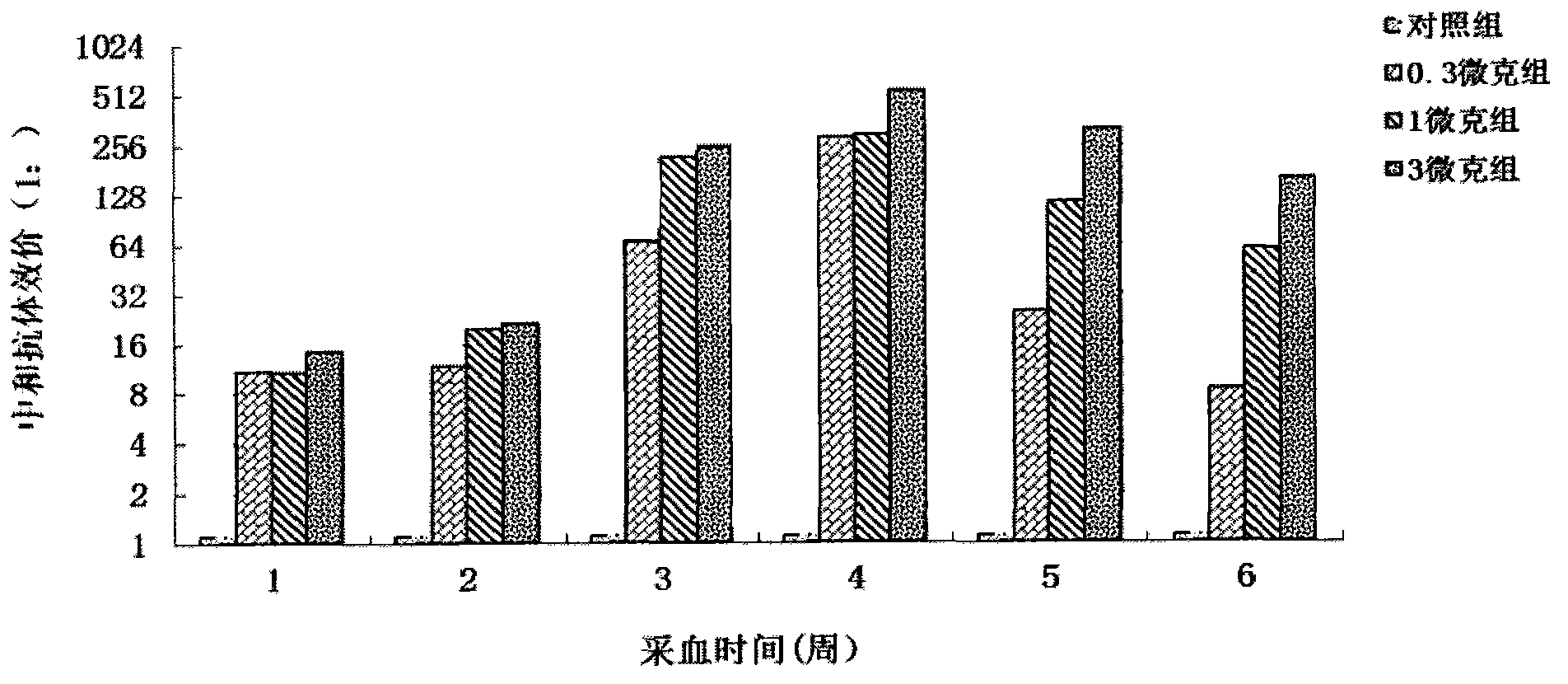
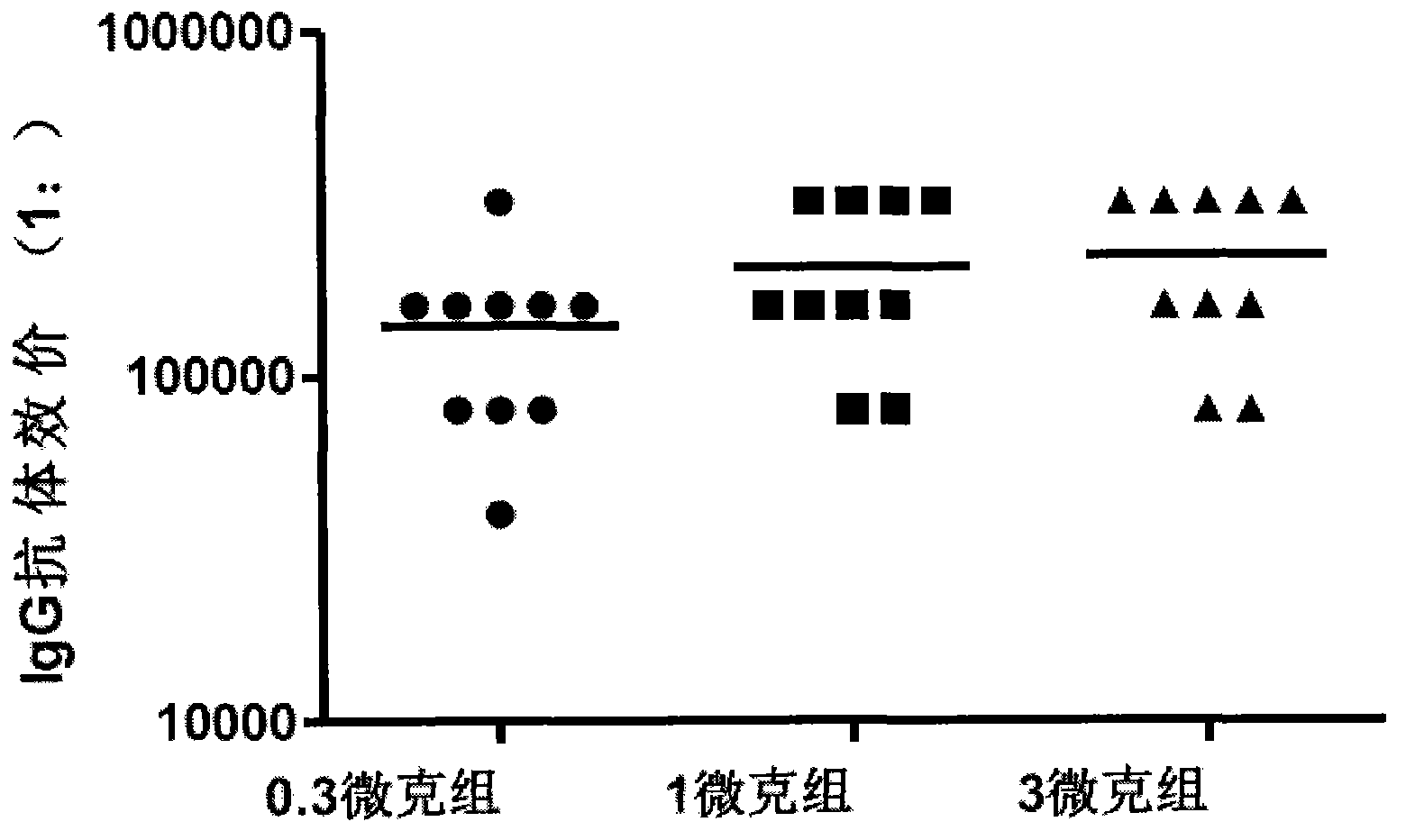
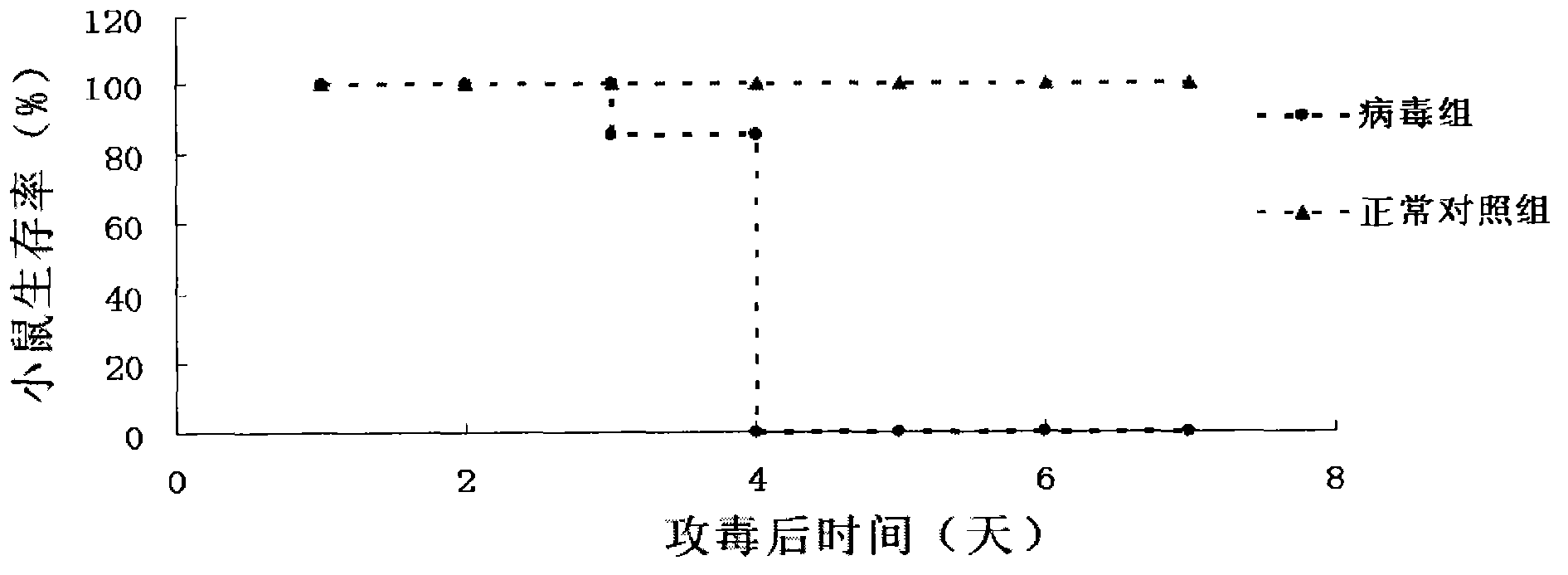
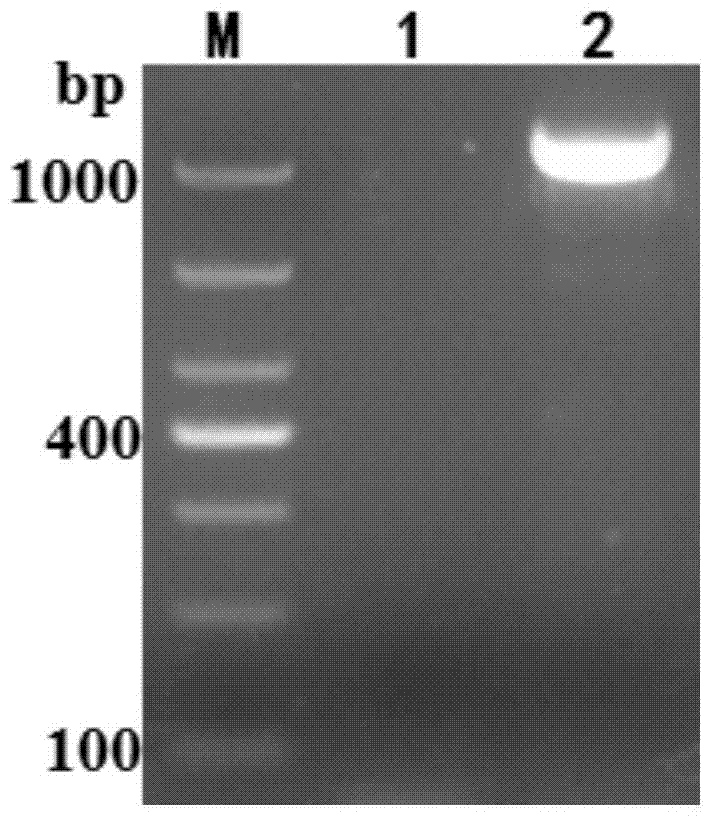
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
195 results about "Viral type" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viral diseases: Types list. The list of types of Viral diseases mentioned in various sources includes: Molluscum contagiosum. HTLV. HTLV-1. HIV/AIDS. Human Papillomavirus. Herpesvirus.
