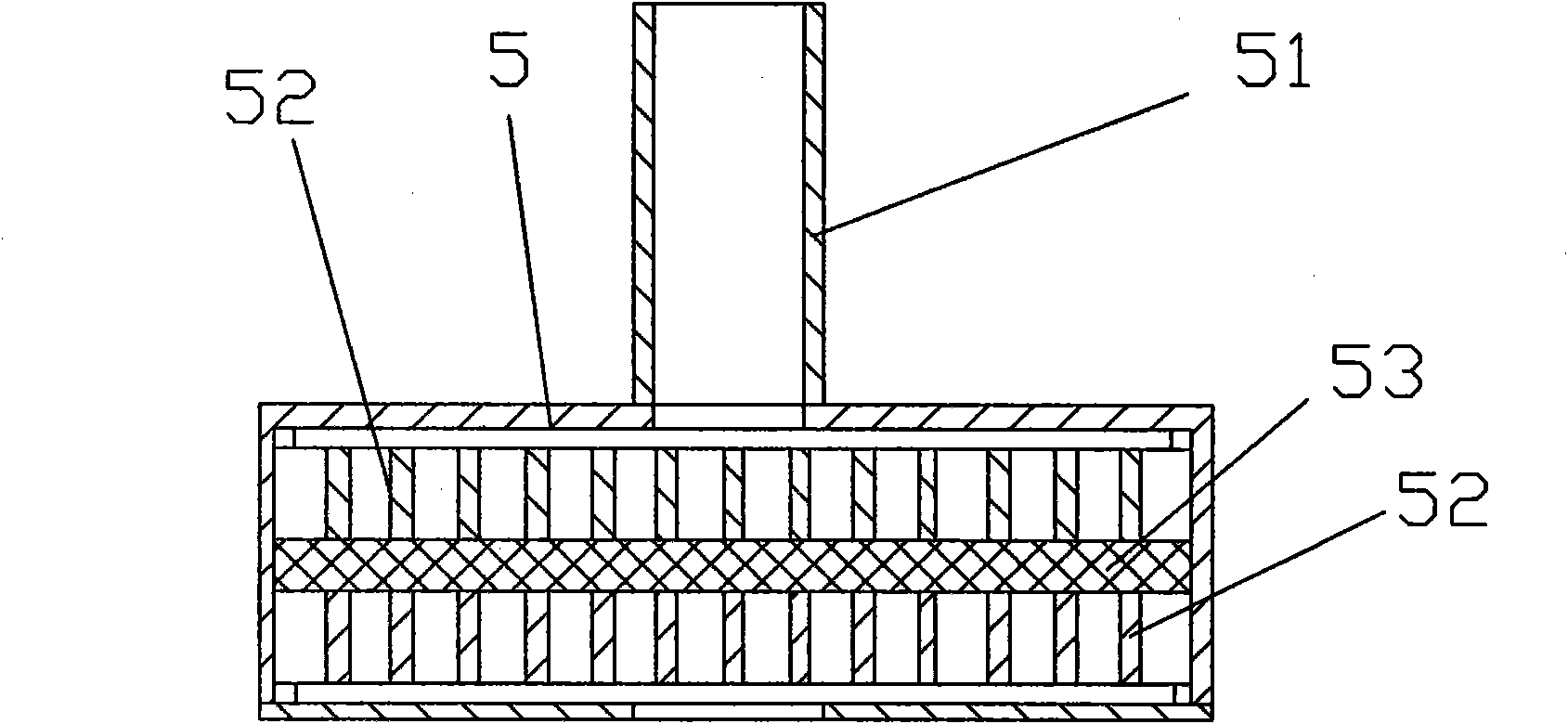
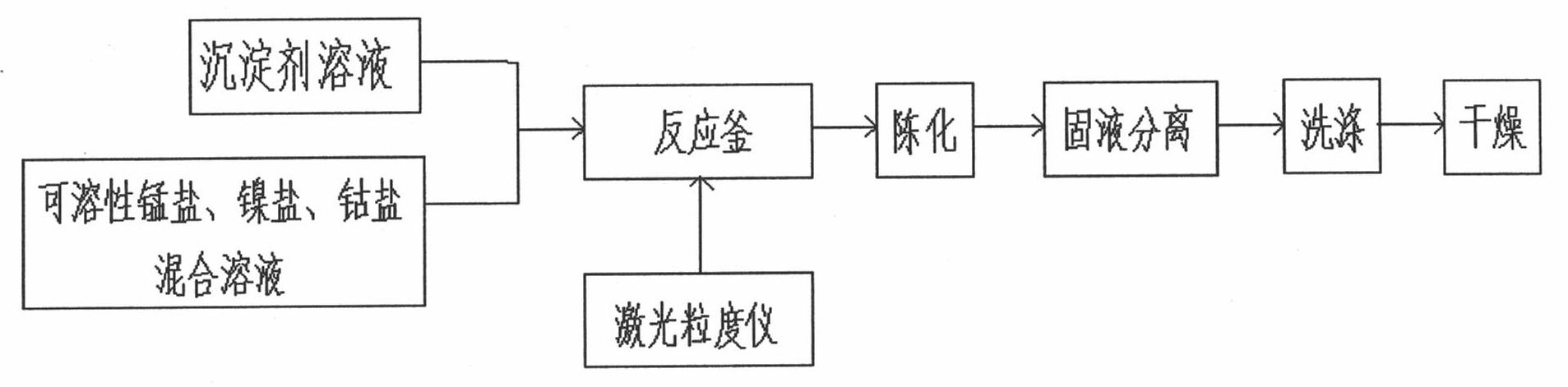
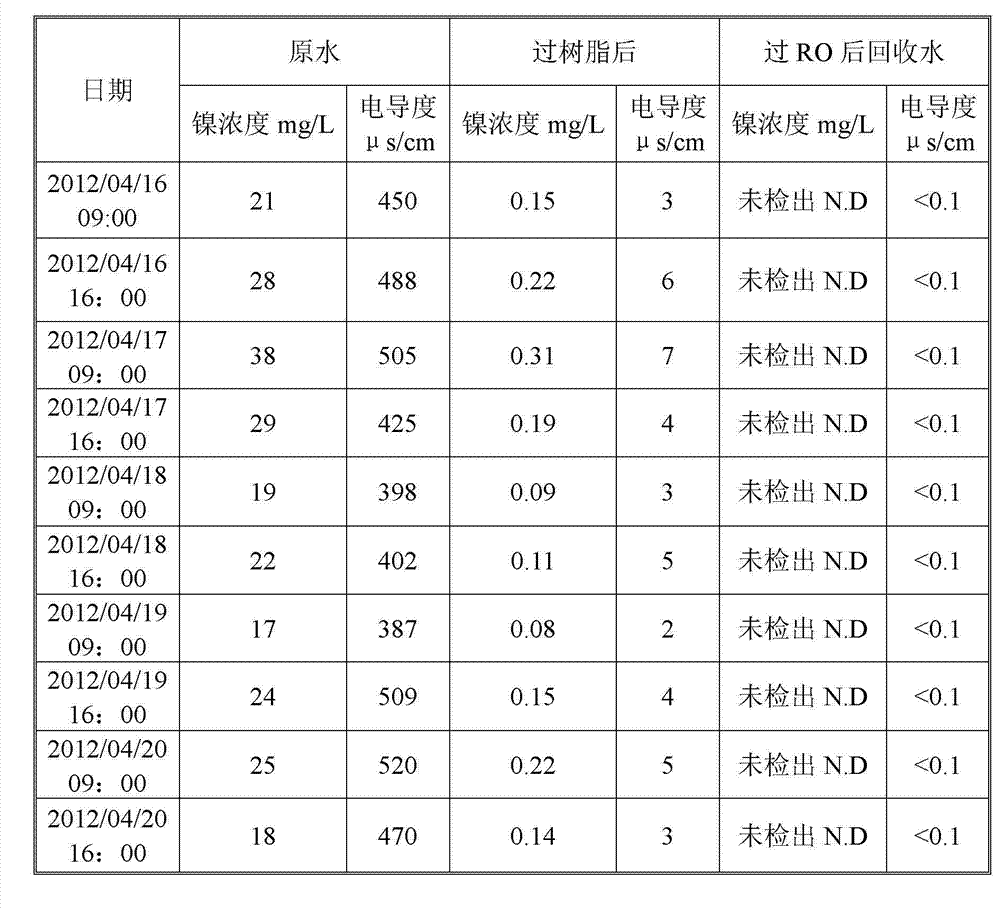
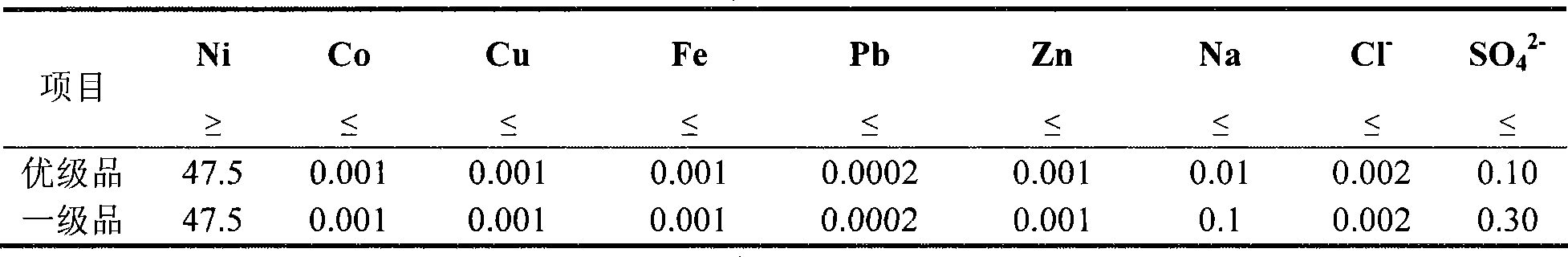
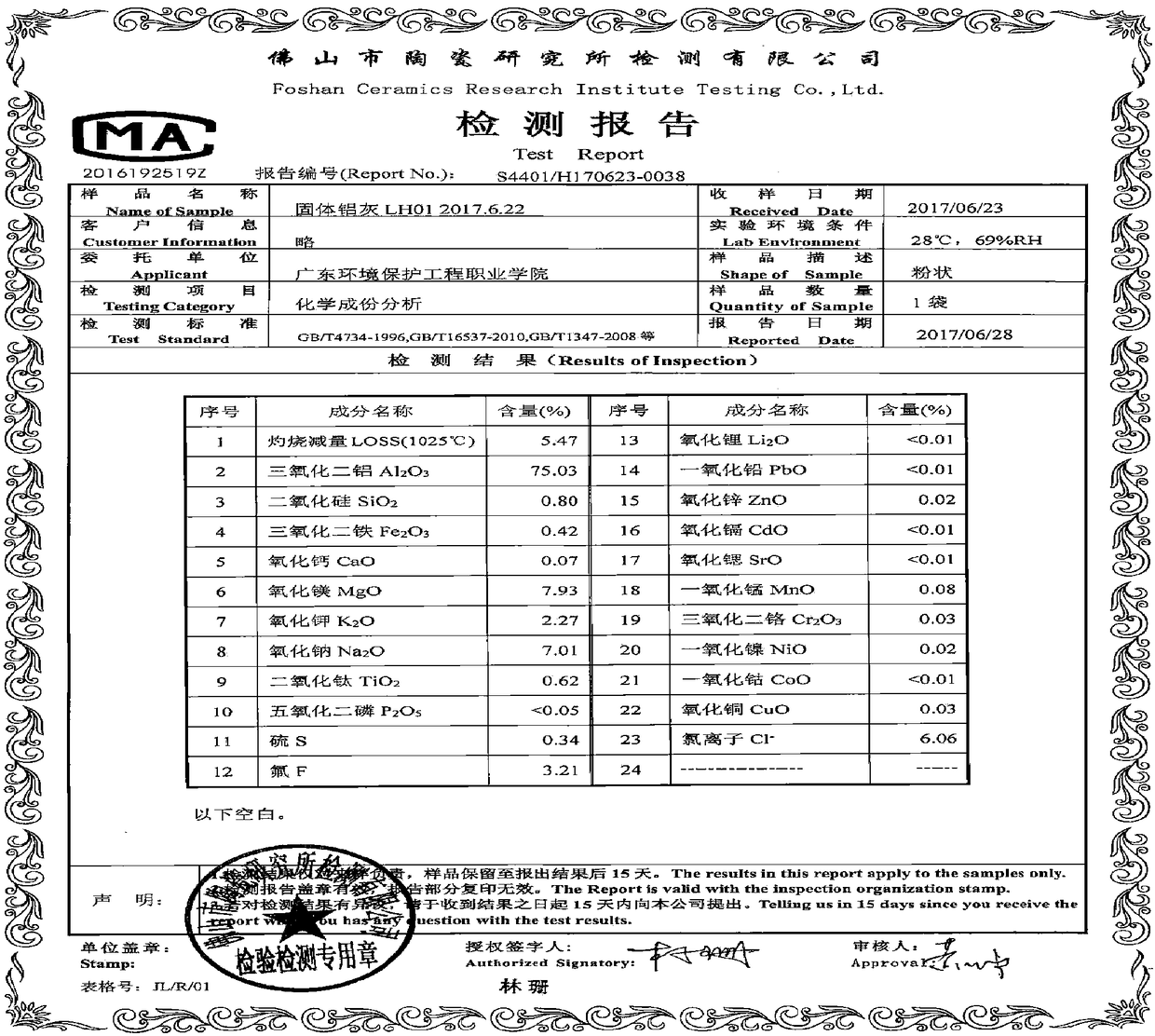
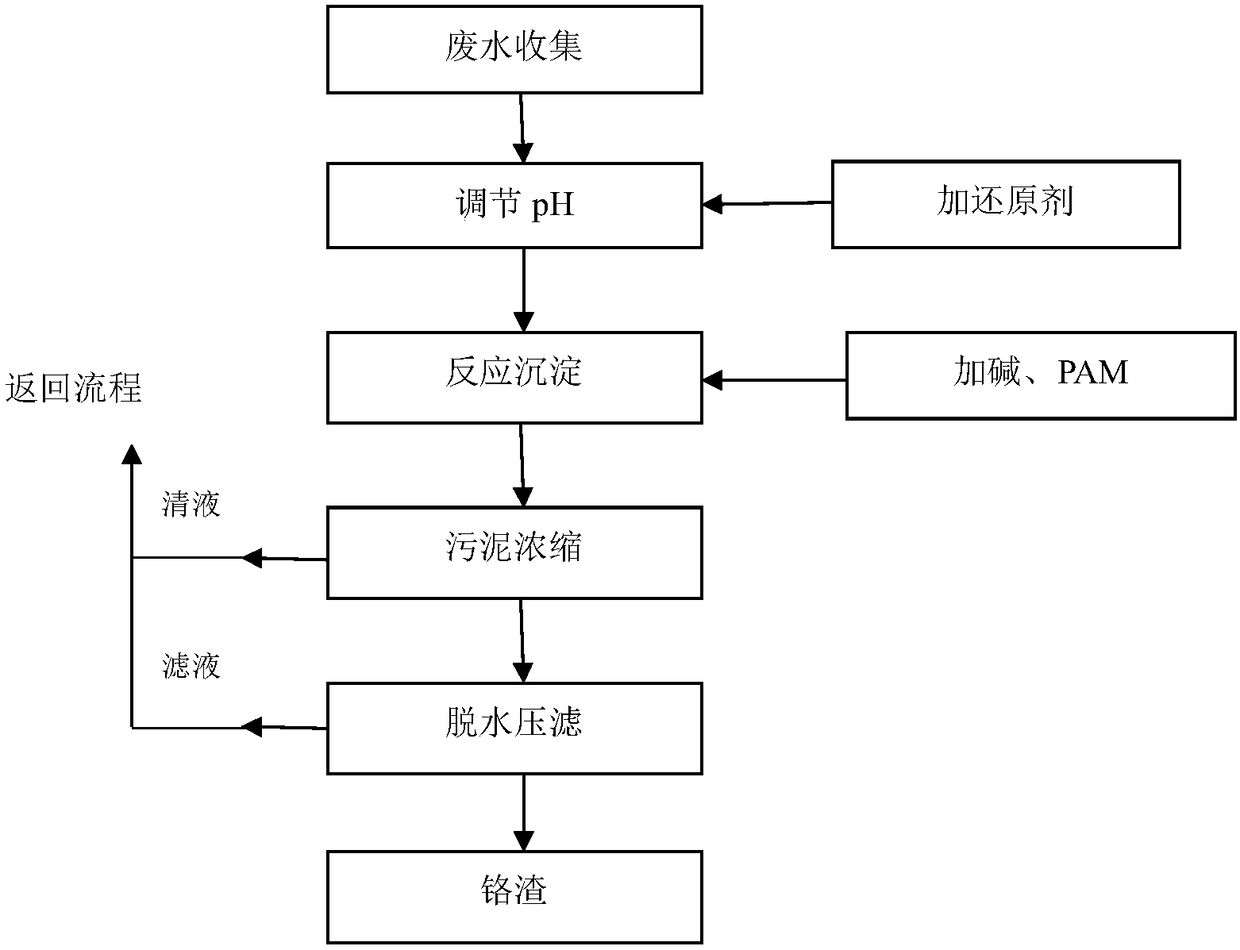
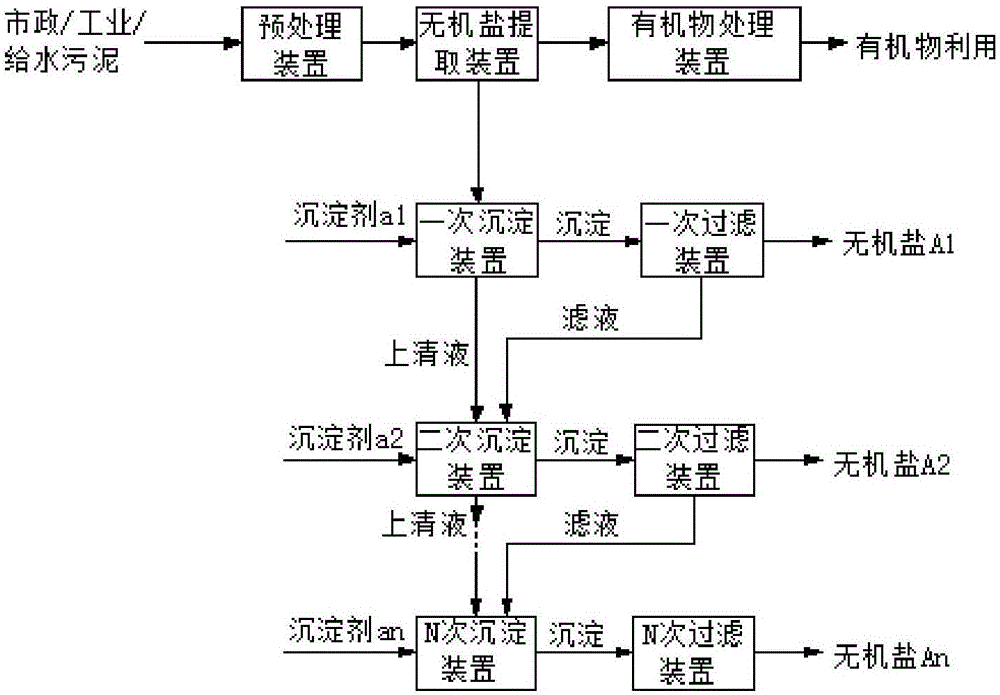
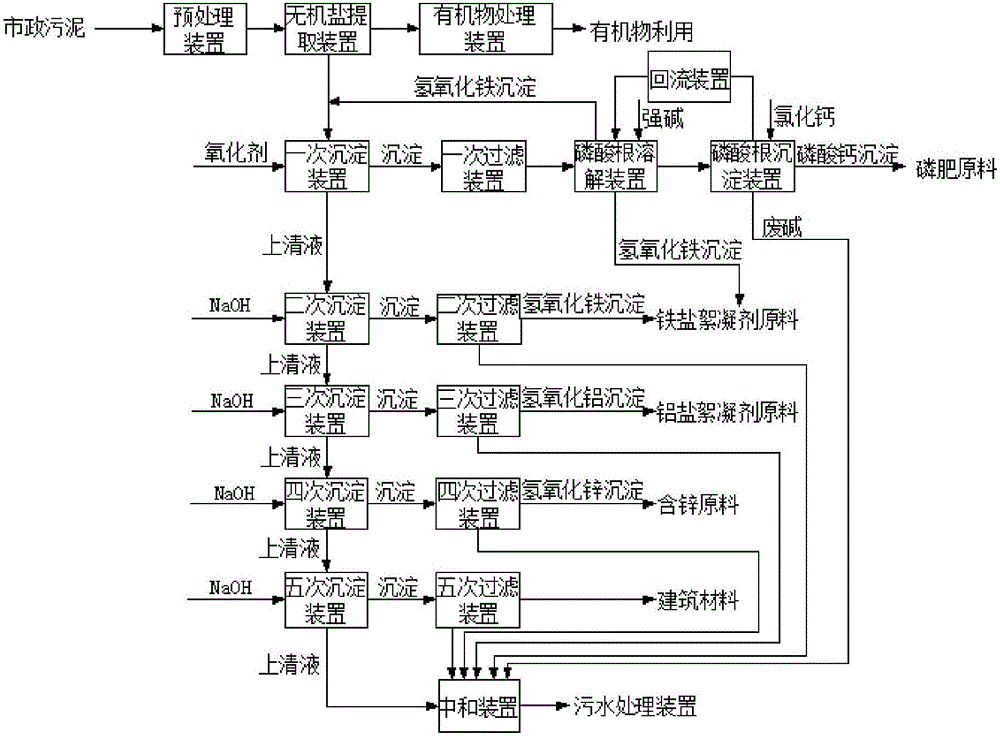
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
160results about "Nickel carbonates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cathode material for lithium secondary battery and method of producing same
InactiveUS20060121350A1Stable supplyImprove sinterabilityOxygen/ozone/oxide/hydroxideElectrode manufacturing processesElectrical batteryPhysical chemistry
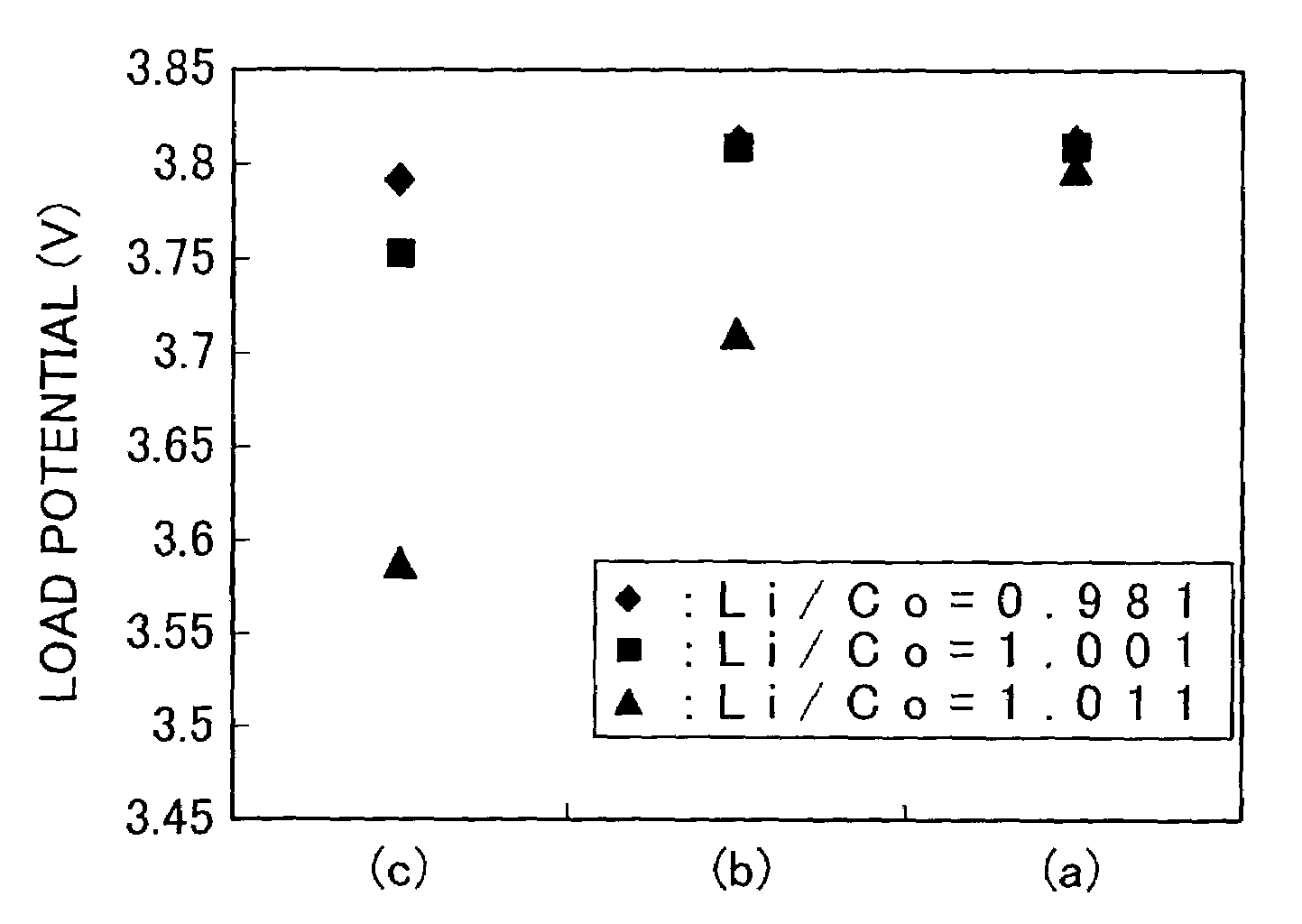
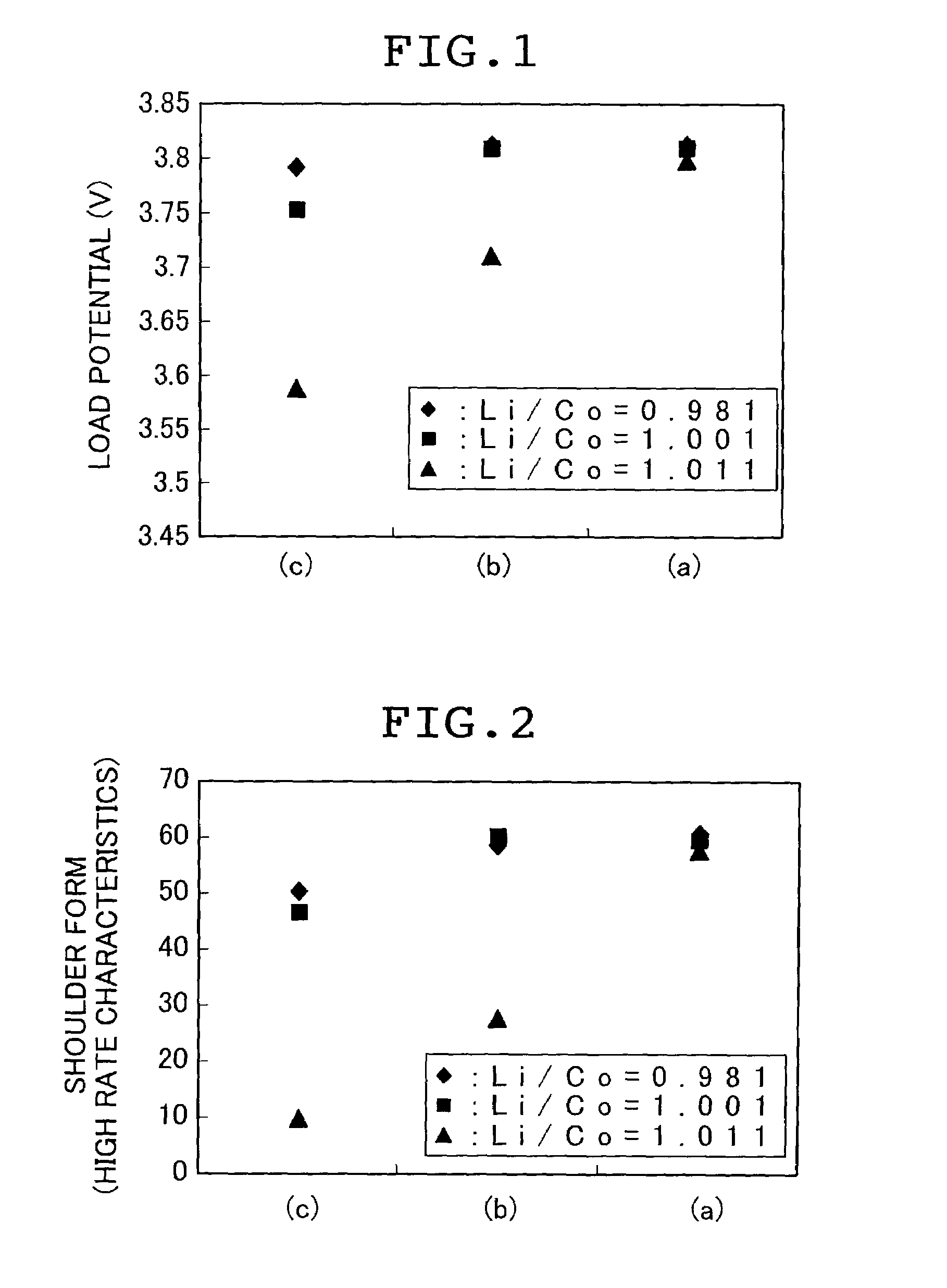
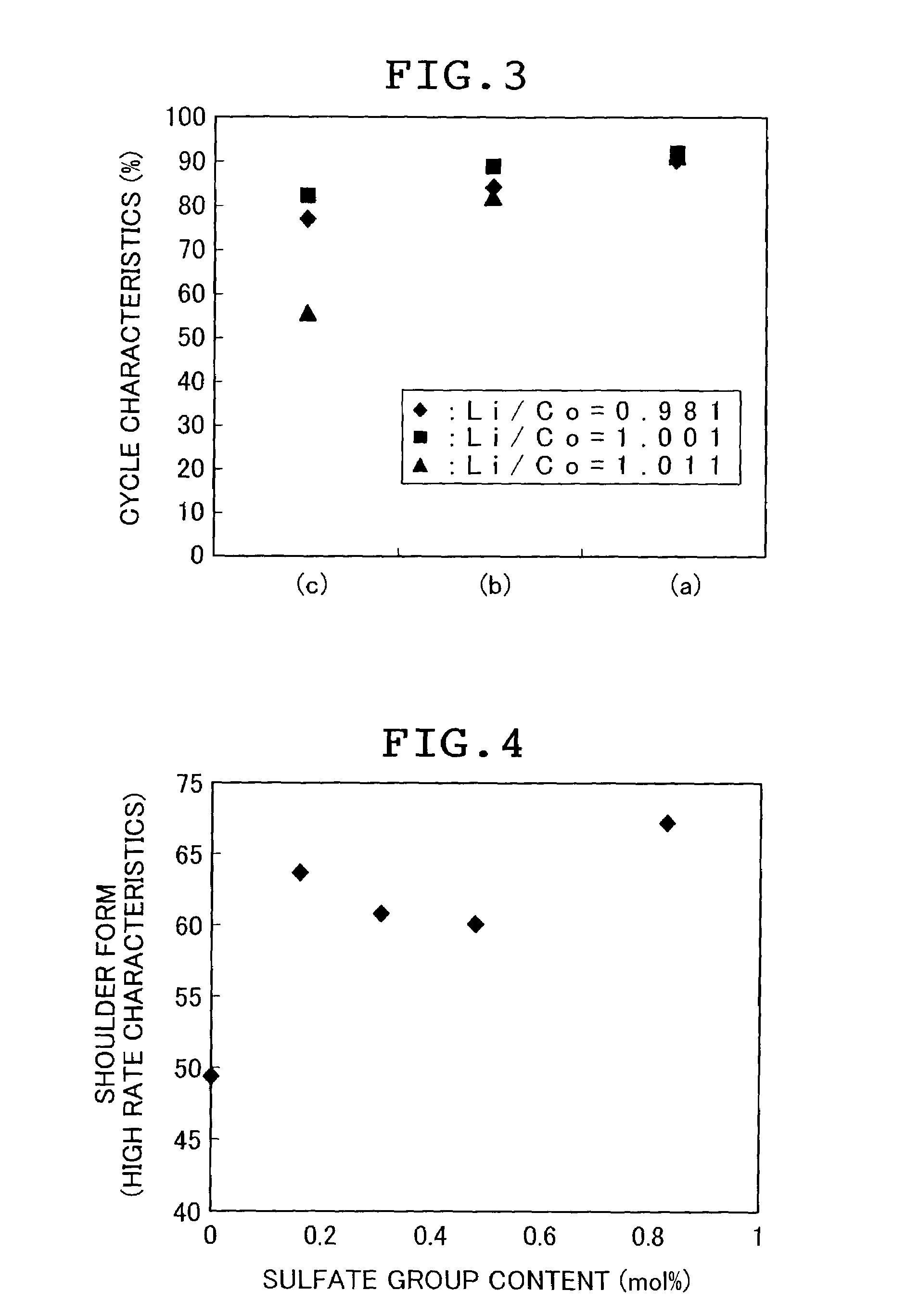
Stable supply of a cathode material for a lithium secondary battery that exels in sinterbility and composition stability and can exhibit satisfactory battery performance is accomplished by reducing to 100 ppm or less both the contents of Na and S being impurity elements in multiple oxides as materials for a cathode material for a lithium secondary battery and carbonic salts as precursor materials for the production of a cathode material for a lithium secondary battery.
Owner:NIKKO MATERIALS CO LTD
Method for recycling copper, nickel, chromium, zinc and iron from plating sludge
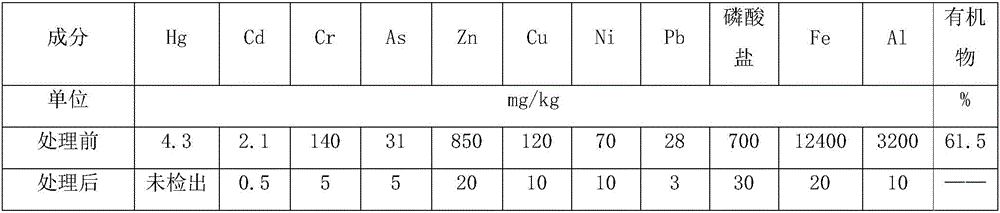
