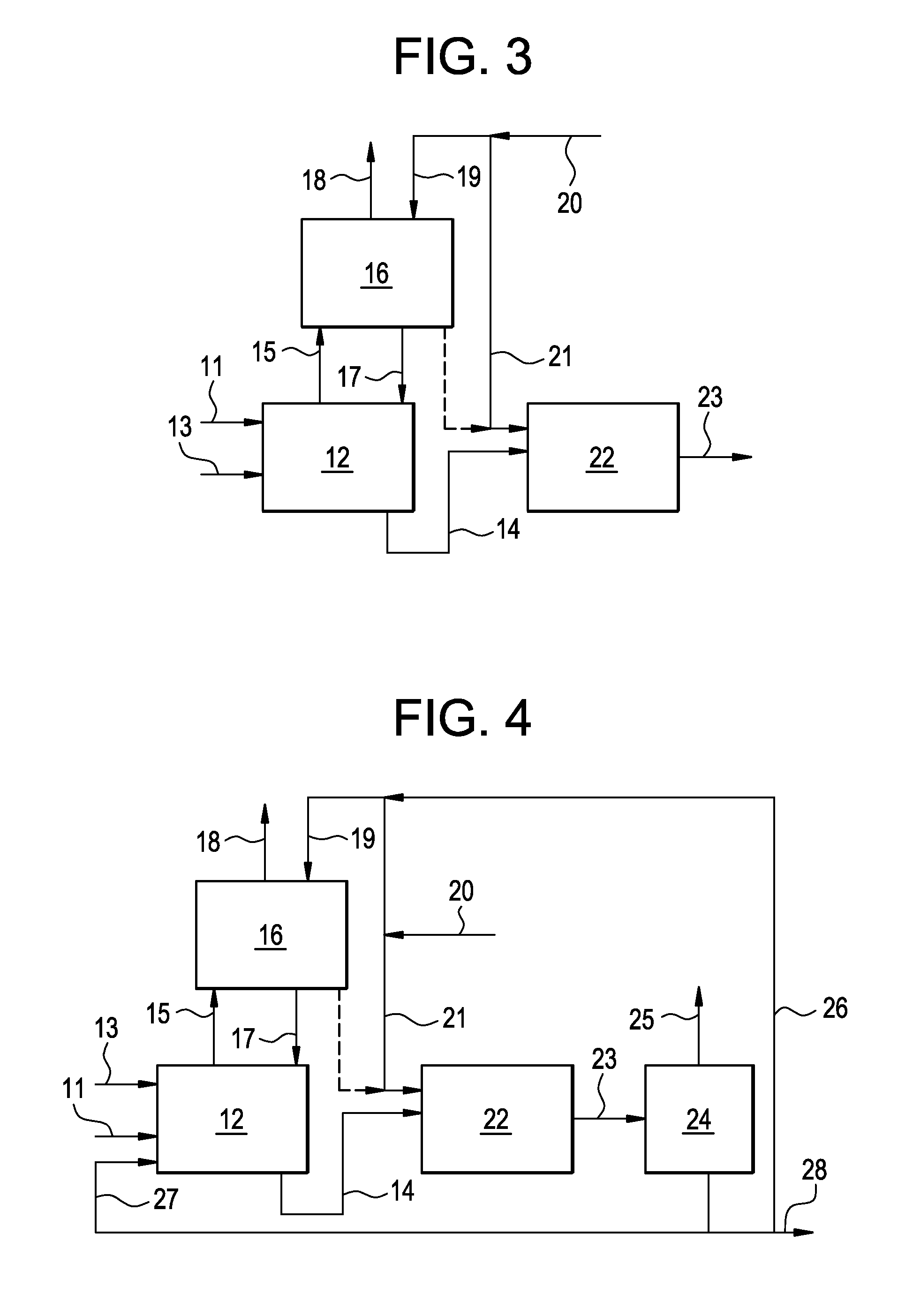
A process for converting multihydroxylated-aliphatic hydrocarbon compound(s) and / or ester(s) thereof to chlorohydrins and / or esters thereof is disclosed in which one or more of multihydroxylated-aliphatic hydrocarbon compound(s) and / or ester(s) thereof and / or monochlorohydrin(s) and / or ester(s) thereof with at least one chlorinating feed stream comprising at least one chlorinating agent and at least one impurity having a boiling point below the boiling point of the chlorohydrin product having the lowest boiling under hydrochlorination conditions, optionally in the presence of water, one or more catalyst(s), and / or one or more heavy byproduct(s) in a reaction vessel under hydrochlorination conditions, wherein the liquid-phase reaction mixture is maintained at a temperature below the boiling point of the chlorohydrin product having the lowest boiling point under hydrochlorination conditions and greater than the boiling point(s) of the at least one impurity and a vapor phase vent stream comprising the at least one impurity is removed from the liquid phase reaction mixture. An apparatus suitable for carrying out the disclosed process is illustrated in FIG. 1 of the drawings. The process and apparatus improve conversion rates and / or provide for recovery of chlorinating agent for lower operating costs.