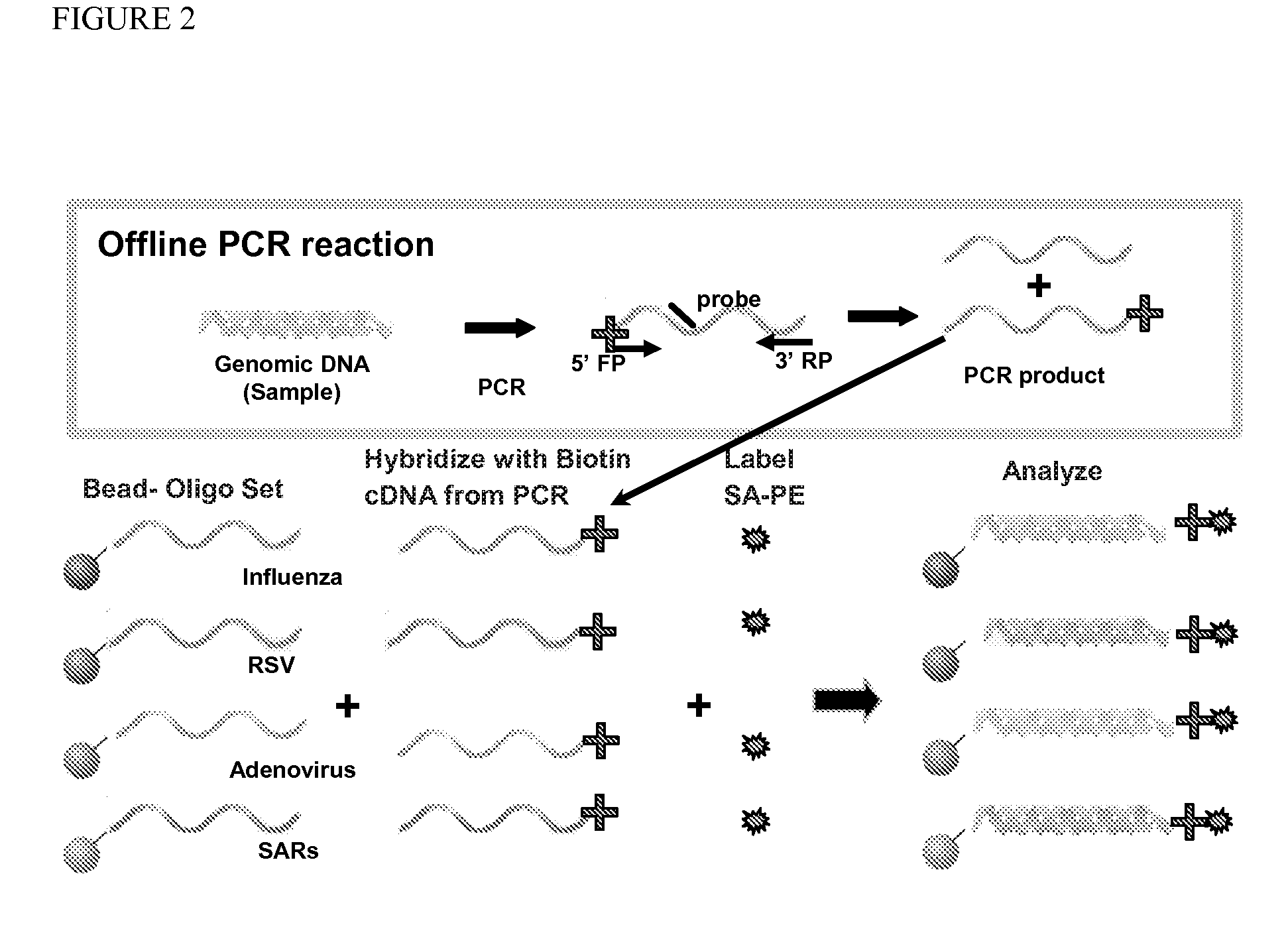
Method for detecting various respiratory viruses and primers and probes thereof
InactiveCN101985665AEasy to operateStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceMicrosphereNucleotide
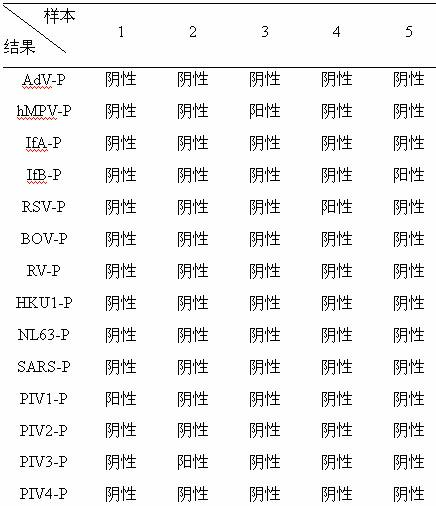
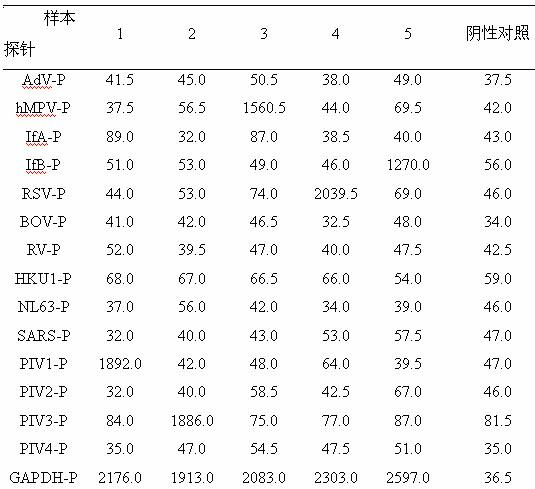
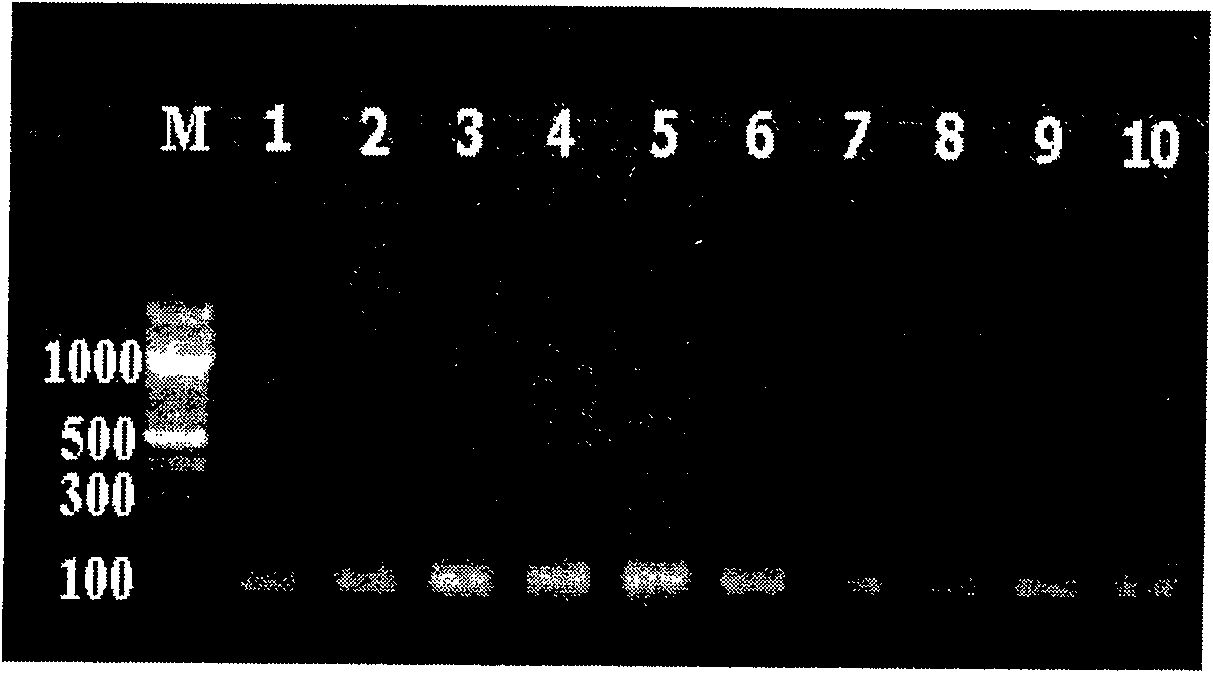
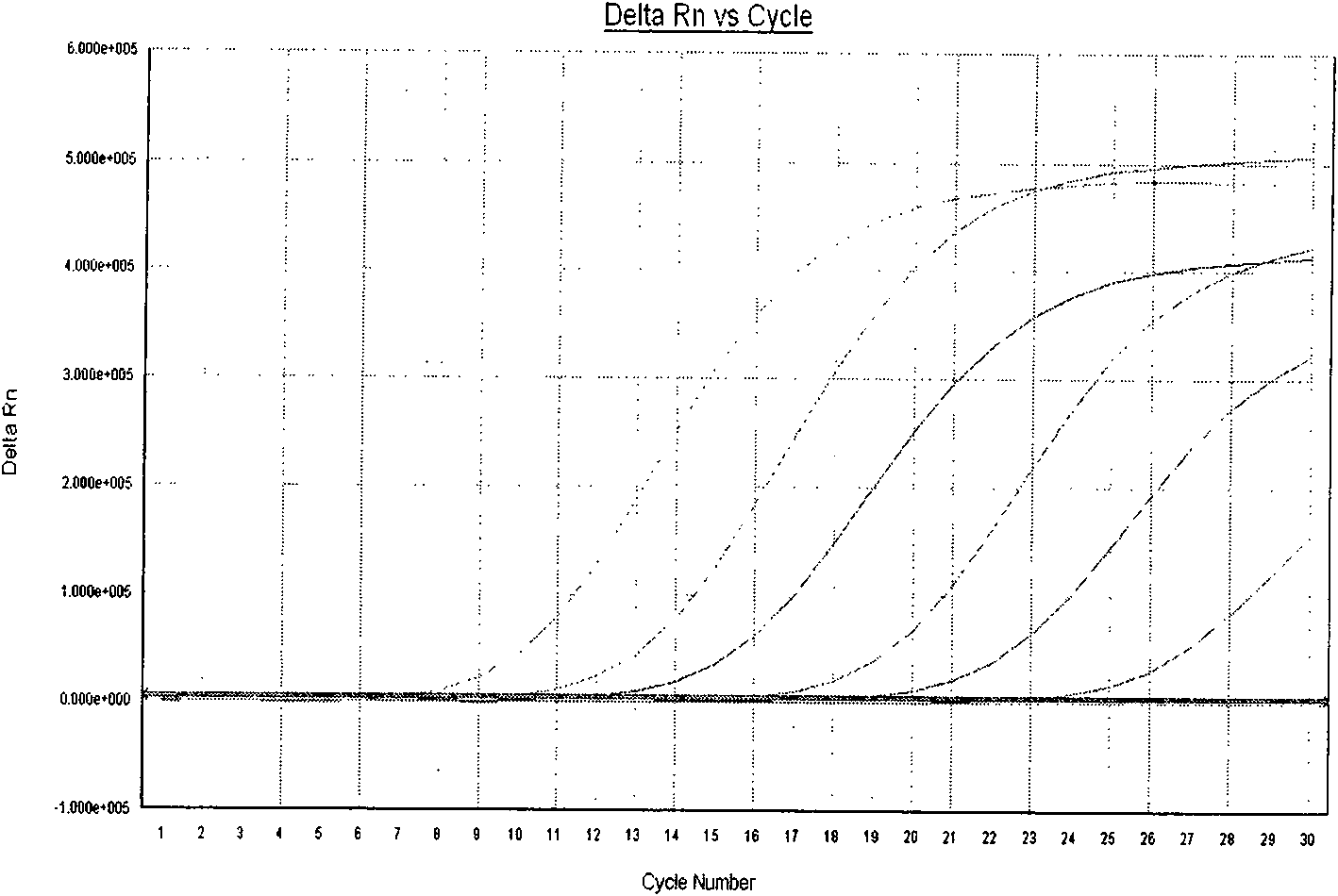
The invention belongs to the technical fields of biochips and diagnostic reagents, and discloses a method for detecting various respiratory viruses, and primers and probes thereof. In the invention, nucleotide sequences of 14 respiratory viruses, namely adenovirus, human metapneumovirus, influenza virus A, influenza virus B, respiratory syncytial virus, bocavirus, rhinovirus, coronavirus (HKU1, NL63 and SARS), and parainfluenza virus (type I, type II, type III and type IV) are analyzed, and corresponding reverse transcription primers, PCR primers and specific probes are designed. Specific gene segments are amplified by reverse transcription and multiple asymmetric PCR methods; a fluorescence-coded microsphere group coupled with the virus specific probes and the PCR amplification product are incubated and hybridized by liquid phase chip technology; and finally the Bio-PlexTM200 is used for detection. The detection method has the advantages of high flux, high specificity and sensitivity, stable results and good repeatability, the detection method is easy to operate, and the detection speed is high.
Owner:FUDAN UNIV +1
Three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as kit thereof
InactiveCN101886138AEasy to detectHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCoxsackievirus a16Reverse transcription polymerase chain reaction