The invention relates to a method for recycling copper, nickel, chromium, zinc and iron from plating sludge, belonging to the technical field of chemical engineering and metallurgy. The method comprises the following steps: acid leaching, vulcanizing for separation and enrichment, hot-pressure leaching, extracting for separation, hot-press oxidizing chromium, purifying chromium solution, extracting ferric chloride and the like. The method has obvious advantages of strong adaptability to different kinds of plating sludge, high utilization of metal resources, high value-added content of product,less process waste residue, thorough deintoxication and the like.
Owner:YANGZHOU NINGDA NOBLE METAL CO LTD
Method for producing fine spherical particles of carbonate or hydroxide of nickel, cobalt or copper
InactiveUS6197273B1Amino compound purification/separationOxide/hydroxide preparationSal ammoniacCobalt metal
The invention provides a process for production of fine spherical particles of a carbonate or a hydroxide of nickel, cobalt or copper which comprises: dissolving a carbonate or a hydroxide of nickel, cobalt or copper having the general formula (I)wherein M represents Ni, Co or Cu, and x and y are numerals satisfying the followings: 0<=x<=2, 0<=y<=2 and x+y=2, in aqueous ammonia, converting the resulting solution to a W / O emulsion containing droplets of the solution in a non-aqueous medium, and then removing volatile components including ammonia from within the droplets, thereby precipitating a basic carbonate or a hydroxide of a metal selected from nickel, cobalt and copperin the droplets.The fine spherical particles of a carbonate or a hydroxide of nickel, cobalt or copper obtained according to