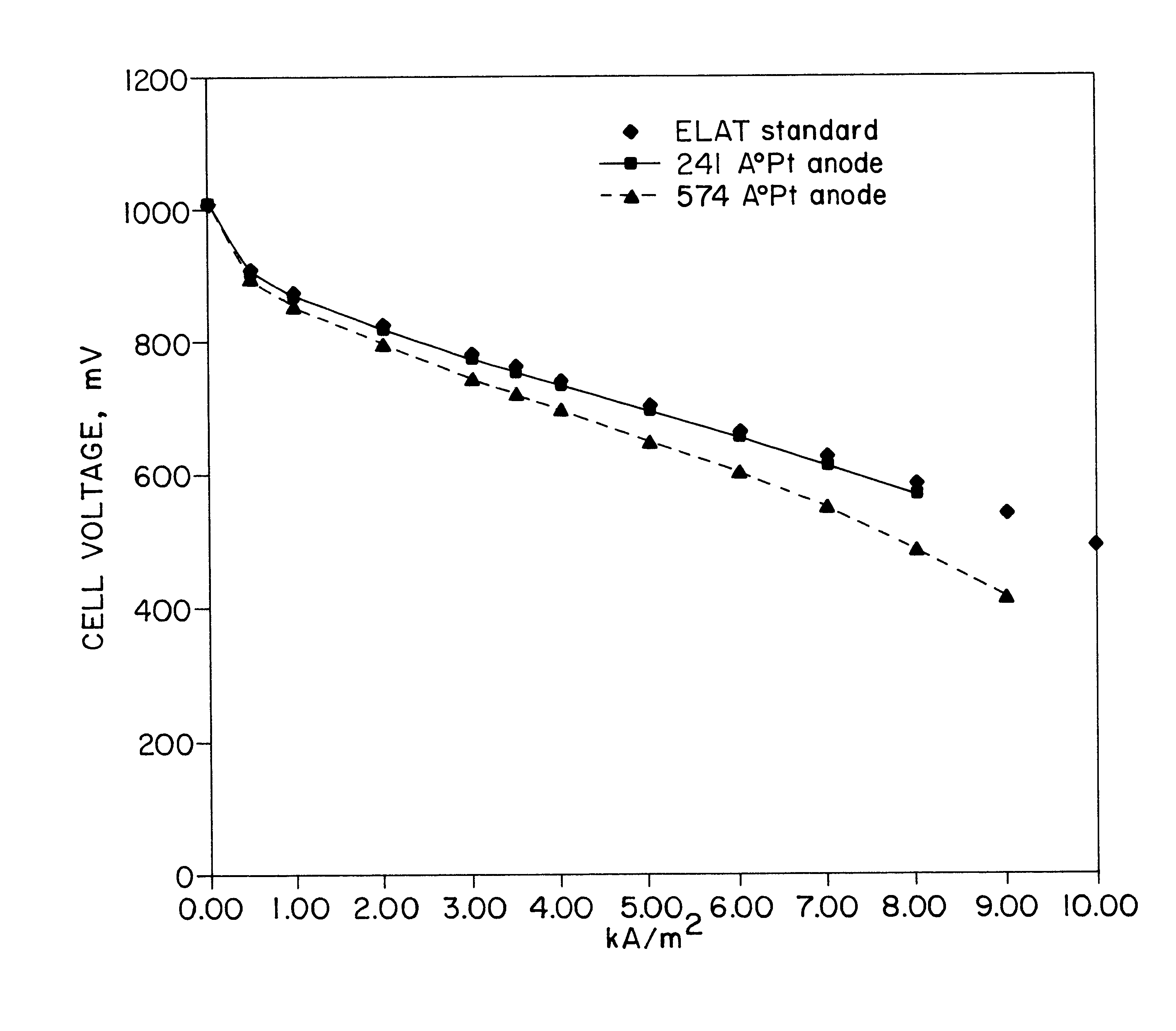
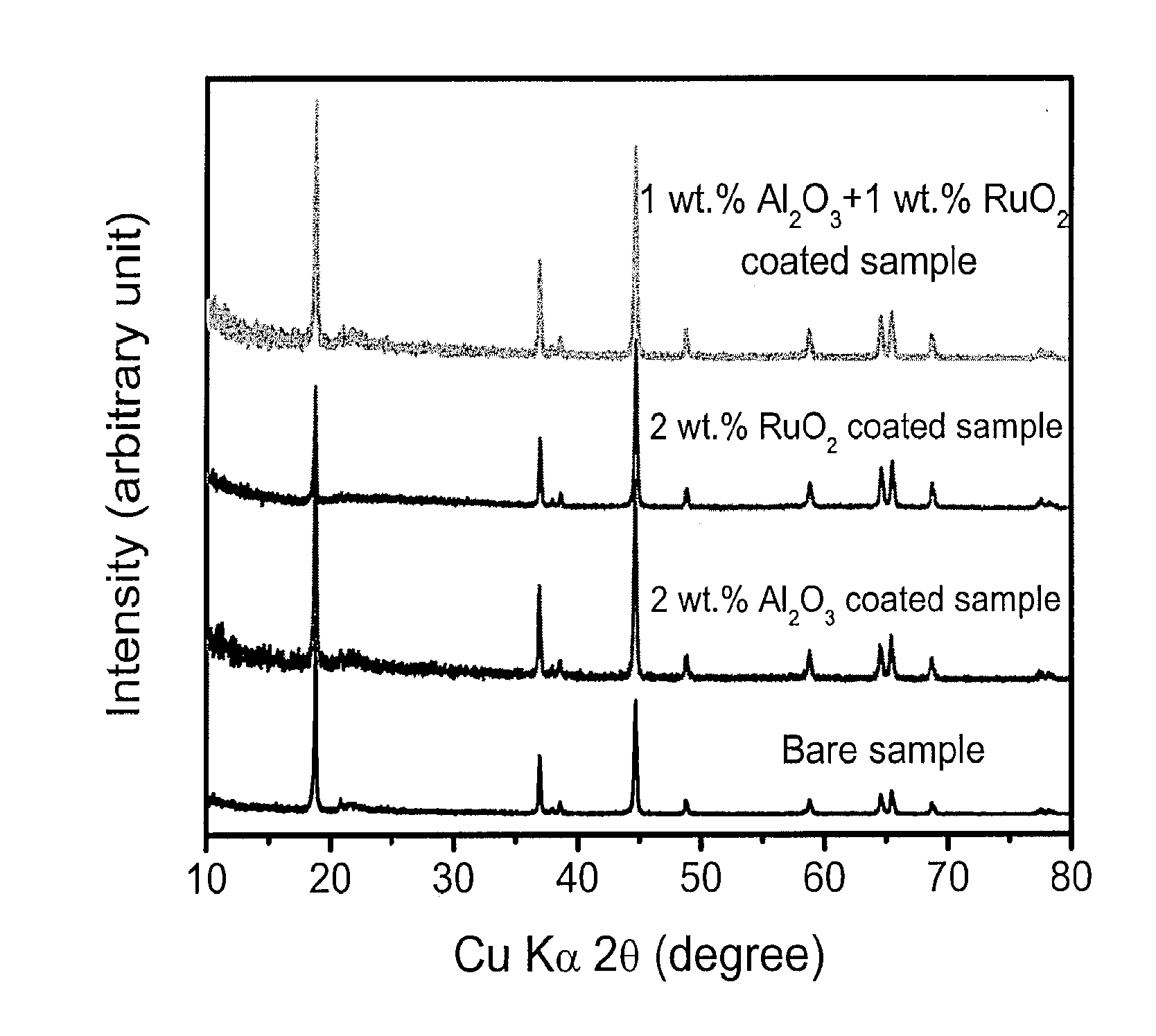
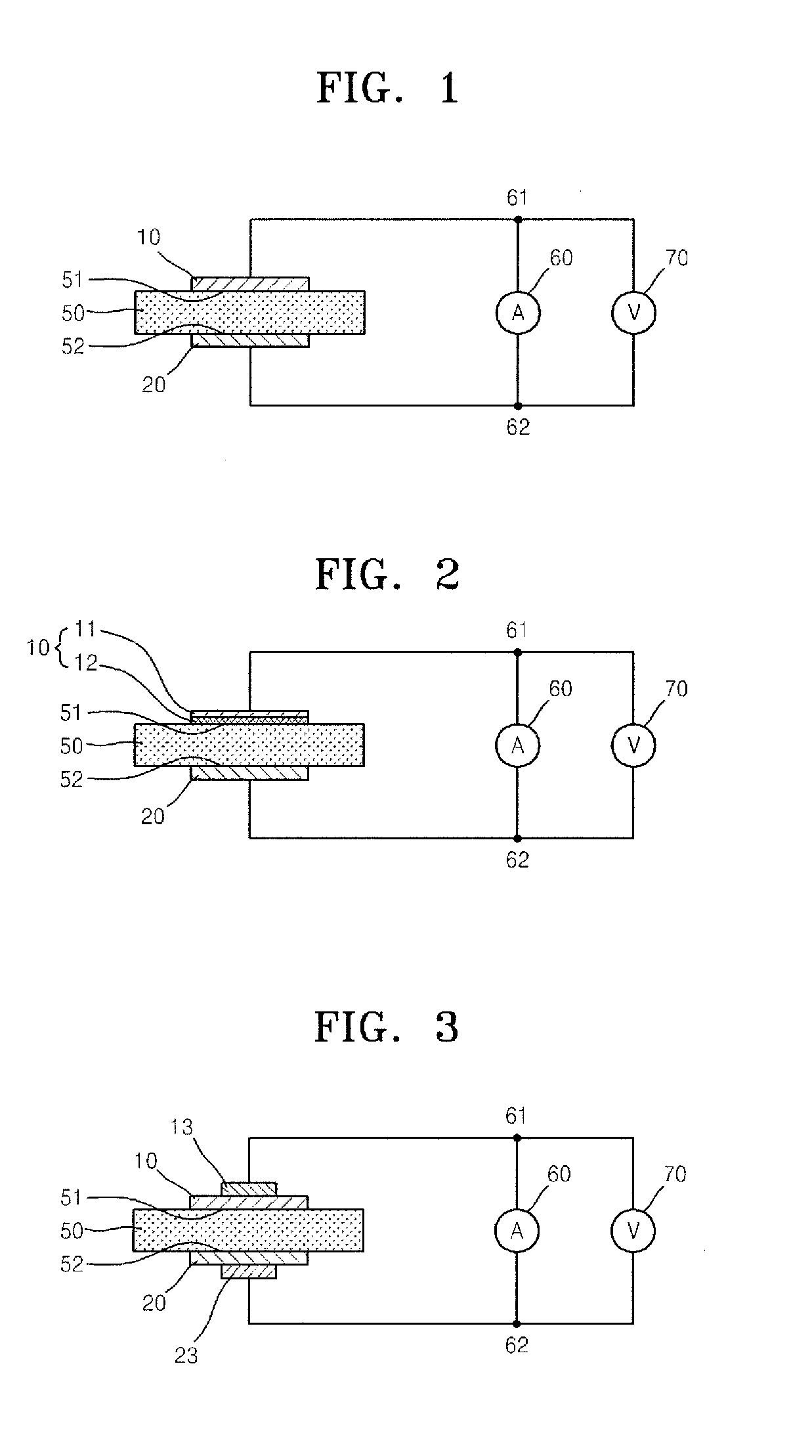
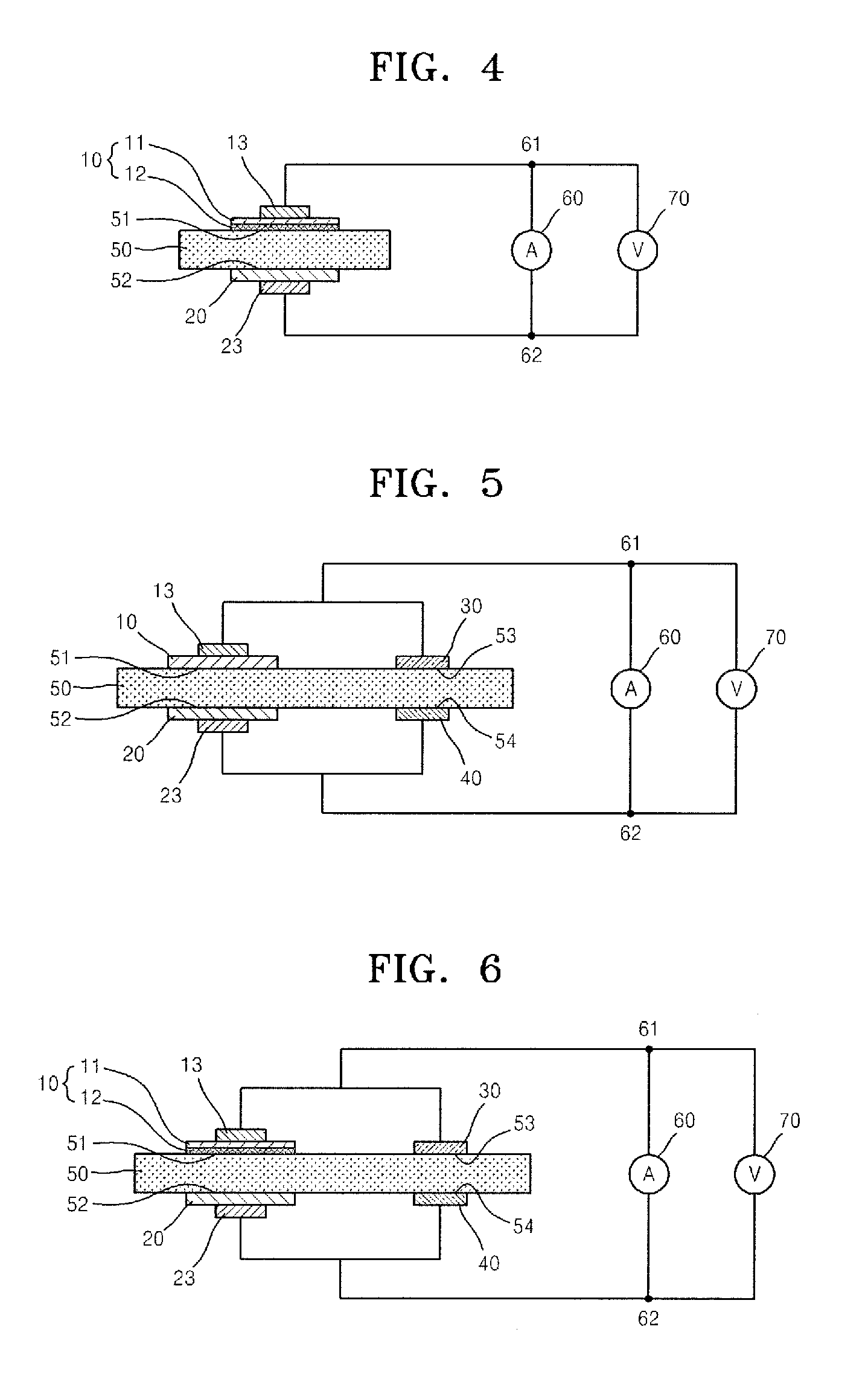
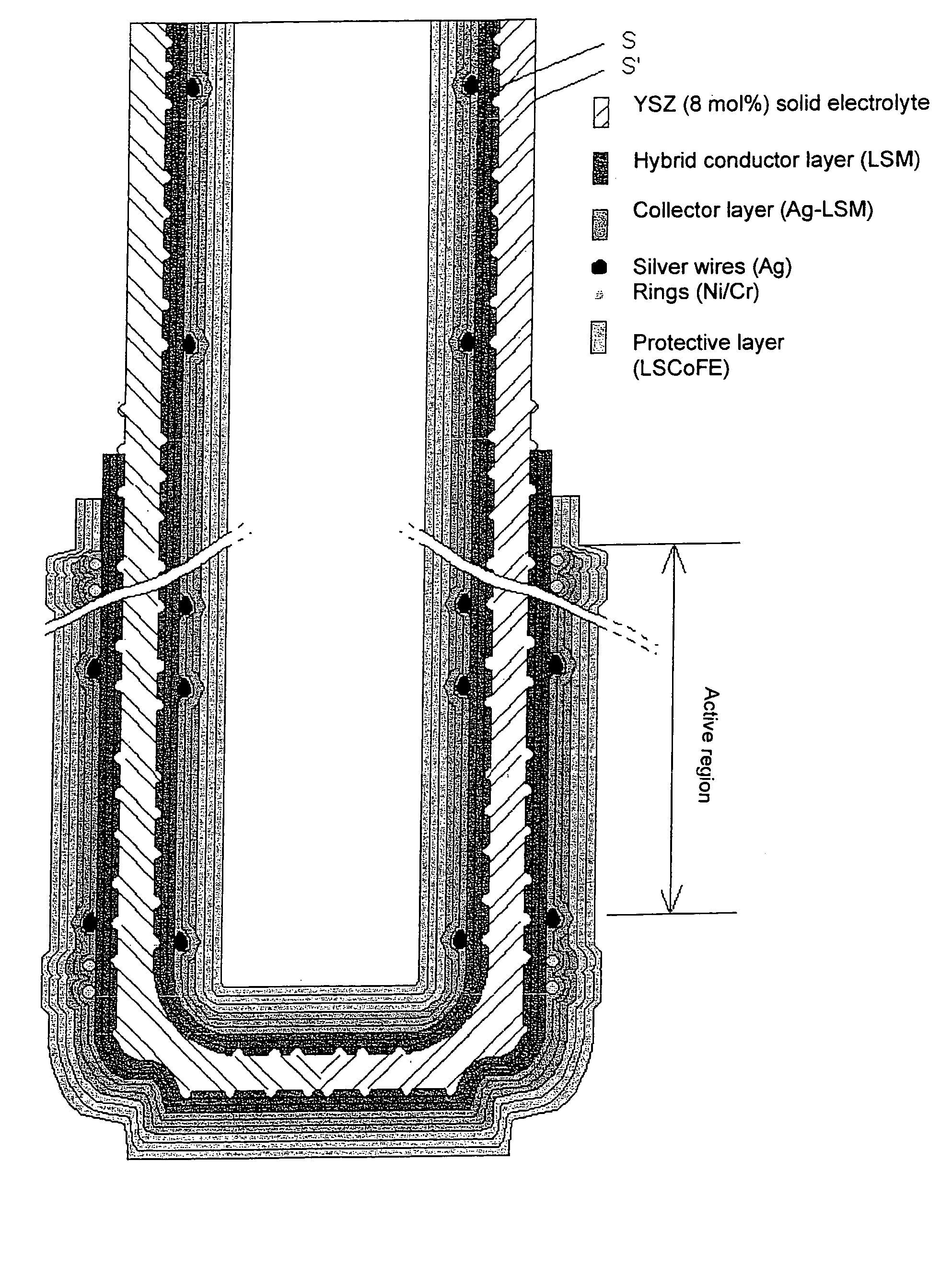
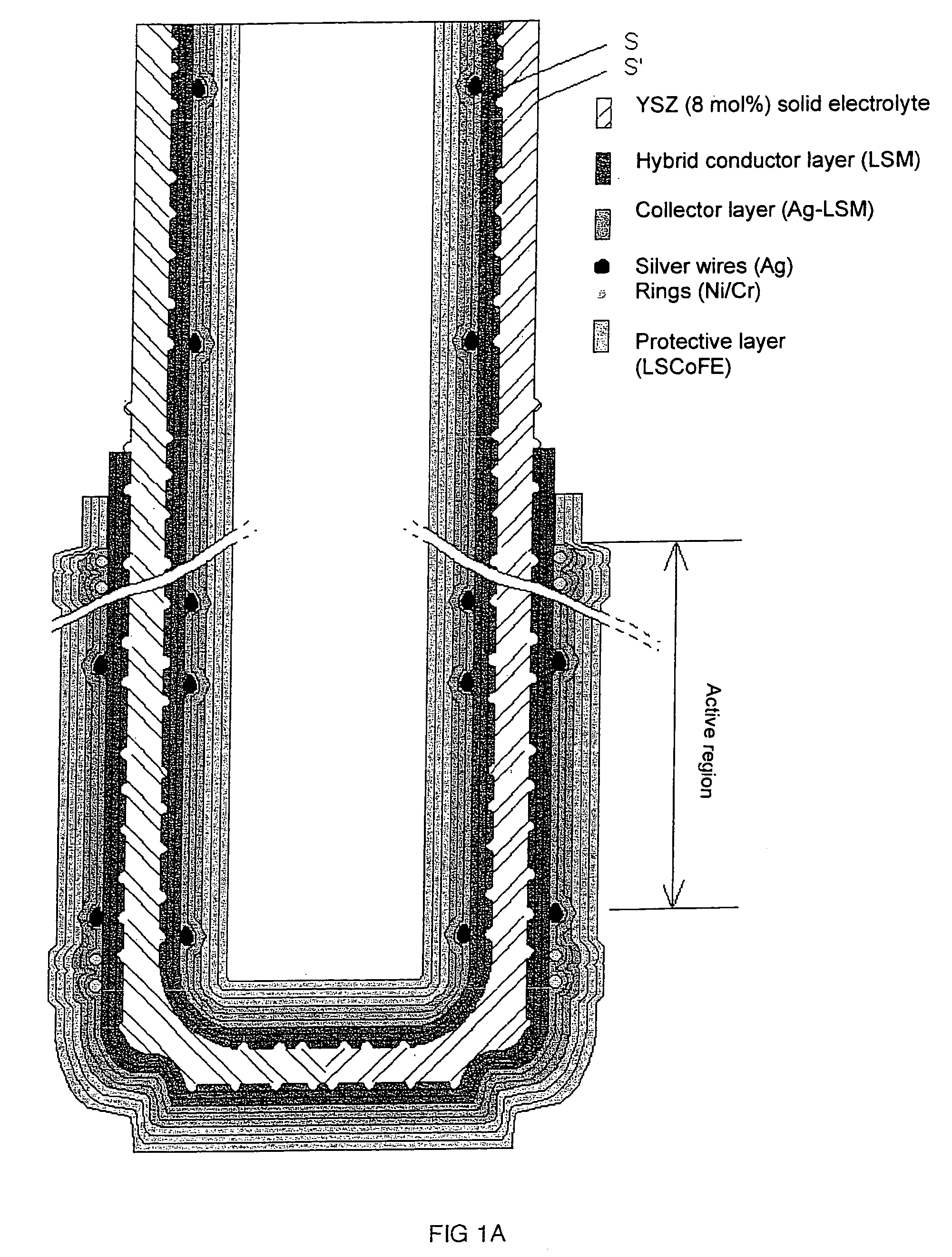
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
90 results about "Oxide ion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Answer Wiki. An oxide ion is a negatively charged oxygen atom. This means it has gained two electrons from another atom. Thus, it is represented as O2- (2- is super scripted). It is also an anion, which is a negatively charged ion.
