Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
88 results about "O-nitroaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
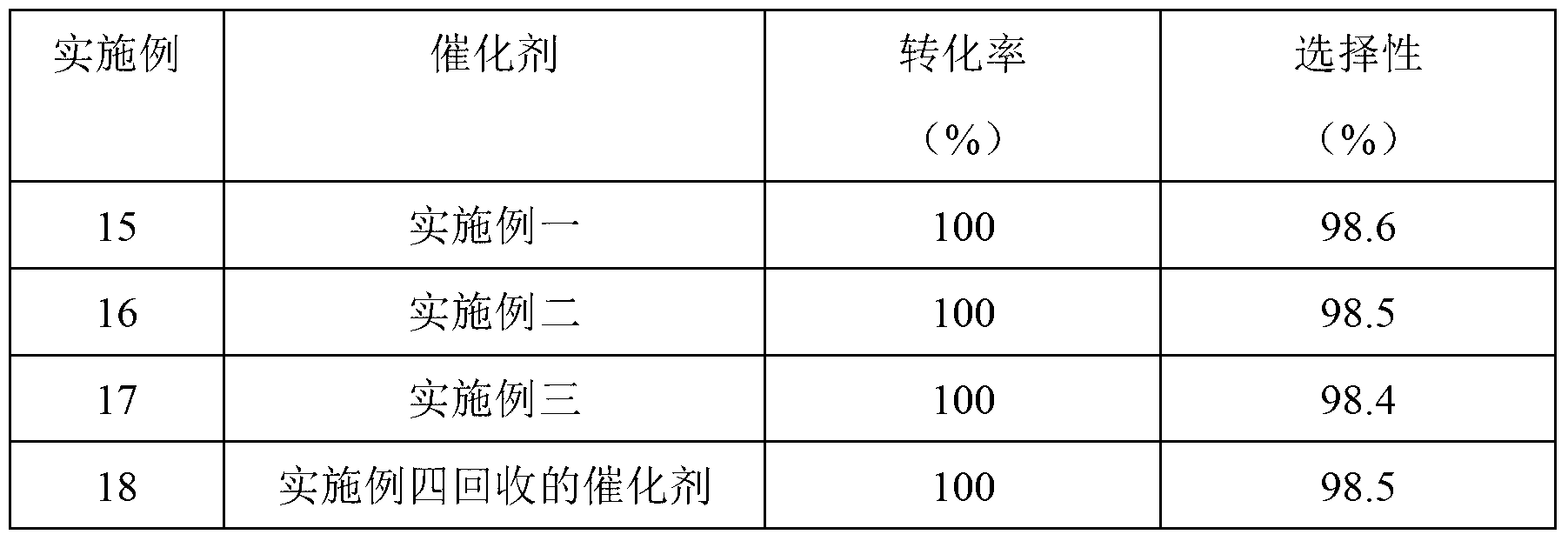
Inventor
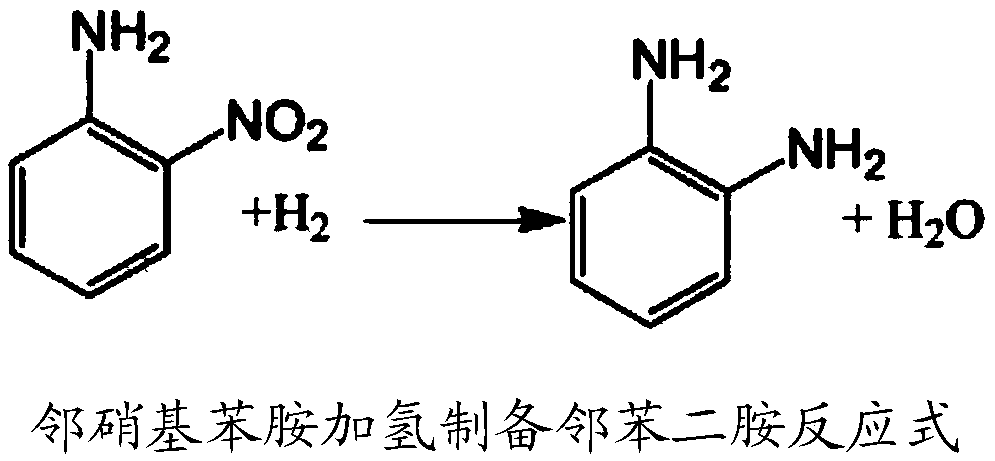
Method for preparing o-phenylenediamine by catalytic hydrogenation of o-nitrophenylamine
InactiveCN102633653ASolve the problem of impuritiesReduce the presence of impuritiesOrganic compound preparationAmino compound preparationPtru catalystHydrogen pressure
The invention discloses a method for preparing o-phenylenediamine by catalytic hydrogenation of o-nitrophenylamine. The method is characterized in that: in the hydrogenation reaction of o-nitrophenylamine, alcohol is used as a solvent, nickel is used as a catalyst, reduction reaction is performed for 2 to 10 hours under the hydrogen pressure of 1.0 to 6 MPa at the temperature of between 40 and 80 DEG C, and the reaction product is rectified to form the while o-phenylenediamine. The method has the advantages that the alcohol is used as the solvent in the catalytic hydrogenation for producing o-phenylenediamine, the alcohol can be reclaimed and directly used for next reaction, and the waste residue produced by distillation can be used as an organic fuel, so that the problem that a large amount of waste water containing organic substances is produced in reduction of iron powder or sodium sulfide in the conventional process is solved; and thick acid and thick alkali used in the conventional process are avoided in the hydrogenation process, so that corrosion of equipment is greatly reduced, pollution is reduced, and almost zero pollution is realized. In addition, compared with the conventional iron powder or sodium sulfide reduction, the catalytic hydrogenation process has the advantages of low pollution, high yield, high quality, short production period and low energy consumption.
