Production method of dichloropropanol
A technology of dichloropropanol and its production method, which is applied in the chemical industry and can solve the problems of increased investment in equipment, increased energy consumption, and ignorance of effective effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
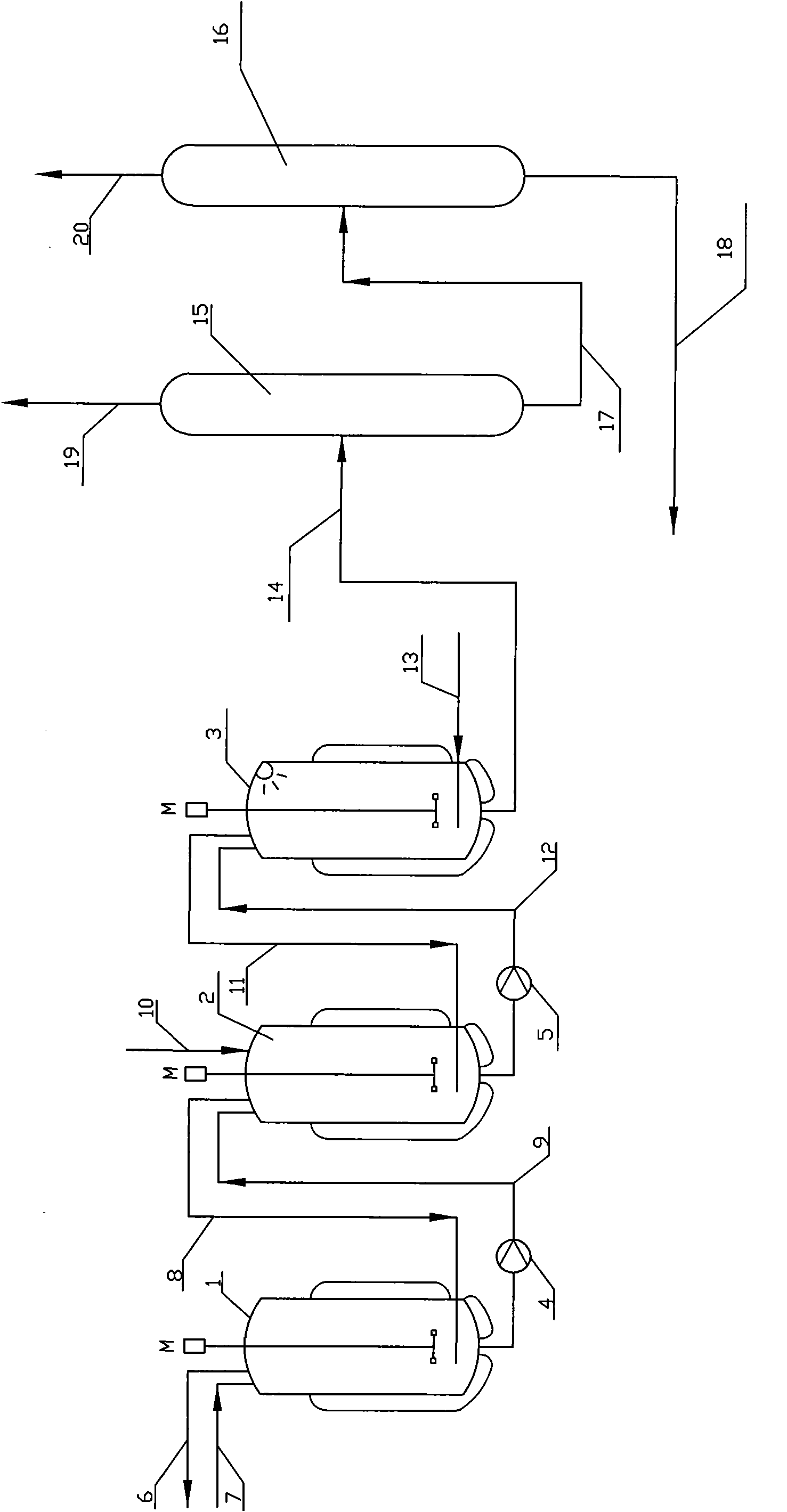
[0105] A tank reactor made of glass-lined carbon steel, with a volume of 11 liters and an inner diameter of 200 mm. The reactor is equipped with a steam heating or water cooling jacket, a magnetically driven stirrer, and the impeller is a six-bladed disc turbine. The blade end distance is 55mm, the motor input power is 22W, the reactor is equipped with a pressure gauge interface and a thermocouple temperature gauge interface, a microwave generator, an inlet for adding raw and auxiliary materials, an exhaust outlet on the top, a liquid outlet on the bottom, and a gaseous HCl nozzle . The raw material gaseous HCl is supplied by gasification of liquefied HCl with a purity of 99.8% in a high-pressure steel gas cylinder, and the supply of HCl is weighed by an electronic scale and measured by a cylinder reduction method.
[0106] 5000 g (54.29 moL) of glycerin and 325 g (2.22 moL) of adipic acid were added to Reactor 1. Under slow stirring and mixing, use industrial steam to pass t...
Embodiment 2
[0133] A low-boiler distillation column (15) with a heating kettle is connected in series with a dichloropropanol product rectification column (16), and the top of the rectification column is equipped with a condenser and a phase separator. , the aqueous phase is refluxed, and the organic phase is the product of dichloropropanol.
[0134] The reaction solution weighing after the reaction of embodiment 1 finishes, gets 9961.5g, and its main component quantity is shown in table 5
[0135] table 5
[0136] components
Weight (g)
The reaction solution
9961.5
3-Chloro-1.2-propanediol
2-Chloro-1.3-propanediol
1.3-Dichloro-2-propanol
2.3-Dichloro-1-propanol
water
hydrogen chloride
other
150.5
164.2
6346.6
157.3
211.3
171.5
1882.7
806.9
70.5
[0137] The reaction solution was continuously sent to the low boilers for distillation, distilled under normal pressu...
Embodiment 3
[0146] Take 2215.7 g of the still feed liquid of the product column (16) obtained in the embodiment of the present invention, and its composition is shown in Table 9.
[0147] Table 9
[0148] composition
[0149] 2215.7 g of the still feed liquid is added to the reactor described in Example 1 of the present invention, and 4000 g (43.44 mol) of glycerin and 24 g (0.164 mol) of adipic acid are added, and the pressure and temperature as described in Example 1 are carried out in three stages Reaction, the first section of reaction took 76 minutes, the second section of reaction took 70 minutes, and the third section of reaction took 64 minutes, the total reaction time was 210 minutes, and a total of 3773.5g (103.5mol) of hydrogen chloride was introduced.
[0150] At the end of the reaction, 10040.9 g of the reaction solution was obtained, and its composition is shown in Table 10.
[0151] Table 10
[0152] composition
[0153] The reaction solution was conti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| critical temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com