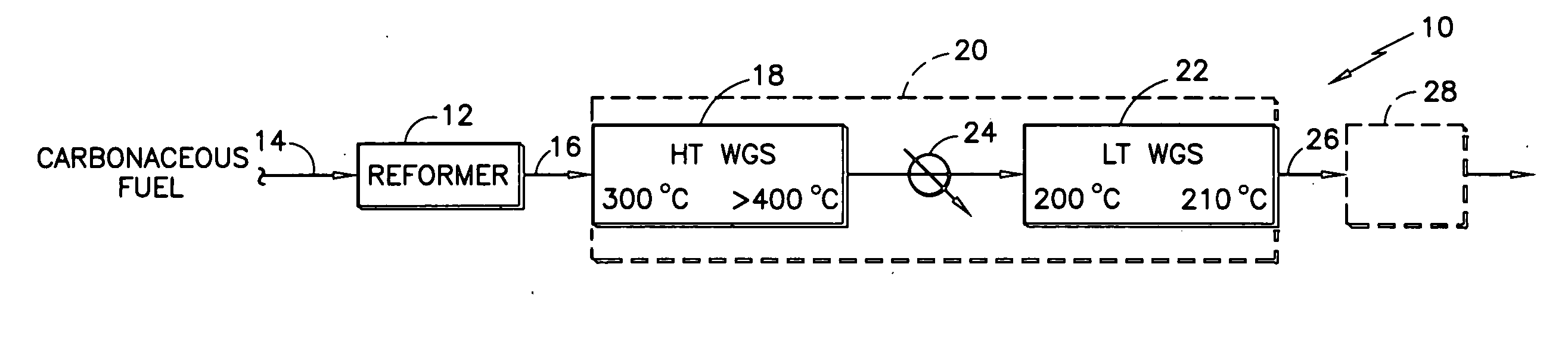
Oxidative reactions using membranes that selectively conduct oxygen
InactiveUS6730808B2Eliminate the problemNot possibleAlkali metal oxides/hydroxidesFunctional group formation/introductionLanthanideOxygen
Reactor membranes for used in oxidation reactions of hydrocarbons involving oxygen comprising a selective oxidation catalyst on a mixed conducting, oxide ion selective ceramic membrane of the composition (Sr1-xCax)1-yAyMn1-zBzO3-delta, whereA is Ba, Pb, Na, K, Y, an element of the lanthanide group or a combination thereof,B is Mg, Al, Ga, In, Sn, an element of the 3d or 4d period or a combination thereof,x is from 0.2 to 0.8,y is from 0 to 0.4,z is from 0 to 0.6, anddelta is a number, dependent on x, y and z, that renders the composition charge neutral.
Owner:BASF AG
Method of forming robust metal, metal oxide, and metal alloy layers on ion-conductive polymer membranes
An ion beam-assisted deposition process for preparing a membrane-electrode structure is described wherein a layer of liquid ionomer is applied to the surface of a carbon cloth gas diffusion electrode structure. The coated structure is heated to form an ionomer film on the cloth electrode and the resulting structure is treated with a metal or metal oxide ion-beam having an energy between 500-2000 eV. The process forms a carbon cloth supported metal or a carbon metal oxide ionomer film membrane-electrode structure.
Owner:BASF FUEL CELL
Electrolyte-electrode assembly and method for producing the same
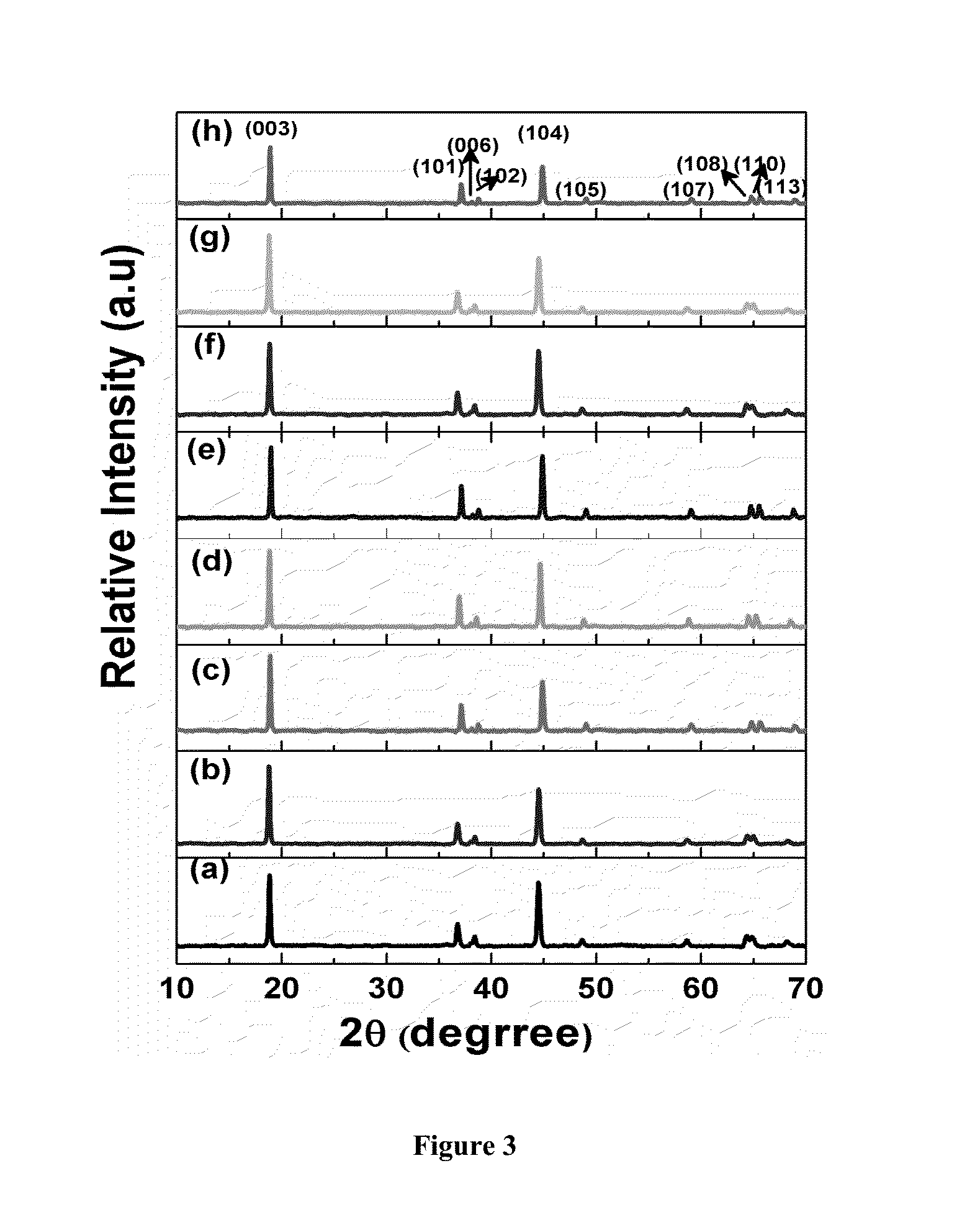
InactiveUS20050100770A1Excellent oxide ion conductivityImprove conductivityPolycrystalline material growthFuel cells groupingSingle crystalElectrolyte
An electrolyte is single crystal composed of La9.33Si6O26 in which the crystal is oriented in the c-axis, and the c-axis is in the thickness direction of the electrolyte. In the electrolyte, the oxide ion is preferentially migrated in the thickness direction. That is, the oxide ion conduction exhibits anisotropy. Isotropic conductive layers composed of YDC, in which the oxide ion conduction exhibits isotropy and the oxide ion conductivity is lower than that of the electrolyte, are provided between the electrolyte and an anode and a cathode.
Owner:HONDA MOTOR CO LTD
High capacity layered oxide cathods with enhanced rate capability
InactiveUS20130040201A1Inhibitory responseIncrease probabilityNon-aqueous electrolyte accumulator electrodesCapacity lossElectrical conductor
The present invention provides a surface modified cathode and method of making surface modified cathode with high discharge capacity and rate capability having a lithium-excess Li[M1-yLiy]O2 (M=Mn, Co, and Ni or their combinations and 0<y≦0.33) cathode surface with a surface modification comprising lithium-ion coated sample conductor, or an electronic conductor, or a mixed lithium-ion and electronic conductor to suppress the elimination of oxide ion vacancies, reduce the solid-electrolyte interfacial (SEI) layer thickness, reduce the irreversible capacity loss in the first cycle, and enhance the rate capability.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
UV target for an environmental air sterilization apparatus
InactiveUS20060144690A1Effectively freshen airEffectively remove odorMechanical apparatusLighting and heating apparatusSuperoxideHigh energy
The present invention is a high volume, wall-mountable air sanitation apparatus for disinfecting and removing VOC's with high energy UV light and ozone. The apparatus has a powerful fan and an elongated UV light source and target for use with the movement of a large volume of air. The target includes a mesh and a secondary target both comprising a target compound which creates hydro-peroxides, super oxide ions and hydroxyl radicals in the presence of water also for removing pollutants in the air.
Owner:FINK RONALD G +2
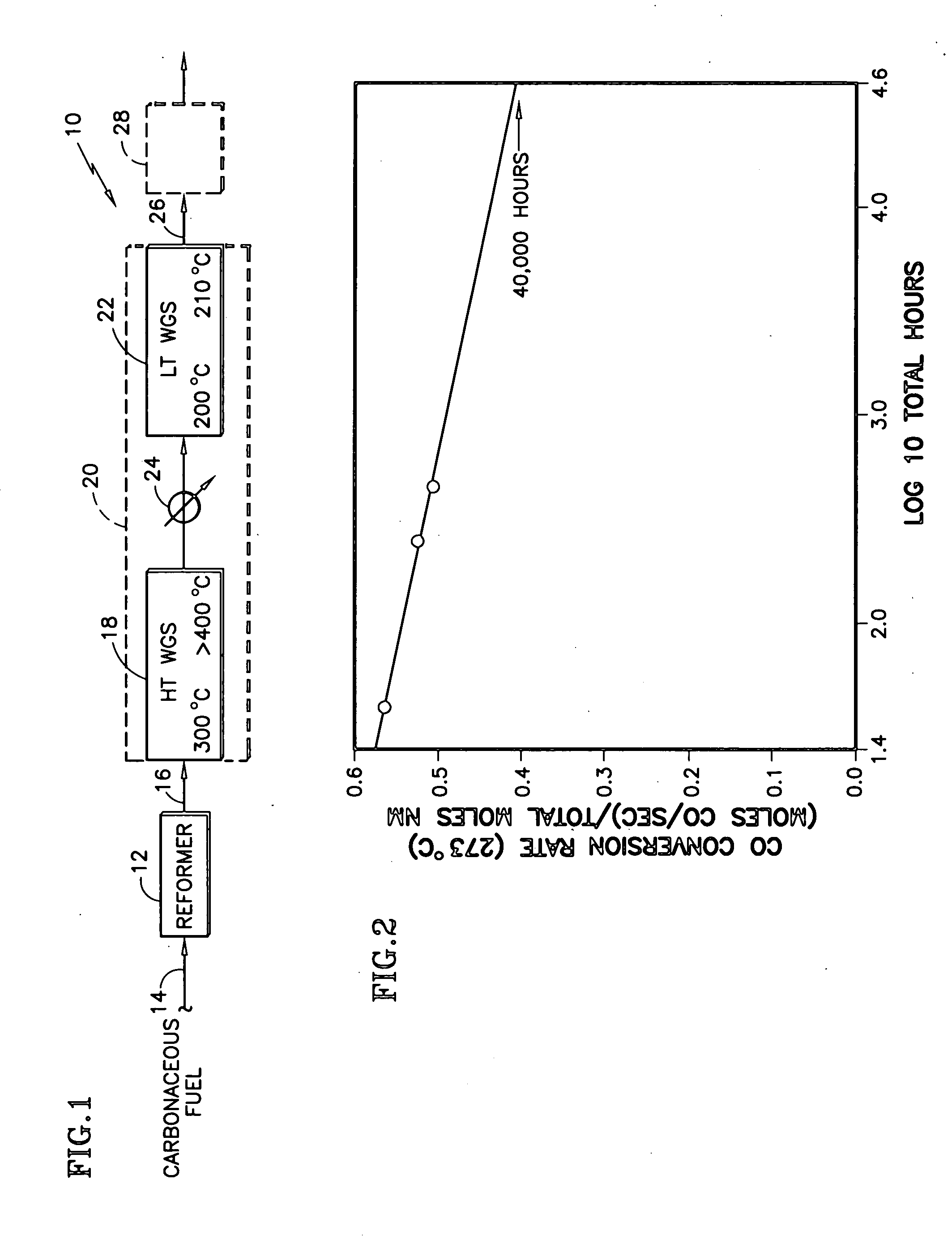
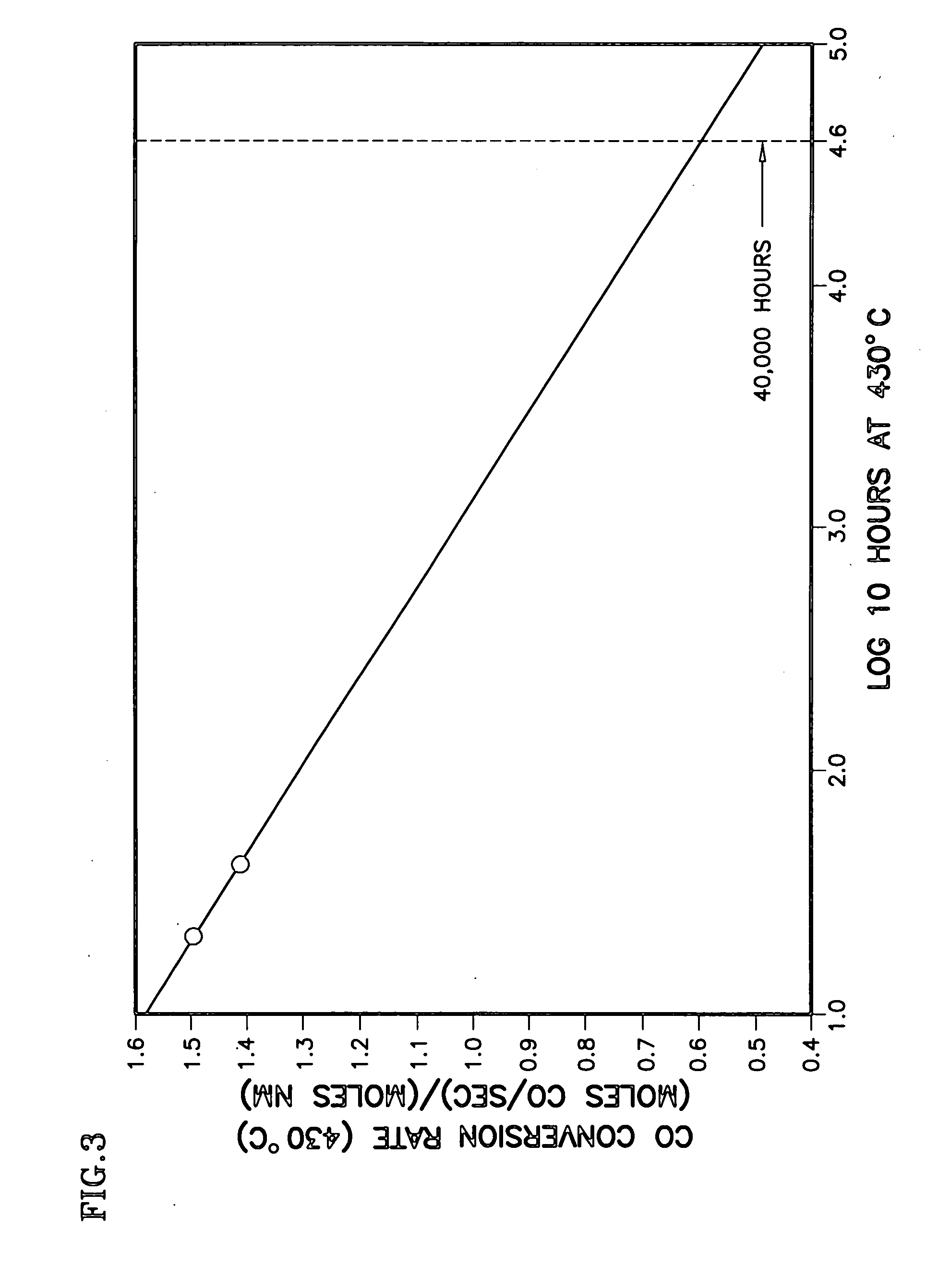
Durable catalyst for processing carbonaceous fuel, and the method of making
InactiveUS20060233691A1Simple structureAvoid turbidityTantalum compoundsHydrogen/synthetic gas productionBoiling pointWater-gas shift reaction
A doped, nanocrystalline, ceria-containing, mixed metal oxide supports a noble metal to provide a thermally-durable catalyst for processing carbonaceous fuels, particularly for the water gas shift reactions. The mixed metal oxide includes Zr and / or Hf and is normally susceptible to oxide ion vacancy ordering at elevated temperature reducing conditions. A dopant is selected to inhibit such oxide ion vacancy ordering. The dopant is preferably selected from the group consisting of W, Mo, Ta, and Nb, most preferably W, for providing a thermally-durable catalyst at operating temperatures exceeding 400° C. The noble metal is preferably Pt and / or Re. The doped ceria-containing mixed metal oxide is prepared from 2 or 3 aqueous solutions variously containing ceria, Zr and / or Hf, the dopant, and urea. The solutions are heated to below boiling, combined in a particular sequence and manner, and brought to boiling to crystallize and precipitate the doped ceria-containing mixed metal oxide.