Owner:JIANGSU KANGHENG CHEM
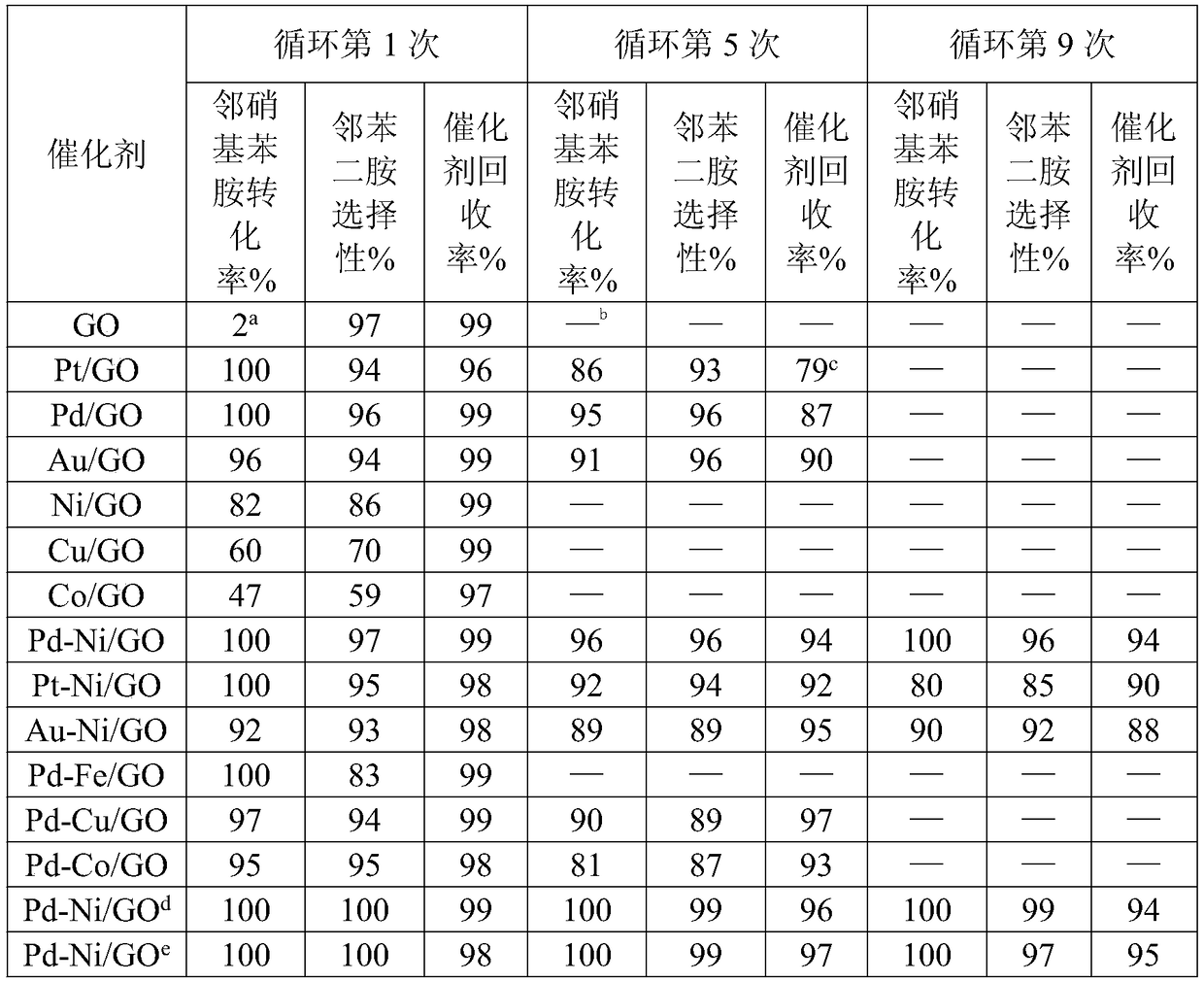
Method for preparing o-phenylenediamine by utilizing catalytic reduction of ortho-nitroaniline
ActiveCN109232271AHigh activityEasy maintenanceOrganic compound preparationAmino compound preparationOrtho-nitroanilineGraphene
The invention provides a method for preparing o-phenylenediamine by utilizing catalytic reduction of ortho-nitroaniline. A bimetal loaded graphene oxide is taken as a catalyst for taking part in hydrogenation reduction in a reaction kettle with a polytetrafluoroethylene liner. According to the scheme, the problems of difficulty in realizing industrial production, difficulty in keeping long-term stable activity of catalyst and the like of the prior art can be solved. The method has a broad industrial application prospect.
Owner:ANHUI DONGZHI GUANGXIN AGROCHEMICAL CO LTD
Green synthetic method of preparing o-phenylenediamine by reducing o-nitroaniline
ActiveCN105130821ARealize green and clean productionHigh reaction yieldOrganic compound preparationAmino compound preparationGreen cleaningO-nitroaniline
The invention relates to a green synthetic method of preparing o-phenylenediamine by reducing o-nitroaniline, wherein the method includes following steps: with o-nitroaniline as a substrate, dissolving the o-nitroaniline in water or in a mixed liquid composed of water and a co-solvent, and adding a supported catalyst under a carbon monoxide atmosphere, wherein the supported catalyst includes a supporter and a catalyst body supported thereon, and performing a reaction to prepare the o-phenylenediamine at 30-150 DEG C. In the method, the supported catalyst is used for preparing the o-phenylenediamine through reduction. The catalyst is high in selectivity and reaction yield and is mild in reaction condition. The method is environment-friendly in production system and less in treatment load of waste water, waste gas and solid waste, achieves green and clean production of the o-phenylenediamine, and is suitable for large-scale popularization.