Owner:BLUE CUBE IP
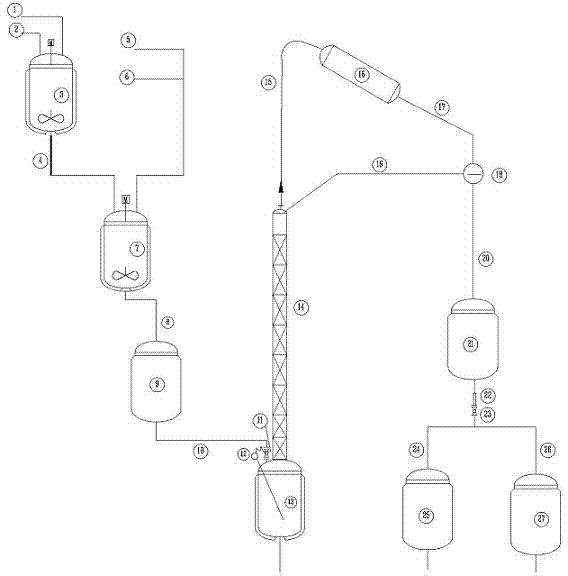
Method for continuous producing dichlorohydrin and apparatus thereof
The invention discloses a method for continuously producing dichlorohydrin. The method uses a hydrochloric acid aqueous solution as a chloridizing agent and acetic acid as an accelerant, continuously chloridizes glycerol by controlling the feed speed and the discharge speed to be same, and can obtain the dichlorohydrin with short residence time and high conversion rate. The invention also discloses a device for continuously producing the dichlorohydrin.
Owner:CHANG CHUN PLASTICS CO LTD
Method for synthesizing dichloropropanol by glycerine
ActiveCN101570471AReduce solubilityReduce corrosionPreparation by halogen introductionOrganic acidGlycerol
The invention provides a method for synthesizing dichloropropanol by glycerine, which comprises the following steps: the glycerine and organic acid are processed through esterification reaction under the catalysis of solid acid functioning as catalyst to generate organic acid glycerine ester, chlorine hydride is guided to the organic acid glycerine ester for chlorination reaction, and the dichloropropanol as the product of reaction is collected after the chlorination reaction finishes. The method for synthesizing dichloropropanol by glycerine is characterized in that firstly, during the esterification reaction of the glycerine and the organic acid under the catalysis of the solid acid, generated water is removed, thus the dissolution of the chlorine hydride during the esterification reaction is lessened, and the corrosion to equipment is reduced; secondly, high chlorination activity and rapid reaction speed are guaranteed through the esterification reaction, and particularly, the reaction time is markedly shortened under pressure; and thirdly, the dosage of the chlorine hydride is reduced and the environmental pollution is alleviated.