Owner:INT FUEL CELLS
All solid state rechargeable oxide-ion battery (ROB) system
ActiveUS20120270088A1Energy efficiencyCost effectiveFuel and secondary cellsFinal product manufactureThermal energyAll solid state
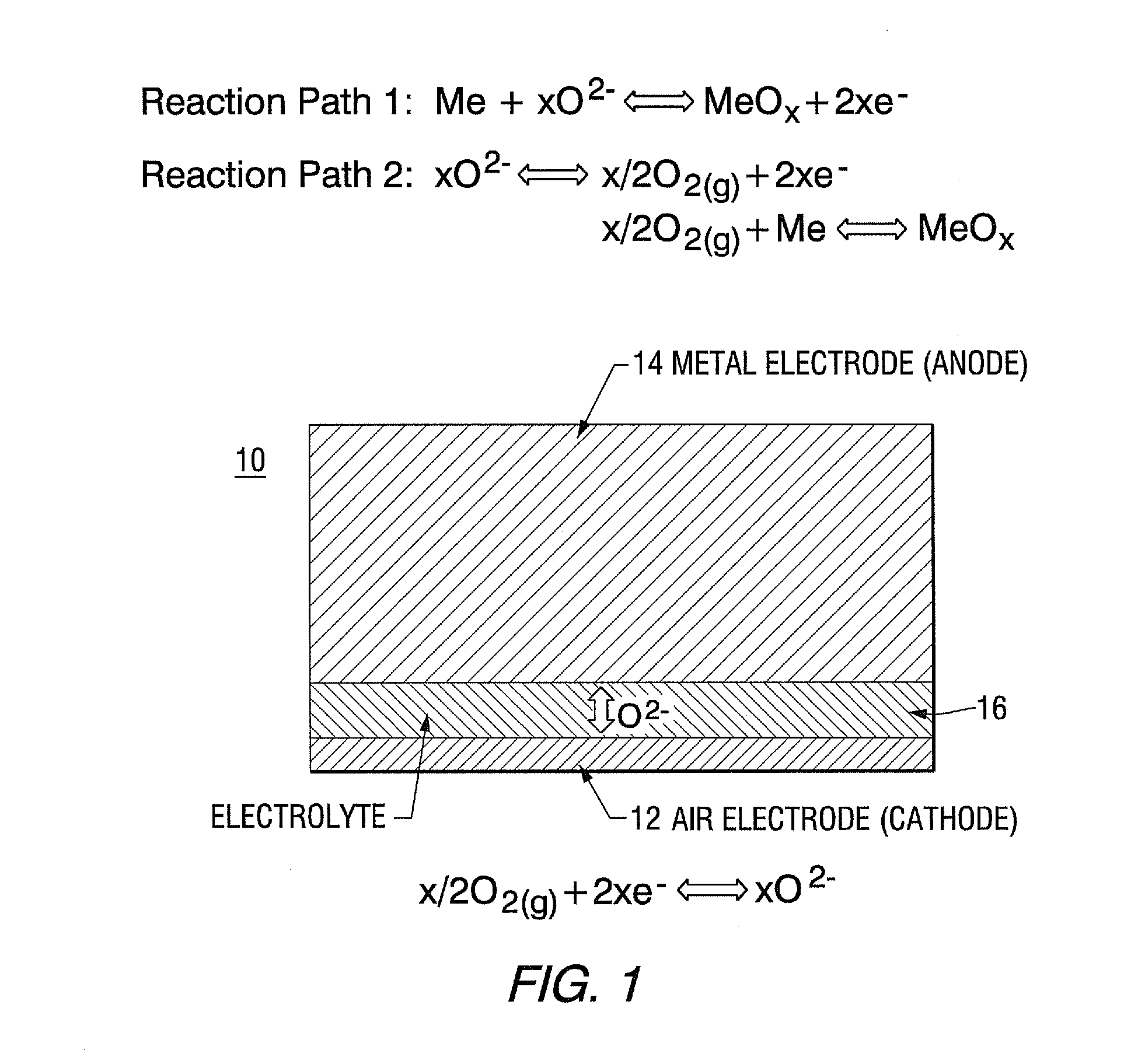
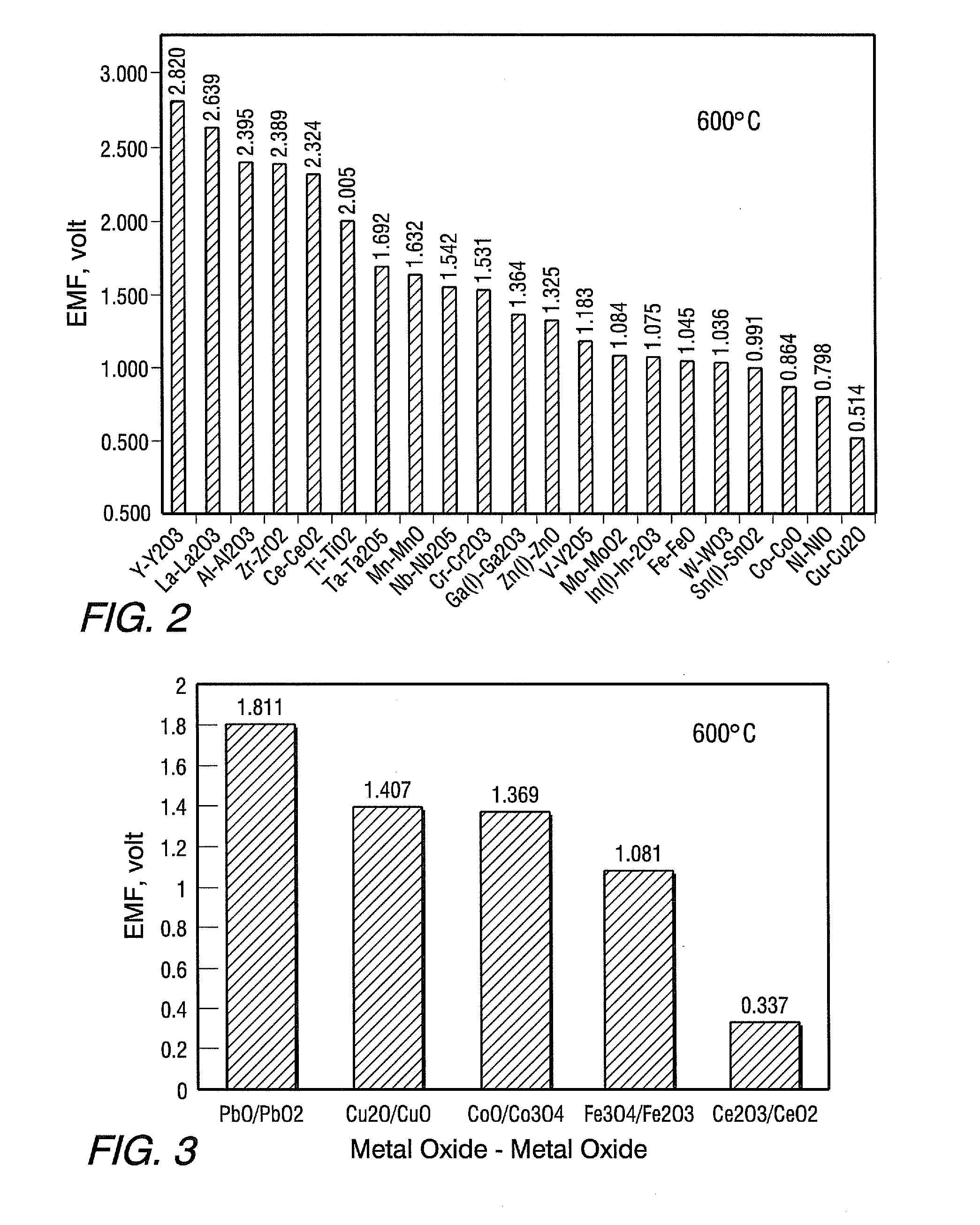
An all solid state rechargeable oxide-ion (ROB) battery (30) has a thermal energy storage (TES) unit (20) between two oxide-ion cells (22, 24) with metal-metal oxide electrodes (34, 36, 40, 42) on opposite sides of an anion conducting solid electrolyte (32,38) where none of the electrodes is contact with air.
Owner:SIEMENS AG
Oxide-ion conductor and use thereof
InactiveUS6844098B1High oxide-ion conductivityImprovement in oxide-ion conductivityWeather/light/corrosion resistanceComponent separationElectrical conductorHeat resistance
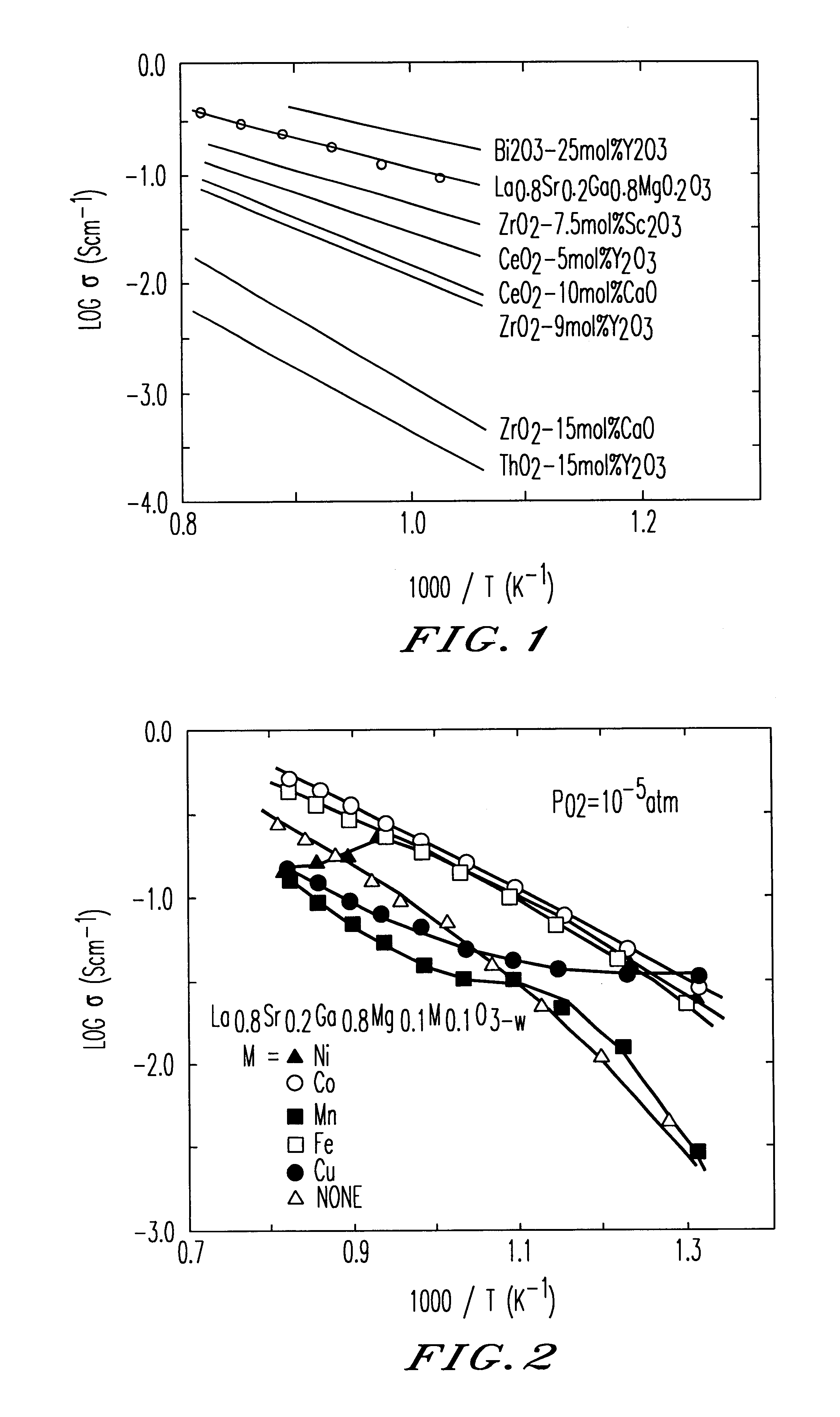
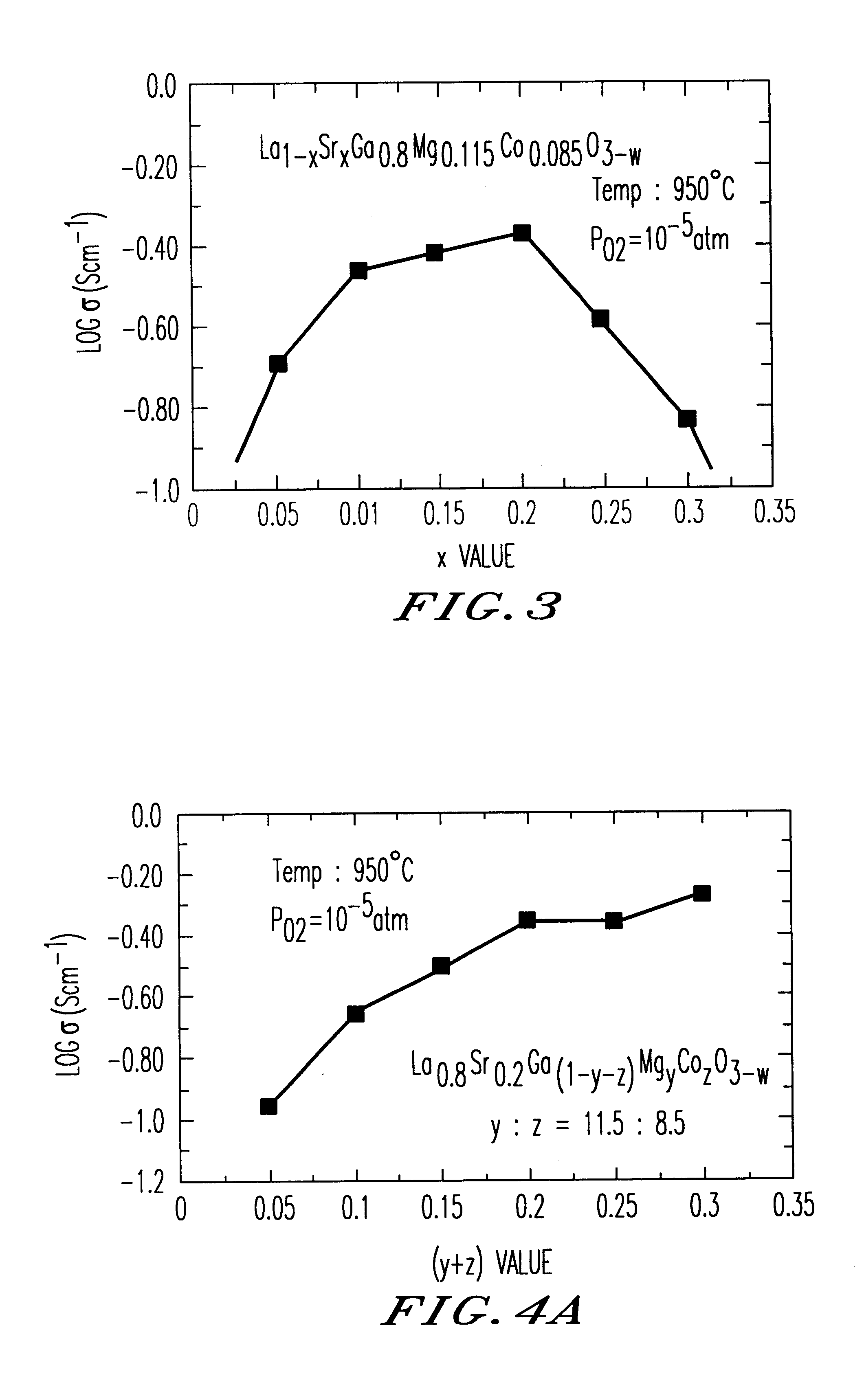
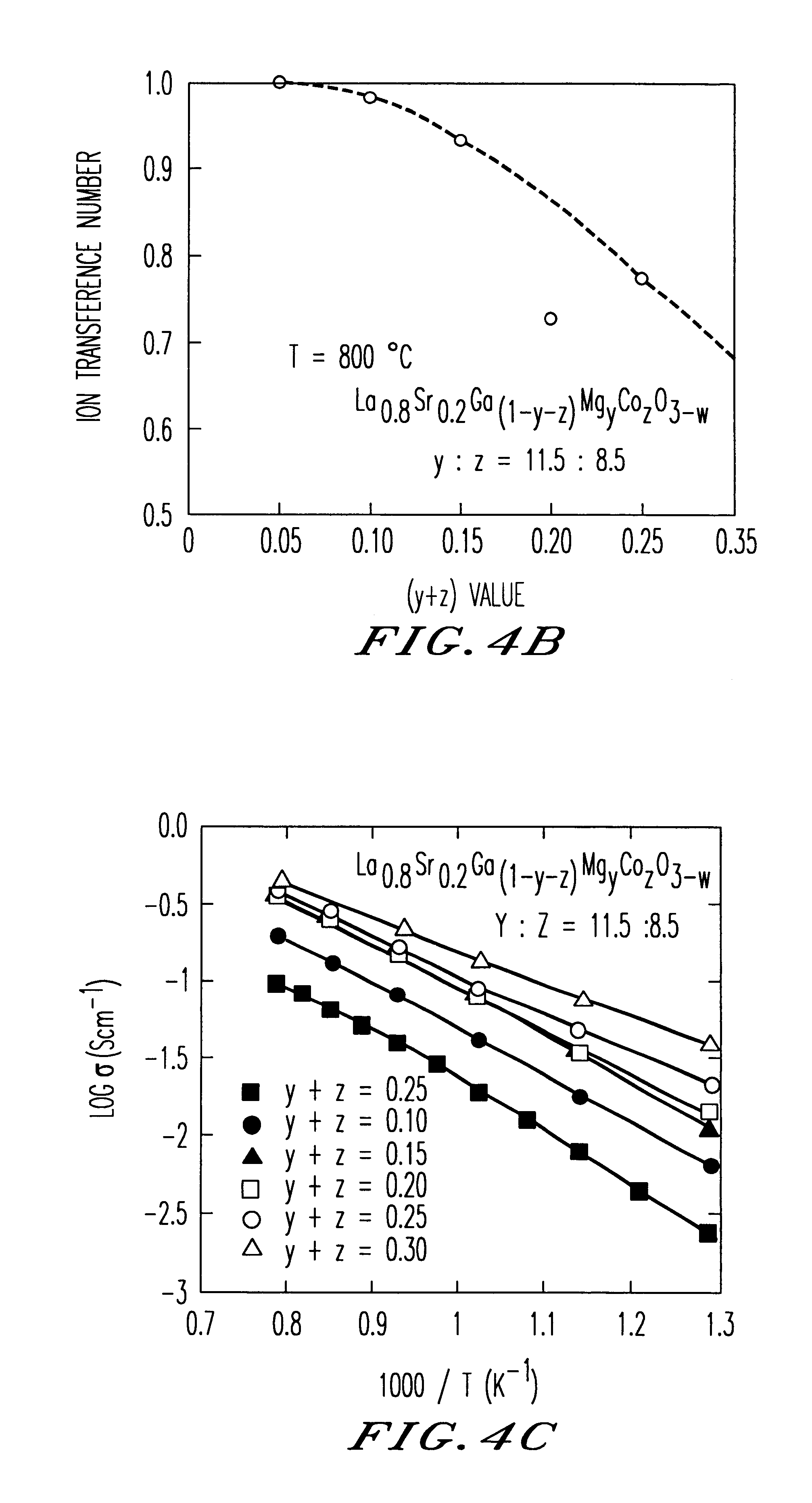
An oxide-ion conductor has the formula Ln1−xAxGa1−y−xB1YB2z oxide, wherein Ln is at least one element selected from the group consisting of La, Ce, Pr, Nd and Sm; A is at least one element selected from the group consisting of Sr, Ca and Ba; B1 is at least one element selected from the group consisting of Mg, Al and In; B2 is at least one element selected from the group consisting of Co, Fe, Ni and Cu; x is 0.05 to 0.3; y is 0 to 0.29; z is 0.01 to 0.3; and y+z is 0.025 to 0.3. The oxide-ion conductor exhibits higher oxide-ion conductivity than stabilized zirconia, excellent heat resistance and also high ion conductivity even at low temperatures, as well as exhibiting low oxygen-partial-pressure dependence of ion conductivity.
Owner:MITSUBISHI MATERIALS CORP +2
Single cell for a solid oxide fuel cell
InactiveUS20040072060A1Improve electrode activityReduce degradationSolid electrolytesFuel cells groupingFuel cellsFlexural strength
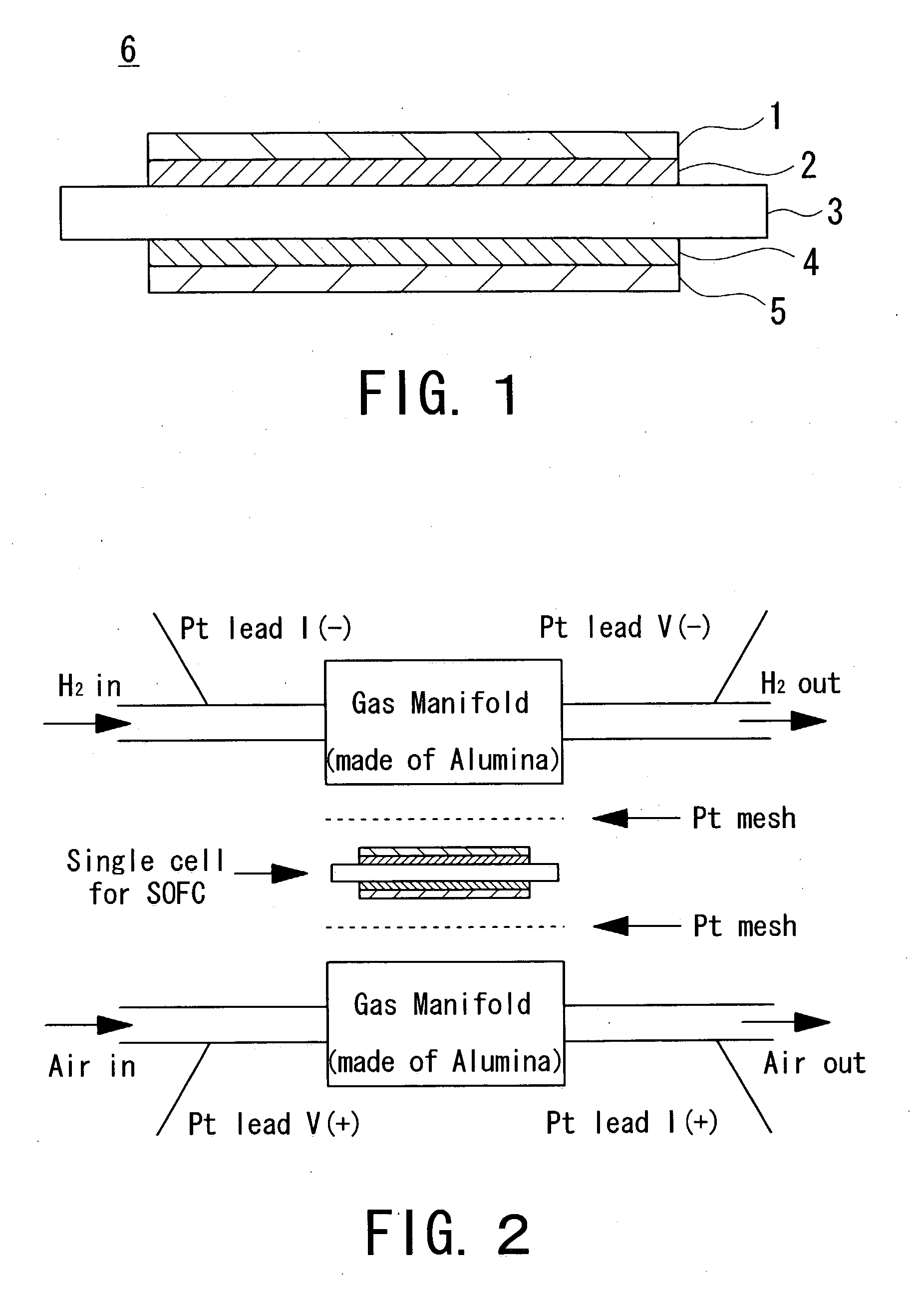
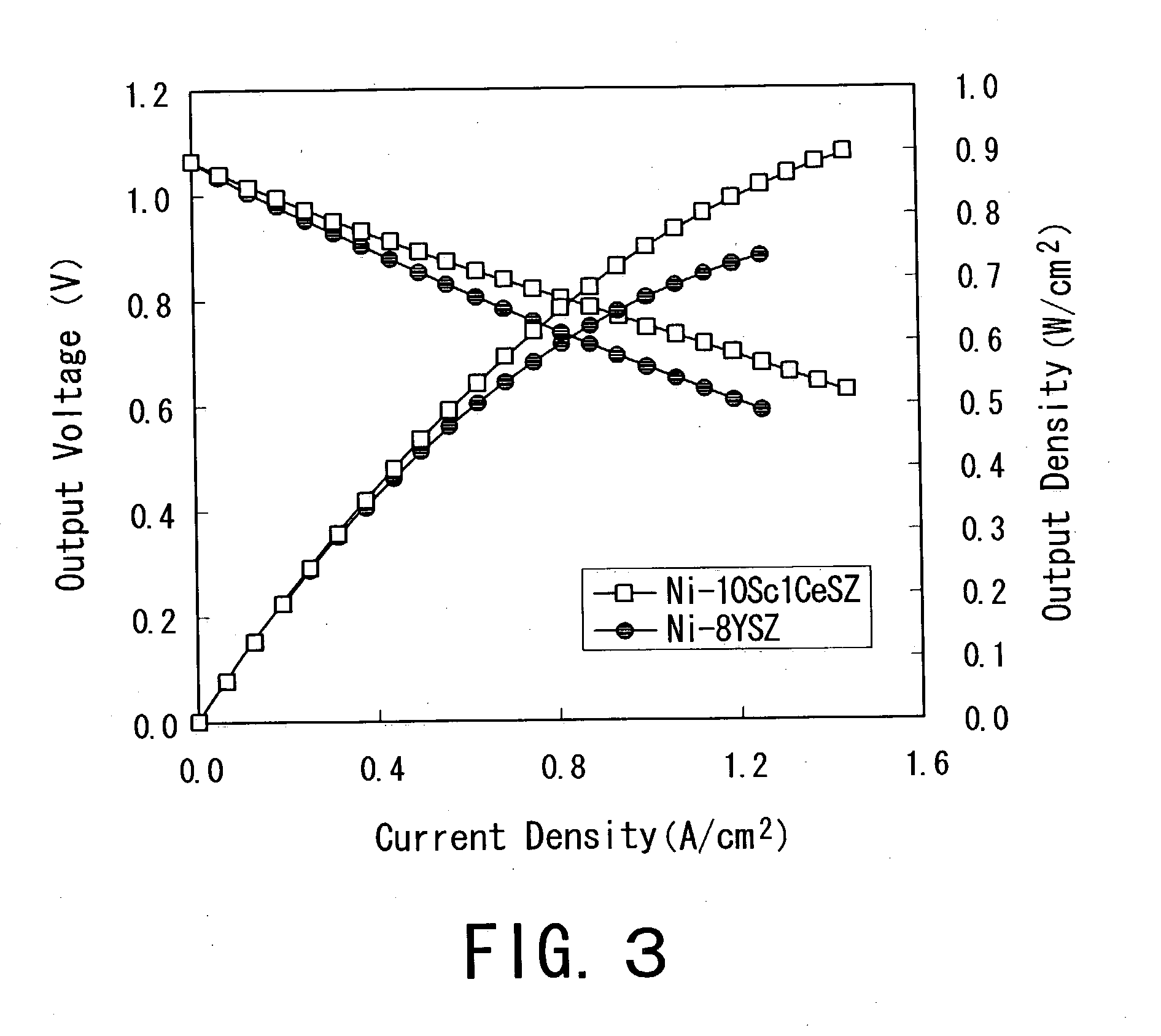
A single cell for a solid oxide fuel cell and a solid oxide fuel cell using the same practicable and excellent in generation performance and durability, wherein a fuel electrode including a cermet of a catalyst and a second solid electrolyte with oxide ion conductivity at 1000° C. of 0.20 S / cm or more is bonded to one side of a solid electrolyte plate with the conductivity of 0.07 S / cm or more and bending strength of 700 MPa or more, and an air electrode including a compound of perovskite type transition metal oxide with a third solid electrolyte is bonded to the other side. A surface of the fuel electrode is coated with a layer, and an air electrode surface is coated with a layer, and an aqueous solution where a noble metal compound is dissolved in water is impregnated into the air electrode.