Owner:江阴市华亚化工有限公司
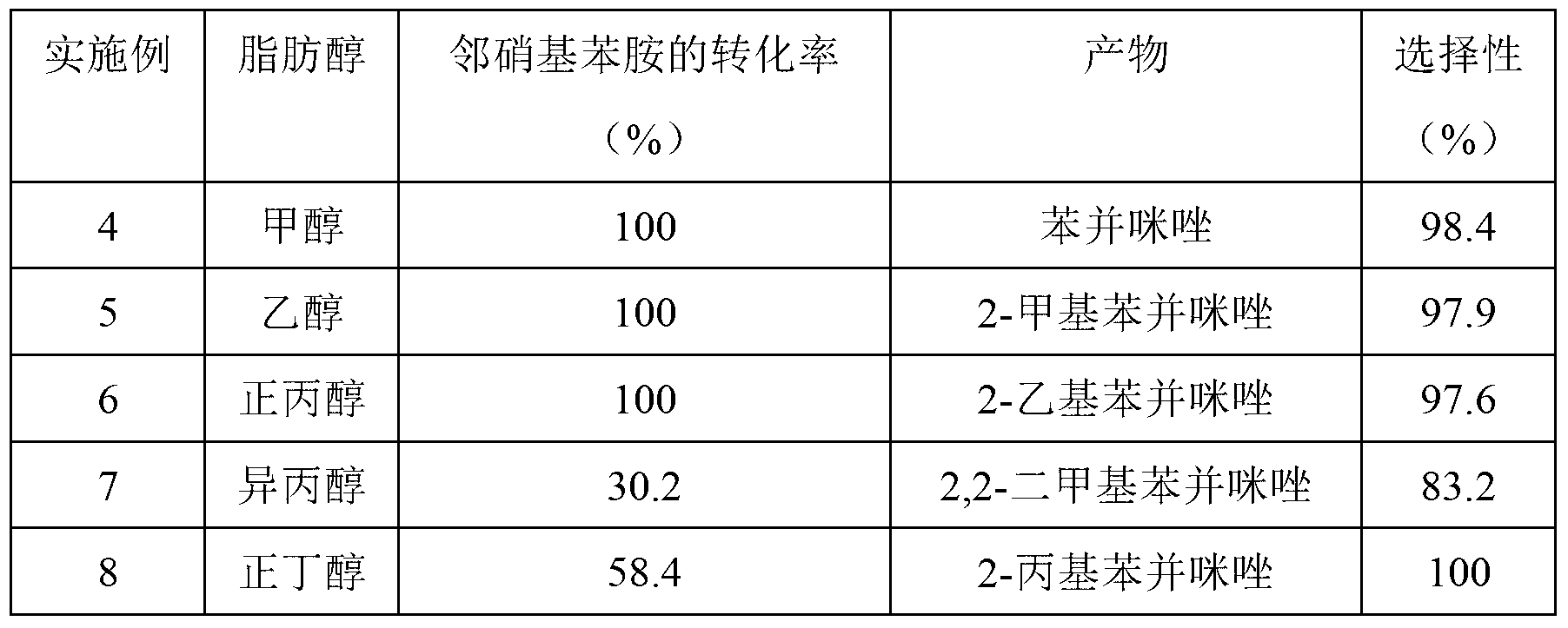
Method for synthesizing benzimidazole compounds
InactiveCN103288743AImprove conversion rateEasy to separateOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsOrtho-nitroanilineOrganic solvent
The invention discloses a method for synthesizing benzimidazole compounds expressed by a formula (III) through a one-pot method. The method comprises the following step of: with ortho-nitroaniline compounds expressed by a formula (I) and fatty alcohol expressed by a formula (II) as raw materials as well as water as a reaction solvent, synthesizing the benzimidazole compounds expressed by the formula (III) through the one-pot method in a shielding gas atmosphere under the action of a supported metal solid catalyst, wherein the supported metal solid catalyst is a Cu-Zn-Pd / Al2O3 catalyst. The method disclosed by the invention has the advantages of being simple in synthesis route, high in product yield, low in production cost, liable to separate the selected solid catalyst, high in activity, good in stability and free of liquid acid and organic solvent, and the like, thereby being an environment-friendly synthesis route. FORMUAL (I), R1CH2OH (II), (III) are described in the specification.
Owner:海宁市盐官工业投资有限公司
Feed additive quinocetone and preparation method thereof
InactiveCN102372678APromote growthEasy to operateAntibacterial agentsOrganic chemistryIntestinal structureDisease
The invention relates to a feed additive, in particular to a feed additive quinocetone and a preparation method thereof and belongs to the technical field of chemical engineering. In the preparation method, o-nitroaniline is used as the starting raw material and the method comprises the following steps: oxidizing with sodium hypochlorite, and then reacting with acetylacetone and benzaldehyde to obtain quinocetone. Quinocetone can inhibit the synthesis of bacterial DNA to inhibit the growth of pathogenic microorganisms in the digestive tract, prevent or reduce diseases, protect good intestine bacteria and intestinal wall from the harms of microorganisms or parasites and promote the digestion and absorption of various nutrients. Therefore, the growth of animals can be promoted and the feed conversion rate can be increased.
Owner:许吉冲
Improved synthetic method for preparing o-phenylenediamine by reducing o-nitroaniline
ActiveCN105017028AHigh recovery rateAvoid security issuesOrganic compound preparationAlkali metal sulfides/polysulfidesReaction temperatureProduction risk
The invention relates to an improved synthetic method for preparing o-phenylenediamine by reducing o-nitroaniline. The method comprises adding o-nitroaniline and sodium sulfide according to the molar ratio of 1:1.5-2 into a reaction vessel, injecting water, controlling the weight ratio of sodium sulfide to water to be 1:1-20, controlling the reaction temperature to be 50-150 DEG C, reacting for 1-5 h, crystallizing and separating o-phenylenediamine, so as to obtain reaction waste water; concentrating the reaction waste water to obtain a solid residue; performing medium-temperature calcining on the residue, concretely adding an inorganic base into the residue and controlling the temperature of medium-temperature calcining to be 300-600 DEG C; performing high-temperature calcining on the residue, concretely mixing with carbon powder, and controlling the temperature of high-temperature calcining to be 900-1200 DEG C; and performing separation filtering, concretely using water to dissolve the post-calcined product, filtering and recovering superfluous carbon powder and obtaining a filtrate which is a sodium sulfide aqueous solution. The method realized continuous efficient wastewater processing, is beneficial for reducing discharge of ' three wastes (waste gas,waste water and industrial residue) ', reduces safe production risk, improves sodium sulfide recovery rate, and is relatively suitable for large-scale production of o-phenylenediamine in industry.
Owner:江阴市华亚化工有限公司
Preparation method of mequindox
The invention discloses a preparation method of mequindox, belonging to the field of medicine chemical industry. The preparation method comprises the following steps: carrying out redox reaction on o-nitroaniline and a sodium hypochlorite solution under alkaline conditions to obtain benzofurazan, and after the reaction finishes, and adding an organic solvent for extraction, thereby obtaining a benzofurazan organic solution; and adding a catalyst and acetylacetone into the benzofurazan organic solution to initiate condensation reaction, thereby obtaining the mequindox. The invention omits the steps of benzofurazan separation, storage, drying, transportation, addition, dissolution and the like, thereby saving the human resources, material resources and financial resources, reducing the loss of benzofurazan in the middle process, and lowering the production cost. Meanwhile, the invention separates water in the benzofurazan by extraction, thereby lowering the water content in the subsequent reaction and increasing the synthesis yield of mequindox.