The invention provides a three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as a kit thereof. The method can rapidly and accurately detect the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of enterovirus in a sample. The method comprises the following steps of: (1) acquiring and conveying a sample of an infected patient or a suspected patient; (2) preprocessing the sample and extracting RNA; (3) detecting the sample by adopting a one-step PCR-three-color fluorescent probe in-vitro amplification method; and (4) analyzing the corresponding sample according to the fluorescence intensity of each amplification reaction after the amplification reaction is finished, thereby judging the existence of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus in the acquired sample and being capable of carrying out accurate quantitation (a figure 3) on the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus. The invention realizes the aim of carrying out rapid and accurate combined detection of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus.
Owner:BEIJING SUOAO BIOTECH
Vaccine for hand-foot-and-mouth disease viruses
ActiveCN101897963AEnsure safetyGood immune effectMicroorganism based processesAntiviralsAdjuvantInfectious Disorder
The invention relates to preparation of a vaccine for hand-foot-and-mouth disease viruses and an application method thereof, belonging to a novel vaccine for preventing infectious diseases. The vaccine of the invention mainly comprises the components of high-purified inactivated human enteropathogenic virus 71 (EV 71) and an aluminum adjuvant. The vaccine, which is prepared according to the method of the invention, has excellent immunogenicity, and after immunity, organisms can selectively generate a high titer serum neutralization antibody, thereby preventing infectious diseases caused by the human EV 71.
Owner:BEIJING LUZHU BIOTECH +1
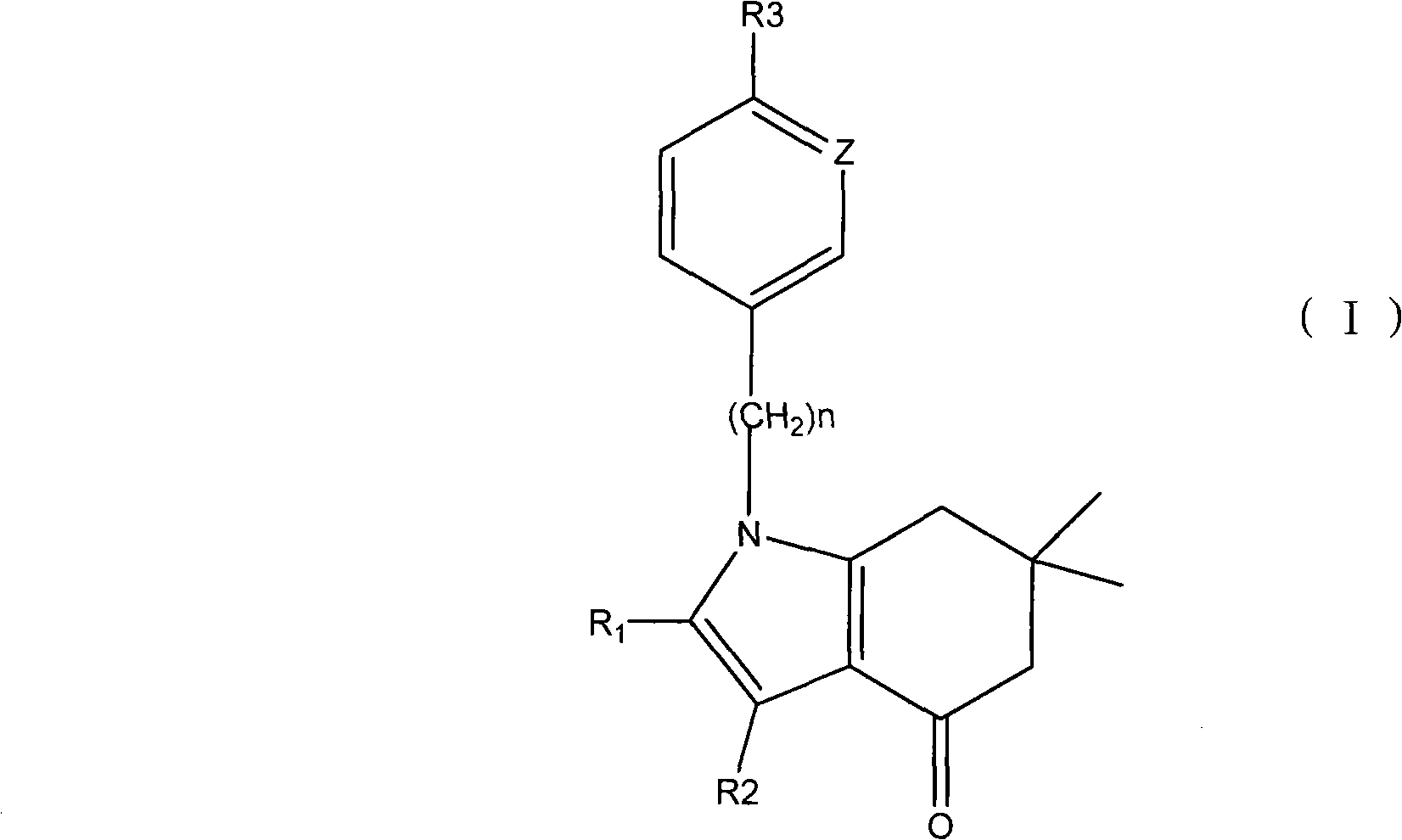
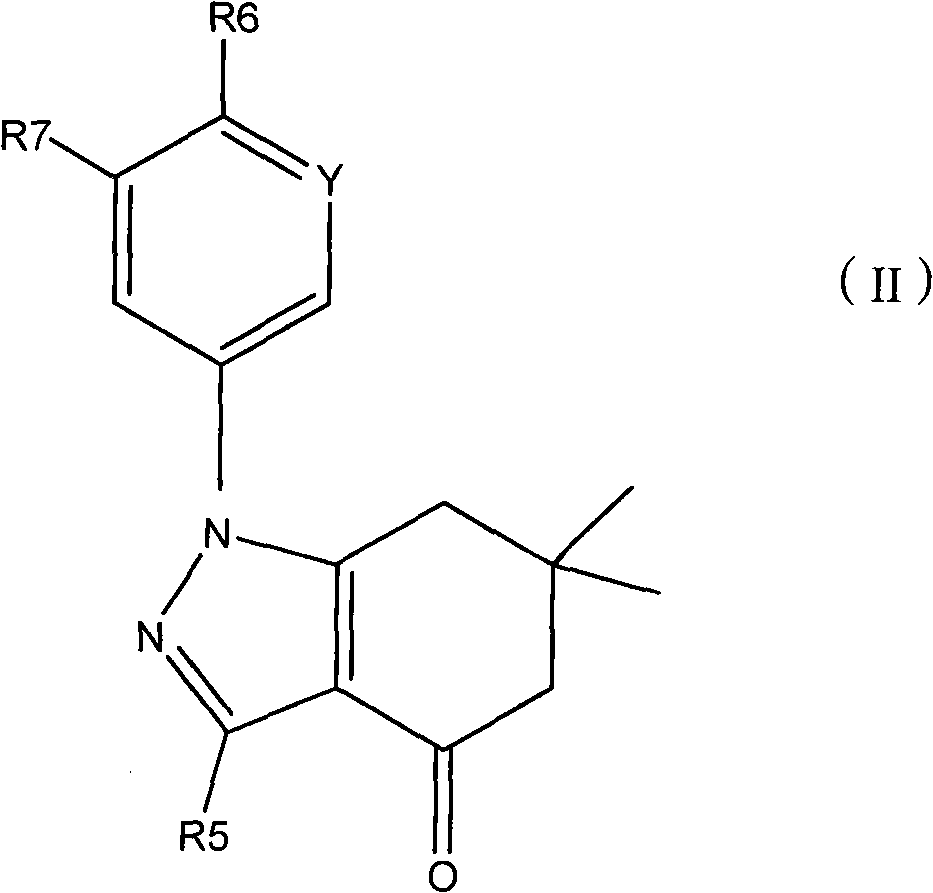
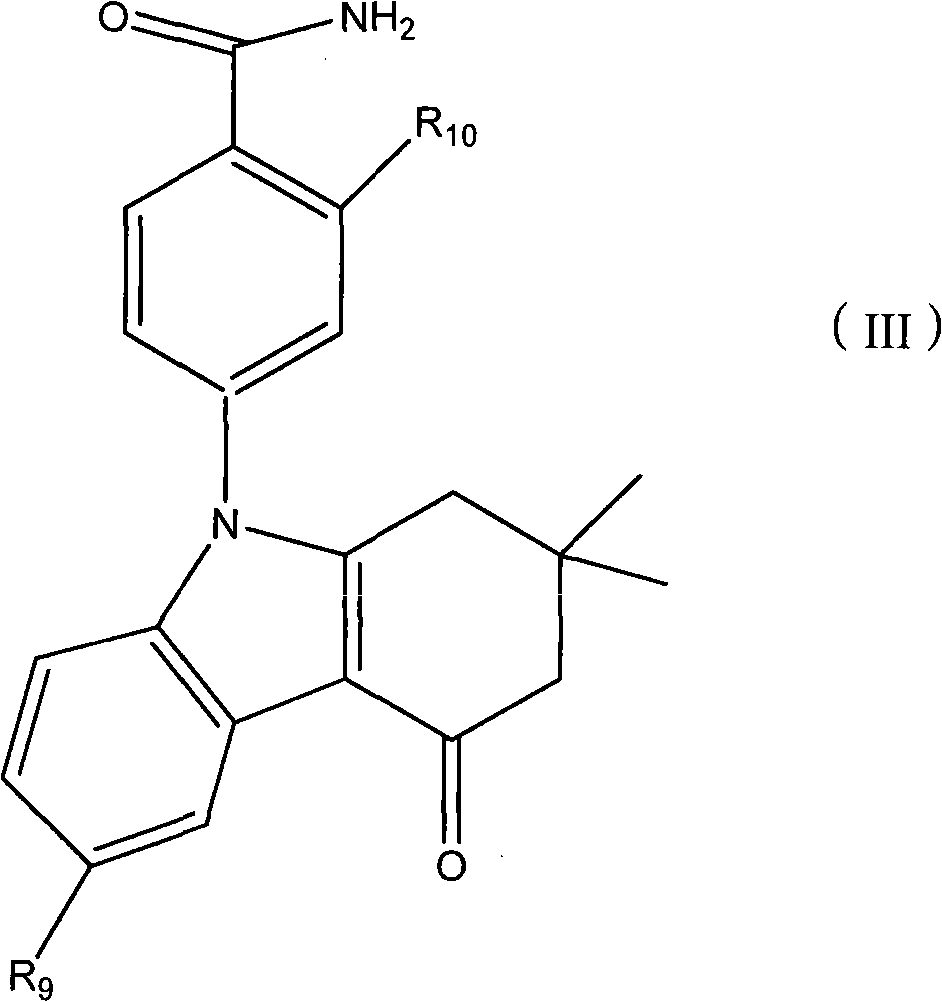
Applications of tetrahydroindolone/tetrahydroindazolone/tetrahydrocarbazole derivatives and salts thereof in preparation of antiviral medicine