Owner:TOHO GAS
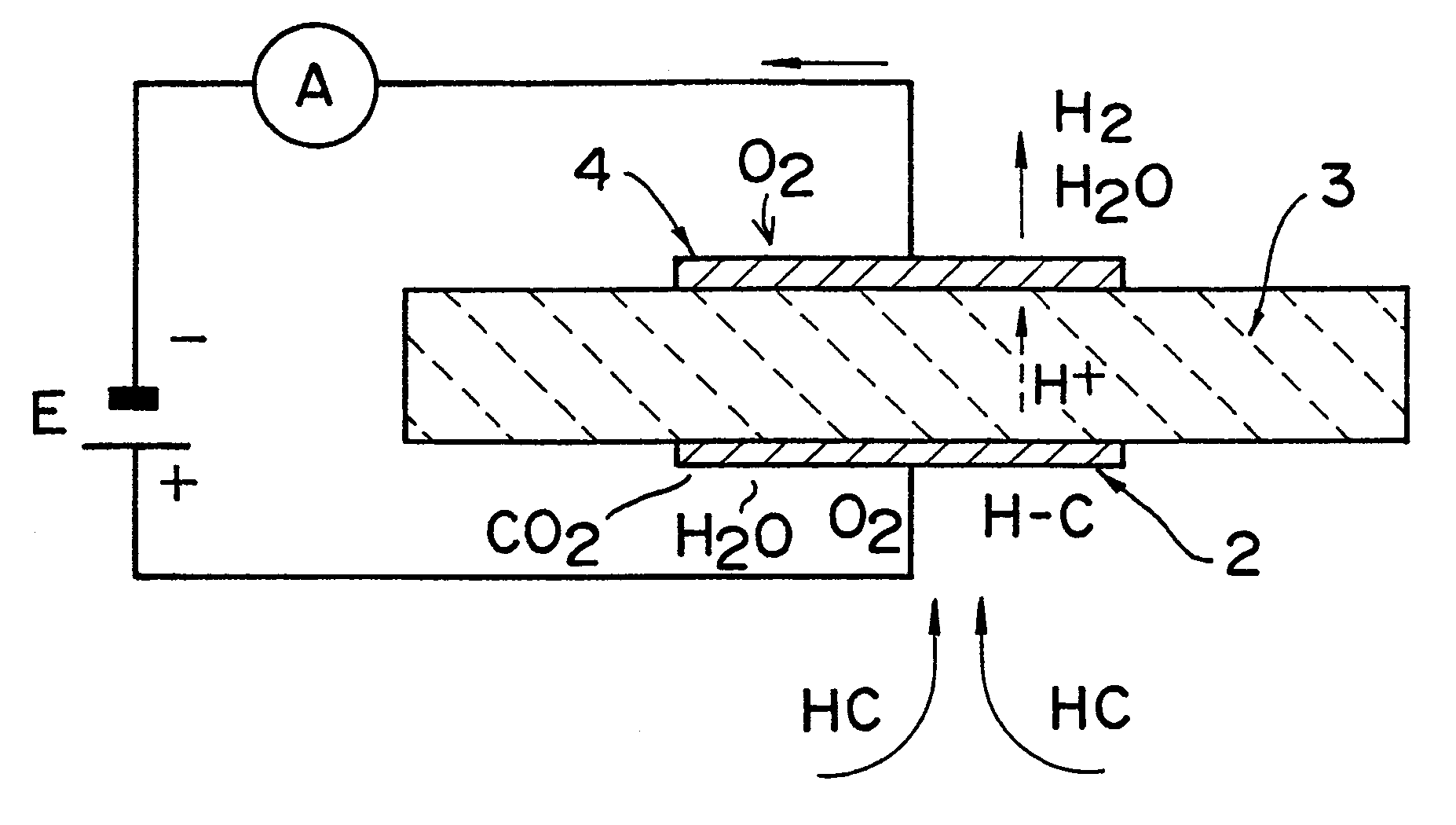
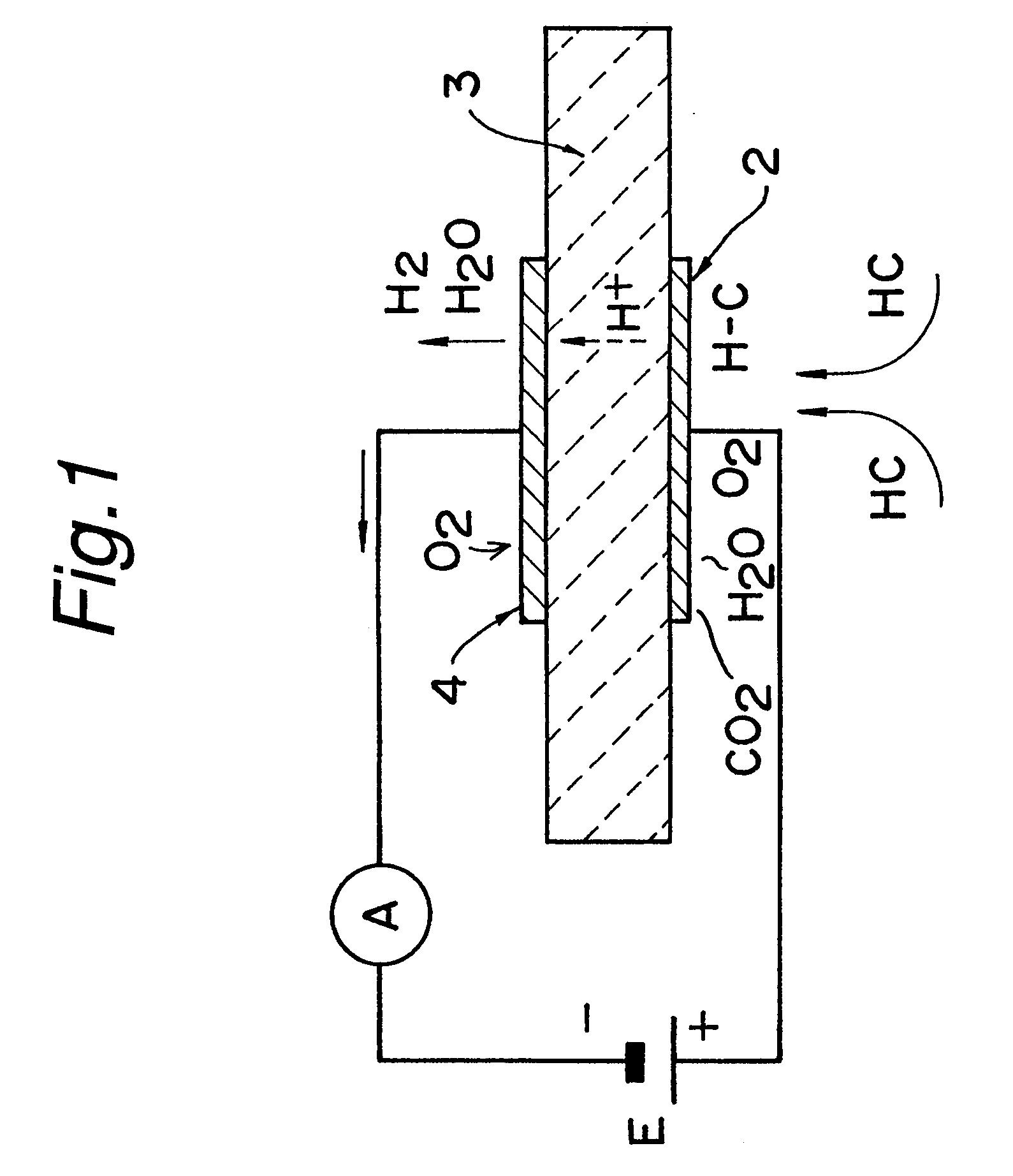
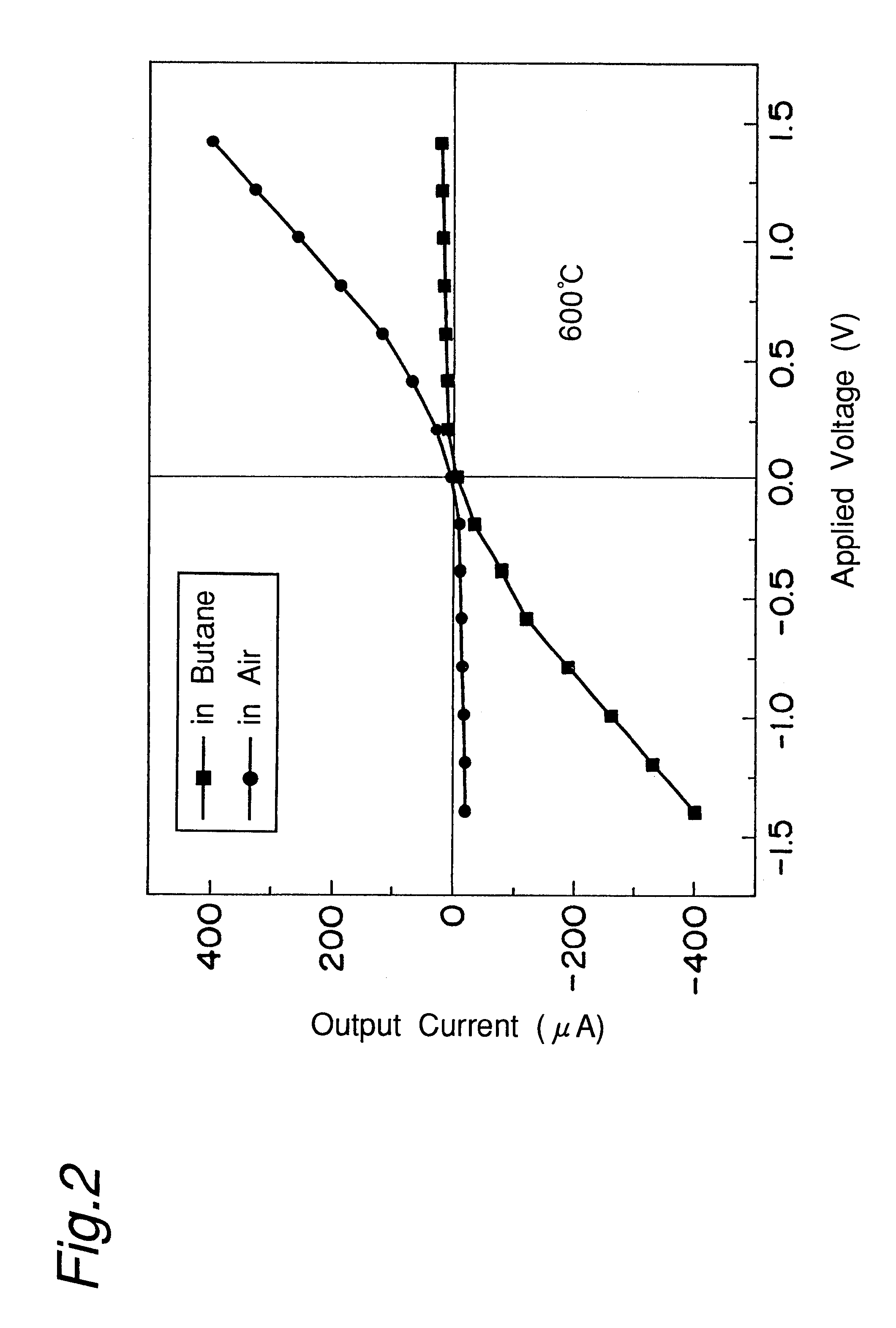
Hydrocarbon sensor
The present invention relates to a limiting-current type hydrocarbon sensor comprising a solid-electrolytic layer made of a Ba-Ce-based oxide, and provides a sensor capable of stably detecting hydrocarbon at high sensitivity, regardless of the concentration of oxygen in an atmosphere. A material mainly containing Al is used for at least one of two electrodes on the solid-electrolytic layer made of a Ba-Ce-based oxide to block oxygen at the electrode, i.e. cathode, whereby a hydrocarbon sensor being stable, high in sensitivity, compact, easy to use and low in cost can be provided. Furthermore, the other electrode, anode, of the limiting-current type hydrocarbon sensor is made of a material mainly containing Ag. The limiting-current type hydrocarbon sensor comprises a thin sensor-use solid-electrolytic layer capable of conducting protons and oxide ions, a pair of sensor-use electrodes formed on both sides of the solid-electrolytic layer, one electrode on each side, and a gas diffusion rate determining layer formed on the side of the cathode, one of the pair of electrodes. On the sensor-use solid-electrolytic layer on the side of the anode, a solid ion pump for transferring oxygen, hydrogen or water vapor is provided between the anode and the atmosphere under measurement.