the process of the invention are especially useful as a precursor for the manufacture of uniform, fine spherical particles of nickel, copper or cobalt metal, as well as useful as themselves as a catalyst for use in organic synthesis, a carrier, a pigment, a filler or a glaze.
Owner:SAKAI CHEM IND CO LTD
Nickel metal compositions and nickel complexes derived from basic nickel carbonates
ActiveUS20110196168A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDicarbonateMetallurgy
Nickel-metal-containing solids for use in manufacturing nickel metal complexes are disclosed. The nickel-metal-containing solids are made by reducing basic nickel carbonates. By varying the molar ratios of carbonates and bicarbonates to nickel salts, the methods provide basic nickel carbonates that produce superior nickel metal-containing solids that react more effectively with phosphorous-containing ligands. The phosphorous containing ligands can be both monodentate and bidentate phosphorous-containing ligands.
Owner:INV NYLON CHEM AMERICAS LLC
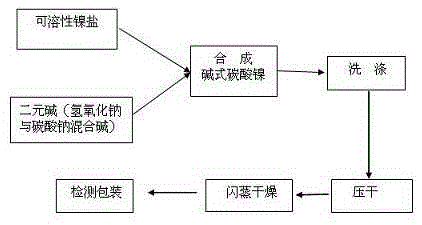
Method of preparing electronic grade nickel carbonate by sodium carbonate deposition
The invention discloses a manufacturing method of electron-grade nickel carbonate through sedimenting sodium carbonate, which is characterized by the following: adopting soluble nickel salt as nickel source and sodium carbonate as sediment; co-flowing nickel salt solution and sodium carbonate solution; controlling pH value, temperature and aging condition; washing; drying; washing again; drying again; grinding into product.