Owner:HUBEI ZHONGMU ANDA PHARMACEUTICAL CO LTD
Method for preparing high-purity p-chloro-m-nitroacetoacetanilide
ActiveCN101177404AImprove solubilityHigh purityOrganic compound preparationCarboxylic acid amides preparationEthyl acetateDiketene
The invention discloses a method for preparing high-purity p-chloro-m-nitroacetoacetanilide, in which adopts the technical proposal that p-chloro-o-nitroaniline and catalyst are added in mixed solvent at 15 to 100 DEG C, and then diketene is dripped within 0.5 to 4 hours for reaction for 0.5 to 6 hours to prepare the p-chloro-m-nitroacetoacetanilide, wherein the mixed solvent can be either the solvent composed of two or more materials among water, acetone, tetrahydrofuran, acetonitrile, ethanol, toluene, benzene, isopropyl alcohol, chloroform, dichloromethane, ethyl acetate, butyl acetate and methyl acetate or circulating mother liquor. The circulating mother liquor can be recycled for as many as 10 times and extra catalyst is not needed with the purity of products exceeding 99% and the yield reaching 97%. The invention has the advantages of simple and controllable process, no need of recrystallization and high purity.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
Green synthesis process for p-chloro-o-nitroacetoanilide
InactiveCN103408452AImprove solubilityNo need to crystallize againOrganic compound preparationCarboxylic acid amides preparationSolubilityDiketene
The invention discloses a green synthesis process for p-chloro-o-nitroacetoanilide. The process comprises the following steps of mixing a novel mixed catalyst system and p-chloro-o-nitroaniline, and enabling the novel mixed catalyst system and the p-chloro-o-nitroaniline to dissolve, wherein the mole ratio of the p-chloro-o-nitroaniline to diketene is 1: (1.05-1.5); finishing the dropwise adding operation of the diketene within 3h; and continuing to react at the temperature of 90 DEG C for 4h to obtain the p-chloro-o-nitroacetoanilide. The solubility of the p-chloro-o-nitroaniline in a solvent is improved, the yield of products is high, the reaction time is shortened, the solvent dosage is reduced, and no solid wastes are generated.
Owner:JIANGSU LONGCHANG CHEM
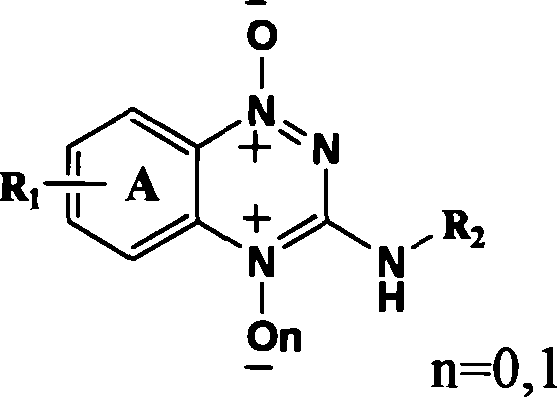
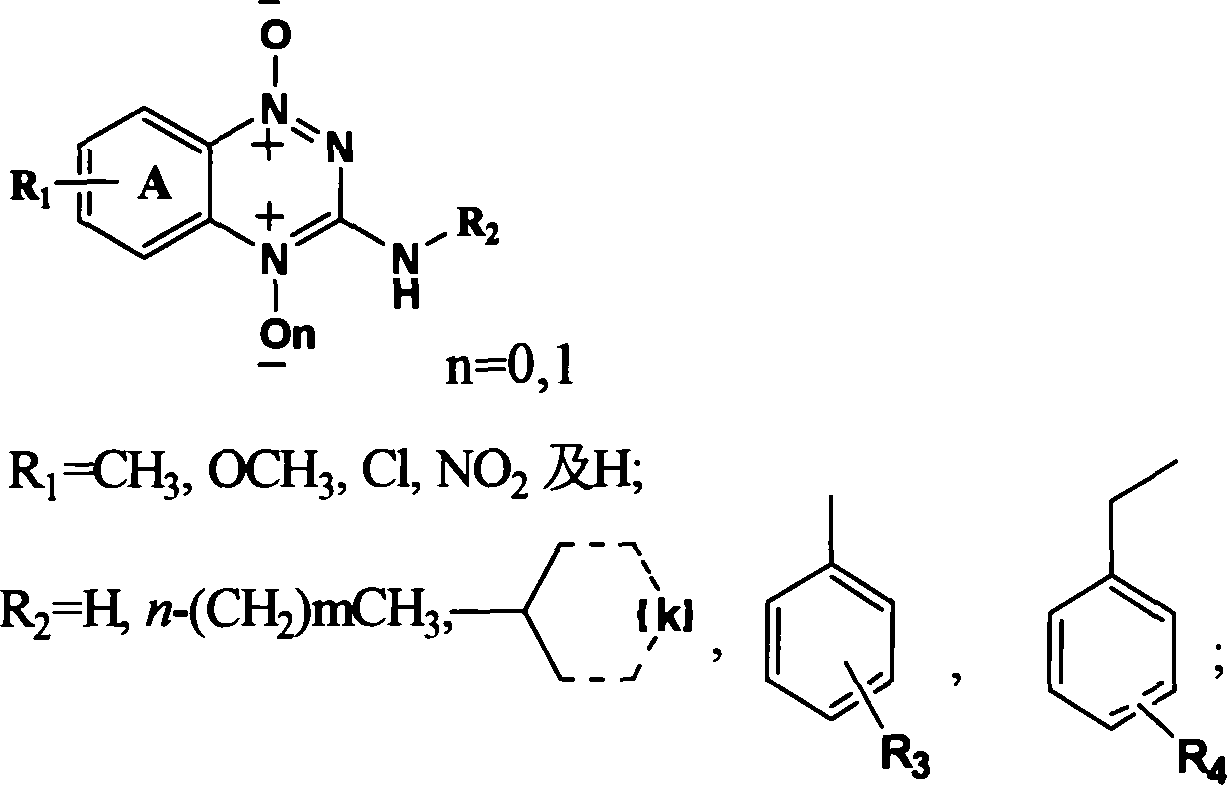
Triazinyl nitroxide and its synthesis and use
InactiveCN1887878AReasonable designSimple and fast operationOrganic chemistryAntineoplastic agentsSide chain2-Nitroaniline
The present invention provides 3-substituted amino-1, 2, 4-substituted phentriazine-1, 4-dioxides in the general expression as shown. The compounds are prepared with substituted one-nitroaniline as main material and through acylation to produce urea, cyclization, chlorination, amino substitution, oxidation and other steps. The tests show that the compounds of the present invention have anticancer activity and hypoxia selectivity higher than those of TPZ and may be used in preparing hypoxia selective anticancer medicine.