Owner:PANASONIC CORP
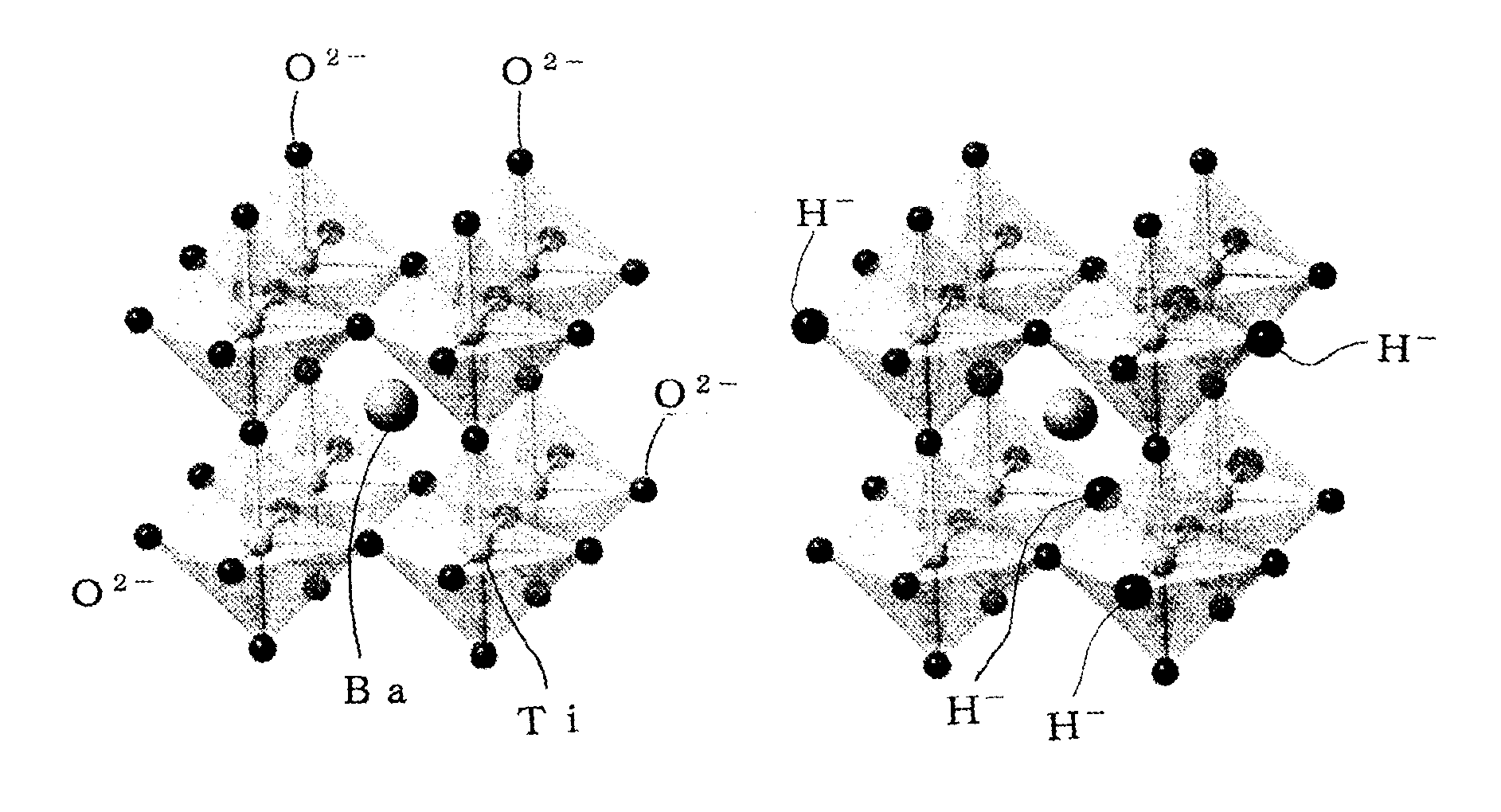
Perovskite oxide containing hydride ion, and method for manufacturing same
ActiveUS20140128252A1Improve featuresAlkaline earth titanatesAlkali titanatesAlkaline earth metalElectrical conductor
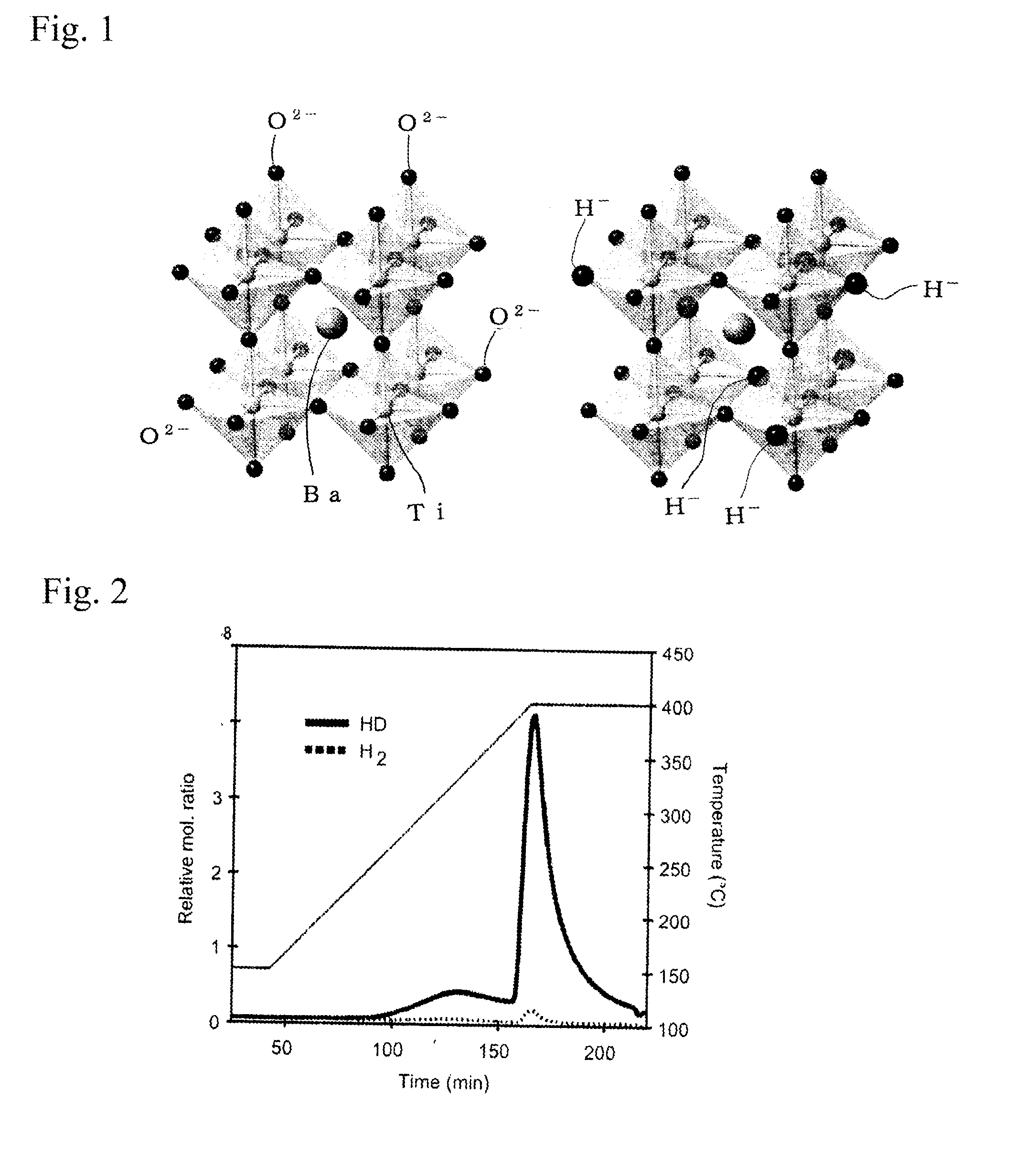
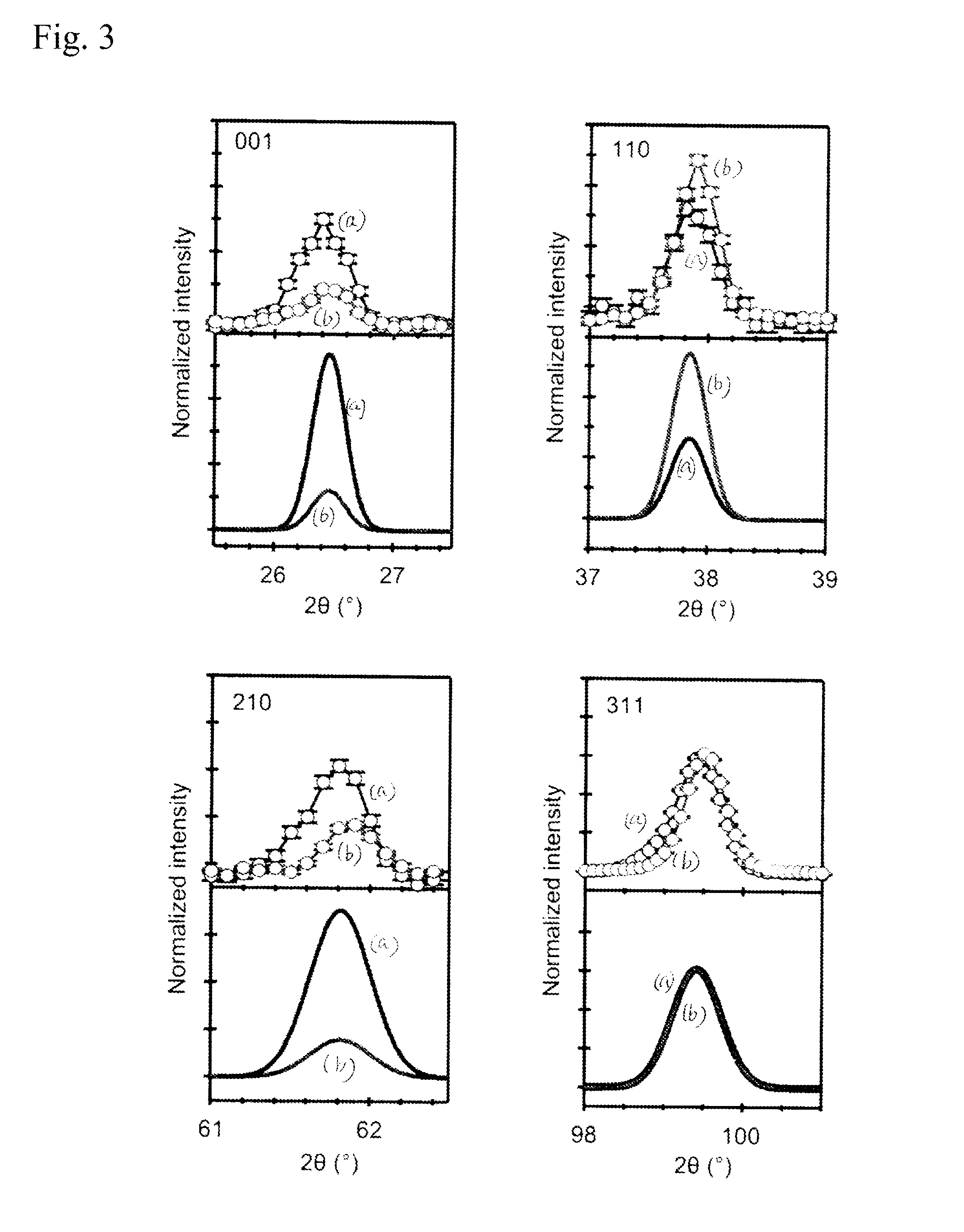
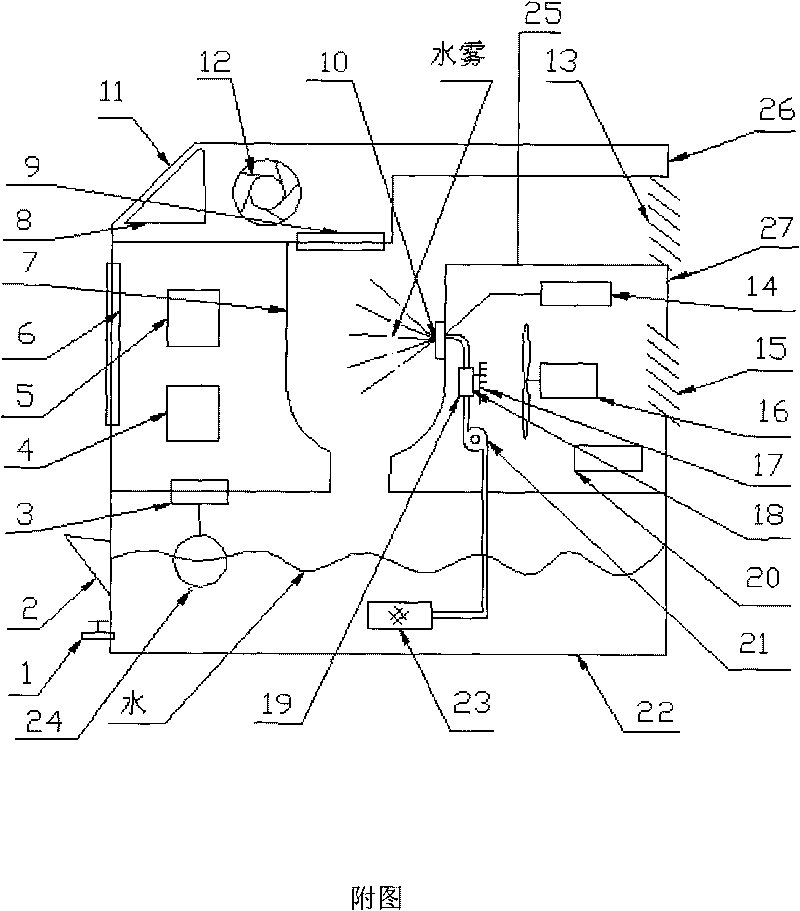
[Problem] Many oxide-ion conductors exhibit high functionality at high temperatures due to the large weight and charge of oxide ions, and it has been difficult to achieve the functionality at low temperatures.[Solution] A perovskite oxide having hydride ion conductivity, at least 1 at % of the oxide ions (O2−) contained in a titanium-containing perovskite oxide being substituted with hydride ions (H−). This oxide, in which negatively charged hydride ions (H−) are used for the ionic conduction, has both hydride ion conductivity and electron conductivity. As a starting material, the titanium-containing perovskite oxide is kept together with a powder of an alkali metal or alkaline-earth metal hydride selected from LiH, CaH2, SrH2, and BaH2 in a temperature range of 300° C. or higher and lower than the melting point of the hydride in a vacuum or an inert gas atmosphere to substitute some of the oxide ions in the oxide with the hydride ions, resulting in the introduction of the hydride ions into oxygen sites.
Owner:JAPAN SCI & TECH CORP
Novel negative ion refrigerating generator
InactiveCN101737884ASuitable for health care cooling needsMachines using electric/magnetic effectsDeodrantsCold airEngineering
The invention relates to a novel negative ion refrigerating generator which uses a semiconductor refrigerating device as a cold source and uses water as a substance for absorbing heat and dust and ionizing; by utilizing high-pressure water to generate air negative ions in the process of spray atomization, a domestic electric appliance product which integrates a refrigerating technology and a negative oxide ion generator is produced. The invention has the advantages of various negative ion generators and simultaneously can generate cold air to meet the demands on health care and temperature reduction.
Owner:洪旗
Optical information recording medium and azo-metal complex dye
InactiveUS20080081286A1Excellent recording/reproducing propertyImprove light resistancePhotosensitive materialsPhotomechanical apparatusHydrogen atomLaser light
An optical information recording medium having a recording layer on which information can be recorded by irradiation with a laser light having a wavelength of 440 nm or less, wherein the recording layer contains at least one azo-metal complex dye derived from a metal ion or a metal oxide ion and an azo dye represented by the following general formula (1-1) or (1-2):wherein Q represents a carbocyclic group or a heterocyclic group, and R6 to R8 independently represent a hydrogen atom or a substituent.
Owner:FUJIFILM CORP
Preparation method for antibacterial finishing agent used for textile fabric
The invention relates to a preparation method for an antibacterial finishing agent used for a textile fabric. The preparation method comprises the following steps: adding deionized water, an aluminum-zirconium coupling agent, a fluorine-silicon coupling agent and nanometer zinc oxide into an agitation vessel, carrying out stirring and heating to 62 DEG C and maintaining the temperature for 30 min so as to prepare nanometer zinc oxide; adding quaternary ammonium salt monomer and deionized water into the reaction vessel, adjusting the pH value of the obtained system to 7 to 8 by using sodium carbonate and carrying out stirring and heating to 65 to 68 DEG C; and adding diallylamine and an aqueous solution of an initiator drop by drop within 2 h, carrying out a reaction for 2 h under a heat preservation condition, adding a solution composed of 0.2 part of silver nitrate and 20 parts of deionized water, carrying out uniform mixing under stirring, rapidly adding a solution composed of 0.2 part of sodium borohydride and 20 parts of deionized water and a carrying out a reduction reaction. Since the quaternary ammonium salt monomer is grafted on the surfaces of modified zinc oxide ions, safety, long-lasting action and high efficacy of the antibacterial finishing agent are realized.
Owner:秦瑶