The invention relates to an application of tetrahydroindolone / tetrahydroindazolone / tetrahydrocarbazole derivatives and salts thereof in the preparation of antiviral drugs. The tetrahydroindolone derivatives, the tetrahydroindazolone derivatives or the tetrahydrocarbazole derivatives, as well as the salts thereof of the invention have anti-viral functions, and the virus comprises I-typed and II-typed herpes virus, coxsackie virus type 3 (CVB3) and hepatitis B virus.
Owner:广州少伯控股集团有限公司 +1
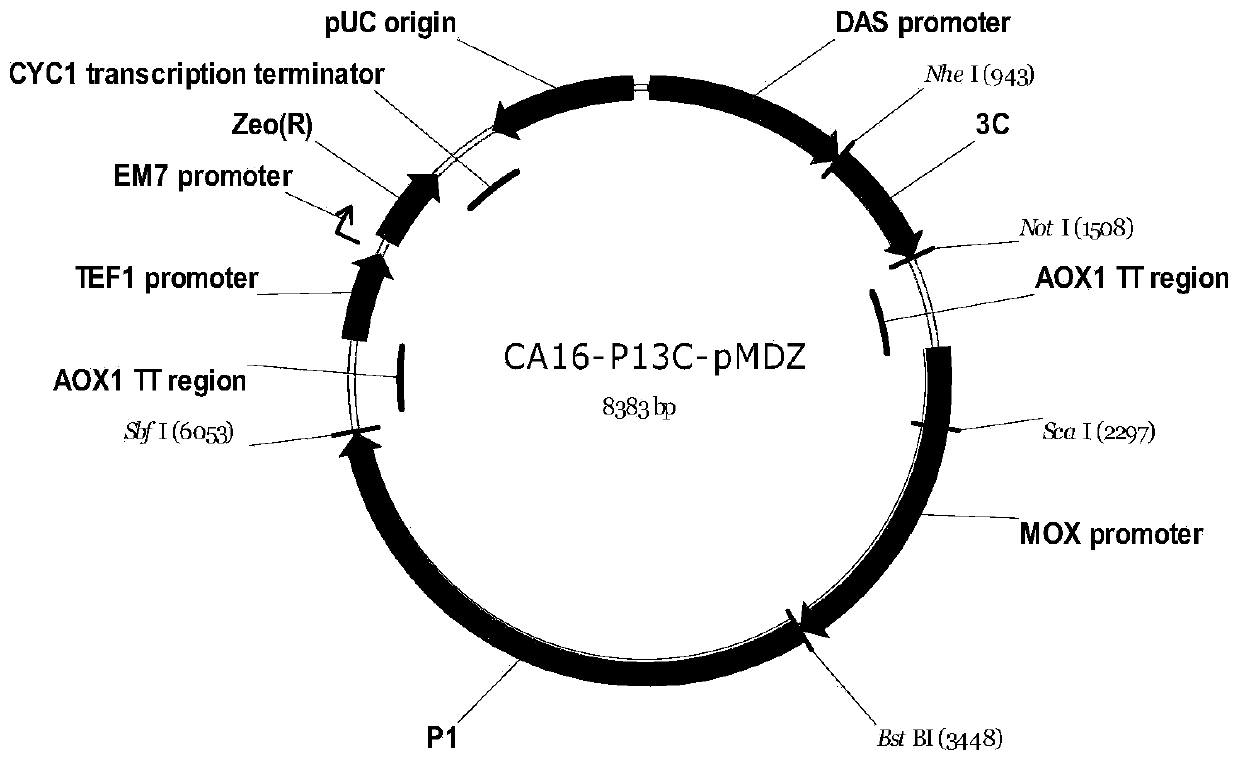
Preparation method for recombinant coxsackie virus A16 like particle and applications thereof
InactiveCN102533797AIncrease productionFungiInactivation/attenuationYeastHand-foot-and-mouth disease
The invention discloses a preparation method for a recombinant coxsackie virus A16 like particle, which comprises the following steps: (1) cloning a P1 gene and a 3CD gene of a coxsackie virus A16 to a target plasmid to obtain a recombinant expression vector; (2) transforming a target yeast cell by using the recombinant expression vector obtained in the step (1) to obtain a recombinant yeast cellfor expressing the P1 gene and the 3CD gene; and (3) cracking the recombinant yeast cell obtained in the step (2), and separating to obtain the recombinant coxsackie virus A16 like particle. The recombinant coxsackie virus A16 like particle can be prepared in a yeast expression system by the method provided by the invention. Compared with a wild-type P1 gene and a wild-type 3CD gene, the yield ofthe coxsackie virus A16 like particle in the yeast expression system is greatly increased through the optimization of codons of the P1 gene and the 3CD gene, and the recombinant coxsackie virus A16 like particle can be further used for producing candidate preventive vaccines and pharmaceutical compositions for infant hand-foot-and-mouth diseases.