Owner:ZHEJIANG UNIV
Method for absorbing nitrogen oxide tail gas and generating by-product p-phenylenediamine by using aniline
ActiveCN102731320AReduce manufacturing costReduce harmPreparation by N-O/N-N bondsP-NitroanilineP-Aminoazobenzene
The invention discloses a method for absorbing nitrogen oxide tail gas and generating by-product p-phenylenediamine by using aniline. The method comprises the following steps of: reacting part aniline with NOx to obtain diazonium salt; reacting the diazonium salt with the non-reacted aniline to obtain 1,3-diphenyltriazene, wherein the reaction products contain less p-nitroaniline and o-nitroaniline; making the 1,3-diphenyltriazene in a rearrangement reactor generate the rearrangement reaction at 30 DEG C-120 DEG C to convert into p-aminoazobenzene, wherein 90% of the 1,3-diphenyltriazene is converted into the p-aminoazobenzene and the remaining 1,3-diphenyltriazene is converted into o-aminoazobenzene and few impurities after the rearrangement reaction; separating the low distillates from the rearranged materials; and carrying out the hydrogenation reaction: using Raney nickel as a catalyst, continuously inputting hydrogen gas and controlling the pressure and the temperature to be 0.2MPa-4MPa and 25 DEG C-150 DEG C respectively to synthesize the p-phenylenediamine. The method provided by the invention reduces the production cost of the p-phenylenediamine and also reduces environmental pollution. A DCS (distributed control system) computer control system is used so that the automation of the whole system is realized and meanwhile the production efficiency is high.
Owner:HANGZHOU LONGSHAN CHEM CO LTD
Azabenzotriazol containing ultraviolet absorber and preparation method thereof
The invention discloses an azabenzotriazol containing ultraviolet absorber the molecular structure of which comprises both a benzotriazole structure and an azacycle structure. Particularly, the invention also discloses the preparation method of the absorber, which comprises the following steps in sequence: 1) carrying out a diazotization reaction on o-nitroaniline derivatives and getting a diazotization solution; 2) carrying out coupling reaction on diazonium salt of the o-nitroaniline derivatives and derivatives of pyrazolone or pyridone and getting a coupling product; and 3) carrying out reduction reaction on the coupling product to finally obtain the azabenzotriazol containing ultraviolet absorber. The molar extinction coefficient of the ultraviolet absorber disclosed by the invention is evidently lower than that of other azabenzotriazol containing ultraviolet absorber with a similar structure.
Owner:ZHEJIANG SCI-TECH UNIV
Composition for simultaneously bleaching and coloring of keratin fibres comprising an anionic or non-ionic dye and an asociative polymer
Composition for the simultaneous (dis)coloration of keratinous fiber comprises a dye comprising an anionic or non-ionic dye preferably 7-(6'-methylphenylazo)-1-acetamido- 3,6-disulfo-8-hydroxy- naphthalene, o-nitro-anilines substituted by meta directing group like amino, quinoline or its derivatives or salts; an associative polymer; a peroxide salt; and an alkaline agent. Independent claims are also included for: a process of discoloring and coloring simultaneously of the keratinous fibers comprising applying the anhydrous composition on the fibers in the presence of an aqueous composition optionally comprising hydrogen peroxide; and a device with several compartments comprising the compositions.
Owner:LOREAL SA
Method for synthesizing mequindox
The invention belongs to the field of organic chemistry synthesis, and particularly to a method for synthesizing mequindox. The method comprises the following steps of: (1), using o-nitrophenylamine as raw material, preparing BFO; and (2), using the BFO and acetylacetone as raw materials, using anhydrous sodium acetate as catalyst, preparing the mequindox. Aiming at the problems of the prior technology that the used catalyst is hard to be dissolved by a solvent, too active after being dissolved, hard to control, easy in generation of side reaction, has great influence to yield, and the like, the invention provides a technology which is easy to control and greatly reduced in side production, so that yield of product can be greatly increased by about 10%.
Owner:衢州伟荣药化股份有限公司
Method for separating nitrochlorobenzene meta-position oil
ActiveCN102134198ALow costImprove qualityOrganic compound preparationAmino compound preparationP-NitroanilineWastewater
The invention discloses a method for separating nitrochlorobenzene meta-position oil, which comprises the following steps of: adding nitrochlorobenzene meta-position oil and aqueous ammonia into an autoclave, heating in the autoclave, and keeping the reaction temperature and the reaction pressure after the reaction starts; slowly reducing the pressure and reducing the temperature after the reaction is completed, and reclaiming ammonia during reducing the pressure and the temperature; pressing the reacted materials to a demixing tank, standing and demixing, and delivering the lower oil material phase to an intermediate tank; pumping the oil phase material in the intermediate tank into a vacuum light component removal tower, removing un-reacted meta-nitrochlorobenzene from the top of the tower, reclaiming the meta-nitrochlorobenzene, recovering a mixed material containing p-nitroaniline and o-nitroaniline from the autoclave, and feeding the mixed material into a mixing tank; and pumping the material in the mixing tank into a vacuum falling film rectifying tower, recovering the o-nitroaniline from the top of the tower, collecting the cooled o-nitroaniline, recovering the p-nitroaniline from the autoclave, and collecting the cooled p-nitroaniline. By the method, the problem of difficult treatment of much wastewater caused by washing with a large amount of water in the conventional production process is solved, and the qualities of the obtain p-nitroaniline and the obtained o-nitroaniline are improved.
Owner:ANHUI BAYI CHEM IND
Medical high-temperature-resistant antistatic film and preparation method thereof
The invention discloses a medical high-temperature-resistant antistatic film and a preparation method thereof. The high-temperature-resistant antistatic film comprises the following components in parts by weight: 30-40 parts of PE (polyethylene), 20-30 parts of PU (polyurethane), 20-30 parts of PC (polycarbonate), 5-10 parts of phenol formaldehyde resin, 5-10 parts of PC, 1-5 parts of p-chloro-o-nitroaniline, 3-8 parts of diethyl phosphite, 0.8-2 parts of antistatic agent, 0.5-2 parts of antioxidant, 0.5-1 part of ultraviolet absorbent, 3-9 parts of pentaerythritol, 2-6 parts of methyl acrylate and 1-3 parts of barium stearate. The preparation method comprises the following steps: adding all the components into a reaction kettle, stirring to react while heating in an ammonia gas environment to obtain a reaction product, carrying out extrusion granulation on the reaction product by a double screw extruder to obtain a master batch, and finally, carrying out extrusion and film blowing on the master batch by a plastic film blowing machine. The high-temperature-resistant antistatic film disclosed by the invention has favorable temperature resistance and antistatic property, and can be better applied to the field of medical treatment.