Owner:JINCHUAN GROUP LIMITED +1
Composite carbonate and method for producing the same
InactiveUS20090194746A1Reduce the amount of wasteGood effectNon-metal conductorsConductive materialManganeseCobalt atom
The present invention provides a nickel atom-, manganese atom- and cobalt atom-containing composite carbonate that is high in specific surface area and large in tap density, and useful as a raw material for producing a lithium nickel manganese cobalt composite oxide to be used in a positive electrode active material for use in a lithium secondary battery, and provides a method for industrially advantageously producing the composite carbonate. The composite carbonate includes nickel atoms, manganese atoms and cobalt atoms, and has an average particle size of 5 μm or more and less than 20 μm, a BET specific surface area of 40 to 80 m2 / g and a tap density of 1.7 g / ml or more.
Owner:NIPPON CHECMICAL IND CO LTD
Process for rapidly producing basic nickel carbonate or basic cobaltous carbonate
InactiveCN101708868AReduce pollutionRapid productionNickel carbonatesCobalt carbonatesChlorideUltimate tensile strength
The invention discloses a process for rapidly producing basic nickel carbonate or basic cobaltous carbonate, comprising the following steps of: dissolving, synthetizing, filtering, water scrubbing and drying. The dissolving proportion by weight of nickel chloride or cobalt chloride in the dissolving process is NiCl2:H2O=1:4-8, CoCl2:H2O=1:4-8, and the dissolving proportion by weight of sodium carbonate is Na2CO3:H2O=1:4-8. Aqueous alkali and a nickel chloride or cobalt chloride solution are added by adopting a spray mode in the synthetic process. The synthetic reaction time is 2-4 hours. The invention not only has simple process, short production cycle, low labor intensity and arbitrary enlargement of production scale but also has simple and practicable operation, low cost, stable quality and high efficiency. The process has the characteristics of fast production, high quality of products and high efficiency; products can be turned out after water scrubbing at one time, and therefore, electricity, water, labor and time are saved. The products have even and superfine granules without crushing and sieving, thereby being beneficial to application.
Owner:江西核工业兴中科技有限公司
Method for preparing supercapacitor electrode material basic nickel-cobalt carbonate through hydrothermal method
InactiveCN106587171AFully moistenedFull penetrationHybrid capacitor electrodesNickel carbonatesNickel saltHigh pressure
The invention discloses a method for preparing a supercapacitor electrode material basic nickel-cobalt carbonate through a hydrothermal method. The method comprises the following steps that metal nickel salt and / or metal cobalt salt and urea are weighed separately and mixed with deionized water, and stirring is performed so that solids can be dissolved to obtain a mixed solution; the mixed solution is poured into a high-pressure reactor, sealing is performed for performing a reaction for 10-16 h under the temperature of 80-110 DEG C, and a reaction product is obtained; the reaction product is separated, washed and dried to obtain basic nickel-cobalt carbonate. According to the method for preparing the supercapacitor electrode material basic nickel-cobalt carbonate through the hydrothermal method, on the basis of control over raw material compatibility and reaction conditions, the three-dimensional porous structure and morphology can be obtained, sufficient wetting and permeation of an electrolyte are promoted, the superficial area of active substances is sufficiently utilized, and the electrochemical performance is improved.
Owner:BEIJING INSTITUTE OF GRAPHIC COMMUNICATION
Process for synthesis of basic nickel carbonate from diacidic base
InactiveCN105384199ACreate pollutionIncrease concentrationNickel carbonatesNickel saltSodium acid carbonate
The present invention discloses a process for synthesis of basic nickel carbonate from a diacidic base, according to the process, a sodium carbonate and sodium hydroxide mixed diacidic base is used for precipitation reaction with a soluble nickel salt, the reaction is one-step room temperature reaction, operation is easy, the base dosage is reduced by 20 to 30% compared with a pure soda base synthesis process, the environment is not polluted, and Na in the obtained basic nickel carbonate product (containing 40% of Ni) can be 100ppm or less.
Owner:JIANGXI NUCLEAR IND XINGZHONG NEW MATERIALS
Nickel compositions for preparing nickel metal and nickel complexes
ActiveUS20110311428A1Organic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsNickel saltPhysical chemistry
Nickel compositions for use in manufacturing nickel metal compositions, and specifically to methods of making basic nickel carbonates used to produce nickel metal compositions are disclosed. By varying the molar ratios of carbonates and bicarbonates to nickel salts, the methods provide basic nickel carbonates that produce superior nickel-containing solids that react more effectively with phosphorous-containing ligands. The phosphorous containing ligands can be both monodentate and bidentate phosphorous-containing ligands.
Owner:INV NYLON CHEM AMERICAS LLC
Method for forming Basic Nickel Carbonate
ActiveUS20160090311A1Alleviate volume expansionImprove conversion rateChemical/physical/physico-chemical processesNickel oxides/hydroxidesParticulatesPotential market
The present disclosure provides a novel method to fabricate the basic nickel carbonate particulates. The nickel content in the basic nickel carbonate particulates fabricated by this invention (51-53 mass %) is higher than the present commercialized products (44-46 mass %). Basic nickel carbonate is an important intermediate to prepare NiO and pure Ni particles, and NiO and pure Ni particles are important materials in electronic industrial. Therefore, basic nickel carbonate has its potential market.