Owner:BEIJING UNIV OF TECH
Human papillomaviruse type hybrid virus-like particles and preparation method thereof
ActiveCN102747047AIncrease production costProne to cross protectionViral antigen ingredientsInactivation/attenuationDiseaseHuman papillomavirus
The present invention relates to human papillomavirus (HPV) type hybrid virus-like particles and a preparation method thereof. The virus-like particles can be used for preventions two or more HPV infections and diseases caused by HPV infections. The present invention further relates to uses of the protein and the virus-like particles in preparations of drug compositions or vaccines, wherein the drug compositions or the vaccines are provided for preventions of HPV infections and diseases caused by HPV infections, and the diseases comprise cervical cancer, condyloma acuminatum, and the like.
Owner:XIAMEN UNIV +1
Coxsackie virus A16 virus strain, uses of strain, vaccine and preparation method of vaccine
ActiveCN104099301AEffective immune activityObvious paralysisSerum immunoglobulinsImmunoglobulins against virusesCoxsackievirus a16Antiviral drug
The invention provides a Coxsackie virus A16 virus strain, uses of the strain, a vaccine and a preparation method of the vaccine. The preservation number of the Coxsackie virus A16 virus strain is CGMCC No.6954. The CA16 virus strain has strong virulence, can be used for evaluating the CA16 vaccine, and can also be used for researching the CA16 virus infection mechanism. A method for establishing a Coxsackie virus A16 infected animal model provided by the invention can provide a stable animal model, and provides a foundation for the development of the Coxsackie virus A16 vaccine, the screening of antiviral drugs and the researches of the CA16 virus infection mechanism. The vaccine prepared by using the CA16 virus has effective immune activity.
Recombinant protein coded by grass carp reovirus (GCRV) type-II S10 gene, polyclonal antibody prepared from recombinant protein and application of recombinant protein
InactiveCN103539842AImproving immunogenicityGood immune protectionViral antigen ingredientsVirus peptidesNucleotideStructural protein
The invention discloses a recombinant protein coded by a grass carp reovirus (GCRV) type-II S10 gene, a polyclonal antibody prepared from the recombinant protein and an application of the recombinant protein. The amino acid sequence of the GCRV type-II S10 gene-coded protein is shown by SEQ ID No:2, and the nucleotide sequence coding the protein is shown by SEQ ID No:1. The recombinant protein disclosed by the invention has good immunogenicity; compared with the proteins of other structures of GCRV, the specific antibody valence generated by inducing an immune animal is higher. Further tests indicate that by immunizing the grass carp with the S10 recombinant protein, the grass carp can be induced to generate high specific antibody, and certain immune protection effect can be realized against the attack of a virulent strain of GCRV. Therefore, the study and application of the GCRV type-II S10 gene-coded protein are of vitally important significance to the development of a novel vaccine for the hemorrhagic disease of grass carp and an immunological detection kit, and a new effective solution is provided for the hemorrhagic disease of grass carp.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Multiple real-time quantitative PCR primer, probe and detection method for identifying viral pathogens relevant to fever with eruption syndrome as infection diseases
ActiveCN102140543ADetection ExpressImprove efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceChickenpoxHerpes zoster virus
The invention discloses multiple real-time quantitative PCR primer, probe and a detection method for identifying viral pathogens relevant to fevers with eruption syndromes as infection diseases, which is used for carrying out multiple real-time fluorescent quantitative PCR detection on varicella-herpes zoster viruses, human small DNA (Deoxyribonucleic Acid) viruses B19, enteroviruses (enteroviruses 71 type and coxsackie viruses A16 type), dengue viruses, rubella viruses and measles viruses. The invention can simultaneously carry out qualitative or quantitative detection on eight kinds of human viruses in various types of samples by multiple double-tubes PCR. The detection method has the advantages of simple operation, short time consumption, high sensitivity and strong specificity, is suitable for field detection, early diagnosis, epidemics detection and research and the like, and takes the actions of assistance and identification diagnosis on the fevers with eruption syndromes.
Owner:SUN YAT SEN UNIV
Method for preparing recombinant coxsackievirus A16 type virus-like particles
ActiveCN103436553AUniform shapeStable traitsInactivation/attenuationAntiviralsOpen reading frameCoxsackievirus a16
The invention relates to the field of immunity technology, and particularly relates to a method for preparing recombinant coxsackievirus A16 type virus-like particles. In the method, the PI protein and 3C protein of CA16 are expressed in a double-expression plasmid through a yeast expression system. In order to realize the preparation of CA16 virus-like particles, according to the method provided by the invention, an expression vector and an expression system for CA16 virus-like particles are established, wherein the expression vector comprises two open reading frames which respectively contain a P1 protein expression sequence and a 3C protein expression sequence of CA16. According to the invention, a method for preparing recombinant coxsackievirus-like particle protein by use of co-expression of 3C protease and P1 protein is established through codon optimization; and the method can obtain virus particles with high purity, uniform form and stable characters, is used for preparing a hand-foot-and-mouth disease vaccine, and has a great market value.
Owner:上海博唯生物科技有限公司 +1
Monoclonal antibody with the capability of neutralizing enterovirus type 71 infection
InactiveUS20060292693A1Enhanced interactionEnhanced antibody-dependent cellular cytotoxicityAnimal cellsMicrobiological testing/measurementEnterovirusMonoclonal antibody
Owner:SCINOPHARM TAIWAN LTD
Evaluating biological material for unassociated virus-size particles having an adenovirus hexon protein epitope
ActiveUS9816912B2Easy to useComplicate to differentiateSsRNA viruses negative-senseSsRNA viruses positive-senseEpitopeAdeno associate virus
A method for evaluating a biological material for unassociated virus-size particles having a particular epitope indicative of an adeno-associated virus viral type or an adenovirus viral type uses a fluorescent antibody stain specific for binding with the epitope and a fluid sample with the virus-size particles and fluorescent antibody stain is subjected to flow cytometry with identification of fluorescent emission detection events indicative of passage through a flow cell of a flow cytometer of unassociated labeled particles of virus size including such a virus-size particle and fluorescent antibody stain.
Owner:SARTORIUS BIOANALYTICAL INSTR INC
A-type antigen polypeptide, fusion antigen polypeptide and vaccine of foot and mouth disease virus
ActiveCN105418738AImproving immunogenicityBroad spectrum immunitySsRNA viruses positive-senseAntibody mimetics/scaffoldsImmunogenicityBroad spectrum
The present invention discloses an A-type antigen polypeptide, a fusion antigen polypeptide and a vaccine of a foot and mouth disease virus, in particular relating to an A-type antigen polypeptide and a fusion antigen polypeptide of the foot and mouth disease virus, and a foot and mouth disease virus vaccine containing the antigen polypeptide and / or a fusion antigen polypeptide. The present invention also provides preparation methods of the antigenic polypeptide, the fusion antigen polypeptide and the vaccine. The present invention further provides applications of the antigen polypeptide, the fusion antigen polypeptide and the vaccine in preventing and controlling of the foot and mouth disease virus infection. The A-type antigen polypeptide, the fusion antigen polypeptide and the vaccine of the foot and mouth disease virus disclosed by the present invention have a broad spectrum immunogenicity, and may produce good immunogenicity on different foot and mouth disease viruses and variants thereof.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP +1
Recombined pig influenza virus NP antigen, its preparing method and use in diagnosis
The present invention discloses recombinant pig influenza virus NP antigen and its preparation process and application in diagnosis. Type-A influenza virus genome consists of 8 segments of RNA, and its nucleoprotein (NP) has type and group specificity and is the basis of typing diagnosis. The recombinant pig influenza virus NP antigen has colibacillus prokaryotic expression system to express complete NP gene of separated pig influenza virus strain in China, and the expressed NP is used as antigen in detecting type-A influenza virus infection produced anti-NP protein antibody. The NP gene is the basis for classifying and diagnosing pig influenza virus with many subtypes. Therefore, the diagnosis technology established based on recombinant SIV NP antigen may be used in detecting type-A influenza virus infection of pig, fowl, horse and human.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Hand-foot-and-mouth disease resistant human immunoglobulin, and preparation and using methods and application thereof
InactiveCN102190725ASignificant effectImprove survival rateImmunoglobulins against virusesAntiviralsTotal proteinPasteurization
The invention relates to a hand-foot-and-mouth disease resistant human immunoglobulin, and preparation and using methods and application thereof in pharmacy. The preparation method comprises the following steps of: separating component I+II+III, I+III and II precipitates in turn by using a low-temperature methanol protein separation method; performing pasteurization on a component II precipitate; refining and purifying; performing dealcholization; preparing; and sterilizing, packaging and performing low-pH incubated inactivation. The product has antibody titer of not less than 1:640 for enteroviruses (including one or more of coxsackie virus, Echo and EV71), the immunoglobulin content is not less than 95.0 percent of the total protein content, and the sum of IgG monomer and dimer is not less than 95 percent; and the using method is that a specific antibody with titer of 160,000-320,000 is intravenously infused. The invention is suitable for industrial production; and the product has high-titer enterovirus resistant specificity, is safe and reliable, and can become an effective medicine for treating the hand-foot-and-mouth disease.
Owner:HUALAN BIOLOGICAL ENG INC
Multivalent immunogenic composition
ActiveCN103394082AImprove securitySave the number of seedsBacterial antigen ingredientsViral antigen ingredientsHemagglutininTetanus toxoids
The invention provides a multivalent immunogenic composition, which includes an inactivated hepatitis A antigen and an inactivated poliovirus. The composition also can further include over one or two of a purified pertussis antigen, diphtheria toxoid, tetanus toxoid, filamentous hemagglutinin, Haemophilus influenzae type b polysaccharide, Neisseria meningitidis capsular polysaccharide, a hepatitis B virus antigen, enterovirus 71 and a coxsackievirus A16 antigen, and a physiologically acceptable carrier. The composition involved in the invention is employed to immunize the inoculated population in the form of a bivalent vaccine or more combined vaccines. Without reducing the immune effects of each immunizing antigen, the inoculation number of times can be reduced at the same time, and the time and human resources can also be saved.