Owner:SUZHOU VIVOTIDE BIOTECH
Material layering process for reduced o-phenylenediamine
ActiveCN105017027AImprove qualityHigh recovery rateAmino compound purification/separationOrganic compound preparationO-nitrochlorobenzeneMan-hour
The present invention provides a material layering process for reduced o-phenylenediamine. The process comprises: firstly, mixing o-nitrochlorobenzene and ammonia to acquire o-nitroaniline; then adding sodium sulphide to the o-nitroaniline, wherein after reduction, a reduction pot contains a hydrate of a mother solution, o-phenylenediamine and a salt; and separating the o-phenylenediamine by using a two-stage separation technique. The present invention employs the self-developed technique for layering reduced o-phenylenediamine, thereby reducing the energy consumption, shortening the man-hour, reducing the rectification residues, providing the necessary condition for subsequent smooth rectification, and acquiring the o-phenylenediamine with high quality; providing a basis for increasing productivity of carbendazim; further improving the product quality of the o-phenylenediamine and the recovery rate in production process, reducing unit consumption, and reducing costs;and improving the operating environment and greatly reducing the labor intensity.
Owner:ANHUI DONGZHI GUANGXIN AGROCHEMICAL CO LTD
Preparation method of fenbendazole intermediate 2-nitro-4-phenylthioaniline
ActiveCN111349032AReduce manufacturing costReduce pollutionThiol preparationSulfide preparationBiotechnologyFenbendazole
The invention relates to the technical field of veterinary drugs, and in particular, relates to a preparation method of fenbendazole intermediate 2-nitro-4-phenylthioaniline. The preparation method comprises the following steps: by taking o-nitroaniline and ammonium thiocyanate as raw materials and an organic solvent as a solvent of a reaction system, uniformly stirring and mixing, introducing chlorine, and filtering after the reaction of the raw materials is finished, so as to obtain 4-thiocyano-2-nitroaniline; taking 4-thiocyano-2-nitroaniline, adding sodium hydroxide and a reaction solvent,stirring and mixing, and filtering after the reaction is finished to obtain a sodium 4-amino-3-nitrothiophenol solution; carrying out a reaction on the sodium 4-amino-3-nitrothiophenol solution withbromobenzene under an alkaline condition, and after the reaction is finished, extracting to obtain the 2-nitro-4-phenylthioaniline. The preparation method is simple in process, the production cost isreduced, the production potential safety hazard can be reduced, the product yield is relatively high, and the environmental pollution is reduced.
Owner:SHANDONG GUOBANG PHARMA +1
Synthesizing method of carbolin compound
The invention discloses a synthesizing method of a carbolin compound. The synthesizing method comprises the following steps of enabling pyridine derivatives, o-nitroaniline and o-nitroaniline derivative thereof to generate coupling and reduction reaction, or directly generate coupling reaction with benzotriazole; converting into a diazotized triazole intermediate; finally, treating the obtained intermediate after reaction under the action of a reagent, so as to obtain substituted carbolin. The synthesizing method has the characteristics that on the basis of the improved Graebe-Ullmann method,the reaction raw materials, reaction reagents and conditions are preferably selected; the selectivity of target products is enhanced, the reaction time is shortened, the reaction temperature is reduced, the number of impurities is fewer, the yield rate is high, and the like; the method is suitable for synthesizing the substituted alpha-carbolin and beta-carbolin, so as to provide a simple and reliable selection plan for the obtaining and utilization of the compound.
Owner:GUIZHOU MEDICAL UNIV
Agent for simultaneously bleaching and coloring of keratin fibres comprising an anionic or non-ionic dye and an inert organic liquid
InactiveCN1899244AImprove stabilityEasy to useCosmetic preparationsHair cosmeticsFiberOrtho-nitroaniline
The present disclosure relates to a composition for simultaneously bleaching and dyeing keratin fibers, comprising at least one direct dye chosen from anionic dyes, nonionic dyes, and addition salts thereof, with the exception of 7-(6'-methylphenylazo)-1-acetamido-3,6-disulfo-8-hydroxynaphthalene, ortho-nitroanilines substituted meta to the amino group, quinoline and quinoline derivatives, and addition salts thereof, at least one inert organic liquid, at least one peroxygenated salt and at least one alkaline agent. The disclosure also relates to process and use for simultaneously bleaching and dyeing keratin fibers using this composition. Especially suitably used for dark hair, capable of being easily used, and capable of rapidly bringing about coloration with a chromatic color.
Owner:LOREAL SA
Novel preparation method of o-phenylenediamine
InactiveCN104086441AEasy to useSimple componentsOrganic compound preparationAmino compound preparationNitrogen gasAir tightness
A preparation method of o-phenylenediamine. The invention relates to the technical field of chemical engineering. The method includes following steps: employing 70-80g of o-nitroaniline, 150-170ml of alcohol or methanol and 1-2g of a catalyst as raw materials; feeding the raw materials into a reaction kettle; closing the reaction kettle and inspecting an air-tightness of the reaction kettle; replacing air in the reaction kettle by nitrogen under 0.3 MPa for three times; replacing the nitrogen in the reaction kettle by hydrogen under 0.3 MPa for three times; increasing the pressure of the hydrogen in the reaction kettle to 1.5 MPa; carrying out a stirring process with a heating process; increasing the temperature in the reaction kettle to 65 DEG C; maintaining the temperature to be constant for 120 min; stopping the reaction when the pressure of the hydrogen in the reaction is not decreased anymore; decreasing the temperature to 85 DEG C; carrying out a material-discharging process, a pressure-decreasing process and a filtering process to obtain a finish product. The method is simple in components of the raw materials, is cheap in raw materials, is convenient and easy to carry out and is convenient to operate. The prepared o-phenylenediamine is good in usage effects and is safe and reliable.
Owner:ANHUI HUARUN PAINTS
Method for treating p-chloro-o-nitroaniline wastewater
InactiveCN102351295AReduce contentSave heatWater contaminantsWater/sewage treatmentActivated carbonWastewater
The invention discloses a method for treating p-chloro-o-nitroaniline wastewater, which is characterized by comprising the following steps of: treating ammoniac p-chloro-o-nitroaniline wastewater to be transparent by using an activated carbon tower, adding quick lime into a replacement reaction kettle, controlling the temperature to be 120 DEG C, reacting for 2 hours and removing free ammonia and ammonia which exists in the form of ammonium chloride. The invention has the advantage that the method is suitable for treating the p-chloro-o-nitroaniline wastewater with different concentration. The ammoniac p-chloro-o-nitroaniline wastewater is treated to be transparent by using the activated carbon tower, the quick lime is added into the replacement reaction kettle, and the free ammonia and the ammonia which exists in the form of the ammonium chloride are removed. The total content of the ammonia in the wastewater is reduced while the ammonia is recovered.
