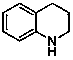
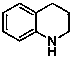
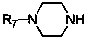Method for preparing amino-acid ester
An amino acid ester and malonate technology, applied in the field of preparation of amino acid derivatives, can solve the problems of difficult industrialization, waste of halides, and high production costs, and achieve the effects of short reaction time, simple post-processing, and avoidance of waste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] The reaction vials were loaded with TBAI (0.4 mmol, 148 mg), compound 1a (2 mmol, 214 mg), compound 3a (4 mmol, 472 mg), NaOAc (4 mmol, 164 mg), TBHP (0.6 mL), water (4.0 mL), acetonitrile (4.0 mL). Then the system was heated at 90°C in air for about 8 hours, quenched with saturated sodium sulfite solution, extracted with ethyl acetate (40 mL × 3), and the product was obtained by simple column chromatography 2a , the yield is 85%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0029] 1 H NMR (300 MHz, CDCl 3 ): δ 7.22 (t, J = 7.5 Hz, 2H), 6.73 (t, J = 7.5 Hz, 1H), 6.67 (d, J = 9.0 Hz, 2H), 4.04 (s, 2H), 3.68 (s, 3H), 3.03 (s, 3H); 13 C NMR (75 MHz, CDCl 3 ): δ 171.0, 147.8, 131.8, 113.9, 109.4, 54.2, 52.0, 39.6; MS: Anal. Calcd. For C 10 h 14 NO 2 : 180, Found: 180 (M+1 + ); IR (KBr, cm -1 ): υ 1749.
Embodiment 2
[0031]
[0032] The reaction bottle is filled with I 2 (0.4 mmol, 102 mg), compound 1a (2 mmol, 214 mg), compound 3a(4 mmol, 472 mg), NaOAc (4 mmol, 164 mg), TBHP (0.6 mL), water (4.0 mL), acetonitrile (4.0 mL). Then the system was heated at 90°C in air for about 8 hours, quenched with saturated sodium sulfite solution, extracted with ethyl acetate (40 mL × 3), and the product was obtained by simple column chromatography 2a , the yield is 80%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0033] 1 H NMR (300 MHz, CDCl 3 ): δ 7.22 (t, J = 7.5 Hz, 2H), 6.73 (t, J = 7.5 Hz, 1H), 6.67 (d, J = 9.0 Hz, 2H), 4.04 (s, 2H), 3.68 (s, 3H), 3.03 (s, 3H); 13 C NMR (75 MHz, CDCl 3 ): δ 171.0, 147.8, 131.8, 113.9, 109.4, 54.2, 52.0, 39.6; MS: Anal. Calcd. For C 10 h 14 NO 2 : 180, Found: 180 (M+1 + ); IR (KBr, cm -1 ): υ 1749.
Embodiment 3
[0035]
[0036] NIS (0.4 mmol, 90 mg), compound 1a (2 mmol, 214 mg), compound 3a (4 mmol, 472 mg), NaOAc (4 mmol, 164 mg), TBHP (0.6 mL), water (4.0 mL), acetonitrile (4.0 mL). Then the system was heated at 90°C in air for about 8 hours, quenched with saturated sodium sulfite solution, extracted with ethyl acetate (40 mL × 3), and the product was obtained by simple column chromatography 2a , the yield is 80%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0037] 1 H NMR (300 MHz, CDCl 3 ): δ 7.22 (t, J = 7.5 Hz, 2H), 6.73 (t, J = 7.5 Hz, 1H), 6.67 (d, J = 9.0 Hz, 2H), 4.04 (s, 2H), 3.68 (s, 3H), 3.03 (s, 3H); 13 C NMR (75 MHz, CDCl 3 ): δ 171.0, 147.8, 131.8, 113.9, 109.4, 54.2, 52.0, 39.6; MS: Anal. Calcd. For C 10 h 14 NO 2 : 180, Found: 180 (M+1 + ); IR (KBr, cm -1 ): υ 1749.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com