Owner:SINOVAC BIOTECH
Method and kit for dual fluorescence quantitative PCR detection of grass carp reovirus types I and II
InactiveCN103484568AImprove accuracySimple and fast operationMicrobiological testing/measurementMicroorganism based processesBiotechnologyViral type
The invention discloses a method for dual fluorescence quantitative PCR detection of grass carp reovirus types I and II. The method can realize that the grass carp reovirus types I and II can be simultaneously detected in one to-be-detected sample, and the load levels of the two reovirus types can be quantified. The invention further provides a kit for dual fluorescence quantitative PCR detection of the grass carp reovirus types I and II. According to the detection method and the kit which are provided by the invention, the operation is simple and convenient, the specificity is strong, the detection sensitivity and accuracy rate are high and reliable, and a wide application prospect is achieved.
Owner:湖南冠牧生物科技有限公司
Multiplex detection of respiratory pathogens
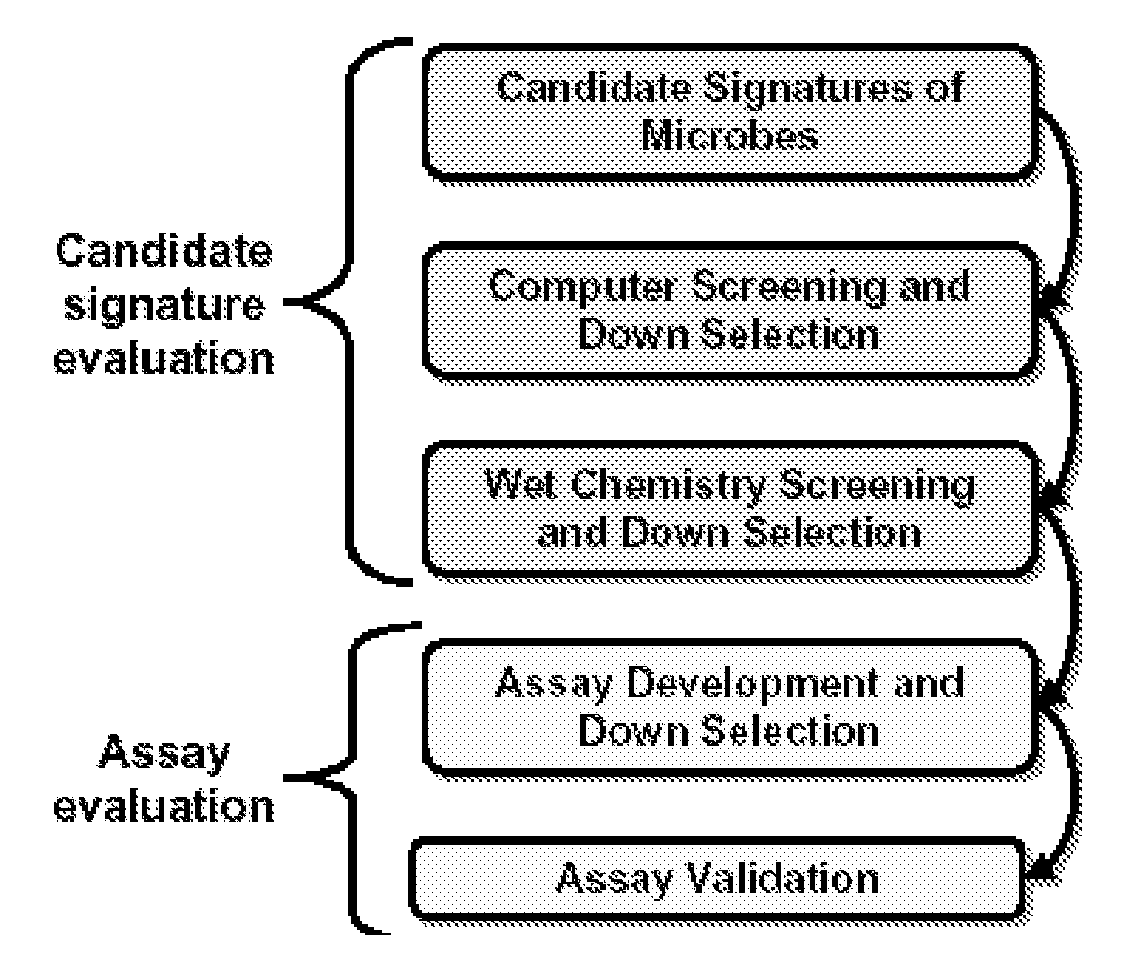
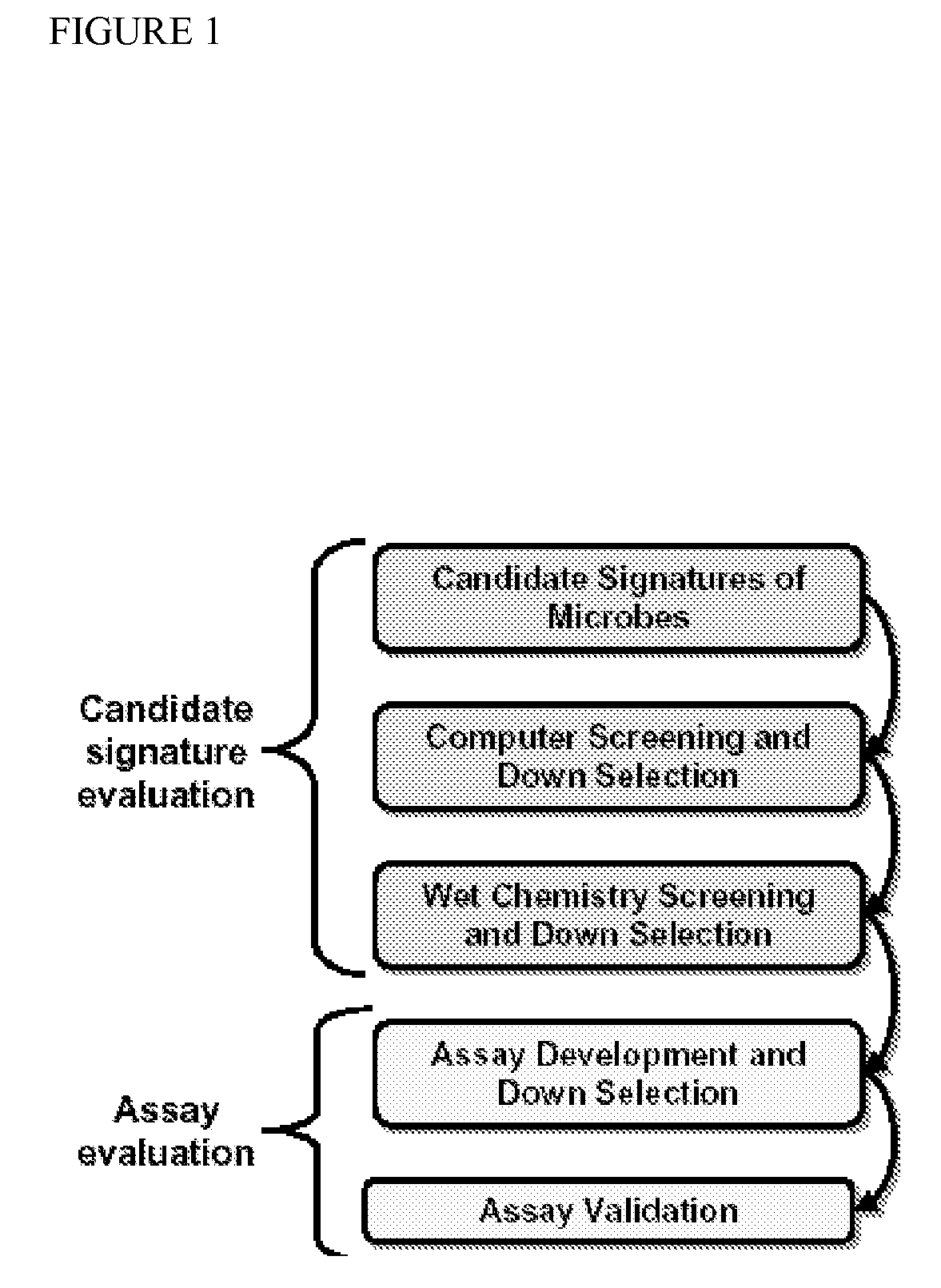
ActiveUS20090305229A1Sugar derivativesMicrobiological testing/measurementViral typeRespiratory pathogens
Described are kits and methods useful for detection of respiratory pathogens (influenza A (including subtyping capability for H1, H3, H5 and H7 subtypes) influenza B, parainfluenza (type 2), respiratory syncytial virus, and adenovirus) in a sample. Genomic sequence information from the respiratory pathogens was analyzed to identify signature sequences, e.g., polynucleotide sequences useful for confirming the presence or absence of a pathogen in a sample. Primer and probe sets were designed and optimized for use in a PCR based, multiplexed Luminex assay to successfully identify the presence or absence of pathogens in a sample.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
A16 type strain of Coxsackie virus and application of the strain
ActiveCN102559606AStable titerImproving immunogenicitySerum immunoglobulinsImmunoglobulins against virusesSequence analysisCoxsackievirus a16
The invention provides an A16 type strain of Coxsackie virus, which has the preservation number of CGMCC No. 5373. When observed with an electronic microscope, the virus is in the shape of an icosahedral three-dimensional symmetrical sphere with the diameter of 23-30nm. VP1 conserved region sequence analysis and mass spectrum analysis are respectively conducted for the strain, and the results indicate that the strain is CA 16 virus which can be efficiently proliferated in Vero cells, and the virus titer can reach 7.01g CCID50 / ml, and furthermore, the strain is free of extraneous contamination, and has good immunogenicity and excellent effects.