Owner:JIANGSU LONGCHANG CHEM
Method for synthetizing 2,6-dichloroquinoxaline by using diketene
The invention discloses a method for synthetizing 2,6-dichloroquinoxaline by using diketene. The method comprises the following steps: taking P-chloro-o-nitroaniline and diketene as primal raw materials, and carrying out condensation, cyclization, reduction, acidification and chlorination reactions in sequence so as to obtain 2,6-dichloroquinoxaline; in the reduction step, taking NaHS as a reducing agent; performing the following steps between the steps of reduction and acidification: (1) adding activated carbon into an reaction system in a reduction step, so as to obtain reaction liquid: (2), heating the reaction liquid while stirring until the reaction liquid reaching 323 to 372 K; and (3) leading air into the reaction liquid for reaction for at least 0.1 h. In the invention, the characteristic of alkalinity of the system when 6-chlorine-2-quinoxaline sodium alkoxide is dissolved in water is used, under the stirring and certain-temperature condition, air is blown into the reaction liquid for a certain time, and polysulfide is enabled to be catalyzed and oxidized. Therefore, no elemental sulfur impurities are produced in the subsequent acidification reaction.
Owner:江苏丰山生化科技有限公司
Synthesis method of substituted nitroaniline
InactiveCN103848706AReduce pollutionMild conditionsOrganic compound preparationCarboxylic acid amides preparationPhenacylNitration
The invention discloses a synthesis method of substituted nitroaniline, comprises the following steps: performing nitration on the substituted aniline and nitrite in a solvent at 20-80 DEG C to obtain the substituted nitroaniline, the structural formula of the substituted aniline is as shown in the specification, wherein R1 is p-Toluenesulfonyl, acetyl, t-butyloxycarbonyl, benzoyl, -COC(CH3)3, benzyl or methyl; R2, R3, R4, R5 and R6 are respectively and independently C1-C4 alkyl, C1-C4 alkoxy, halogen, H, NO2, OCF3 or aryloxy, at least one of R2, R4 and R6 is H, the nitration means that at least one of R2, R4 and / or R6 with value of H on the substituted aniline is substituted by NO2. The synthesis method disclosed by the invention is free from high-temperature and high-pressure, the condition is gentle, the operation is safer, the strong acid is avoided, the post-treatment is simple, and the pollution to the environment is small, and the harm is light.
Owner:LANZHOU UNIVERSITY
Preparation method of mequindox
PendingCN114044756AReduce releaseProlong the action timeCatalyst carriersOrganic chemistry methodsPtru catalystNitrobenzene
The invention relates to the technical field of veterinary drug synthesis, and provides a preparation method of mequindox. According to the invention, o-nitroaniline and acetylacetone are used as raw materials, mequindox is prepared through an oxidation reaction and a condensation reaction, and the oxidation reaction is carried out under the conditions of an oxidizing agent, a dispersing agent and a catalyst; the dispersing agent can make the solution more uniform, and after the oxidation reaction is completed, benzofurazan does not need to be separated, and the next condensation reaction is directly carried out, so production procedures are reduced, and production efficiency is improved; a KOH / eggshell compound is used as the catalyst, KOH is adsorbed in eggshells and can be slowly released in a reaction process, so the KOH has a slow release effect, and the action time of the catalyst is prolonged; and the waste eggshells are used as a catalyst carrier, so a material source is rich, cost is extremely low, and the catalyst is green and environment-friendly.
Owner:BAYECAO HEALTH IND RES INST (XIAMEN) CO LTD +1
Method for separating nitrochlorobenzene meta-position oil
ActiveCN102134198BLow costImprove qualityOrganic compound preparationAmino compound preparationP-NitroanilineWastewater
The invention discloses a method for separating nitrochlorobenzene meta-position oil, which comprises the following steps of: adding nitrochlorobenzene meta-position oil and aqueous ammonia into an autoclave, heating in the autoclave, and keeping the reaction temperature and the reaction pressure after the reaction starts; slowly reducing the pressure and reducing the temperature after the reaction is completed, and reclaiming ammonia during reducing the pressure and the temperature; pressing the reacted materials to a demixing tank, standing and demixing, and delivering the lower oil material phase to an intermediate tank; pumping the oil phase material in the intermediate tank into a vacuum light component removal tower, removing un-reacted meta-nitrochlorobenzene from the top of the tower, reclaiming the meta-nitrochlorobenzene, recovering a mixed material containing p-nitroaniline and o-nitroaniline from the autoclave, and feeding the mixed material into a mixing tank; and pumping the material in the mixing tank into a vacuum falling film rectifying tower, recovering the o-nitroaniline from the top of the tower, collecting the cooled o-nitroaniline, recovering the p-nitroaniline from the autoclave, and collecting the cooled p-nitroaniline. By the method, the problem of difficult treatment of much wastewater caused by washing with a large amount of water in the conventional production process is solved, and the qualities of the obtain p-nitroaniline and the obtained o-nitroaniline are improved.