Owner:CHINA PETROLEUM & CHEM CORP +1
System for preparing dichloropropanol by autocatalytic reaction of glycerol and hydrogen chloride
ActiveCN102040479AReduce usageEliminate formationPreparation by halogen introductionBoiling pointGlycerol
The invention discloses a process for preparing dichloropropanol by an autocatalytic reaction of glycerol and hydrogen chloride. The process comprises the following steps: the glycerol and the hydrogen chloride gas perform the autocatalytic reaction at the temperature of between 105 and 160 DEG C and pressure of between 0.05 and 0.5MPa, and generated water is distilled and removed during the reaction. The invention also discloses a system for preparing the dichloropropanol by the autocatalytic reaction of the glycerol and the hydrogen chloride. The invention adopts the technology of the autocatalytic reaction of the glycerol and the hydrogen chloride without using a catalyst, so that the production cost is remarkably reduced, formation of high boiling side products is eliminated, and the reaction selectivity is improved. The invention has the advantages of simple process and low production cost. The system can ensure that the glycerol is completely converted and greatly improves the selectivity of the dichloropropanol, so that the purity of the finally prepared dichloropropanol is more than 99.5 percent.
Owner:NANTONG TIANSHI CHEM
Method for preparing dichlorohydrin by glycerol hydrochlorination
ActiveCN101323555ALess investmentEasy to operate and controlPreparation by halogen introductionComing outGas phase
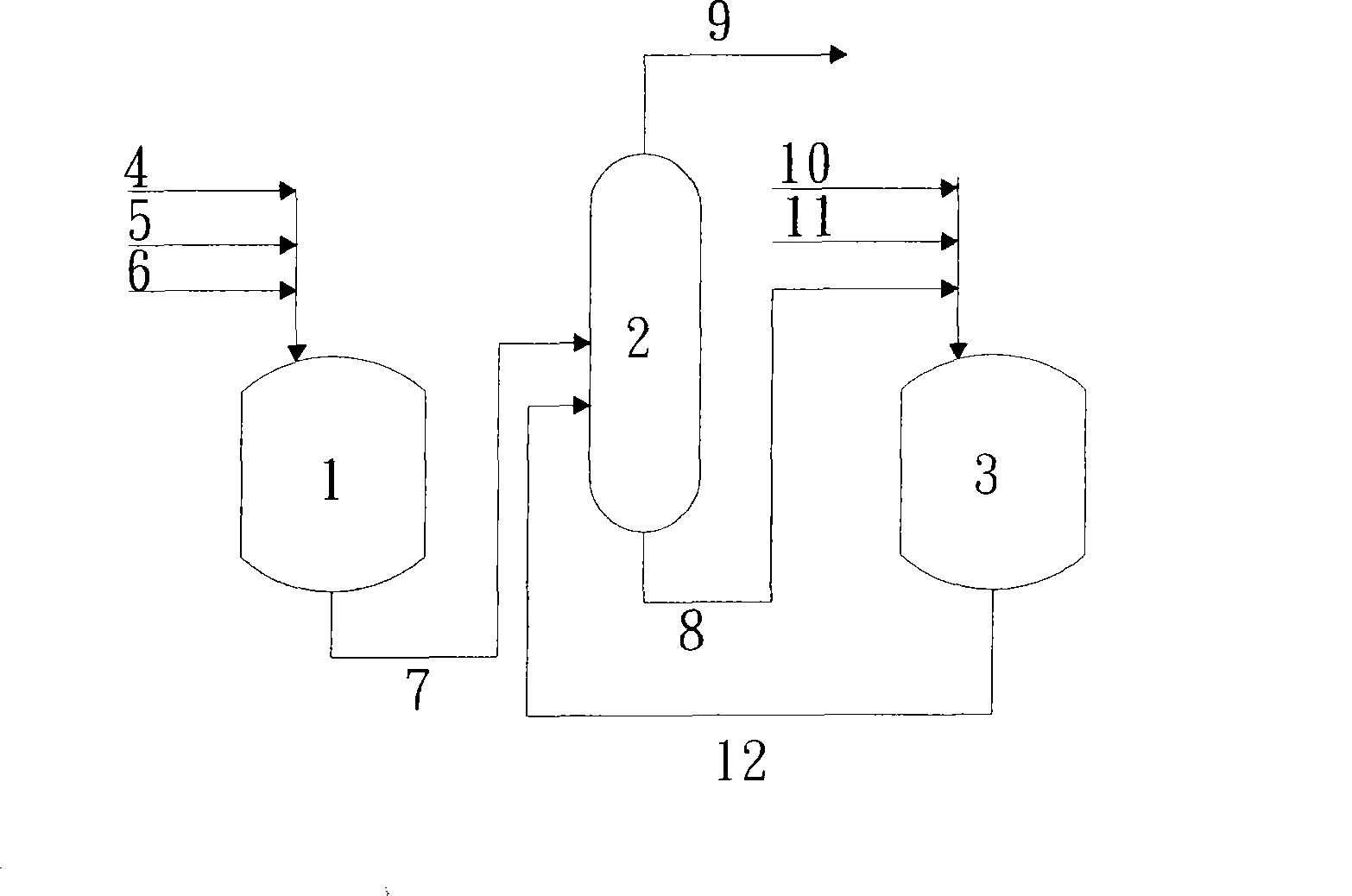
The invention provides a method for preparing dichloropropanol from glycerol through hydrochlorination, which comprises the steps as follows: (1) the mixing raw material liquid of activator and the glycerol is poured into an absorption reaction tower from the top thereof, hydrogen chloride is put into the absorption reaction tower from the bottom thereof, the materials at the bottom of the absorption reaction tower are partially sent back to the absorption reaction tower and parts are put into the first stirring vessel of n stirring vessels; (2) the hydrogen chloride is let into the first stirring vessel for carrying out reaction and then reaction solution enters into the second stirring vessel of the n stirring vessels to react with the hydrogen chloride, and the reaction solution reacts with the hydrogen chloride in the n stirring vessels in turn according to the process; (3) the condensate of the gaseous phase mixtures braised out from the tops of the stirring vessels are gaseous phase products and the mother liquor comes out from the nth stirring vessel is aqueous phase product. The method of the invention is low in investment on equipment, simple in operation and control and high in reaction speed, thus being easy for expanding production with the yield of dichloropropanol per pass higher than 90 percent and the use ratio of the hydrogen chloride higher than 70 percent.