Owner:CHUNG YUAN CHRISTIAN UNIVERSITY
Method for preparing spherical carbonate
ActiveCN101857278AReduce processing stepsExcellent indicatorsNickel carbonatesCobalt carbonatesManganateSlurry
The invention discloses a method for preparing spherical carbonate serving as a cell fuel precursor. At present, the preparation of a nickel-cobalt lithium manganate anode material mainly adopts a co-precipitation method, and the obtained materials are generally irregular granules with low practical value. The method is characterized by comprising the following steps of: taking tail ammonium chloride solution as reaction base solution, adding metal chlorate solution and ammonium carbonate into the base solution at the same time with stirring to produce precipitation reaction at the temperature of between 40 and 80 DEG C, ensuring that the pH value of the solution at the end point is between 7.0 and 7.5, preserving heat and ageing the solution, and washing and drying the obtained slurry to obtain a spherical carbonate product, wherein the metal chlorate solution is one or mixture of cobalt chloride, nickel chloride and manganese chloride, and the concentration of the ammonium chloride is 10 to 30 grams per liter. The tail solution is used as the base solution, and the pH value of the solution at the end point is between 7.0 and 7.5 by using the liquid-phase precipitation method so that the spherical carbonate product with large grain diameter is obtained and all indexes are excellent.
Owner:ZHEJIANG HUAYOU COBALT
Method for continuously compounding high-purity alkali nickel carbonate
ActiveCN102923794ALow impurity contentFlat surfaceNickel carbonatesTemperature controlReaction temperature
The invention discloses a method for continuously compounding high-purity alkali nickel carbonate. The method comprises the steps as follows: a nickeliferous stock solution, ammonium salt and a precipitant Na2CO3 are continuously conveyed to a multifunctional reactor for carrying out continuous compounding reaction, and resultant slurry is continuously discharged from a material outlet; during the continuous compounding reaction, the reaction temperature is controlled within 50-80 DEG C with a real-time monitoring system, the reaction pH value is controlled within 7.5-8.5, and the average residence time of a reaction system in the multifunctional reactor is controlled within 20 h-30 h; and a reaction discharging is filtered, filter residue is aged, and then the reaction discharging enters into a multi-level washing tower to be washed, when the flow conductance value of the multi-level washing tower bottom is less than 600 ms / cm, a washed product is filtered and dried to obtain the high-purity alkali nickelous carbonate. The invention has the advantages of simple process, low equipment investment, environment protection, complete product structure, good quality and the like.
Owner:CHANGSHA RES INST OF MINING & METALLURGY
Method for preparing nano nickel bicarbonate
The invention relates to a method for preparing nano nickel bicarbonate, which comprises the following steps of: adding water into urea or urotropine for dissolving, adding nickel salt or nickel hydroxide and stirring, wherein the mole ratio of the urea or the urotropine to nickel is 1:1-16:1, and the concentration of the nickel salt in solution is 0.1-1.0mol / L; shifting the obtained solution into a hydrothermal reactor; after reacting at 90-240 DEG C for 1-96 hours, cooling; and filtering obtained reaction mixed liquid, washing with distilled water and anhydrous ethanol and placing in a vacuum drying box at 60 DEG C for drying to obtain a cubic phase nano nickel bicarbonate square block with the size of 100nm or so. The invention uses the urotropine or the urea as a precipitator, NH3 andCO2 generated by the precipitator dissolve nickel hydroxide, and the pure cubic phase Ni(HCO3)2 nano crystal is prepared finally.
Owner:NANJING UNIV OF TECH
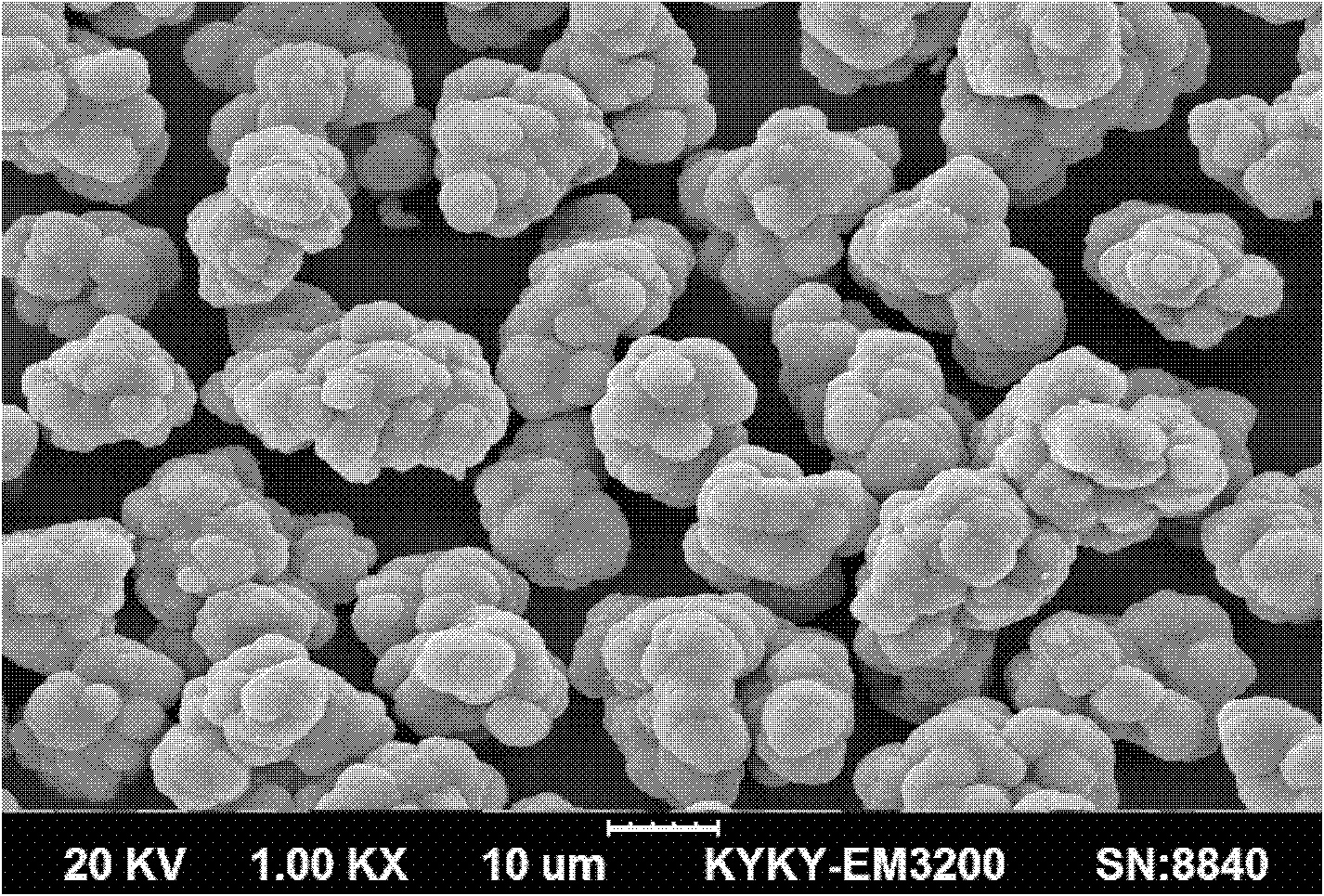
Preparation method of microporous ultrafine high activity nickel carbonate
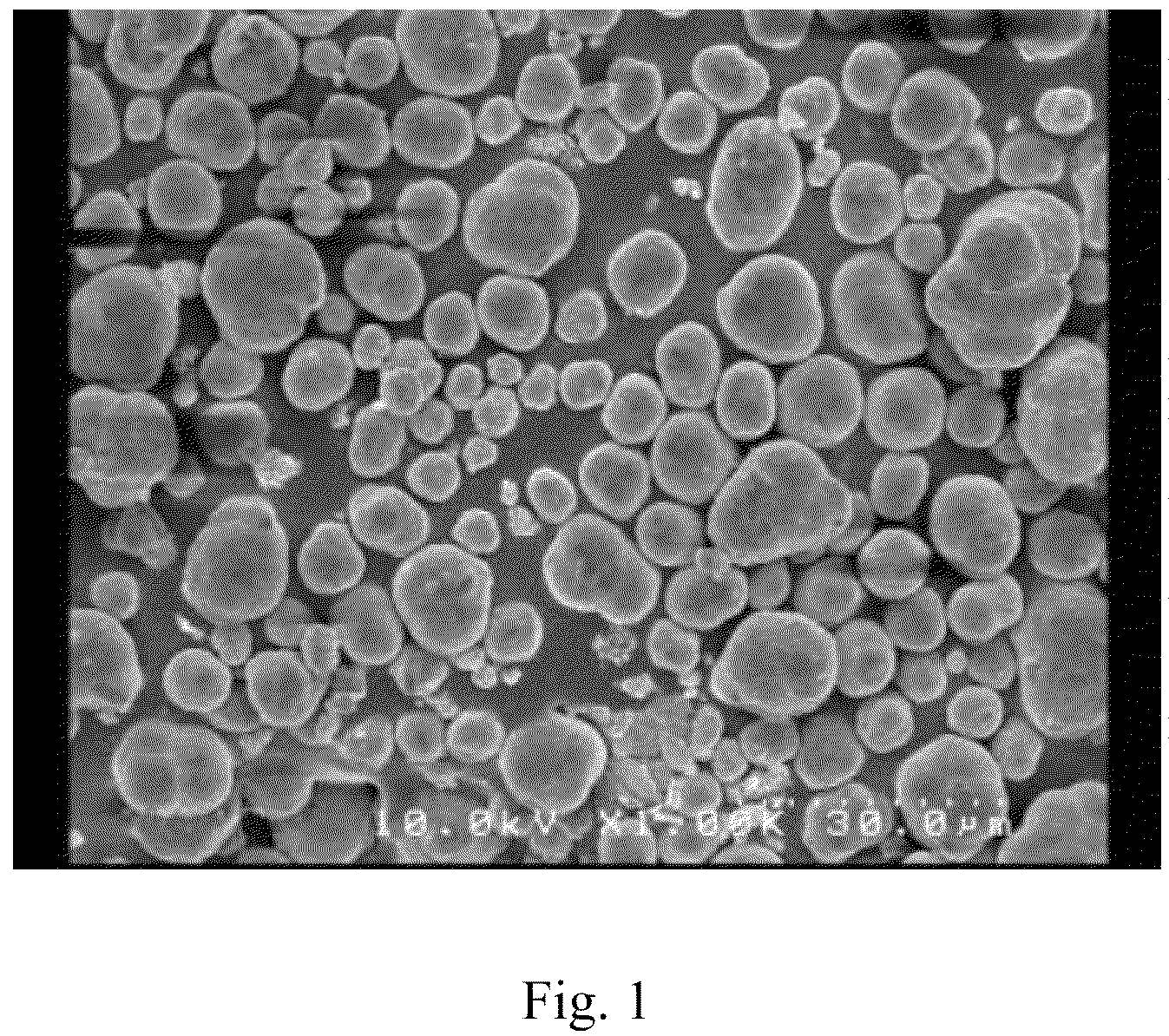
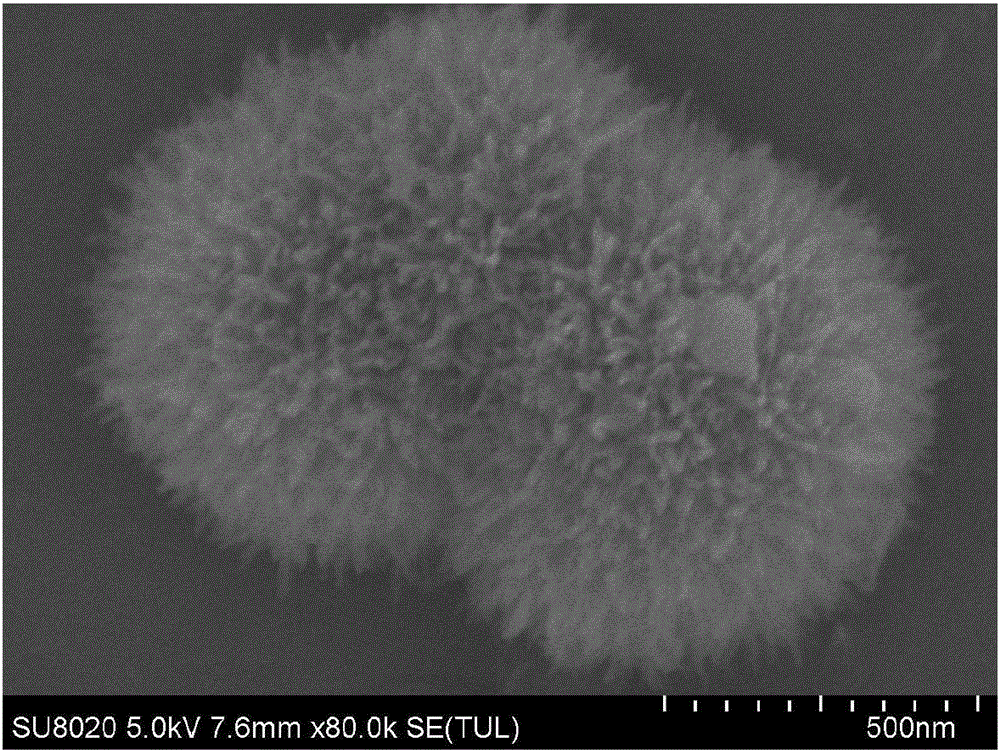
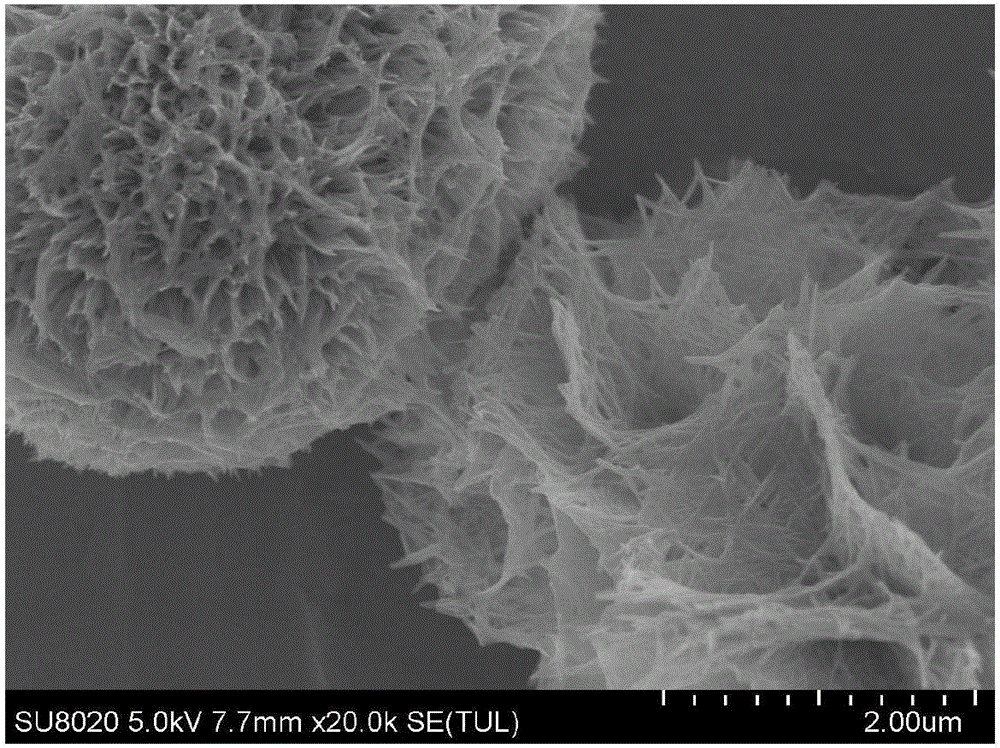
InactiveCN101863520AFine granularityNarrow and uniform particle size distributionNickel carbonatesSem micrographsHigh pressure
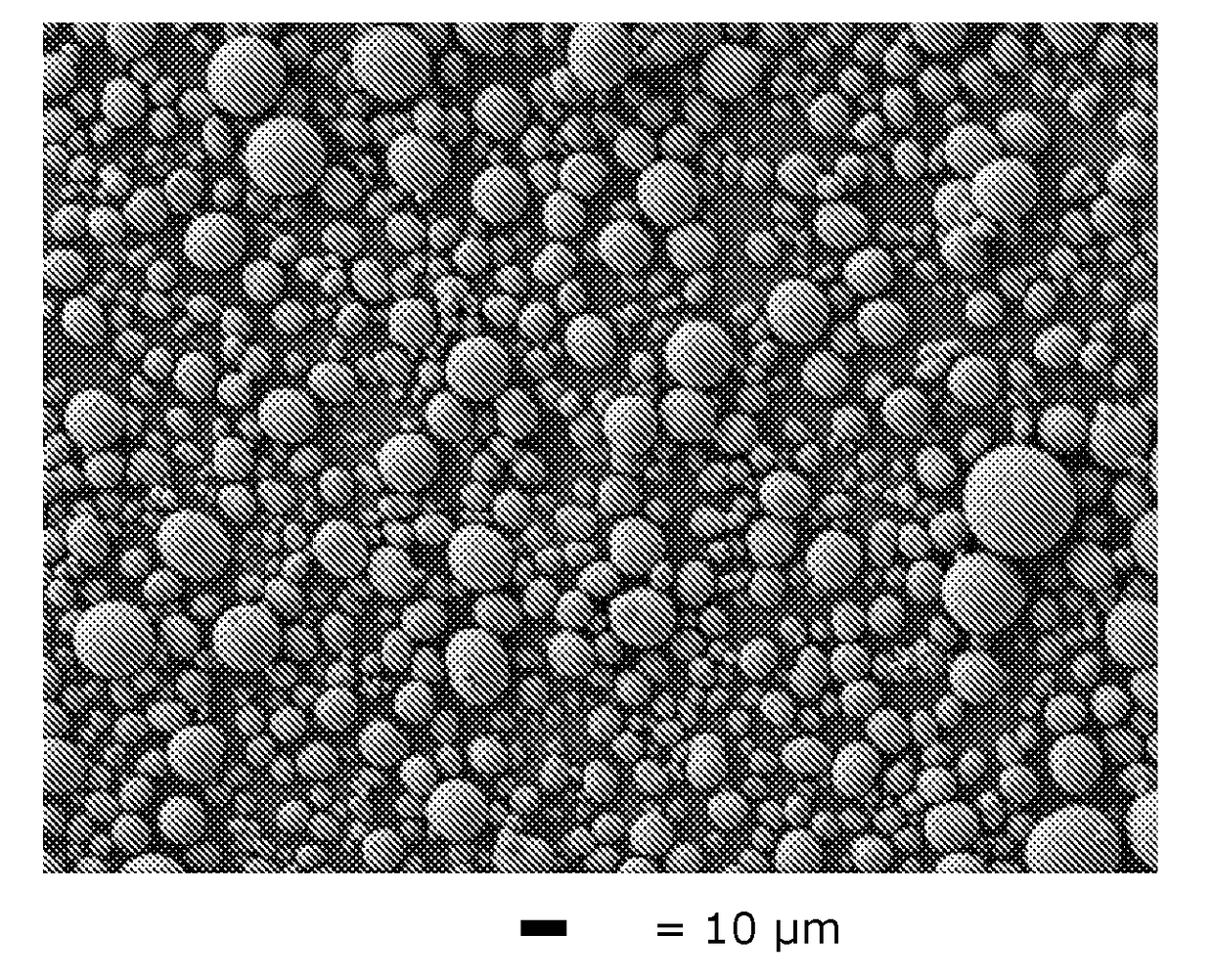
The invention discloses a preparation method of microporous ultrafine high activity nickel carbonate, comprising the following steps of adopting mixed acid solution prepared from nitric acid, sulfuric acid and hydrochloride to dissolve metal nickel to prepare composite nickel salts used as raw materials; carrying out precipitation reaction with the composite nickel salts by using mixed percipitant of sodium carbonate, ammonium carbonate or ammonium bicarbonate and sodium hydroxide in a sealed drum type reaction pot under the action of high pressure CO2; and preparing the nickel carbonate by crushing and drying precipitates by adopting an integrative machine of crushing and drying after washing the precipitates. Shown through a particle image analyzer, a full-automatic specific surface area voidage analyzer and an SEM (Scanning Electron Microscope) photograph result, the nickel carbonate has fine size, the particle size of D50 can achieve 1-5 microns, the particle size specific surface area can achieve more than 300m2 / g, the content of carbon dioxide is larger than 20 percent, the nickel carbonate has multiple and small micropores, the powder nickel carbonate with the nickel content of 49-51 percent has narrow and uniform particle size distribution, wherein the particle size of D10 is 0.93 microns, the particle size of D50 is 1.81 microns and the particle size of D90 is 2.82 microns, and the dispersion of the powder nickel carbonate is favorable.