Owner:SINOVAC BIOTECH
Enterovirus 71 antigen detection test strip (colloidal gold method)
InactiveCN102243237AImprove featuresHigh sensitivityMaterial analysisCase fatality ratePulmonary edema
The invention relates to the field of biomedicine, and specifically relates to an enterovirus 71 antigen detection test strip (colloidal gold method) and a preparation method and application thereof. Enterovirus 71 can cause hand-foot-and-mouth disease, which has largegeneration proportion of severe infections (viral encephalitis, meningomyelitis virus and pulmonary edema), and a high death rate reaching 10%-25%. The test strip of the invention is used for rapid diagnosis of EV(enterovirus)71 infection. A virus separation and an RT-PCR (reverse transcription-polymerase chain reaction) are methods first used for EV71 antigen detection, but are not suitable for primary clinic usage due to defects of difficult operation and high costs, etc. The invention overcomes the above insufficiencies and provides a reagent, which is highly demanded in clinic detection, simply operated, suitable for various medical disease control sections, and capable of detecting EV71 antigens in human oropharyngeal swabs, bubble liquid, serum or excrement, and also provides the preparation method and application thereof. A technical scheme is as follows: a specimen is dropped on a sample pad, and the EV71 antigen wherein combines with a gold-labeled EV71 polyclonal antibody in a gold-labeled pad and migrates along a chromatography membrane. A detected line captures colloidal gold particles to form a red line visible to naked eyes, so as to realize detection of the EV71 antigen.
Owner:BEIJING BEIER BIOENG
Application of lycorine in preparing medicament for treating diseases caused by human enterovirus 71 type infection
ActiveCN102178678AReduce mortalityRelieve symptomsOrganic active ingredientsNervous disorderViral MyocarditisPulmonary edema
The invention belongs to the field of medicaments, discloses application of lycorine, lycorine salt, lycorine hydrate, lycorine optical isomer or lycorine prodrug in preparing medicaments for treating diseases caused by human enterovirus 71 type infection, preferably treating hand-foot-and-mouth disease, herpangina, viral meningitis, viral encephalitis, flaccid paralysis, pulmonary edema and vital myocarditis. In vitro and in vivo tests prove that the lycorine can inhibit replication and lesion of enterovirus (EV) 71 in cells, has excellent function of inhibiting the EV 71 virus, and has clinical application prospect.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Enterovirus 71 type latex agglutination detection kit, preparation and application
The invention relates to an enterovirus 71 type latex agglutination detection kit, preparation and application. The kit comprises the following substances: (1) a carboxylated latex reagent sensitizing a prokaryotic expression enterovirus 71 type VP1 specific antigen; (2) a carboxylated latex reagent sensitizing three strains of monoclonal mixed antibodies aiming at enterovirus 71 type VP1; (3) enterovirus 71 type positive-negative standard serum, an inactivated virus solution and PBS; and (4) a toothpick or a plastic cement rod which contains a glass slide platform for using the sentization latex to perform aggregation reaction and is used to uniformly mix latex and to-be reacted serum. The kit can detect EV71 antigen in samples of different sources, and overcomes the disadvantages that the sensitized antigen is less in amount and unstable, sensitized protein is easy to fall off, and the like when protein sensitizes common latex. The detection method is applicable to enterovirus 71 type serum epidemiological investigation, clinic assistant diagnosis, regulation and control on EV71 virus replication, anti-virus therapy medicine screening and other scientific research fields.
Owner:绍兴市疾病预防控制中心
Recombinant protein coded by GCRV (grass carp reovirus) II type S9 genes and application thereof
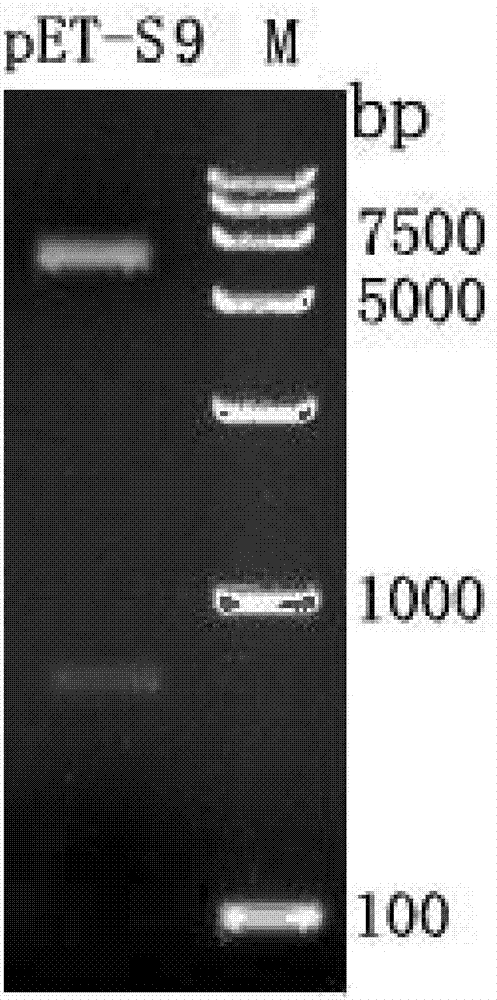
InactiveCN106866796AImproving immunogenicityEasy to identifyViral antigen ingredientsVirus peptidesDiseaseNucleotide
The invention discloses a recombinant protein coded by GCRV (grass carp reovirus) II type S9 genes and application thereof. The amino acid sequence of the recombinant protein is shown as SEQ ID NO:2. The nucleotide sequence for coding the protein is shown as SEQ ID NO:1. The obtained recombinant protein has better immunogenicity. Compared with CGRV proteins of other structures, the recombinant protein has the advantage that the valence of specific antibodies generated by immune animals induced by the protein is higher. Further experiments show that when VP6 proteins are used for immunizing grass carps, the grass carps can be induced to generate higher specific antibody; a certain immune protection effect is achieved on the GCRV strong virus plant attack. Therefore the development and the application of the GCRV II type VP6 protein have very important significance on developing novel grass carp hemorragic disease vaccine and immunological detection kits, and a novel effective solution path is provided for grass carp hemorragic diseases.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
ELISA (enzyme-linked immunosorbent assay) kit for EV (enterovirus) 71 inactivated vaccine antigen
The invention relates to an ELISA (enzyme-linked immunosorbent assay) kit for an EV (enterovirus) 71 inactivated vaccine antigen. The detection kit comprises an EV71 pre-coated polyclonal antibody ELISA plate, a sample diluent, a second antibody, an enzyme-labeled antibody, a concentrated cleaning solution, an enzyme substrate solution and a stop buffer, wherein the ELISA plate is pre-coated with a polyclonal antibody prepared by taking recombinant EV71 structural protein 1 as an immune source; the second antibody adopts a monoclonal antibody prepared by taking keyhole limpet hemocyanin coupled polypeptide sequence FGEHKQEKDL as the immune source; the enzyme-labeled antibody adopts a horse radish peroxidase labelled goat anti-mouse immunoglobulin antibody; and an antibody standard is placed in the kit. The ELISA kit has better sensitivity when measuring the titer of the EV71 inactivated vaccine antigen, and has better linearity in the range of 5.9-750 ng / ml, and the linearly dependent coefficient r2 is larger than 0.99. The kit for measuring the titer of the EV71 inactivated vaccine antigen simply and conveniently has good specificity, accurate quantification, high sensitivity and good repeatability.
Owner:ZHEJIANG PUKANG BIOTECH
Genetic markers for discrimination and detection of viruses causing infectious aquatic organism diseases, and method of discriminating and detecting the viruses using the same
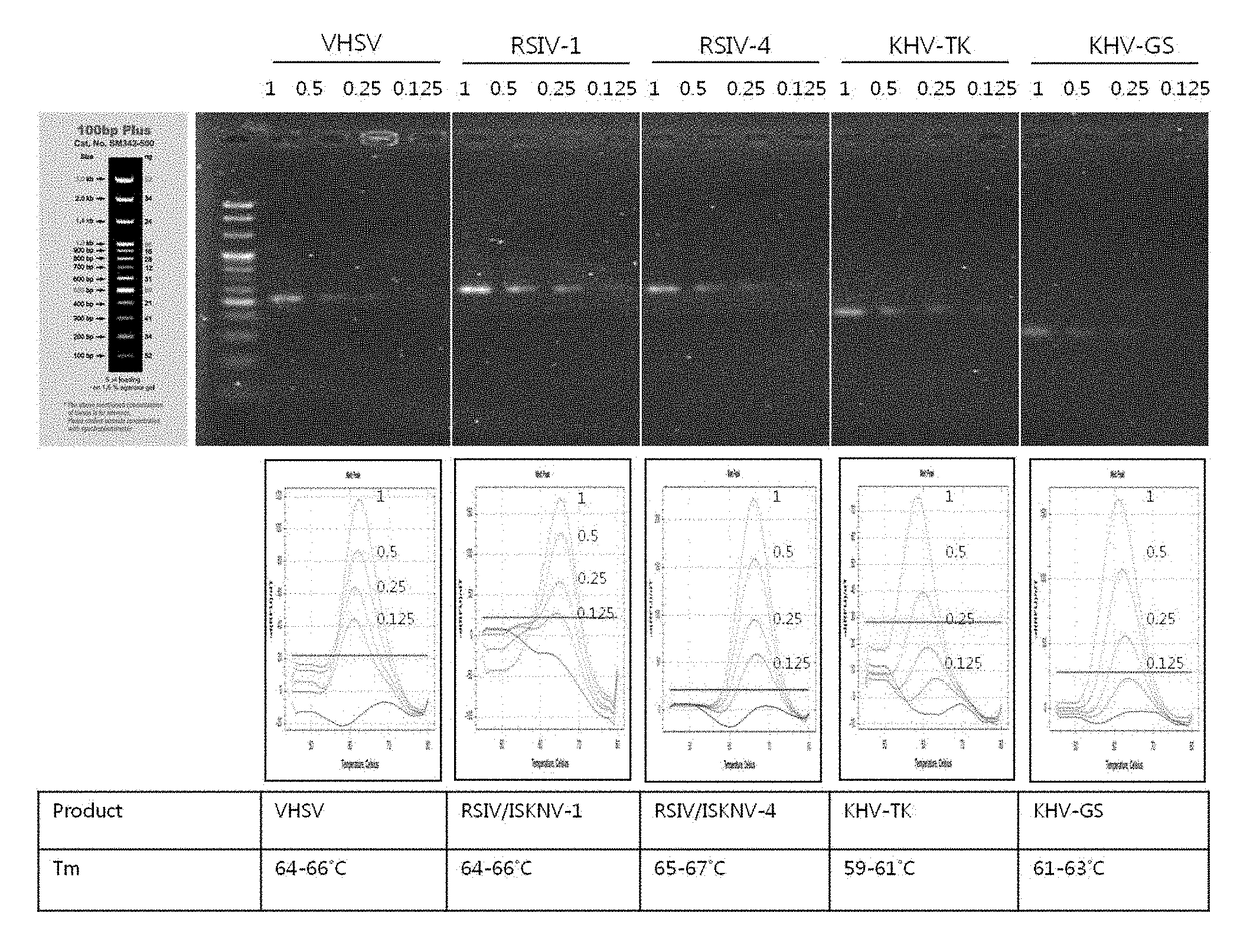
The present invention relates to genetic markers for discrimination and detection of viruses causing infectious aquatic organism diseases, and a method of discriminating and detecting the viruses using the same, and more particularly to a method for discriminating or detecting viruses causing infectious aquatic organism diseases, the method comprising: selecting and amplifying a DNA nucleotide sequence encoding a gene specific for viral hemorrhagic septicemia virus (VHSV), red sea bream iridovirus (RSIV) or infectious spleen and kidney necrosis virus (ISKNV), which is a virus causing red sea bream iridovirus disease, or Koi herpesvirus (KHV); hybridizing a peptide nucleic acid (PNA) that specifically recognizes the amplification product; controlling the temperature of the hybridization product to obtain a temperature-dependent melting curve; and discriminating the viral type or detecting whether or not fish would be infected with the viral type by analyzing the obtained melting curve to determine a melting temperature.