Owner:SHANGHAI CHLOR ALKALI CHEM
Epoxychloropropane and production method of midbody dichlorohydrin
InactiveCN101195607AReduce consumptionReduce the amount requiredPreparation by halogen introductionSimple Organic CompoundsBiodiesel
The invention provides a method for using by-product glycerin of recyclable material bio-diesel as material to produce organic compounds as dichlorohydrin and epichlorohydrin. In the presence of catalyst carboxyl acid or relative derivative, glycerin is reacted with chlorizating agent as hydrochloride or alcaine to produce dichlorohydrin, and the dichlorohydrin in the presence of alkali is dewatered to produce epichlorohydrin.
Owner:BALING PETRO CHEM CO LTD SINOPEC
Method of preparing dichloropropanols from glycerine
ActiveUS7473809B2Increase productionOrganic compound preparationPreparation by halogen introduction1-PropanolGlycerol
Owner:SPOLEK PRO CHEMICKOU A HUTNI VYROBU
Method for synthesizing 1,3-diphenyl-1-propanol compound
ActiveCN102146020AWidely distributedHigh yieldOrganic compound preparationPreparation by halogen introduction1-PropanolSolvent
The invention relates to a method for synthesizing a 1,3-diphenyl-1-propanol compound, belonging to a method for synthesizing a compound. P-substituted 1-phenyl ethanol and p-substituted benzyl alcohol are used as raw materials, wherein R and R' are hydrogen atoms, halogen atoms Cl, Br, alkyl and alkoxy, and the adding ratio of the substituted 1-phenyl ethanol to the substituted benzyl alcohol is1: (1-2). The method comprises the following steps of: sequentially adding a dry catalyst, alkali, substituted1-phenyl ethanol and substituted benzyl alcohol into an anhydrous solvent under the condition of nitrogen, placing a reactor into an oil bath of 125 to 135 DEG C, and stirring for 8 to 24 hours; cooling, neutralizing the reaction solution, performing extraction by using ethyl acetate, andconcentrating the extract in vacuum till no ethyl acetate smell; and performing chromatographic purification by using a positive silica gel column of 100 to 200 meshes. The invention has the advantages of simple, convenient and feasible method, low production cost, reduced difficulty of purification and post treatment and weakened influence on the environment.