Owner:郑景宜
Method for preparing spherical carbonate ternary precursor
InactiveCN102070179AEasy production controlIncrease productivityCell electrodesNickel carbonatesAlkalinityManganese
The invention discloses a method for preparing a spherical nickel-manganese-cobalt carbonate ternary precursor, which comprises the following steps of: continuously adding nickel-manganese-cobalt metal salt mixed solution of certain concentration and precipitant into reaction equipment, controlling the pH of the reaction system to be between 6.5 and 7.5, controlling the alkalinity at 5 to 40g / L, controlling the solid content of the slurry at 70 to 130g / L, and aging, washing and drying the slurry to obtain the spherical nickel-manganese-cobalt carbonate ternary precursor. The prepared spherical nickel-manganese-cobalt carbonate ternary precursor granules are relatively uniform, have good spherical degree, smooth spherical surface, good liquidity and controllable size, and are suitable for industrialized continuous production.
Owner:ANHUI ALAND NEW ENERGY MATERIALS
Chemical nickel plating waste water circulating reclamation and nickel resource circulating reutilization method
InactiveCN102826678ALow conductivityImprove water qualityWater/sewage treatment by ion-exchangeMultistage water/sewage treatmentSuspended particlesWater cycling
The invention discloses a chemical nickel plating waste water circulating reclamation and nickel resource circulating reutilization method which comprises the following steps: (1) sending the chemical nickel plating waste water into a strongly-alkaline anion exchange resin to carry out complex adsorption and decomplexation treatment, thereby destabilizing nickel complexation; (2) adsorbing nickel ions contained in the nickel plating waste water treated by the step (1) with a strongly-acidic cation exchange resin; (3) adsorbing residual anions in the waste water treated by the step (2) with a strongly-alkaline anion exchange resin to lower the electrical conductivity; (4) filtering to remove suspended particles, colloidal matters or organic matters in the waste water obtained in the step (3); and (5) treating the waste water treated by the step (4) with a reverse osmosis apparatus, wherein the produced water can be recycled, and thick water generated by the reverse osmosis apparatus can be circulated back to the step (1). Since zero water is discharged in water circulating reclamation, the problem of overproof discharge water does not exist, and the nickel in the raw waste water is prepared into basic nickel carbonate as a recycled resource.
Owner:宋洪华 +1
Method for continuously synthesizing nickel carbonate
InactiveCN101519229AImprove product qualityAvoid problems that are not easy to removeNickel carbonatesSulfateElectronic industry
The invention relates to a method for continuously synthesizing nickel carbonate, which is characterized by comprising the synthesizing processes: adding and combining a nickel sulfate solution with the concentration of nickel ions from 50 g / l to 55 g / l and a sodium carbonate solution with the concentration from 120 g / l to 125 g / l into a synthesizing kettle according to a flow rate of 1 to 1.2:1, and controlling the pH value of a synthesizing process to be 8.3-8.6 to synthesize the nickel carbonate. The method strictly controls the adding rate of the two raw solutions and the pH values, effectively solves the problems in the prior art that because the excess sodium carbonate solution is added, more local complex salts (Ni1-x / 2-y / 2Nax(CO3)1-a-b / 2(SO4)a(OH)b are generated, the impurity content of the prepared nickel carbonate is higher, the impurities, such as sodium, chlorine, and the like are not easy to be removed in a washing process, thereby effectively improving the product quality of the nickel carbonate, and meeting the requirements of electronic-grade nickel protoxide production and electronic industry.
Owner:JINCHUAN GROUP LIMITED
Process for Heap Leaching of Nickeliferous Oxidic Ores
InactiveUS20110150729A1Easy to processReduce consumptionSolvent extractionIron compoundsPregnant leach solutionFerrous
A process for the recovery of nickel and cobalt from a nickeliferous oxidic ore by heap leaching and / or atmospheric agitation leaching, the process including the steps of: mixing a sulfur containing reductant selected from reductants that do not include copper into a nickeliferous oxidic ore; leaching the reductant / ore mixture with an acidic leach reagent to produce a pregnant leach solution including nickel, cobalt, iron substantially in a ferrous form and other acid soluble impurities; and recovering the nickel and cobalt from the pregnant leach solution.
Owner:BHP BILLITON SSM TECH PTY LTD
Positive electrode active material for lithium ion secondary battery
ActiveUS7026068B2Excellent battery characteristicsImprove featuresNon-aqueous electrolyte accumulator electrodesVehicular energy storageHigh rateElectrical battery
Disclosed is a positive electrode active material for a lithium ion secondary battery, including lithium-transition metal composite oxide of a layer crystal structure, in which the lithium-transition metal composite oxide contains an element that improves conductivity of electrons in the lithium-transition metal composite oxide. Use of this positive electrode active material can improve cycle characteristics, high rate characteristics and thermal stability of lithium ion secondary batteries. Furthermore, by use of this positive electrode active material, gas generation in batteries can be decreased.
Owner:NICHIA CORP
Method of precipitation of metal ions
ActiveUS20110280778A1Low production costLow priceAluminium hydroxide preparationMercury oxidesCalcium bicarbonateIndium
The present invention relates to a method of precipitation of metal ions. Mineral(s), oxide(s), hydroxide(s) of magnesium and / or calcium are adopted as raw materials, and the raw material(s) is processed through at least one step of calcination, slaking, or carbonization to produce aqueous solution(s) of magnesium bicarbonate and / or calcium bicarbonate, and then the solution(s) is used as precipitant(s) to deposit rare earth, such as nickel, cobalt, iron, aluminum, gallium, indium, manganese, cadmium, zirconium, hafnium, strontium, barium, copper and zinc ions. And at least one of metal carbonates, hydroxides or basic carbonates is obtained, or furthermore the obtained products are calcined to produce metal oxides. The invention takes the cheap calcium and / or magnesium minerals or their oxides, hydroxides with low purity as raw materials to instead common precipitants such as ammonium bicarbonate and sodium carbonate etc. The calcium, magnesium, carbon dioxide etc are efficiently and circularly used, and the environment pollution by ammonium-nitrogen wastewater, high concentration salts wastewater is avoided, and both of the discharge of greenhouse gas carbon dioxide and the production cost of metal are decreased.
Owner:GRIREM ADVANCED MATERIALS CO LTD
Recovery system and method of single nickel salt coloring and moderate temperature hole sealing agents in aluminum processing
ActiveCN108191107AReduce environmental costsConserve waterWater/sewage treatment by centrifugal separationWater contaminantsNickel saltWastewater
The invention relates to a recovery system and a method of single nickel salt coloring and moderate temperature hole sealing agents in aluminum processing. The system comprises a nickeliferous wastewater entrapment unit, a nickeliferous wastewater collection unit and a basic nickel carbonate recovery unit, wherein a running water wash trough 18# in the nickeliferous wastewater entrapment unit is externally connected with tap water; running water wash troughs 14#, 15#, 17# and 18# are in series-opposing connection; a single nickel salt coloring trough 13# is compatible with a moderate temperature hole sealing trough 16#; the nickeliferous wastewater collection unit comprises a nickeliferous wastewater collection pool A and a nickeliferous wastewater collection pool B; a pipeline connected with the nickeliferous wastewater collection pool A and the nickeliferous wastewater collection pool B is arranged at a water outlet of the running water wash trough 14#; and the basic nickel carbonaterecovery unit comprises a pump 1#, a recovery tank, a centrifuge, a secondary crystallization tank and a pump 2#. According to the system and the method, nickeliferous wastewater can be separately collected on line; a nickeliferous product, namely basic nickel carbonate, is recovered and converted into a moderate temperature hole sealing agent on a basis of agent compatibility; and reclamation and recycling of toxic waste are achieved.
Owner:FOSHAN SANSHUI XIONGYING INNOVATION CENT FOR ALUMINUM SURFACE TECH
Method and device for respectively extracting and using inorganic matters in sludge
InactiveCN106007292ALow impurity contentHigh puritySludge treatmentLead hydroxidesInorganic saltsResource utilization
The invention provides a method for respectively extracting and using inorganic matters in sludge, and belongs to the technical field of sludge treatment. The method comprises the following steps: (1) pretreating the sludge; (2) extracting inorganic salt from a sludge solution subjected to pretreatment; (3) adding a precipitant a1 into the inorganic salt extracting solution obtained in the step (2) till precipitates are separated, and forming inorganic salt A1 for recycling; (4) adding a precipitant a2 into precipitate-removed supernate in the step (3) till precipitates are separated, and forming inorganic salt A2 for recycling; (5) by parity of reasoning, adding a precipitant an into the precipitate-removed supernate in the former step till precipitates are separated, forming inorganic salt An for recycling. According to the method, the inorganic matters in the sludge are respectively recycled, so that weight reduction for the sludge is realized, and resource utilization of the sludge is also realized; the method is simple in process, high in applicability, simple and convenient to operate, easy to control, low in operating cost, good in treatment effect, wide in application range and easy to popularize and use.