Owner:REPUBLIC OF KOREA (NAT FISHERIES RES & DEV INST)
Coxsackievirus A6-type strain and application thereof
ActiveCN113564131AImprove abilitiesToxicSsRNA viruses positive-senseViral antigen ingredientsStructural proteinGenotype
The invention relates to the technical field of biology, in particular to a coxsackievirus A6-type strain and application thereof. The amino acid sequence of P1 structural protein of the coxsackievirus A6-type strain is shown as SEQ ID NO.1. The strain has high cross-neutralization capacity in genotypes and between genotypes, is high in toxicity, has high pathogenic and lethal capacity on mice, and has good immunogenicity and high titer and stability. The strain can be used for immunogenicity evaluation or protective evaluation of coxsackievirus A6-type vaccines, the accuracy and repeatability of vaccine immunogenicity evaluation are improved, and the strain can also be used for preparing coxsackievirus infection animal models and has good application prospects.
Owner:BEIJING MINHAI BIOTECH
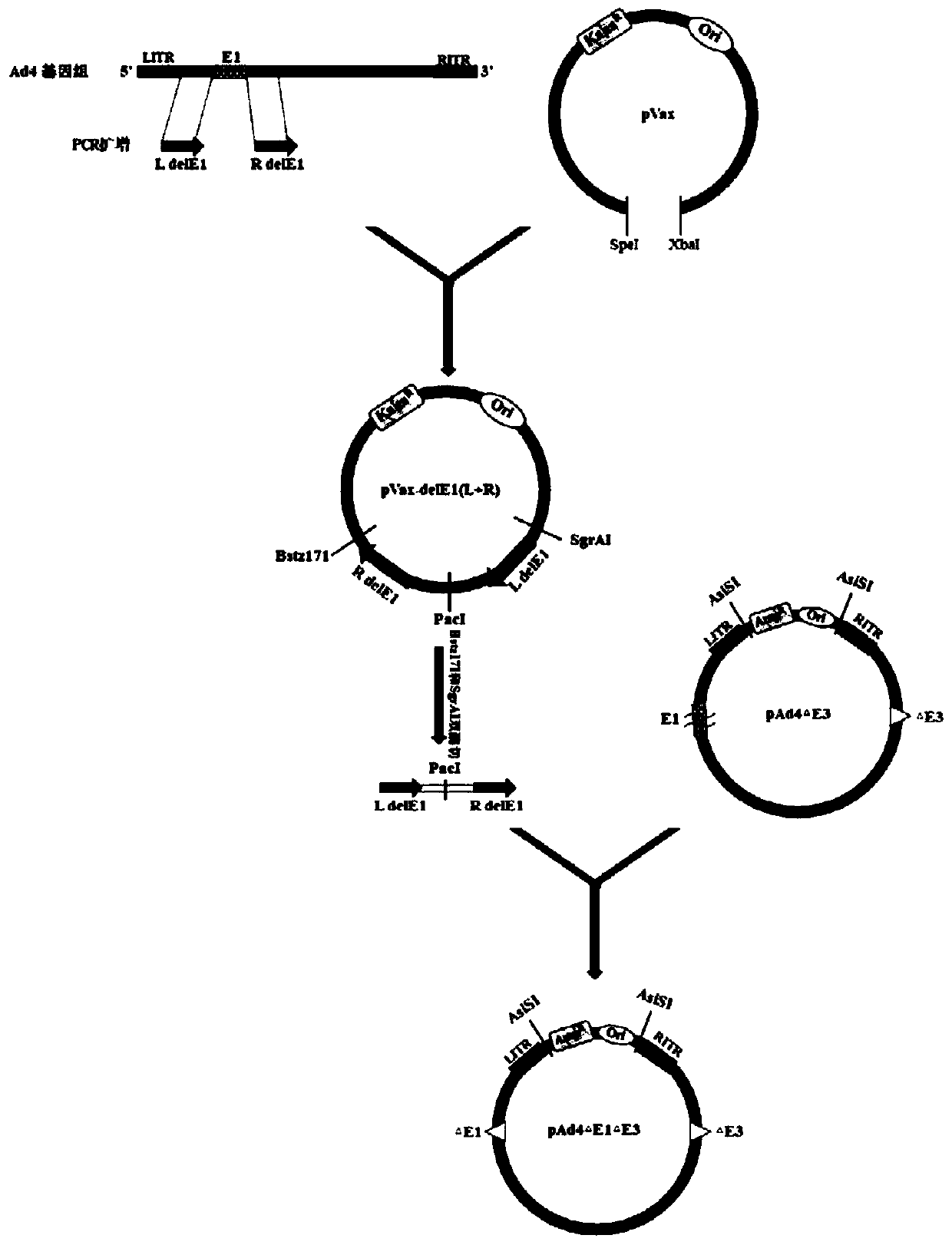
Adenovirus bivalent vaccine
InactiveCN111166875AImprove securityStrong replicabilityViral antigen ingredientsAntiviralsViral typeTGE VACCINE
The invention discloses an adenovirus bivalent vaccine. The vaccine comprises a replication-deficient human adenovirus type 4 and a replication-deficient human adenovirus type 7. E1 and E3 genes of the replication-deficient human adenovirus type 4 and the replication-deficient human adenovirus type 7 are deleted, and partial coding frames of the E4 gene are replaced by corresponding coding framesof E4 gene of human adenovirus type 5. The vaccine can effectively stimulate an organism to generate a humoral immune response and a cellular immune response, generate specific neutralizing antibodieswith high titer, and is used for preventing infection of pathogens.
Owner:GUANGZHOU N BIOMED LTD
Preparation method and application of recombination coxsackie virus B3-type virus-like particles
The invention discloses a preparation method of recombination coxsackie virus B3-type virus-like particles. The method disclosed by the invention comprises the following steps that 1, the P1 gene and 3CD gene of the coxsackie virus B3 type are cloned to a target plasmid to obtain a recombination expression vector; 2, the target yeast cell is transformed by the recombination expression vector which is obtained in the step 1 to obtain a recombination yeast cell which expresses the P1 gene and the 3CD gene; and 3, the recombination yeast cell which is obtained in the step 2 is cracked, and the recombination coxsackie virus B3-type virus-like particles are obtained after the separation. Experiments prove that after the method disclosed by the invention is utilized, the recombination coxsackie virus B3-type virus-like particles are successfully produced in the yeast expression system, and can be further used for candidate prophylactic vaccine and medicine combination for vital myocarditis and diabetes mellitus type I.
Owner:BEIJING UNIV OF TECH
Hand-foot-mouth disease detection reagent kit and its detection method
InactiveCN102643927ASuitable for field applicationStrong specificityMicrobiological testing/measurementHand-foot-and-mouth diseaseCoxsackievirus a16
The invention belongs to biological field, and relates to a reagent kit for detecting main pathogen Coxsackievirus A16 (Cox A16) and enterovirus 71 of hand-foot-mouth disease, construction method and uses thereof. The reagent kit comprises reaction solutions for detecting four primers of Coxsackievirus A16 and four primers of enterovirus 71.As detected, the reagent kit has a good performance of specificity, sensitivity, rapid amplification, high efficiency and simple identification. The detection system of the invention can detect Coxsackievirus A16 and enterovirus 71 quickly, conveniently, high-effectively, high-specifically and highly-sensitive under temperature of 64 DEG C without need of complicated instruments, which can preferably satisfy clinical detection for hand-foot-mouth disease and be easily popularized in large scope.
Owner:刘志学 +1
Interfering RNA (Ribonucleic Acid) for suppressing hand-foot-and-mouth disease virogene, vector containing the same and application thereof
InactiveCN102399780AElimination of clinically manifest symptomsImprove targetingGenetic material ingredientsAntiviralsA-DNAExperimental animal
The invention provides a method for suppressing virogene expression causing hand-foot-and-mouth disease, in particular two interfering RNA (Ribonucleic Acid) for two main pathogens of enterovirus 71 and coxsackie virus A16 genomes and a DNA sequence thereof, a carrier containing the interfering RNA and an application of the carrier transcribed interfering RNA in preparation of medicines for preventing and curing hand-foot-and-mouth diseases. It is shown by experiment result that the carrier transcribed interfering RNA can be used for obviously suppressing copying of two viruses and eliminating clinical symptoms of the hand-foot-and-mouth diseases at the same time of reducing pathogen enterovirus 71 and coxsackie virus A16 gene expressions on cell level and experimental animals. The carrier transcribed interfering RNA and the carrier containing the same, provided by the invention, can play am important role in preventing and curing the hand-foot-and-mouth diseases.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com