Benzotriazole compounds and a preparing method thereof
A technology for benzotriazole and compounds, which is applied in the field of organic compound synthesis, can solve the problems of complex operation of benzotriazole compounds and insufficient mild reaction conditions, and achieve the effects of simple post-processing, convenient operation and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
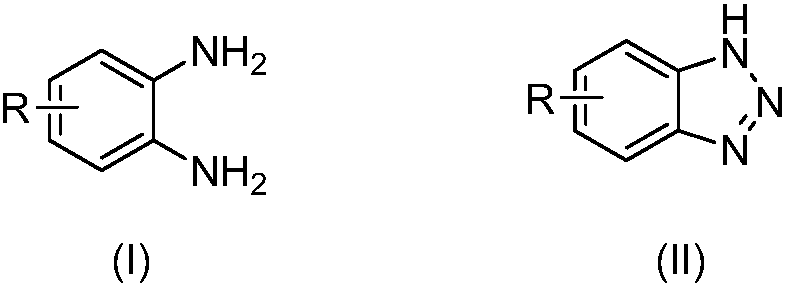
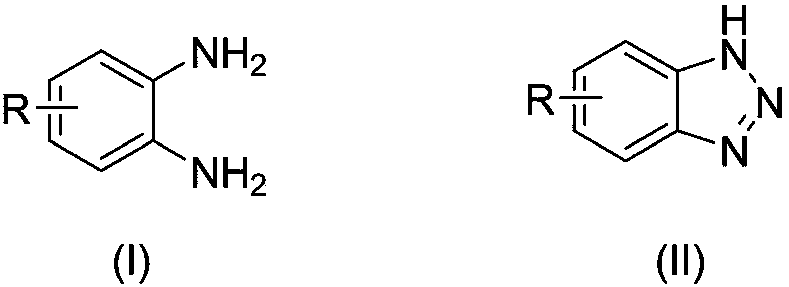
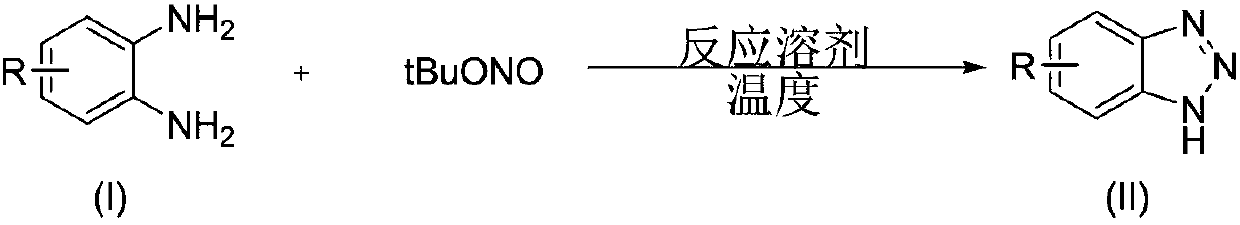
[0037] A kind of preparation method of benzotriazole compound, take o-phenylenediamine or o-phenylenediamine compound and tert-butyl nitrite as raw materials with the structure shown in formula (I), through intramolecular The diazotization reaction obtains the benzotriazole compound shown in formula (II);
[0038] Above-mentioned reaction process, available following reaction equation expression:
[0039]
[0040] (1) The molar ratio of o-phenylenediamine or o-phenylenediamine compound and t-butyl nitrite of structure shown in the formula is 1:(1~5), preferably 1:(1~3).
[0041] (1) O-phenylenediamine or o-phenylenediamine compounds
[0042] O-phenylenediamine or o-phenylenediamine compound has the structure shown in formula (I):
[0043]
[0044] In formula (I), each R is independently selected from H, chlorine, bromine, trifluoromethyl, tert-butyl and methyl.
[0045] (2) Reaction solvent
[0046] The reaction solvent used in the present invention is dimethyl sulfo...
Embodiment 1
[0061] Synthesis of Benzotriazole
[0062]
[0063] At room temperature, o-phenylenediamine (0.3mmol, 1equiv) and tertbutyl nitrite (0.45mmol, 1.5equiv) were added to the reaction tube, and 50mmol of H 2 O, stirred at 25°C reaction temperature for 15min, and the reaction solution was obtained after the reaction; then the reaction solution was cooled and extracted with 10ml of ethyl acetate to obtain ethyl acetate containing the extract; then the ethyl acetate containing the extract was obtained by adding Excessive anhydrous sodium sulfate was dried, filtered, and concentrated under reduced pressure to obtain a concentrate; finally, the concentrate was separated by column chromatography (using 300 mesh silica gel), using petroleum ether and ethyl acetate at a volume ratio of 3:1 as the eluent, The eluent was collected, and the solvent was spun off to obtain a white solid, that is, the benzotriazole compound represented by formula (II), with a yield of 99%.
[0064] The data...
Embodiment 2
[0072] Synthesis of 5,6-dichlorobenzotriazole
[0073]
[0074] At room temperature, 4,5-dichloro-o-phenylenediamine (0.3 mmol, 1 equiv) and tertbutyl nitrite (1.5 mmol, 5 equiv) were added to the reaction tube, and 300 mmol of H 2 O, stirred at 40°C reaction temperature for 30min, and the reaction solution was obtained after the reaction was completed; then the reaction solution was cooled and extracted with 10ml of ethyl acetate to obtain ethyl acetate containing the extract; then the ethyl acetate containing the extract was obtained by adding Excessive anhydrous sodium sulfate was dried, filtered, and concentrated under reduced pressure to obtain a concentrate; finally, the concentrate was separated by column chromatography (using 400 mesh silica gel), using petroleum ether and ethyl acetate at a volume ratio of 3:1 as the eluent, The eluate was collected, and the solvent was spun off to obtain a white solid, that is, the benzotriazole compound represented by formula (II...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com