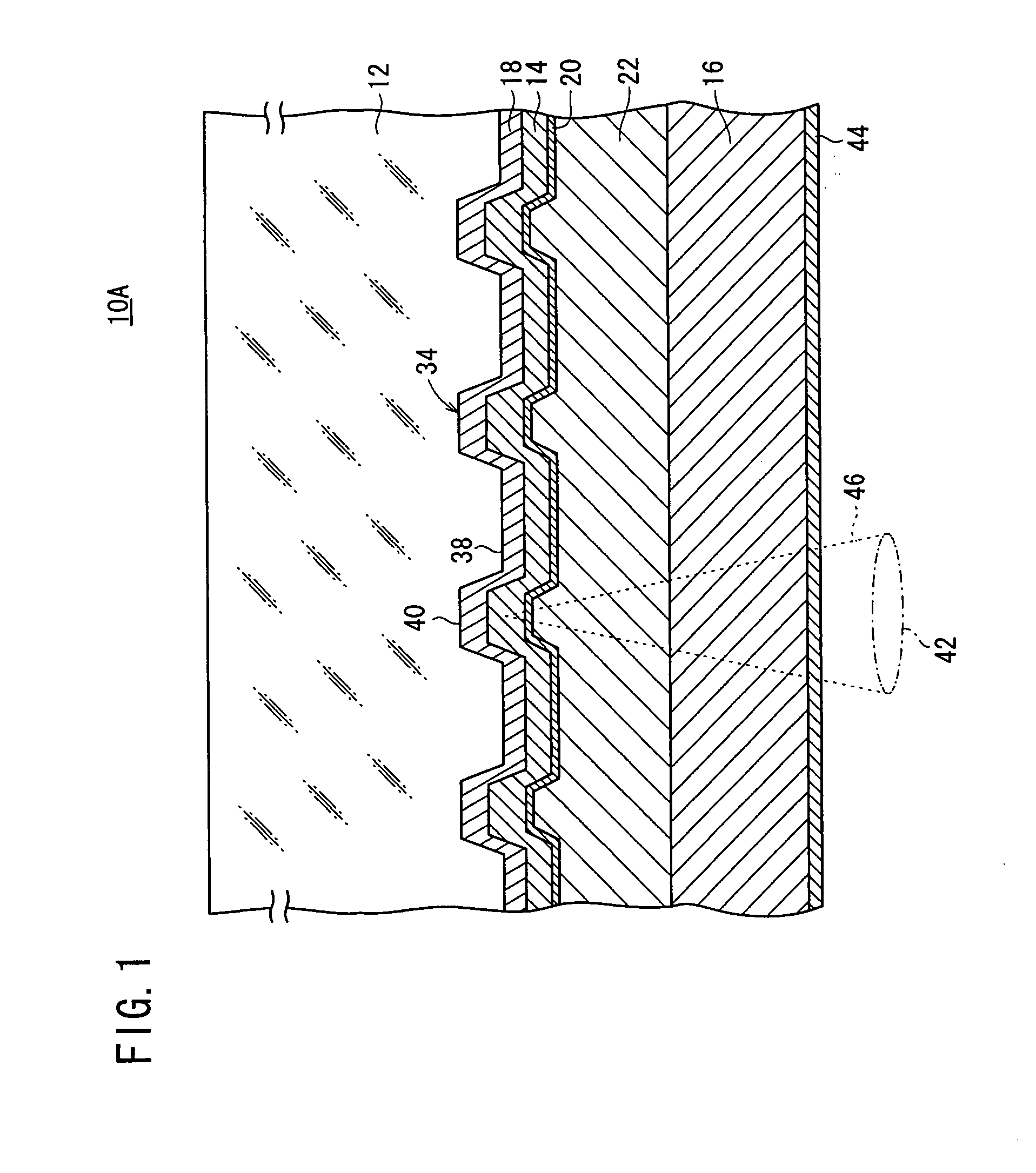
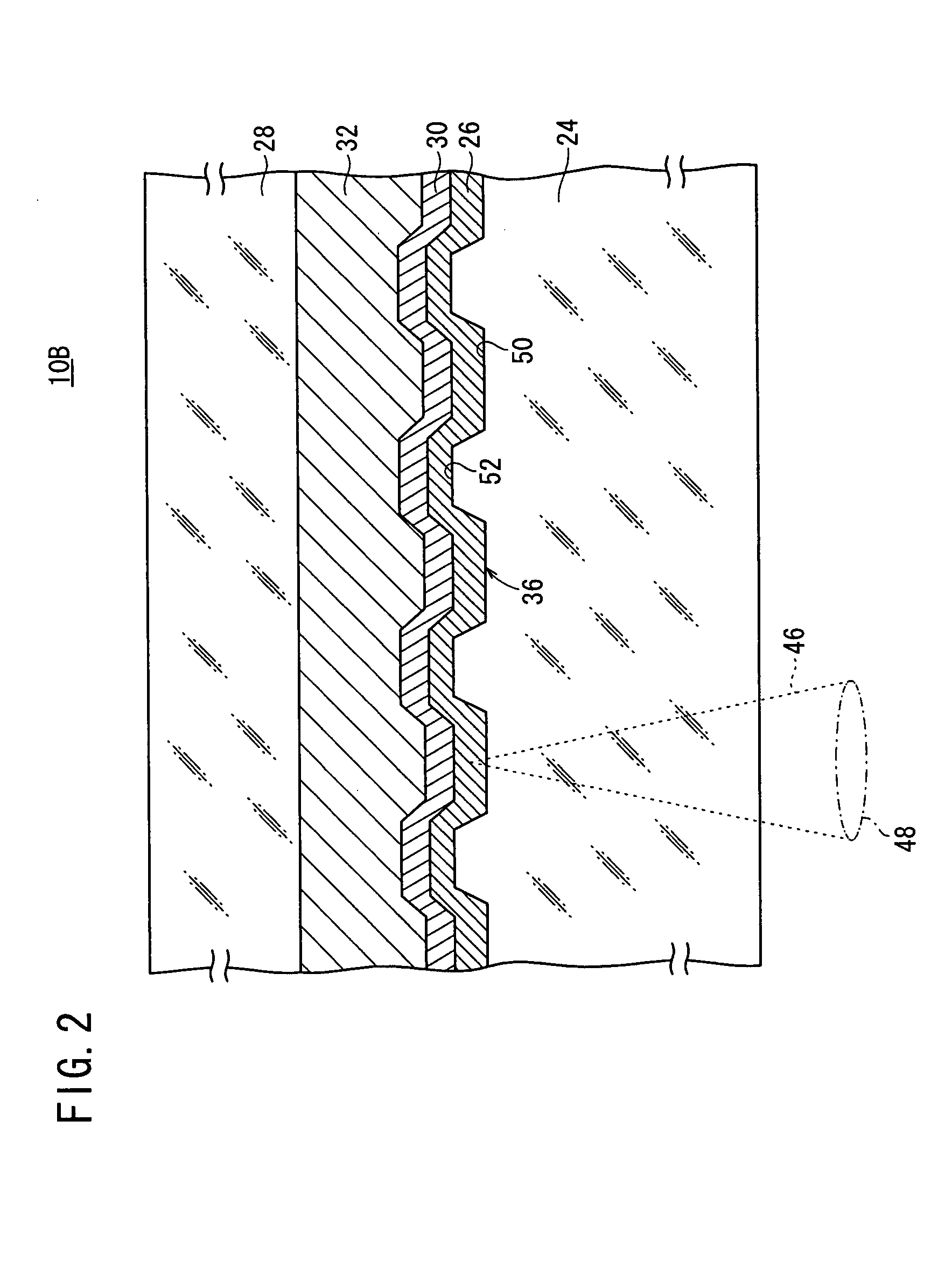
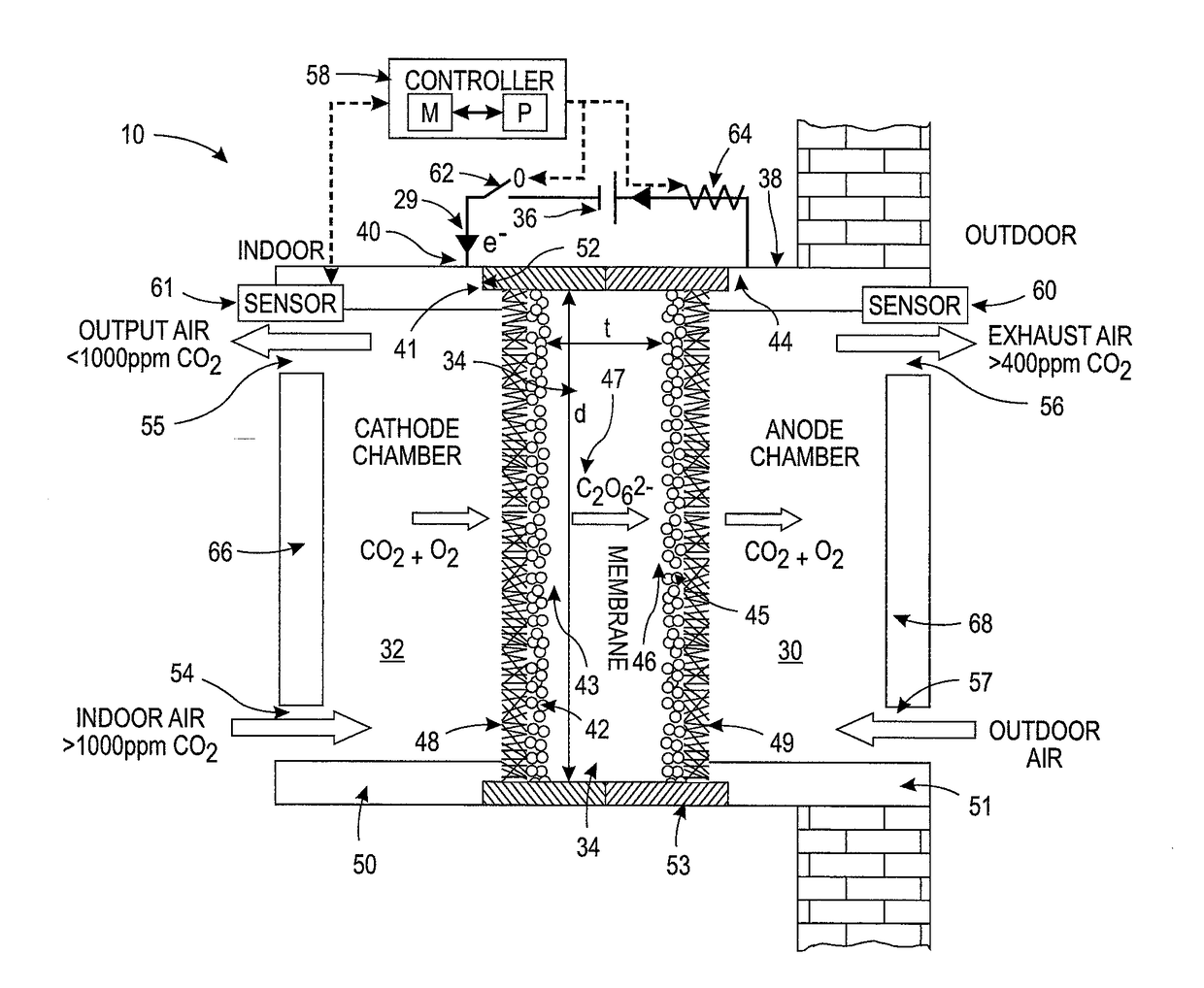
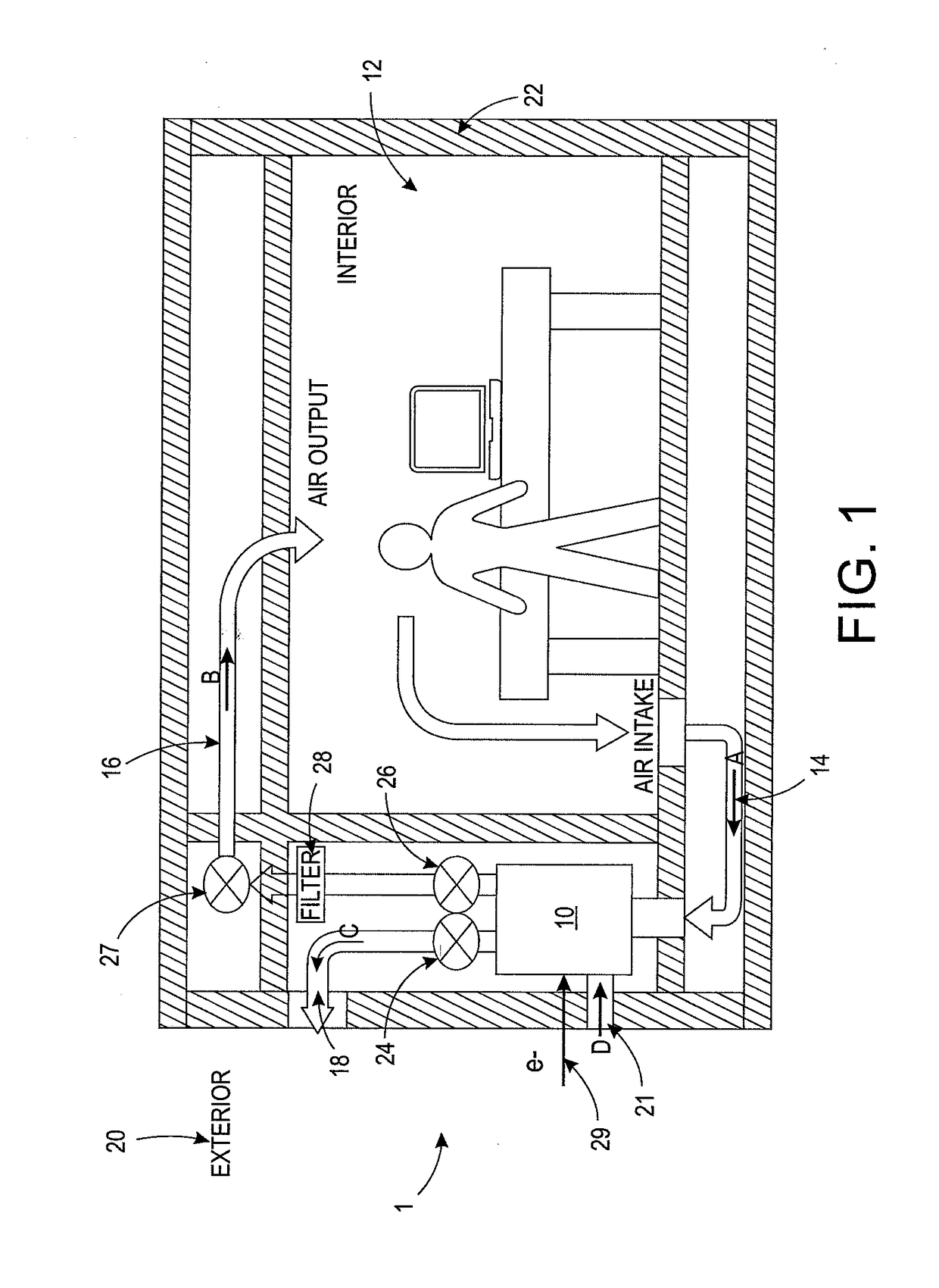
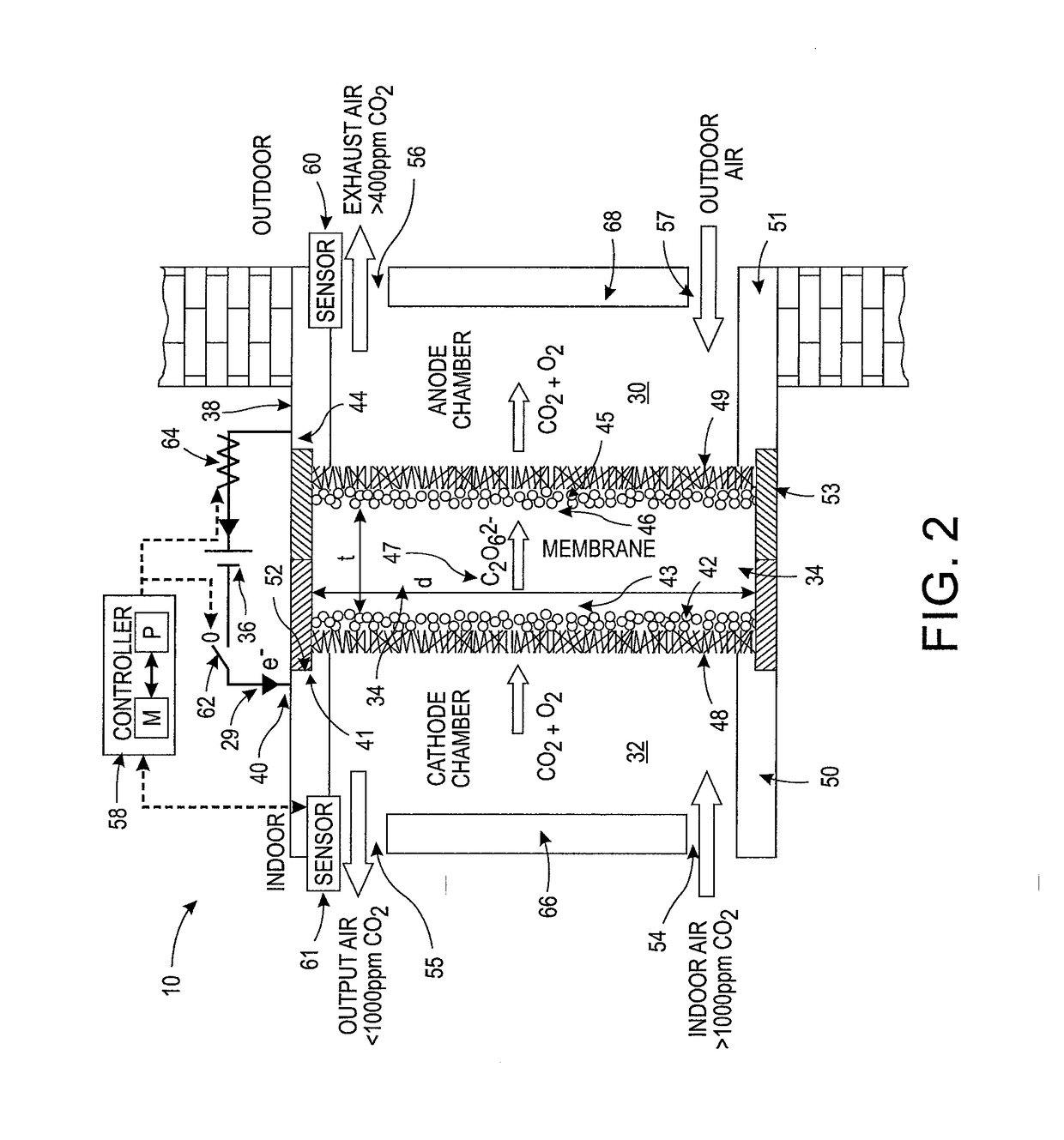
System and method for adjusting carbon dioxide concentration in indoor atmospheres
An electrochemical device suited to modifying a carbon dioxide concentration in an interior space includes a cathode chamber with an inlet which receives a feed gas containing carbon dioxide. A reduction catalyst layer in the cathode chamber reduces carbon dioxide in the gas to form an ionic carrier species. An anode chamber with an outlet outputs a gas comprising carbon dioxide. A solid electrolyte membrane spaces the anode chamber from the cathode chamber and transports the ionic carrier species between the cathode chamber and the anode chamber. The membrane includes an ionic liquid. An oxidation catalyst layer in the anode chamber oxidizes the ionic carrier species to form carbon dioxide. A voltage source provides a voltage difference across the membrane.
Owner:XEROX CORP
Single cell for a solid oxide fuel cell
InactiveUS7108938B2Excellent generating performance and durabilityThe process is feasibleSolid electrolytesFuel cells groupingFuel cellsFlexural strength
Owner:TOHO GAS
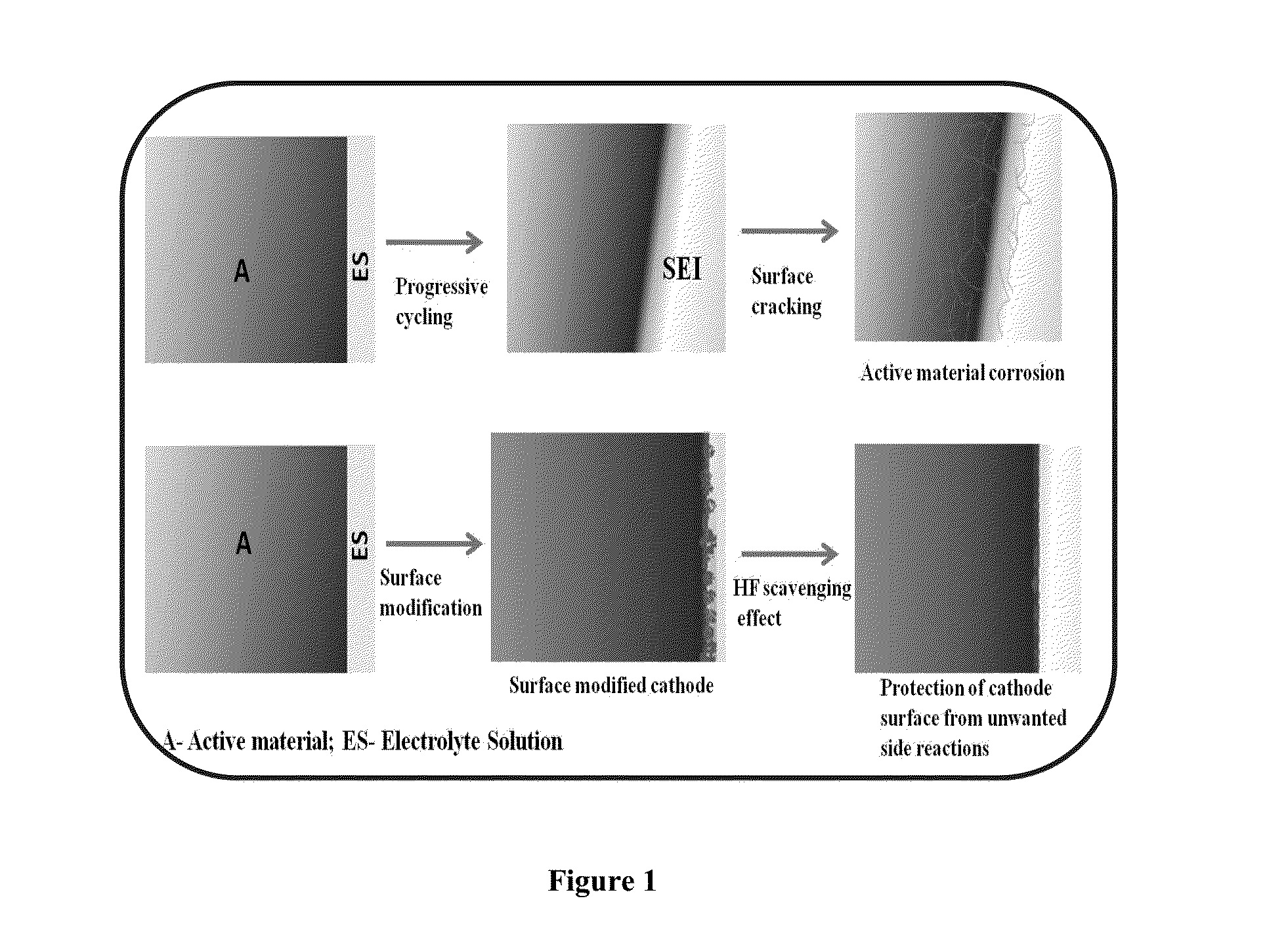
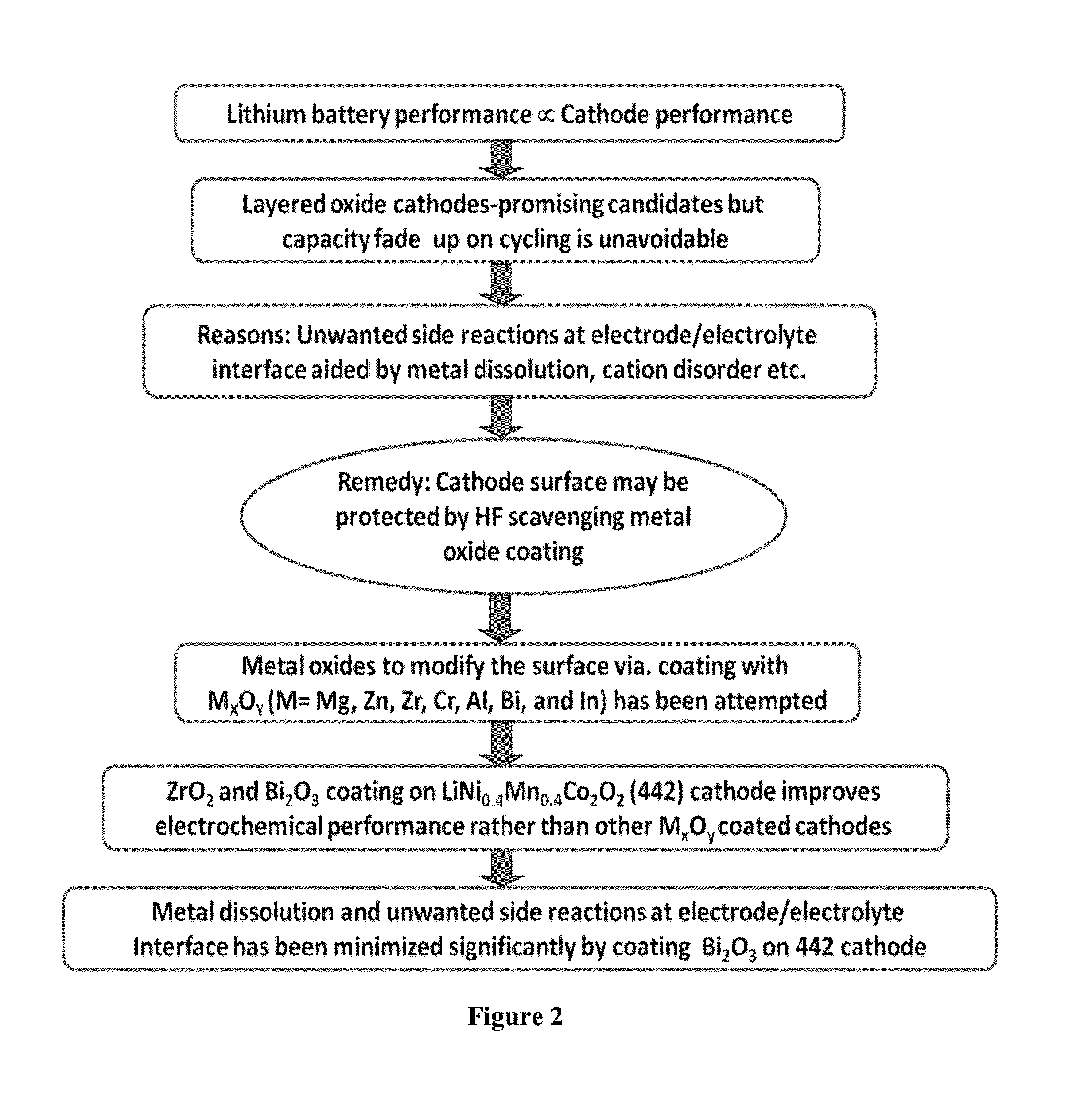
Surface modified cathode with improved lithium intercalation behavior
ActiveUS20160043387A1Improved lithium intercalation behaviourEnhance intercalationSecondary cellsPositive electrodesHigh rateCapacity value
Surface modification of LiNi0.4Mn0.4Co0.2O2(442)compound with certain inert (MxOy) metal oxides viz., Al2O3, Bi2O3, In2O3, Cr2O3, ZrO2, ZnO, MgO has been attempted with a view to improve the structural and cycling stability, especially upon high voltage and high rate cycling conditions. In addition to HF scavenging effect, the protective metal oxide inter-connect layer restricts the number of oxide ion vacancies eliminated during the initial cycling of cathode, resulting in the reduced irreversible capacity loss of the first cycle. Among the surface modified cathodes, Bi2O3 coated LiNi0.4Mn0.4Co0.2O2 cathode exhibits appreciable specific capacity values of 196 (Qdc1) and 175 (Qdc100) mAh g−1 with 89% capacity retention, thus evidencing the superiority of Bi2O3 modifier in improving the electrochemical behavior of pristine LiNi0.4Mn0.4Co0.2O2 cathode. Further, suitability of Bi2O3 coated LiNi0.4Mn0.4Co0.2O2 cathode for high voltage (5.0 V) and high rate (3 C) lithium intercalation and de-intercalation applications has been demonstrated up to 100 cycles.
Owner:COUNCIL OF SCI & IND RES
Inductively coupled plasma mass spectrometry used for determining trace aluminum molybdenum vanadium titanium niobium in silicon steel
InactiveCN104614434AOvercoming distractionsOvercome the shortcoming that the detection limit cannot reach the product control standardMaterial analysis by electric/magnetic meansNiobiumDecomposition
The invention discloses an inductively coupled plasma mass spectrometry used for determining trace aluminum molybdenum vanadium titanium niobium in silicon steel, and simultaneous determination of trace aluminum, molybdenum, vanadium, titanium, and niobium in silicon steel via inductively coupled plasma mass spectrometry (ICP-MS) is discussed. Determination parameters are optimized via condition experiments, and it is determined that RF power is 1400W, pump speed is 30rpm, sampling depth is 140, and atomization pressure is 0.90. Sample decomposition is realized using nitric acid, Be and Y mixed inner standard method is used for correcting signal drift caused by high matrix in measuring processes. Isotope 27Al, 98Mo, 51V, 47Ti, and 93Nb are taken as measuring isotopes based on mass spectrum interference conditions in determination; and at the same time, yield of double charged ions and oxide ions is adjusted to be lowest by adjusting instrument parameters so as to reduce influences. Calibration solutions are prepared via matrix matching, working curve is established via standard addition method, and reagent blank is deducted. Determination lower limit of the elements reaches 1<mu>g / g, and element RSD is less than 5.2% when the inductively coupled plasma mass spectrometry is used for determining silicon steel standard samples.
