Production process for refining dichlorohydrin by glycerol reaction distillation
A technology of dichloropropanol and reactive distillation, applied in the field of fine chemical industry, can solve the problems of low conversion rate, difficult scale, large consumption of raw materials and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
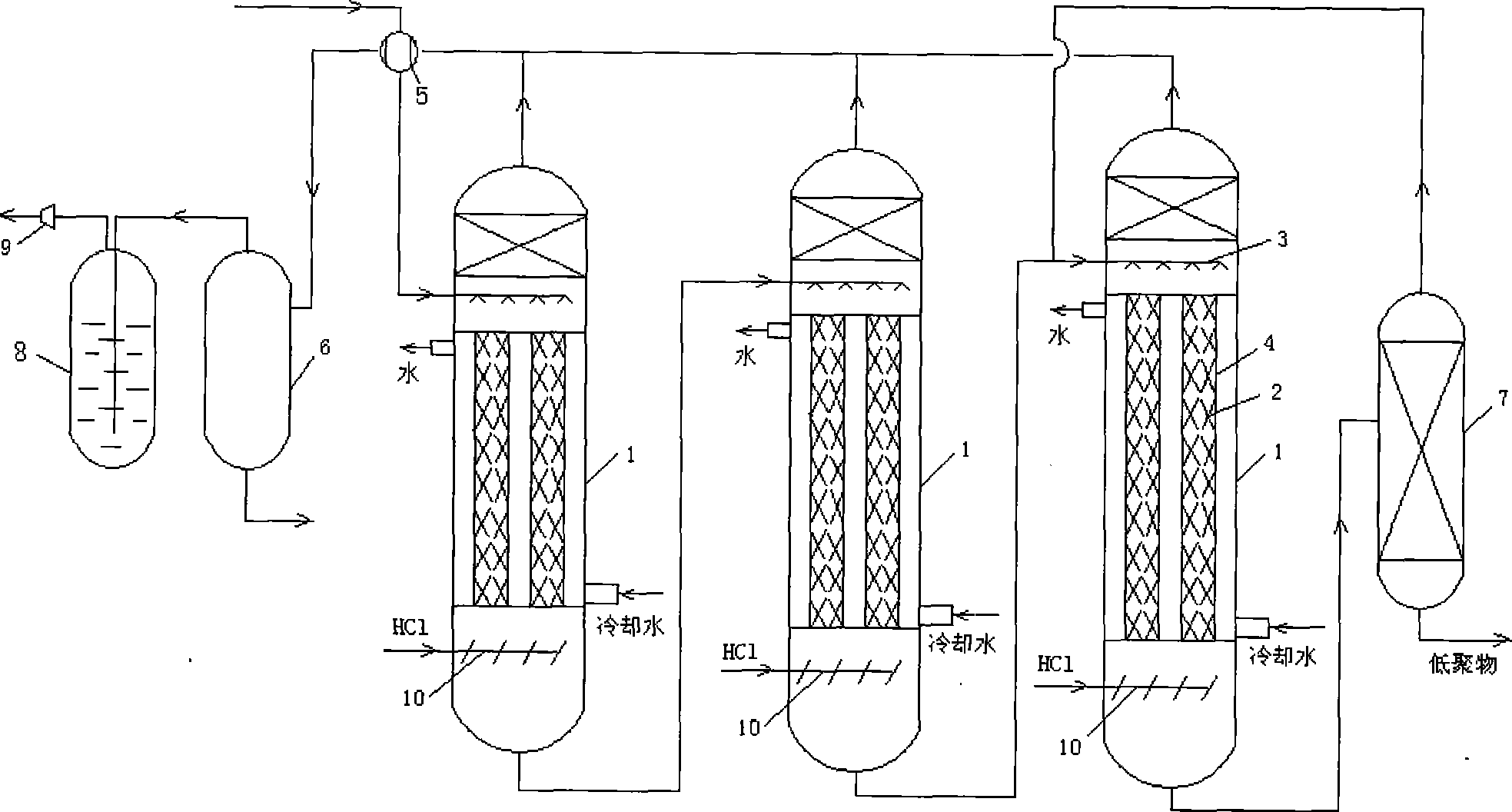
[0015] Example 1: After the heat exchange, the raw material glycerin containing the homogeneous catalyst enters from the top of the tubular reaction rectification tower 1, and hydrogen chloride gas enters from the lower part of the packing 2 section of the tubular reaction rectification tower 1 through the gas distributor 10. The raw material glycerin and hydrogen chloride gas coming down from the liquid distributor 3 countercurrently pass through the 2 layers of packing and undergo absorption chlorination reaction rectification at 90~120℃ and 0.001~0.1MPa (absolute pressure); the heat of reaction passes through the cooling water between the tubes 4 Remove and maintain the reaction temperature; the monochloropropanediol, glycerol and glycerol oligomers at the bottom of the tubular reaction rectification tower 1 are sent to the heavy component separation tower 7 to recover the monochloropropanediol and glycerol under reduced pressure and return to the raw glycerin; The product gas ...
Embodiment 2
[0016] Example 2: After heat exchange, the raw material glycerin containing homogeneous catalyst enters from the top of the first-stage tubular reaction rectification tower 1, and hydrogen chloride gas passes through the gas distributor 10 from the lower part of the packing 2 of the tubular reaction rectification tower 1 Enter, the raw material glycerin and hydrogen chloride gas coming down from the liquid distributor 3 countercurrently pass through the 2 layers of packing, and undergo absorption chlorination reaction rectification at 90~120℃ and 0.001~0.1MPa (absolute pressure); the heat of reaction passes through the tube 4 The cooling water is removed and the reaction temperature is maintained; the liquid at the bottom of the first-stage tubular reactive distillation tower 1 is sent to the top of the second-stage tubular reactive distillation tower 1 as feed; The liquid is sent to the top of the three-stage tubular reactive distillation tower 1 as feed; the monochloropropanedio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com