Owner:JILIN UNIV
Preparation method of dichloro propanol from glycerin
ActiveCN100509726CIncrease profitIncrease reaction rateOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by halogen introductionReaction rateDistillation
The invention relates to a method for preparing dichloropropanol from glycerol. Firstly, glycerin, HCl and carboxylic acid catalyst are added into a mixer, continuously pressed into a tubular reactor for chlorination reaction, and the glycerol is rapidly transformed through the reaction in the tubular reactor. The reactant then enters the HCl bubbling tank to continue the reaction. Here, the water generated by the reaction forms an azeotrope with dichloropropanol, HCl and some catalysts and evaporates from the upper part of the bubbling kettle. The product dichloropropanol is obtained at the top of the distillation tower, and the bottom liquid of the rectification tower is sent to the circulation reaction. The reactor combination method proposed by the present invention is designed according to the characteristics of the different stages of the reaction, so that the reaction rate is increased, the utilization rate of the equipment is improved, and the glycerol is completely converted within 12 to 16 hours, and the yield of dichloropropanol can be greater than 90%. , HCl utilization increased to over 70%.
Owner:溧阳常大技术转移中心有限公司
Copper fluoride (I) reagent as well as preparation method and application thereof
InactiveCN103254219AHigh reactivitySimple and fast operationCarboxylic acid nitrile preparationOrganic compound preparationHydrogen fluorideCopper fluoride
The invention discloses a copper fluoride (I) reagent as well as a preparation method and an application thereof. The copper fluoride (I) reagent is prepared through the following steps: stirring cuprous chloride and sodium tert-butoxide in a tetrahydrofuran solvent at room temperature to react, and adding a nitrogen ligand and triethylamine trihydrofluoride so as to obtain the copper fluoride (I) reagent which is stable in air. The triethylamine trihydrofluoride serves as a fluorine element source of the reagent, and the reagent can efficiently catalyze haloalkane to form hydrofluoroalkane. The high-purity and high-yield copper fluoride (I) reagent can be directly obtained through utilizing the industrial low-cost cuprous chloride, and has good reactivity with the hydrofluoroalkane containing a plurality of important functional groups; and a metal fluorinated reagent can be prepared without using toxic compounds and precious metals, the cost and operation requirements can be greatly lowered, and the copper fluoride (I) reagent has a good industrial application prospect.
Owner:FUZHOU UNIV
Method for synthesizing 1-iodo-alkyne in high-selectivity manner
ActiveCN106831283AAvoid Metal ResidueAvoid toxicitySilicon organic compoundsOrganic compound preparationEnvironmental resistanceAlkyne
The invention belongs to the technical field of synthetic chemistry and particularly relates to a method for synthesizing 1-iodo-alkyne in a high-selectivity manner. The method is mild in reaction condition, controllable in reaction product, single in product, easy in product purification, high in chemical selectivity, simple in synthesizing step, safe, reliable, green, environmentally friendly, applicable to various terminal alkyne reaction substrates and suitable for industrial production, and the synthesizing yield of the method reaches up to 99%.
Owner:GUANGDONG UNIV OF TECH
Method for preparing dichloropropanol with chemical reaction-pervaporation coupling method
InactiveCN102887816AReduce wasteReduce waste greatlyPreparation by halogen introductionHydroxy compound separation/purificationHigh concentrationChemical reaction
The invention discloses a method for preparing dichloropropanol with a chemical reaction-pervaporation coupling method, wherein the method provided by the invention comprises the following steps of: directly adding glycerol and a liquid catalyst into a chemical reactor (if a solid catalyst is used, putting the solid catalyst into the reactor in advance) after pre-heating, simutaneously feeding a hydrogen chloride gas into the reactor, chlorinating the glycerol with the hydrogen chloride in the reactor, instantly introducing the reaction product into a pervaporation device, wherein a permeable film in the device preferably permeates and removes water generated in the reaction to accelerate chlorination reaction efficiently, thereby finally obtaining the dichloropropanol with high concentration. The method provided by the invention couples the pervaporation device with the chemical reactor to instantly remove the water generated in the reaction, thereby improving the efficiency of the chlorination reaction, reducing the hot load in the subsequent dehydration process of a dichloropropanol rectifying column, and improving the utilization efficiency of hydrogen chloride.
Owner:NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com