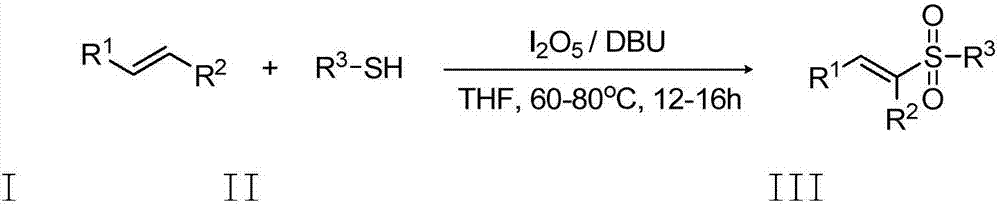
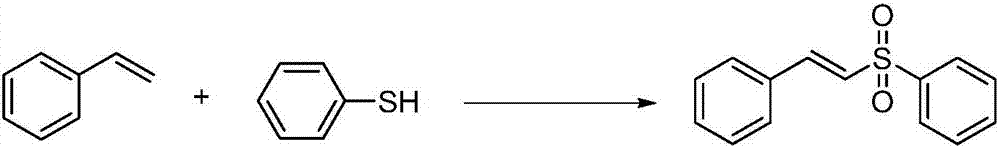Preparation method of E-vinyl sulfones compound
A compound, alkenyl sulfone technology, applied in the field of organic synthetic chemistry, to achieve the effects of wide substrate adaptability, stable process conditions, and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] At room temperature, add styrene (1.15ml, 10mmol), p-methylthiophenol (2.48g, 20mmol), diiodine pentoxide (4.00g, 12mmol), DBU (1.50ml, 10mmol) into a 50mL reaction flask in sequence and tetrahydrofuran (20 mL). Then, the reaction mixture was stirred at 80° C. for 12 hours (reaction detected by TLC). Then, the reaction was stopped and concentrated under reduced pressure to obtain a crude product. Finally, it was washed with a mixed eluent of petroleum ether and ethyl acetate, and flash column chromatography (silica gel column) obtained the corresponding product E-alkenyl sulfone compound (white solid 2.25 g, yield 87%).
[0031] NMR data: 1 H NMR (CDCl 3 ,500MHz,ppm):δ7.85(d,J=8.3Hz,2H),7.68(d,J=15.5Hz,1H),7.51-7.49(m,2H),7.42-7.41(m,3H), 7.37(d, J=8.1Hz, 2H), 6.87(d, J=15.4Hz, 1H), 2.46(s, 3H); 13 C NMR (CDCl 3 , 125MHz, ppm): δ144.4, 141.9, 137.8, 132.5, 131.1, 130.0, 129.1, 128.5, 127.7, 127.6, 21.6.
Embodiment 2
[0033]
[0034] At room temperature, styrene (1.15ml, 10mmol), thiophenol (2.05ml, 20mmol), diiodine pentoxide (4.00g, 12mmol), DBU (1.50ml, 10mmol) and THF ( 20mL). Then, the reaction mixture was stirred at 80° C. for 12 hours (reaction checked by TLC). Then, the reaction was stopped and concentrated under reduced pressure to obtain a crude product. Finally, it was washed with a mixed eluent of petroleum ether and ethyl acetate, and flash column chromatography (silica gel column) obtained the corresponding product E-alkenyl sulfone compound (yellow solid 2.22 g, yield 91%).
[0035] NMR data: 1 H NMR (CDCl 3 ,500MHz,ppm):δ7.96(d,J=7.4Hz,2H),7.70(d,J=15.4Hz,1H),7.64-7.61(m,1H),7.55(t,J=7.3Hz, 2H), 7.50-7.48(m, 2H), 7.42-7.38(m, 3H), 6.86(d, J=15.4Hz, 1H); 13 C NMR (CDCl 3, 125MHz, ppm): δ142.5, 140.7, 133.4, 132.4, 131.3, 129.4, 129.1, 128.6, 127.7, 127.3.
Embodiment 3
[0037]
[0038] At room temperature, add styrene (1.15ml, 10mmol), 3-methylthiophenol (2.38ml, 20mmol), diiodine pentoxide (4.00g, 12mmol), DBU (1.50ml, 10mmol) into a 50mL reaction flask in sequence ) and tetrahydrofuran (20 mL). Then, the reaction mixture was stirred at 80° C. for 12 hours (reaction checked by TLC). Then, the reaction was stopped and concentrated under reduced pressure to obtain a crude product. Finally, it was washed with a mixed eluent of petroleum ether and ethyl acetate, and flash column chromatography (silica gel column) obtained the corresponding product E-alkenyl sulfone compound (yellow oily liquid 1.88g, yield 73%).
[0039] NMR data: 1 H NMR (CDCl 3 ,500MHz,ppm):δ7.75-7.74(m,2H),7.68(d,J=15.4Hz,1H),7.50-7.48(m,2H),7.44-7.38(m,5H),6.86(d ,J=15.4Hz,1H),2.44(s,3H); 13 C NMR (CDCl 3 , 125MHz, ppm): δ142.3, 140.5, 139.7, 134.2, 132.4, 131.2, 129.2, 129.1, 128.6, 128.0, 127.4, 124.8, 21.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com