Owner:TIANJIN ENEW ENVIRONMENTAL PROTECTION ENGCO LTD
Process for Complete Utilisation of Olivine Constituents
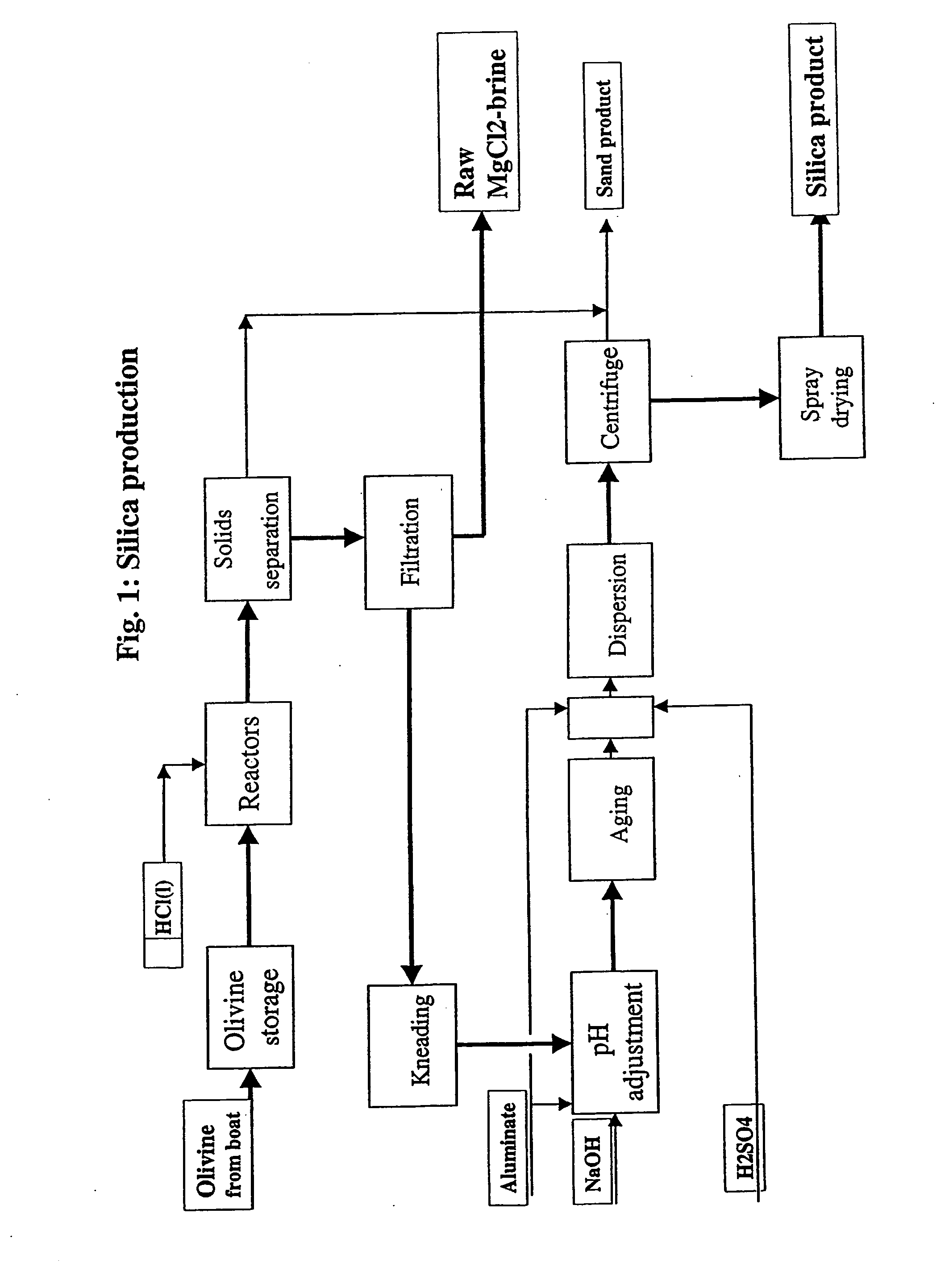
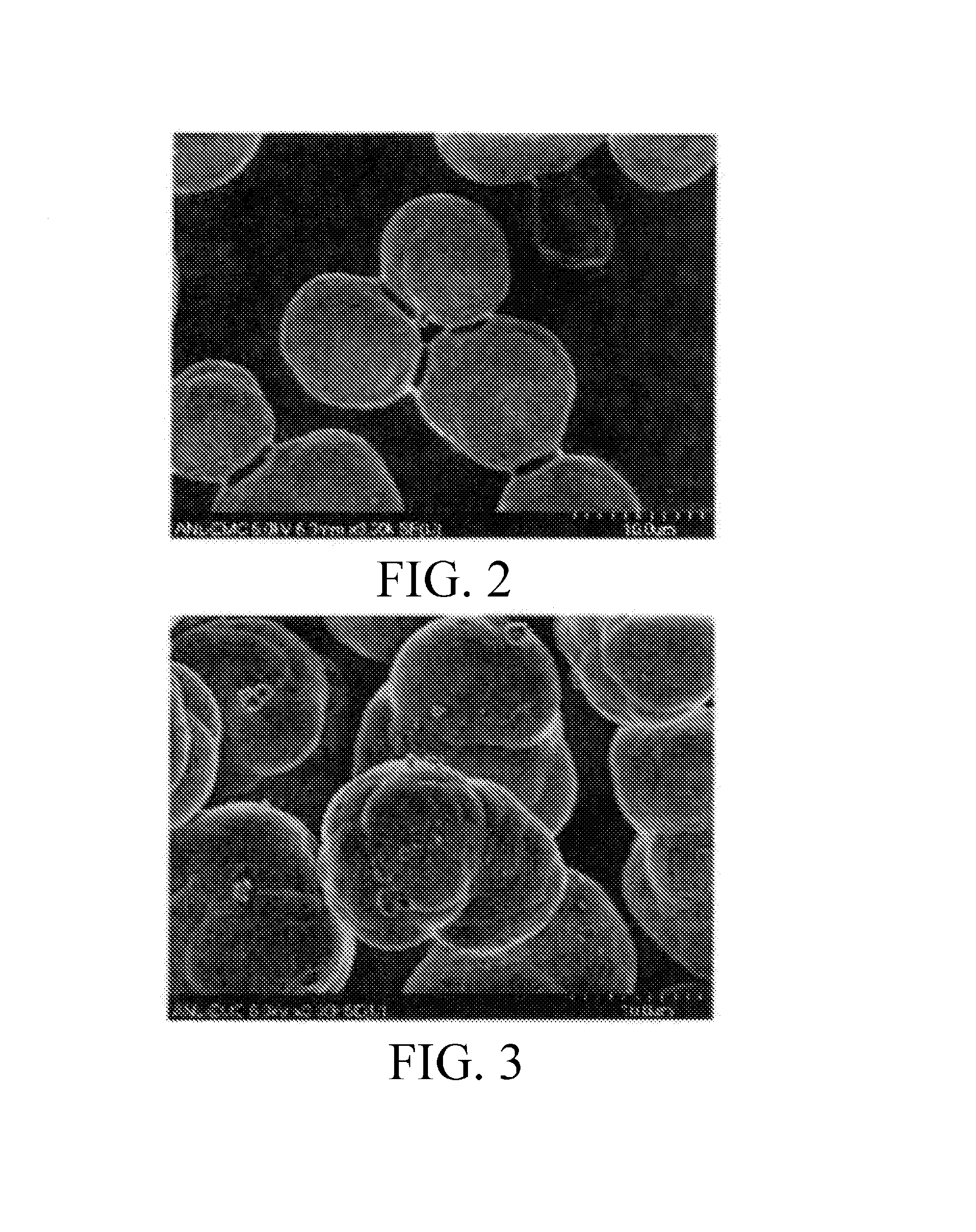
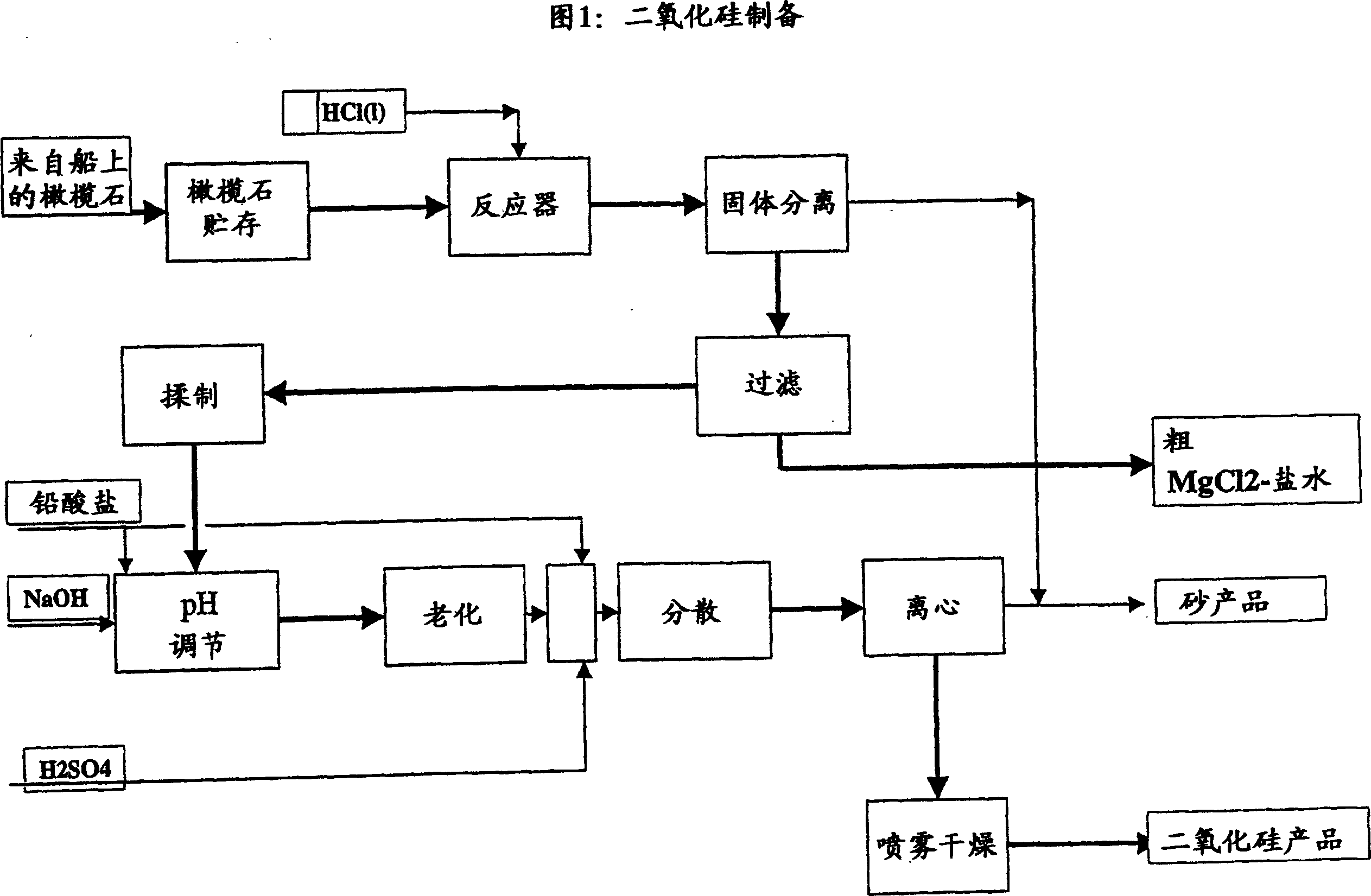
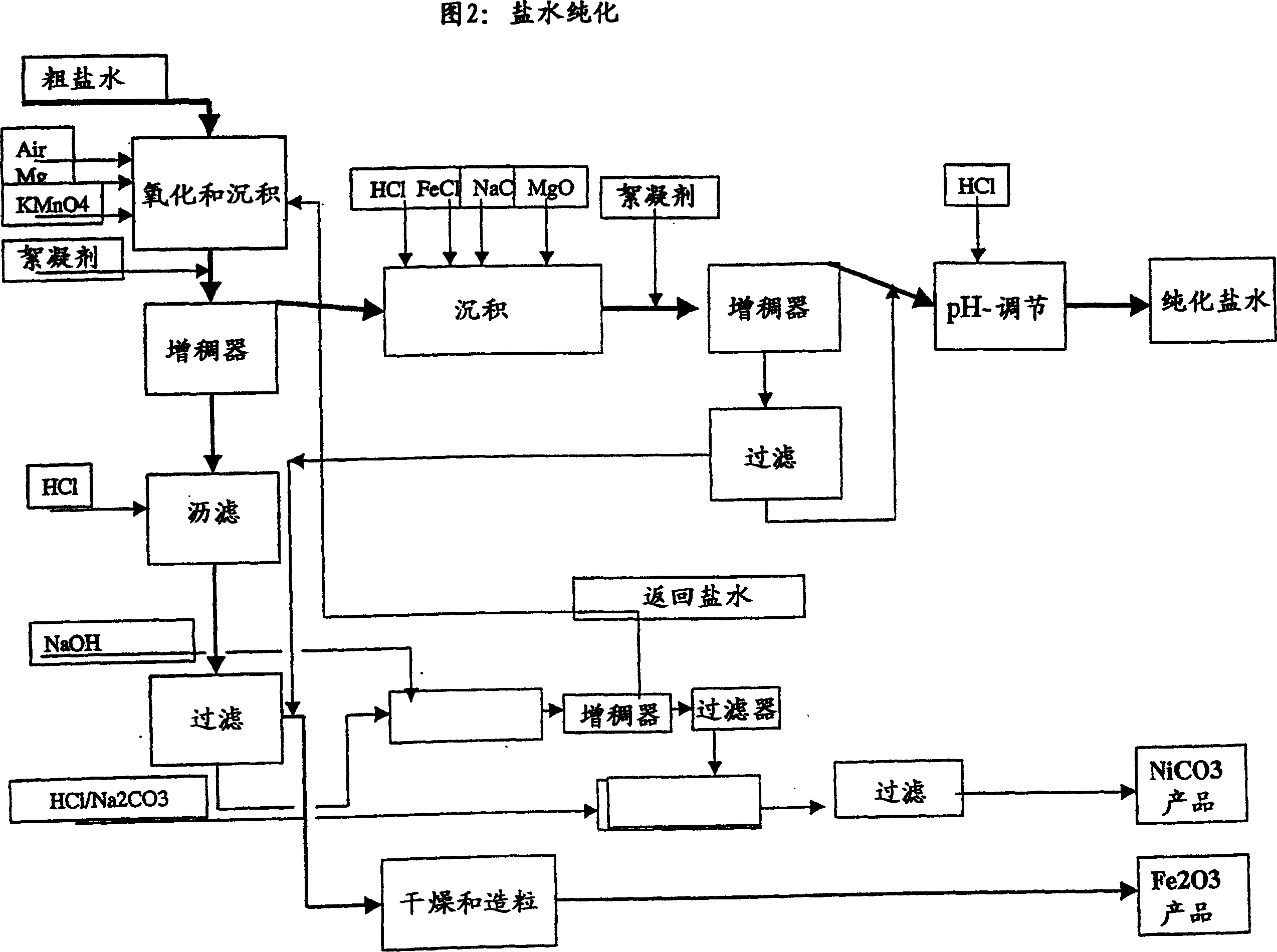
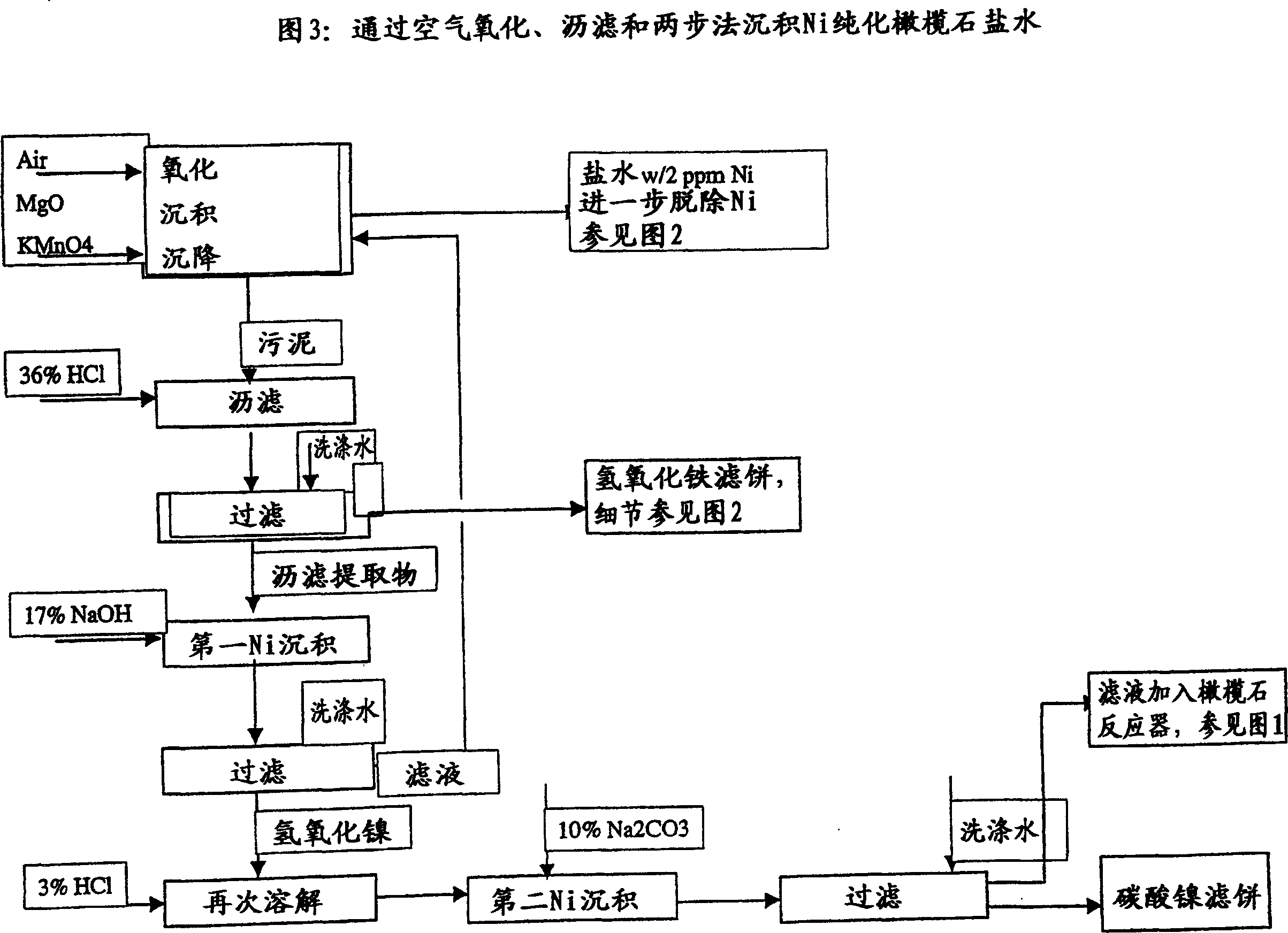
ActiveUS20080075645A1Efficient separationLow costOxide/hydroxide preparationSolvent extractionNickel compoundsOlivine
A novel process for complete utilization of olivine is based on purification of brine by oxidation and precipitation of iron and nickel compound.