Owner:QINGDAO TIANHENG MACHINERY
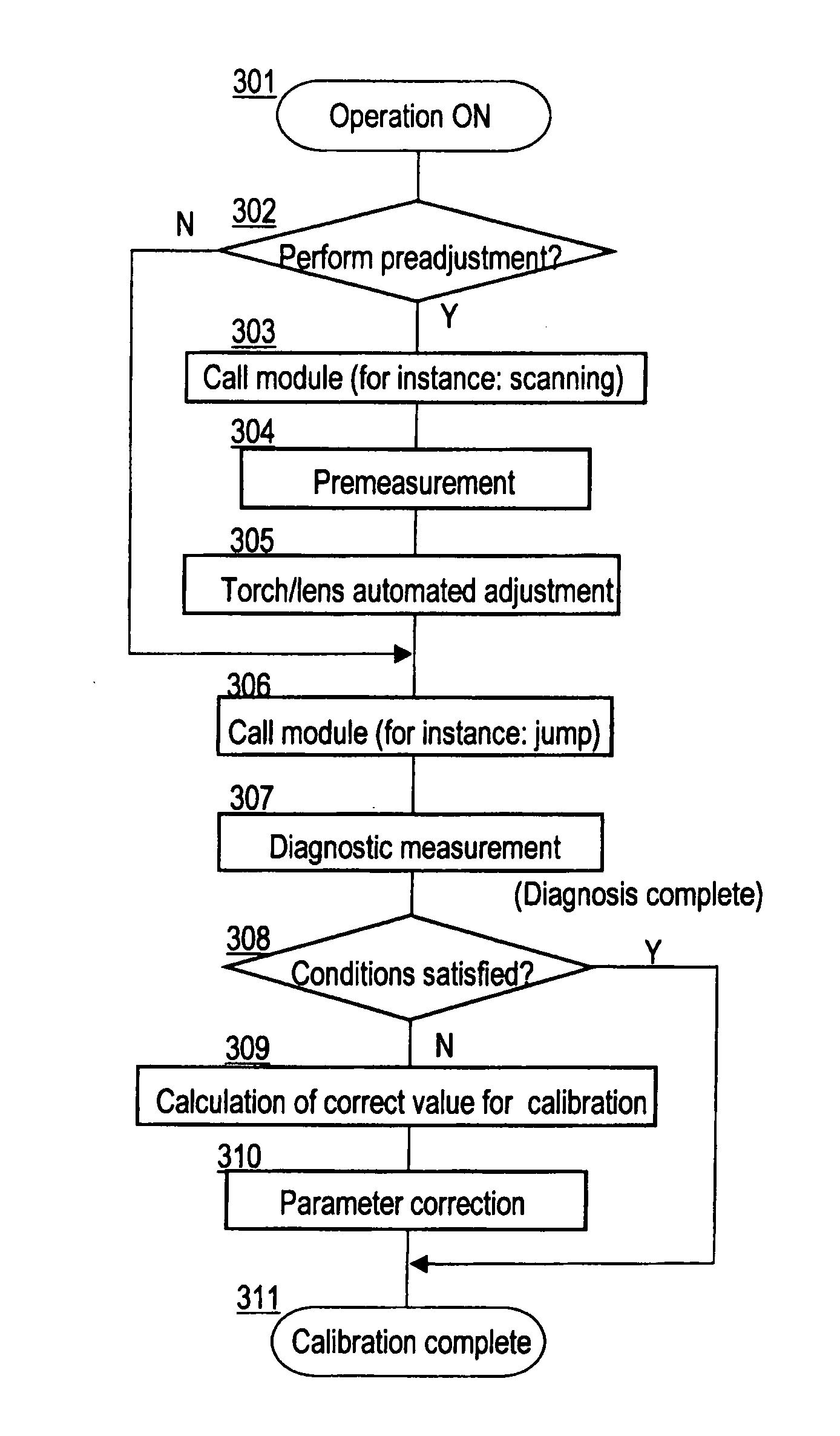
Diagnosis and calibration system for ICP-MS apparatus
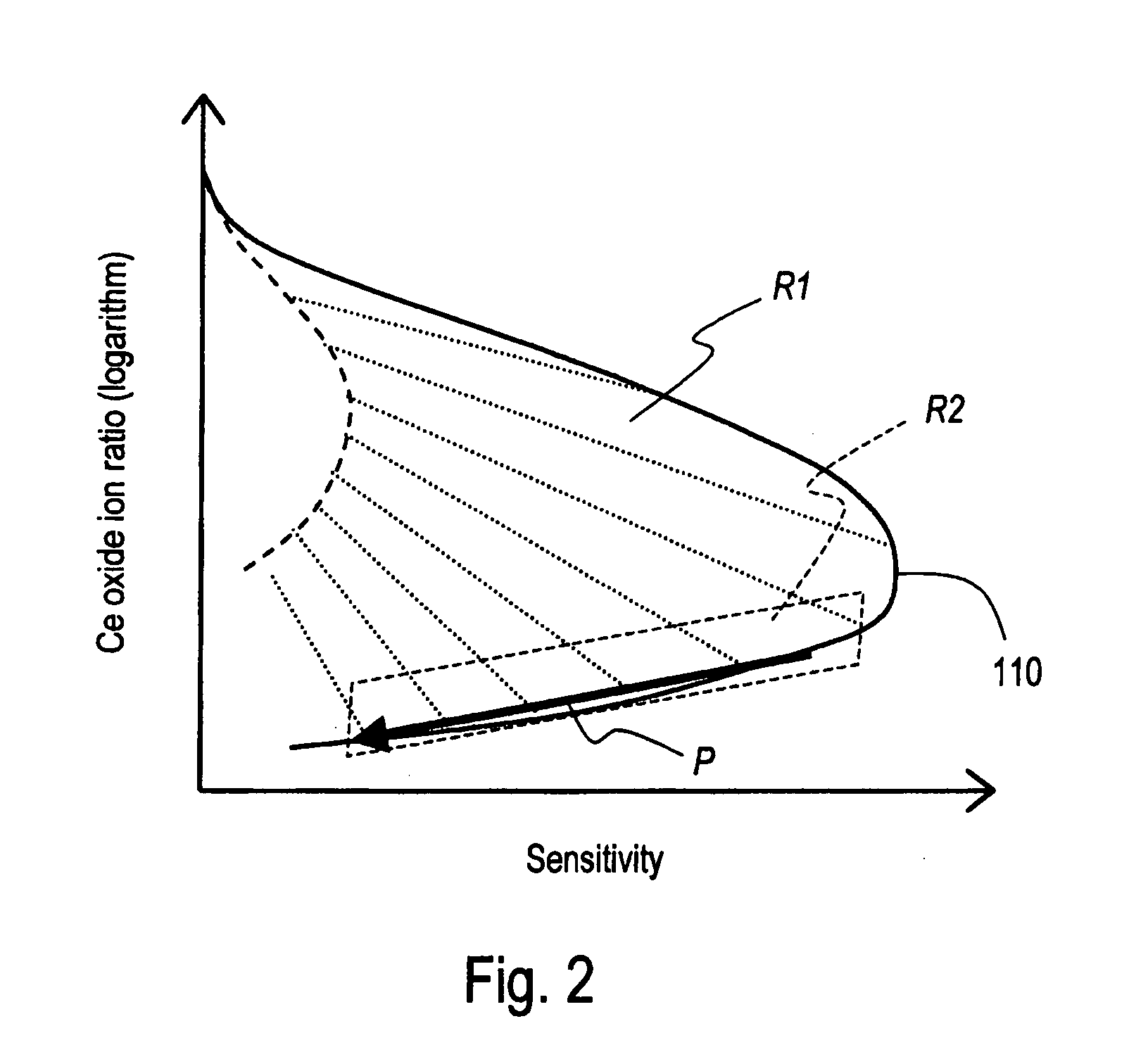
ActiveUS20080099671A1Easily property of deviceGood reproducibilityParticle separator tubesDigital computer detailsHigh frequency powerDevice Properties
A diagnostic system designed such that an aggregate of parameter combinations is stored, which is an aggregate of combinations of parameters consisting of a first parameter for determining the output of the high-frequency power source, a second parameter for determining the flow rate of the carrier gas in the aerosol, and a third parameter for determining the distance between the plasma torch and the interface, and which forms a specific array such that the measurement points corresponding to the respective combinations are lined up in order along the direction of length of an envelope that forms the end on the high-sensitivity side of a graph drawn as an aggregate of all measurement points on a sensitivity-oxide ion ratio graph, and a diagnostic measurement is performed with a specific diagnostic sample using the parameter value of each combination of the above-mentioned parameter combinations that form the aggregate such that the device properties can be confirmed from the position on the envelope on the sensitivity-oxide ion ratio graph of the actual measurement points corresponding to each combination.
Owner:AGILENT TECH INC
Rare earth metal-based permanent magnet having corrosion-resistant film and method for producing the same
InactiveUS6884513B2Improve corrosion resistanceEffectively suppresses the corrosionMagnetic materialsDomestic articlesMolybdic acidChemical conversion
The chemical conversion film containing, at least as the constituent components thereof, (a) at least one of the metals selected from molybdenum, zirconium, vanadium, and tungsten; (b) a rare earth metal constituting the magnet; and (c) oxygen, which is formed on the surface of a rare earth metal-based permanent magnet according to the present invention, contains a composite metal oxide provided on the surface of the R-rich phase having a lower oxidation-reduction potential through a preferential reaction of the metallic ions that are present in the form of complex ions or oxide ions, such as of molybdenum, contained in the treatment solution, with the rare earth metals that elute from the magnet. Thus formed composite metal oxide reduces the difference in corrosion potential as to realize a uniform surface potential, and effectively suppresses the corrosion based on potential difference. Furthermore, the chemical conversion film thus formed exhibits excellent corrosion resistance even if it is provided as a thin film. The production method thereof can be implemented at low cost and by a simple process comprising treating the surface of the magnet by using a treatment solution containing a molybdate and the like.
Owner:HITACHI METALS LTD
Device and method for adjusting oxygen concentration in liquid lead-bismuth alloy by using solid lead oxide
InactiveCN103499983ANot generatedOxygen ion exchange efficiency is highRatio controlLead bismuthLead oxide
The invention discloses a device and a method for adjusting oxygen concentration in a liquid lead-bismuth alloy by using a solid lead oxide, and belongs to the technical field of nuclear engineering. The device comprises a solid oxide ion exchanger bypass, a solid oxide ion exchanger mounting hole, a liquid lead-bismuth alloy inlet, a liquid lead-bismuth alloy outlet, a bypass heat exchanger and a solid oxide ion exchanger, wherein the solid oxide ion exchanger mounting hole is formed in the solid oxide ion exchanger bypass; the liquid lead-bismuth alloy inlet is formed in the solid oxide ion exchanger bypass; the liquid lead-bismuth alloy outlet is formed in the solid oxide ion exchanger bypass; the bypass heat exchanger is wound on the outer wall of the solid oxide ion exchanger bypass; the solid oxide ion exchanger is arranged in the solid oxide ion exchanger bypass. The method comprises the following steps: arranging the solid oxide ion exchanger and the solid oxide ion exchanger bypass respectively; adjusting the oxygen concentration of the liquid lead-bismuth alloy by adjusting the cooling power of the bypass heat exchanger. According to the device and the method, the oxygen content in the liquid lead-bismuth alloy is adjusted by controlling the solid lead oxide, so that the defects existing in the prior art are overcome.
Owner:NORTH CHINA ELECTRIC POWER UNIV (BAODING)
Nitrogen-oxide gas sensor with long signal stability
ActiveUS8399883B2Semiconductor/solid-state device detailsSolid-state devicesElectricityNitrogen oxides
The present invention provides a nitrogen-oxide gas sensor that is able to measure nitric oxide and nitrogen dioxide at the same time and ensure measurement accuracy and long stability. For these purposes, the nitrogen-oxide gas sensor includes: an oxide ion conductive solid electrolyte; a primary film that contacts the solid electrolyte and is made of a p-type semi-conductor metal oxide; a secondary film that contacts the solid electrolyte and is made of a p-type semiconductor metal oxide; an n-type semiconductor metal oxide that is included in at least one of the primary and secondary films; a power source that applies electric power to the primary and secondary films by electrically connecting a primary node to the primary film and a secondary node to the secondary film; and a measurement unit that measures the electric potential difference between the primary and secondary nodes.
Owner:LOTTE ENERGY MATERIALS CORP +1
Solid oxide fuel cell components tuned by atomic layer deposition
InactiveUS20080311455A1Low costEnhanced surface exchange rateCell electrodesFinal product manufactureFuel cellsCatalytic metal
A reduced cost solid oxide fuel cell having enhanced surface exchange rates and diffusivity of oxide ions is provided. The invention cell includes a first porous electrode and a second porous electrode, where the porous electrodes have a layer of electronically conductive porous non-precious metal, and the porous non-precious metal layer is a gas diffusion layer. The porous electrodes further include at least one atomic layer of catalytic metal deposited on the non-precious metal layer, and an electrolyte layer disposed between the first porous electrode and the second porous electrode. The electrolyte layer includes a first dense ion-conductive doped oxide film layer, and a second dense ion-conductive doped oxide film layer deposited on the first doped oxide film layer, where the catalytic metal layer on the conductive porous non-metal layer enhances surface exchange rates and diffusivity of the oxide ions, thus the material costs of the fuel cell are reduced.
Owner:HONDA MOTOR CO LTD +1
Composite ceramic powder, process for production of same and solid oxide fuel cell
InactiveCN102066263AImprove the distribution effectImprove distributionMaterial nanotechnologyCell electrodesComposite ceramicControllability
Provided are a composite ceramic powder which is excellent in distribution at nanometer level, controllability of composition, formation of oxide ions, and electronic conductivity; a process for the production of same, and a solid oxide fuel cell. The composite ceramic powder is a powder which contains either an oxide represented by formula: A1-xBxC1-yDyO3 or nickel oxide, and zirconia and which is produced by adding a zirconia acidic dispersion containing both yttria-stabilized zirconia particles and either ions of one or more elements selected from the group consisting of the elements defined for A, B, C and D or nickel ions to an alkali solution to form a precipitate via neutralization, and heat-treating the precipitate at 200 DEG C or above. In the formula, A is one or two elements selected from the group consisting of La and Sm; B is one or more elements selected from the group consisting of Sr, Ca and Ba; C is one or two elements selected from the group consisting of Co and Mn; D is one or two elements selected from the group consisting of Fe and Ni; 0.1 = x = 0.5 and 0 = y = 0.3.
Owner:SUMITOMO OSAKA CEMENT CO LTD
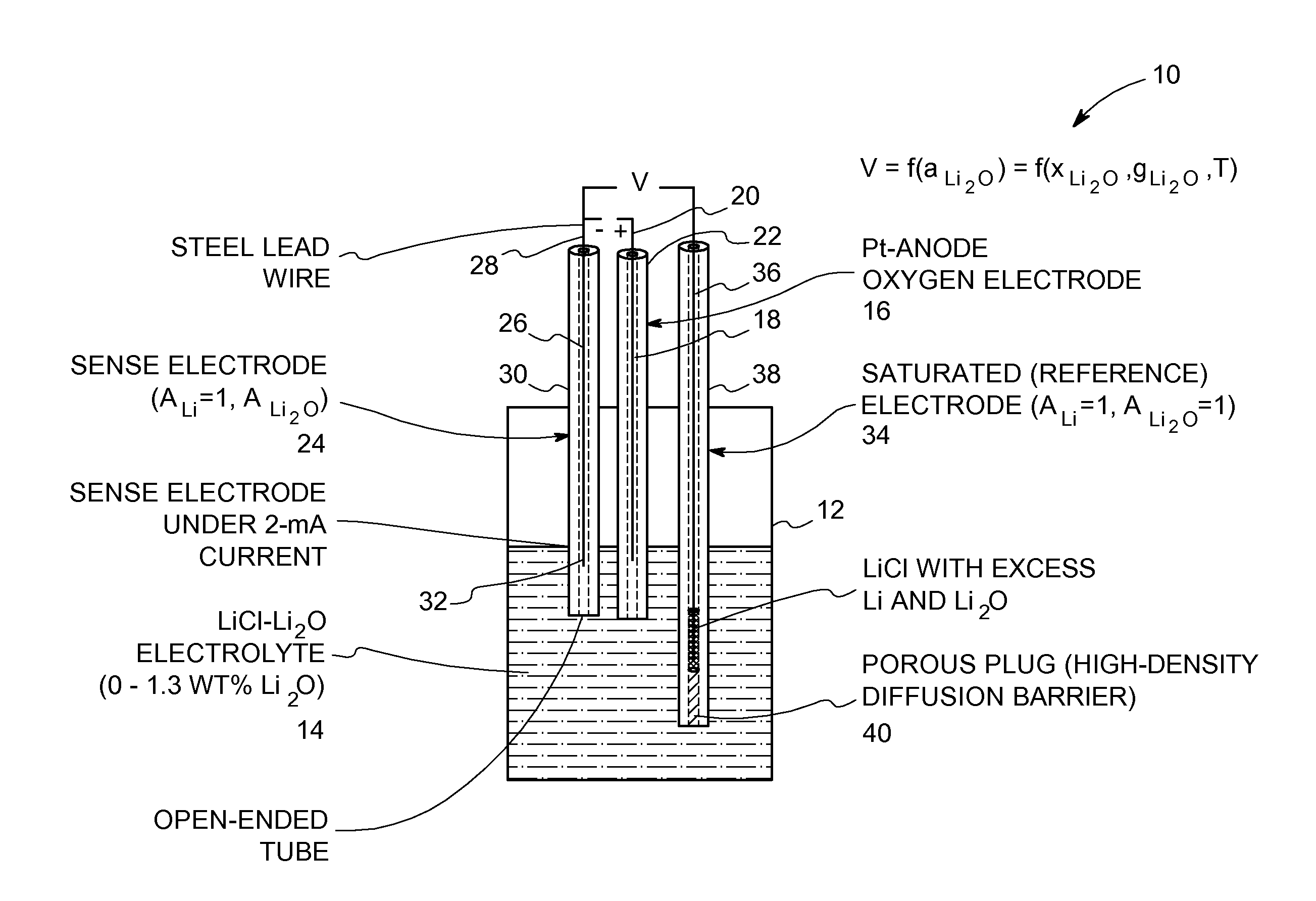
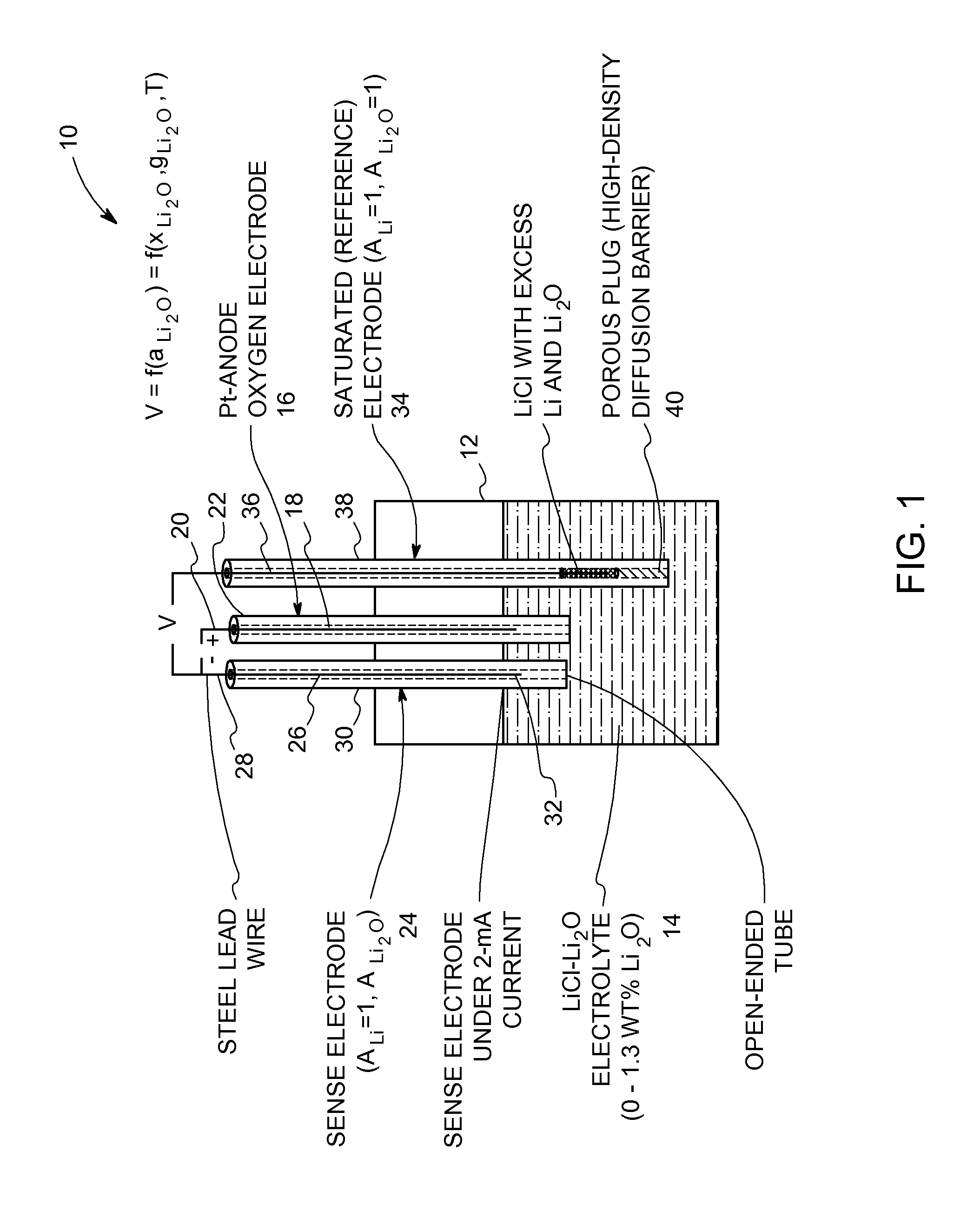
Oxide-ion sensor for use in a molten-salt based electrochemical reduction process
InactiveUS20110108439A1Weather/light/corrosion resistanceVolume/mass flow measurementEquilibrium potentialMolten salt
An oxide-ion sensor includes an oxygen electrode, a sense electrode and a saturated (reference) electrode. The sense electrode is operated at a substantially constant current for determining an instantaneous value of a dissolved oxide-ion concentration in the molten salt electrolyte. The saturated electrode is used to determine a reference value of the dissolved oxide-ion concentration in the molten salt electrolyte. A dissolved oxide-ion concentration in the molten salt electrolyte is continuously monitored in-situ during the molten-salt based electrochemical reduction process by determining an equilibrium potential between the sense electrode and the saturated electrode with the sense electrode carrying a small current in a circuit that is completed using the oxygen electrode. In another embodiment, the dissolved oxide-ion concentration in the molten salt electrolyte is continuously monitored in-situ by determining an electrochemical impedance of the molten salt electrolyte using a pair of bare current-carrying conductors and a frequency response analyzer.
