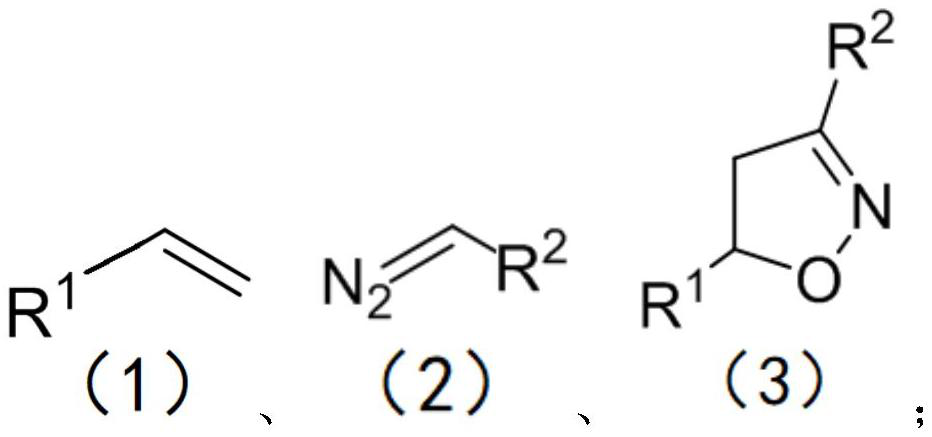
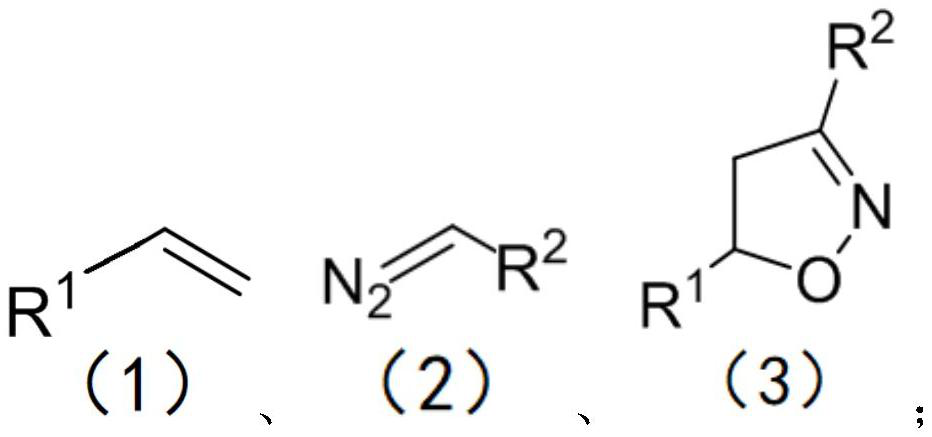
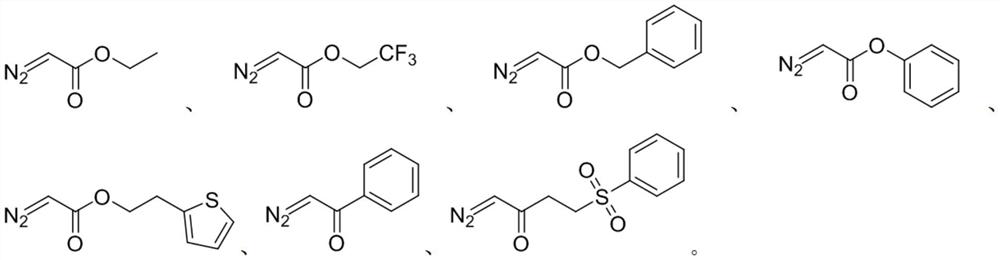
Method for preparing isoxazoline
An isoxazoline and a selected technology, applied in the field of preparing isoxazoline, can solve problems such as limited application, and achieve the effects of good reaction, cheap and easily available raw materials, and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] Add acetonitrile (40mL), compound 1a (10mmol, 1.78g), compound 2a (20mmol, 2.40g), compound 3 (20mmol, 2.17g), catalyst boron trifluoride diethyl ether (1mmol, 263μL) into the reaction flask in sequence; Then react under the condition of 25 ℃ in the air for 12 hours; The mixed solvent of petroleum ether was subjected to column chromatography to obtain the product 4a with a yield of 80%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0042] 1 H NMR (400MHz, CDCl 3 )δ7.40(d, J=8.4Hz, 2H), 7.26(d, J=8.4Hz, 2H), 5.76(dd, J=11.6, 9.0Hz, 1H), 4.36(q, J=7.1Hz, 2H), 3.60(dd, J=17.8, 11.6Hz, 1H), 3.23(dd, J=17.8, 9.0Hz, 1H), 1.37(t, J=7.1Hz, 3H), 1.31(s, 9H). ; 13 C NMR (101MHz, CDCl 3 )δ160.5, 151.7, 151.1, 136.3, 125.69, 125.66, 84.9, 62.0, 41.1, 34.5, 31.2, 14.0; HRMS (ESI-TOF): Anal. Calcd. For C 16 h 21 NO 3 +H + :276.1594,Fou...
Embodiment 2
[0044]
[0045]Add acetonitrile (2.0mL), compound 1b (0.5mmol, 53.2mg), compound 2a (1.0mmol, 108μL), compound 3 (1.0mmol, 121μL), catalyst boron trifluoride diethyl ether (0.05mmol , 14 μL); then reacted for 12 hours at 25°C in the air; after the reaction was completed, it was quenched with saturated sodium chloride solution, extracted with ethyl acetate, the solvent was removed by a rotary evaporator, adsorbed on silica gel, and finally used The mixed solvent of ethyl acetate and petroleum ether was subjected to column chromatography to obtain the product 4b with a yield of 74%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0046] 1 H NMR (400MHz, CDCl 3 )δ7.47-7.21(m,5H),5.77(dd,J=11.6,8.9Hz,1H),4.35(q,J=7.1Hz,2H),3.63(dd,J=17.8,11.6Hz,1H ),3.21(dd,J=17.8,8.9Hz,1H),1.37(t,J=7.1Hz,3H).; 13 C NMR (101MHz, CDCl 3 )δ160.4, 151.0, 139.4, 128.7,...
Embodiment 3
[0048]
[0049] Add acetonitrile (2.0mL), compound 1c (0.5mmol, 61.1mg), compound 2a (1.0mmol, 108μL), compound 3 (1.0mmol, 121μL), catalyst boron trifluoride diethyl ether (0.05mmol , 14 μL); then reacted for 12 hours at 25°C in the air; after the reaction was completed, it was quenched with saturated sodium chloride solution, extracted with ethyl acetate, the solvent was removed by a rotary evaporator, adsorbed on silica gel, and finally used The mixed solvent of ethyl acetate and petroleum ether was subjected to column chromatography to obtain the product 4c with a yield of 67%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0050] 1 H NMR (400MHz, CDCl 3 )δ7.35-7.28(m,2H),7.14-7.00(m,2H),5.77(dd,J=11.6,8.9Hz,1H),4.36(q,J=7.1Hz,2H),3.64(dd ,J=17.8,11.6Hz,1H),3.18(dd,J=17.8,8.9Hz,1H),1.38(t,J=7.1Hz,3H); 13 C NMR (101MHz, CDCl 3 )δ162.7(d, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com