Owner:AMG ADVANCED METALLURGICAL GRP INVESTMENT BV
Composite carbonate and method for producing the same
ActiveUS20110114900A1Reduce the amount of wasteConductive materialCarbonate/bicarbonate preparationManganeseCobalt atom
The present invention provides a method for producing a nickel atom-, manganese atom- and cobalt atom-containing composite carbonate that is high in specific surface area and large in tap density, and useful as a raw material for producing a lithium nickel manganese cobalt composite oxide to be used in a positive electrode active material for use in a lithium secondary battery. The composite carbonate includes nickel atoms, manganese atoms and cobalt atoms, and has an average particle size of 5 μm or more and less than 20 μm, a BET specific surface area of 40 to 80 m2 / g and a tap density of 1.7 g / ml or more.
Owner:NIPPON CHECMICAL IND CO LTD
Preparation method of graphene/nickel bicarbonate nanometer cubic three-dimensional composite material and application of composite material to energy storage
InactiveCN107640761AImprove electrochemical performanceSimple methodHybrid capacitor electrodesCell electrodesFreeze-dryingHYDROSOL
The invention provides a preparation method of a graphene / nickel bicarbonate nanometer cubic three-dimensional composite material. The preparation method comprises the following steps: preparation ofgraphene oxide hydrosol: preparing graphene oxide with a Hummer' s method, and preparing the graphene oxide hydrosol from the prepared graphene oxide; adding urea into the prepared graphene oxide hydrosol, stirring uniformly, and slowly adding nickel acetate to obtain a precursor solution; transferring the precursor solution into a reaction kettle, placing the reaction kettle into a drying oven, and reacting at a constant temperature for a certain period of time to obtain graphene / nickel bicarbonate aerogel; performing vacuum freeze drying on the obtained graphene / nickel bicarbonate aerogel toobtain the graphene / nickel bicarbonate nanometer cubic three-dimensional composite material. The prepared graphene / nickel bicarbonate nanometer cubic three-dimensional composite material is applied to power lithium batteries and super batteries as an electrode material, and has excellent electric performance. The preparation method provided by the invention has the advantages of simple reaction process, easiness in operation, low cost, energy saving and environmental friendliness, and is suitable for industrial application.
Owner:ZHENGZHOU UNIV
Composite carbonate and method for producing the same
InactiveUS7897069B2Reduce the amount of wasteNon-metal conductorsConductive materialManganeseCobalt atom
The present invention provides a nickel atom-, manganese atom- and cobalt atom-containing composite carbonate that is high in specific surface area and large in tap density, and useful as a raw material for producing a lithium nickel manganese cobalt composite oxide to be used in a positive electrode active material for use in a lithium secondary battery, and provides a method for industrially advantageously producing the composite carbonate. The composite carbonate includes nickel atoms, manganese atoms and cobalt atoms, and has an average particle size of 5 μm or more and less than 20 μm, a BET specific surface area of 40 to 80 m2 / g and a tap density of 1.7 g / ml or more.
Owner:NIPPON CHECMICAL IND CO LTD
Carbonate Precursors for Lithium Nickel Manganese Cobalt Oxide Cathode Material and the Method of Making Same
ActiveUS20170309894A1Reduce energy densityLow tap densityElectrode thermal treatmentLi-accumulatorsManganeseSlurry
A method for producing a M-carbonate precursor of a Li-M oxide cathode material in a continuous reactor, wherein M=NixMnyCozAn, A being a dopant, with x>0, y>0, 0≦z≦0.35, 0≦n≦0.02 and x+y+z+n=1, the method comprising the steps of: —providing a feed solution comprising Ni-, Mn-, Co- and A-ions, and having a molar metal content M″ feed, —providing an ionic solution comprising either one or both of a carbonate and a bicarbonate solution, the ionic solution further comprising either one or both of Na- and K-ions, —providing a slurry comprising seeds comprising M′-ions and having a molar metal content M′ seeds, wherein M′=Nix′Mny′Coz′A′n′, A′ being a dopant, with 0≦x′≦1, 0≦y′≦1, 0≦z′≦1, 0≦n′≦1 and x′+y′+z′+n′=1, and wherein the molar ratio M′ seeds / M″ feed is between 0.001 and 0.1, —mixing the feed solution, the ionic solution and the slurry in the reactor, thereby obtaining a reactive liquid mixture, —precipitating a carbonate onto the seeds in the reactive liquid mixture, thereby obtaining a reacted liquid mixture and the M-carbonate precursor, and —separating the M-carbonate precursor from the reacted liquid mixture.
Owner:UMICORE AG & CO KG +1
Method for producing size selected particles
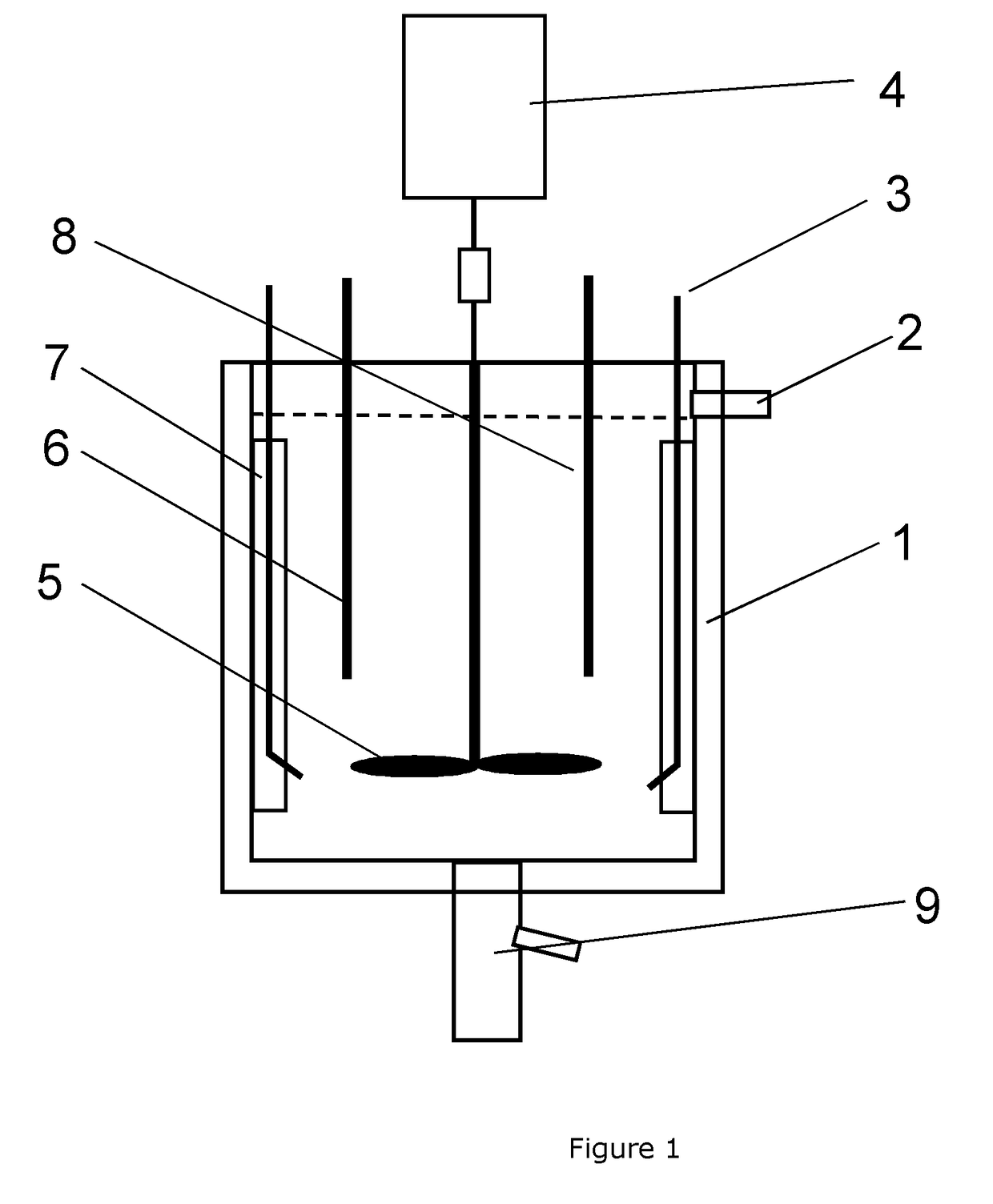
ActiveUS20140341797A1Improve featuresHigh tap densityCell electrodesLiquid-gas reaction processesSufficient timeProcess engineering
The invention provides a system for preparing specific sized particles, the system comprising a continuous stir tank reactor adapted to receive reactants; a centrifugal dispenser positioned downstream from the reactor and in fluid communication with the reactor; a particle separator positioned downstream of the dispenser; and a solution stream return conduit positioned between the separator and the reactor. Also provided is a method for preparing specific sized particles, the method comprising introducing reagent into a continuous stir reaction tank and allowing the reagents to react to produce product liquor containing particles; contacting the liquor particles with a centrifugal force for a time sufficient to generate particles of a predetermined size and morphology; and returning unused reagents and particles of a non-predetermined size to the tank.
Owner:UCHICAGO ARGONNE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com