Owner:GENERAL ELECTRIC CO
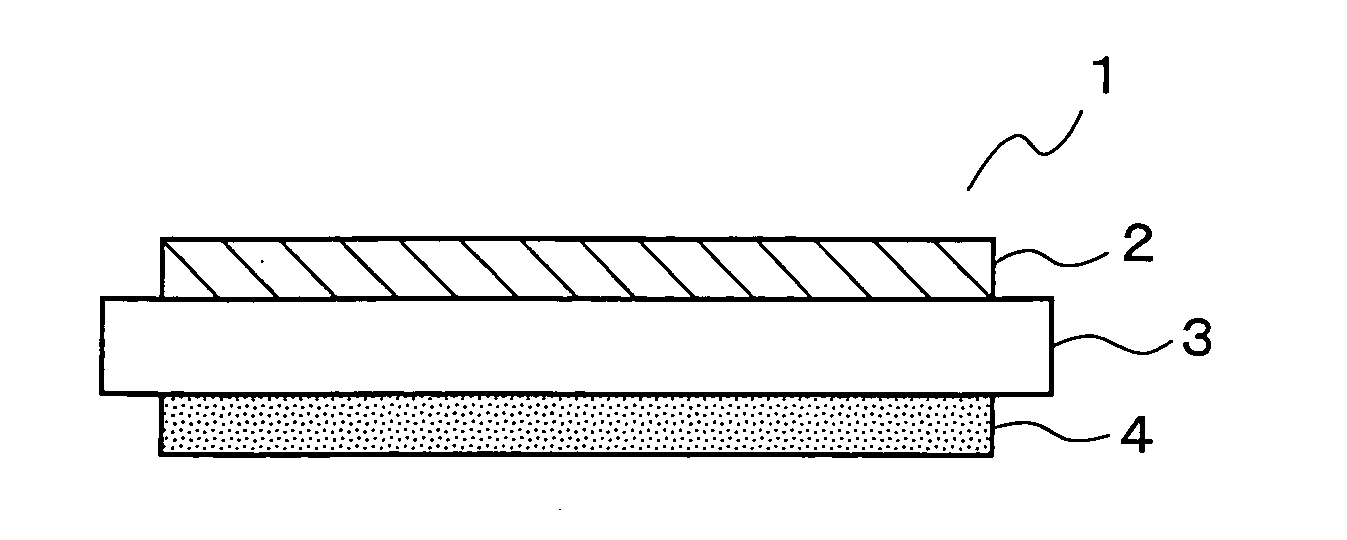
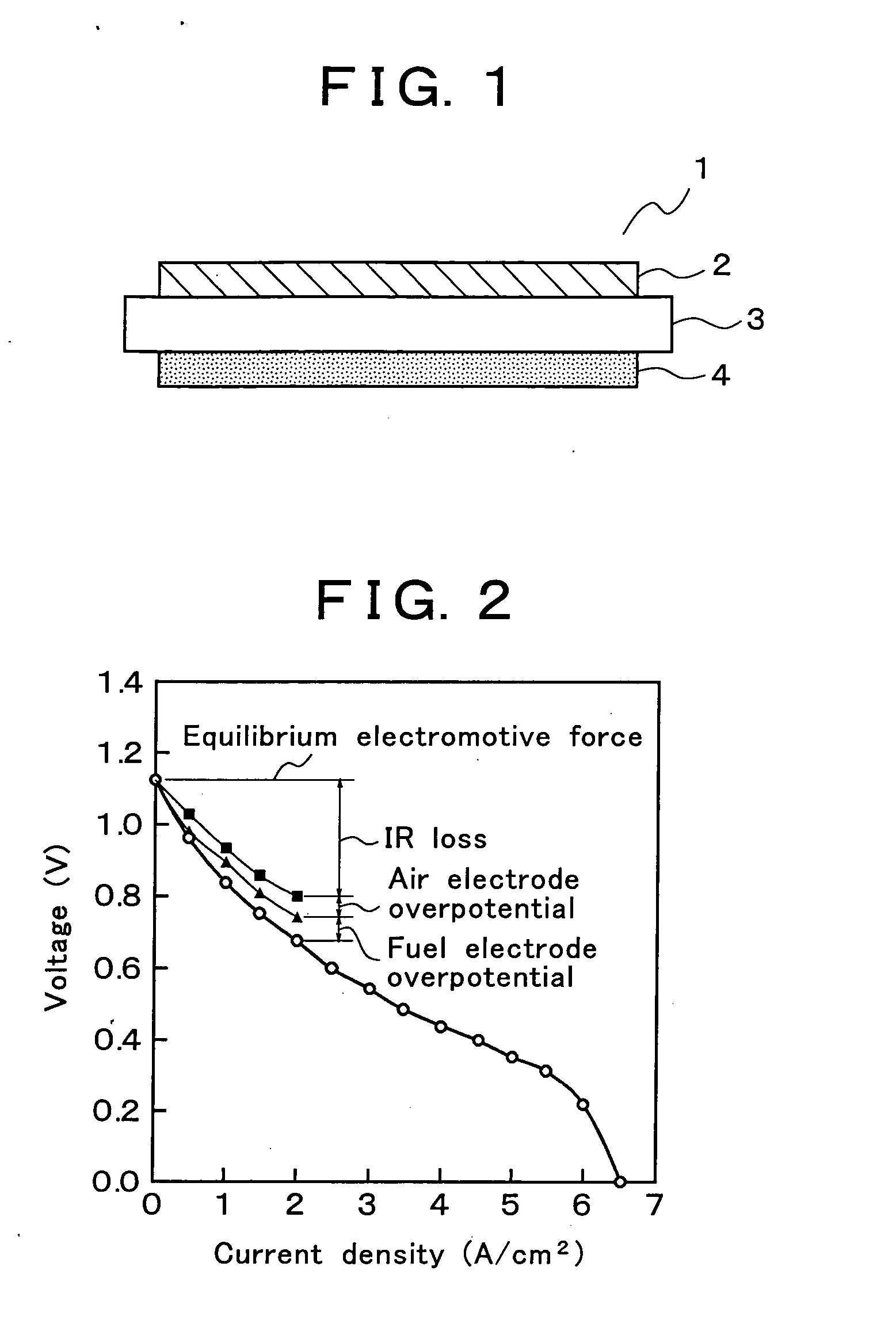
Solid oxide fuel cell and manufacturing method thereof
InactiveUS20100021792A1Improve performanceImprove powerFinal product manufactureGallium/indium/thallium compoundsFuel cellsNetwork structure
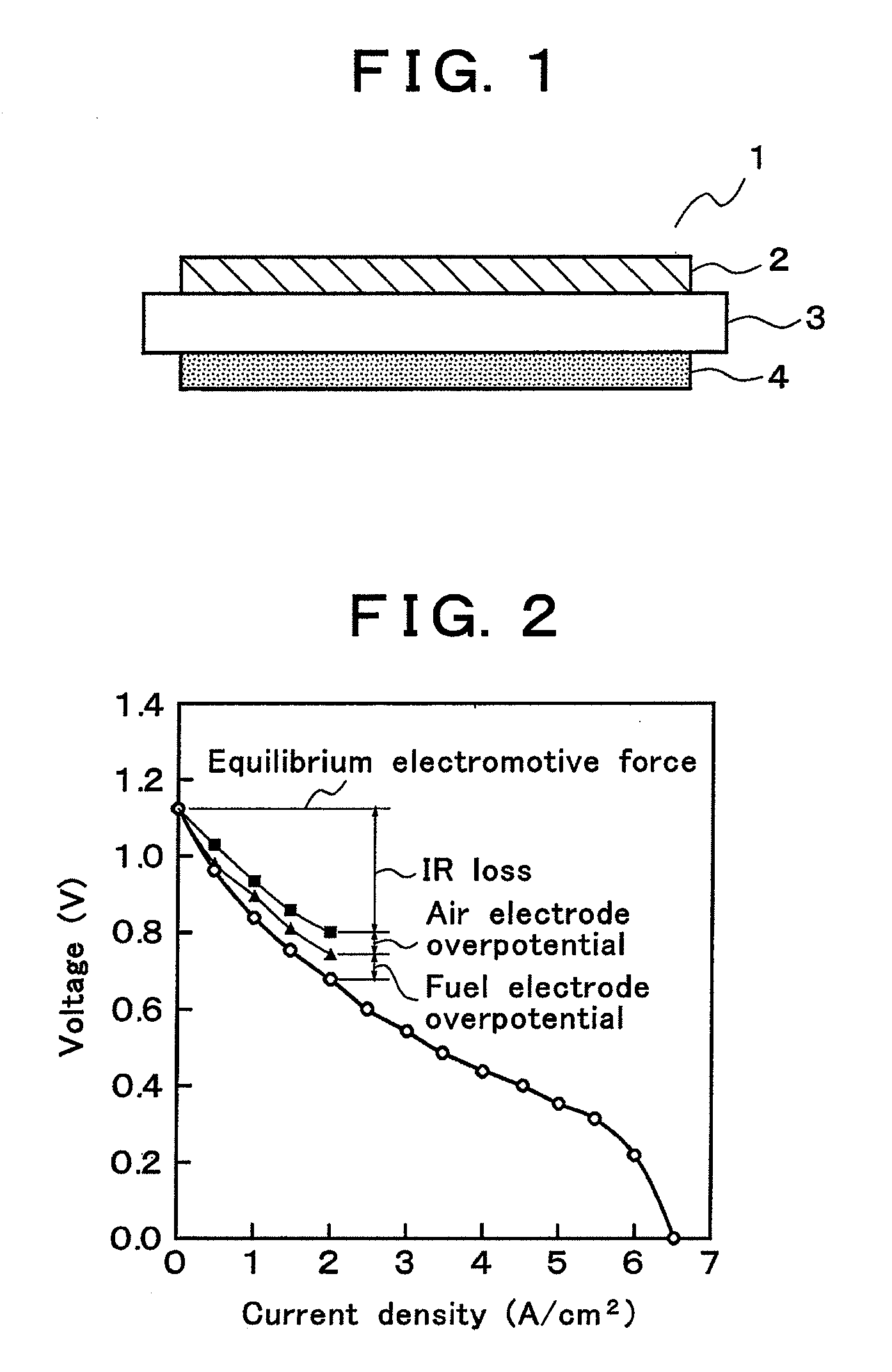
An electric power generation cell 1 is constituted by arranging a fuel electrode layer 4 on one side of a solid electrolyte layer 3 and an air electrode layer 2 on the other side of the solid electrolyte layer 3. The solid electrolyte layer 3 is constituted of an oxide ion conductor mainly composed of a lanthanum gallate based oxide. The fuel electrode layer 4 is constituted of a porous sintered compact having a highly dispersed network structure in which a skeletal structure formed of a consecutive array of metal grains is surrounded by mixed conductive oxide grains. For the air electrode layer 2, a porous sintered compact mainly composed of cobaltite is used. This configuration reduces the overpotentials of the respective electrodes and the IR loss of the solid electrolyte layer 3, and accordingly can actualize a solid oxide type fuel cell excellent in electric power generation efficiency.
Owner:THE KANSAI ELECTRIC POWER CO +2
Nitrogen-oxide gas sensor with long signal stability
ActiveUS20110163314A1Semiconductor/solid-state device detailsSolid-state devicesNitrogen oxidesSolid mass
The present invention provides a nitrogen-oxide gas sensor that is able to measure nitric oxide and nitrogen dioxide at the same time and ensure measurement accuracy and long stability. For these purposes, the nitrogen-oxide gas sensor includes: an oxide ion conductive solid electrolyte; a primary film that contacts the solid electrolyte and is made of a p-type semi-conductor metal oxide; a secondary film that contacts the solid electrolyte and is made of a p-type semiconductor metal oxide; an n-type semiconductor metal oxide that is included in at least one of the primary and secondary films; a power source that applies electric power to the primary and secondary films by electrically connecting a primary node to the primary film and a secondary node to the secondary film; and a measurement unit that measures the electric potential difference between the primary and secondary nodes.
Owner:ILJIN COPPER FOIL +1
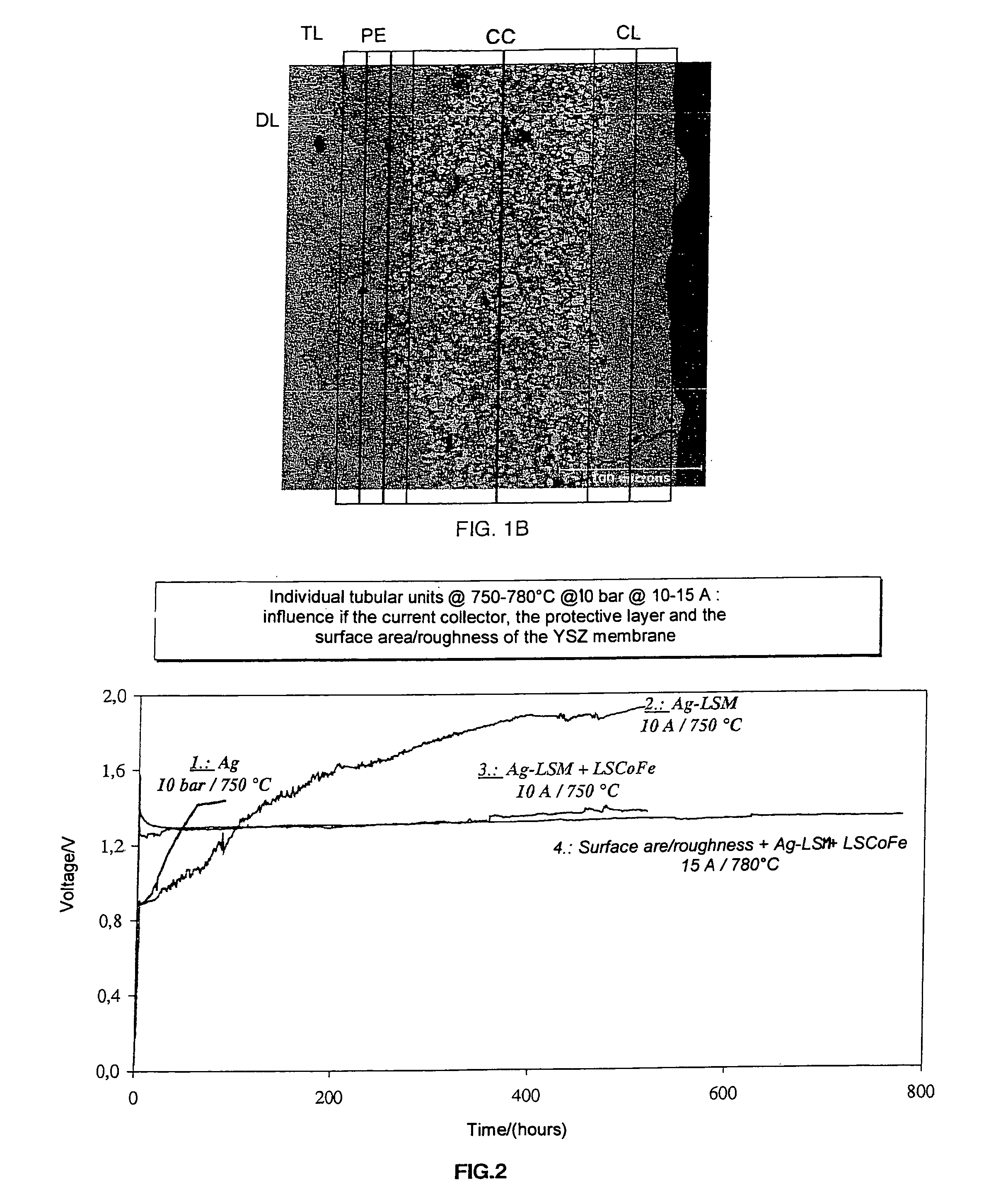
Oxide ion conductive ceramic membrane stacked microstructures; use for separating oxygen from air
InactiveUS7300561B2Improve relationshipImprove electrochemical performanceSemi-permeable membranesFuel cell auxillariesPorous layerPorous electrode
The invention concerns an oxide ion conductive ceramic membrane, characterized in that it comprises a non-null finite volume with non-null total thickness E, comprising a dense layer (CD) of a solid electrolyte, a so-called bonding layer (CA), of two porous electrodes (EP) and (EP′), two porous current collectors (CC) and (CC′), and at least at least a coating porous layer (ER), characterized in that the thickness E of the volume of said membrane, is equal to the sum of the thickness of each of said elements. The membrane is used for separating oxygen from air or from a gas mixture containing same.
Owner:LAIR LIQUIDE SOCR ANONYME A DIRECTOIRE & CONSEIL DE SURVEILLANCE POUR LETUD & LEXPL DES PROCEDES GEORCLAUDE
Solid oxide fuel cell and manufacturing method thereof
InactiveUS20050064277A1Reduce overpotentialImprove power generation efficiencyFinal product manufactureGallium/indium/thallium compoundsElectrical batteryEngineering
An electric power generation cell 1 is constituted by arranging a fuel electrode layer 4 on one side of a solid electrolyte layer 3 and an air electrode layer 2 on the other side of the solid electrolyte layer 3. The solid electrolyte layer 3 is constituted of an oxide ion conductor mainly composed of a lanthanum gallate based oxide. The fuel electrode layer 4 is constituted of a porous sintered compact having a highly dispersed network structure in which a skeletal structure formed of a consecutive array of metal grains is surrounded by mixed conductive oxide grains. For the air electrode layer 2, a porous sintered compact mainly composed of cobaltite is used. This configuration reduces the overpotentials of the respective electrodes and the IR loss of the solid electrolyte layer 3, and accordingly can actualize a solid oxide type fuel cell excellent in electric power generation efficiency.
Owner:THE KANSAI ELECTRIC POWER CO +2
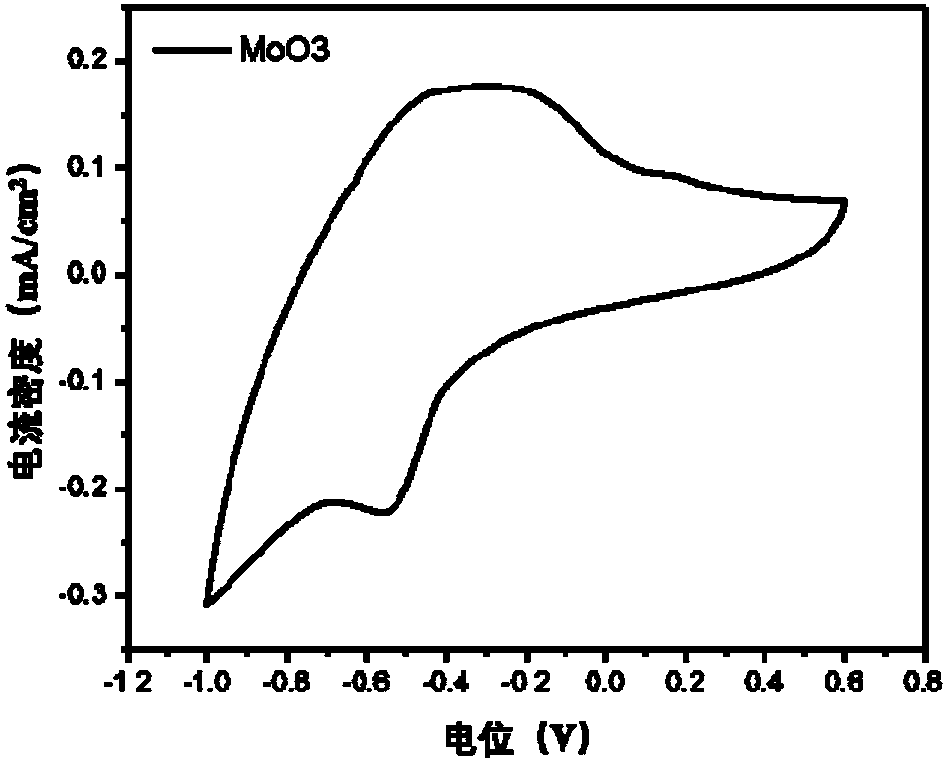
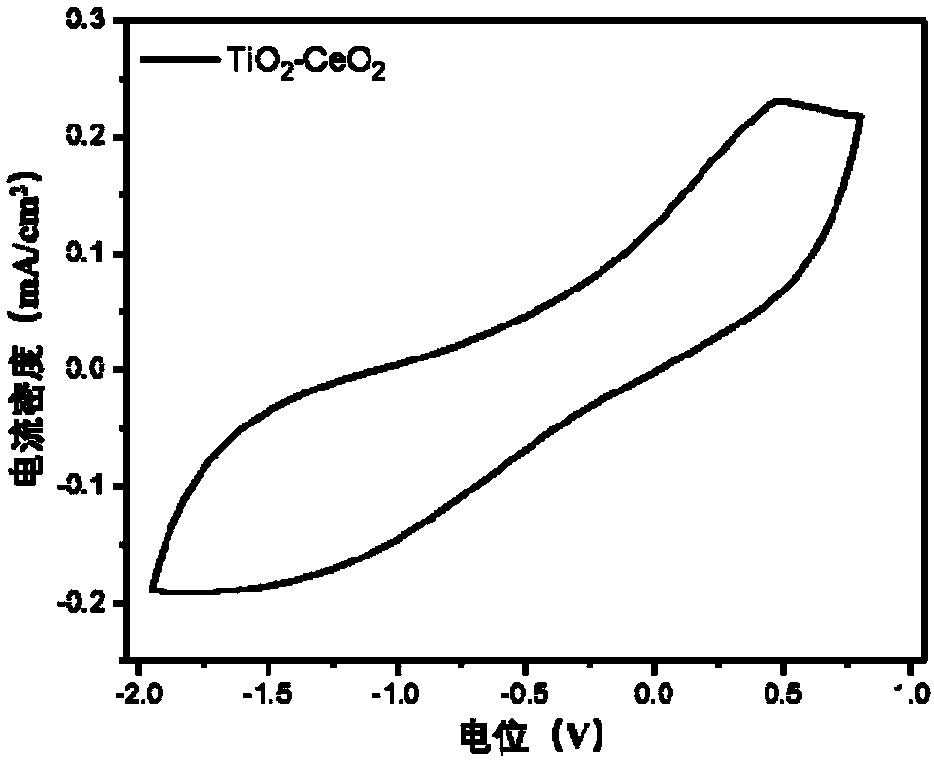
Inorganic metal oxide ion storage layer, processing method and application of low-temperature solution of inorganic metal oxide ion storage layer
ActiveCN108279540AReduce manufacturing costExcellent ion storage layer capacityNon-linear opticsSolventInorganic materials
The invention discloses an inorganic metal oxide ion storage layer, a processing method and application of a low-temperature solution of the inorganic metal oxide ion storage layer, and belongs to thetechnical field of inorganic material preparation. The processing method of the solution of an inorganic metal oxide layer comprises the following steps that (1) precursor salt of the inorganic metaloxide, polar solvent and ligand are mixed to obtain a precursor solution; (2) film is formed by adopting the precursor solution, heat treatment is conducted, and the inorganic metal oxide layer is obtained after the polar solvent is volatilized. According to the inorganic metal oxide ion storage layer, the processing method and application of the low-temperature solution of the inorganic metal oxide ion storage layer, under the condition that the concentration and components of the precursor are regulated, after low-temperature post-treatment, obtained metal thin film has high stability and high ion storage capacity. The method does not need complex high-temperature vacuum environment, only needs to use the precursor of different oxides, and the metal oxide thin film with an excellent property of different types prepared at a lower temperature can be taken as the ion storage layer.
Owner:SHENZHEN GUANGYI TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com