Owner:ANHUI BAYI CHEM IND
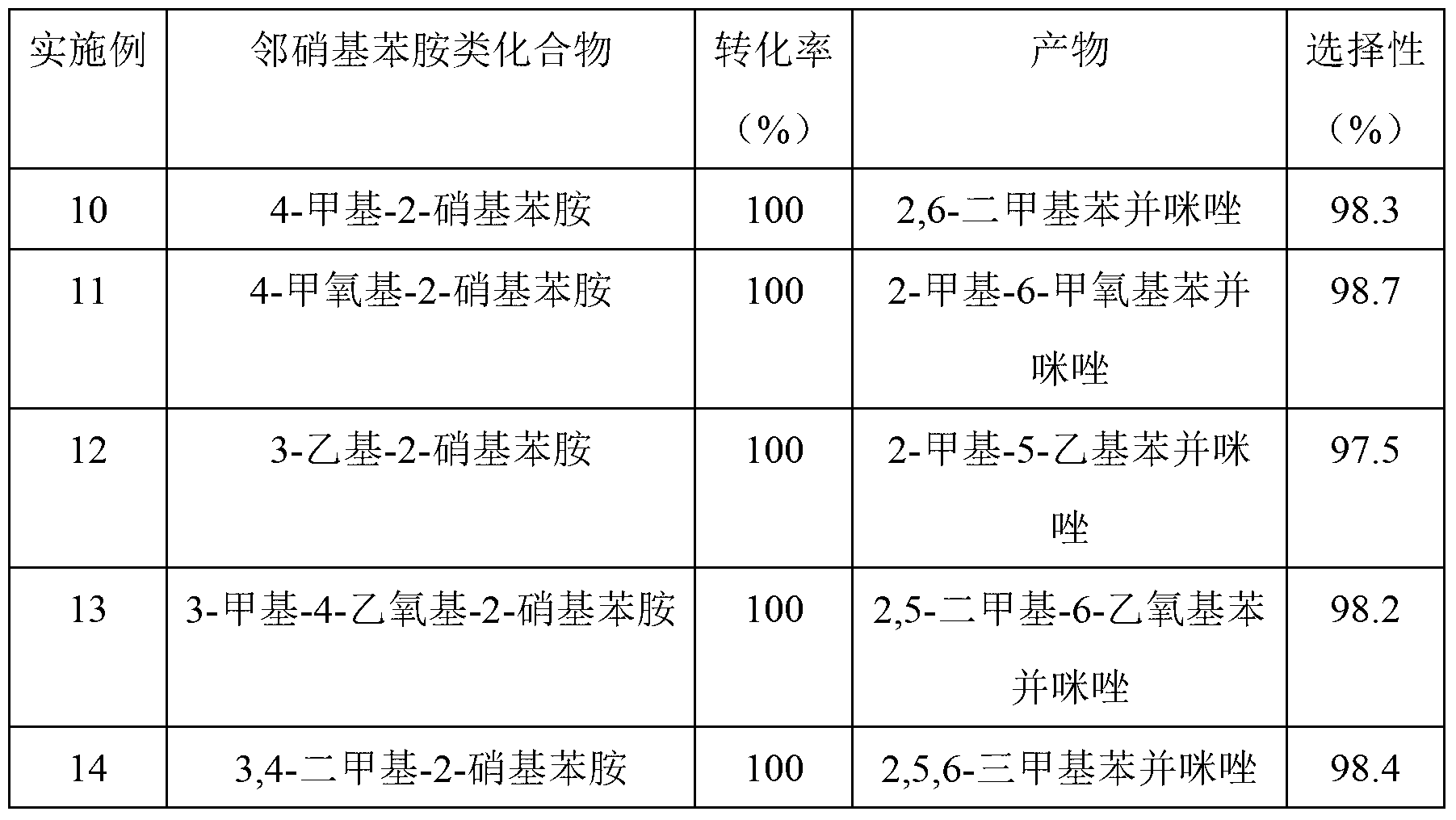
Method for synthesizing bis-benzimidazole compound through one-pot method
ActiveCN105777650AEasy to separateReduce generationOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsOrtho-nitroanilineAlcohol
The invention provides a method for synthesizing a bis-benzimidazole compound through a one-pot method.According to the method, a bis(ortho-nitroaniline) compound shown in a formula II-1 or II-2 or II-3 and alcohol shown in a formula III are used as raw materials, water and organic solvent are added, and under the effect of a load type multi-metal solid catalyst, the bis-benzimidazole compound shown in the corresponding formula II-1 or II-2 or II-3 is synthesized through the one-pot method, wherein X1, X2, X3, X4, X5, X6, X7 and X8 are H, F, C1, CH3, CH2CH3 and OCH3 or OCH2CH3 independently; X9 is O or CO or CH2 or NH; R1 is H or alkyl or phenyl or alkyl phenyl or alkoxy phenyl, and the number of carbon atoms of alkyl and alkoxy is 1-3; in Y1 and Y2, Y3 and Y4, Y5 and Y6, Y7 and Y8, Y9 and Y10, and Y11 and Y12, one in each group is amino, and the other in each group is nitryl.The method has the advantages that the synthetic route technology is simple, the product yield is high, the production cost is low, the catalyst is easy to separate, high in activity and good in stability, and liquid acid is not used.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing N substituted o-phenylenediamine
InactiveCN101607912AInhibitionHigh purityOrganic compound preparationAmino compound preparationAlkaneAlcohol
The invention provides a method for preparing N substituted o-phenylenediamine with the general formula (1), wherein R represents H, C1-C4 alkyl, C1-C4 alkoxyl and C1-C4 alkyl sulphonyl, and R1represents C1-C4 alkyl and phenyl. The method takes water, alcohol and other alkanes as solvent, and uses Ranery nickel to catalyse, hydrogenate and deoxidize N substituted o-nitroaniline with the general formula 2 into N substituted o-phenylenediamine in the presence of ammonia or amine. The method has the advantages of high purity, high yield, easy industrialization, no solid waste discharge and no environment pollution.
Owner:沈阳感光化工研究院有限公司
Method for preparing fenbendazole intermediate 2-nitro-4-thiophenyl aniline
The invention discloses a method for preparing a fenbendazole intermediate 2-nitro-4-thiophenyl aniline, and solves the problems that the existing preparation method is unreasonable; and the technicalproblems of high raw material price, complex operation, high reaction equipment condition requirements, high cost, poor yield and unsuitability for industrial production exist in the prior art can besolved. The invention relates to a method for preparing a fenbendazole intermediate 2-nitro-4-thiophenyl aniline. Under the substitution reaction conditions, substitution reaction is carried out on the N-phenylthio acetanilide and o-nitroaniline, thus synthesizing the fenbendazole intermediate 2-nitro-4-thiophenyl aniline; the whole novel synthesis method is mild in reaction conditions, the operation is simple and convenient, the safety is high, the product yield is high, the atom utilization rate is high, the three wastes are few, the operation is simple and convenient, the cost is low, thesustainable development requirements are met, and the method is suitable for industrial production; the method can be widely applied to the technical field of fenbendazole organic synthesis.
Owner:SHANDONG GUOBANG PHARMA +1
Synthetic method of 2-bromocarbazole and intermediate thereof
ActiveCN105315194AHigh reaction yieldReduce pollutionOrganic chemistryOrganic compound preparationTriethylphosphiteNitrite
The invention discloses a synthetic method of 2-bromocarbazole and an intermediate thereof. The synthetic method includes the steps of 1) with dichloromethane as a solvent and tert-butyl nitrite as a diazotization reagent, diazotizating o-nitroaniline and performing a coupling reaction to the diazotizated o-nitroaniline and bromobenzene to prepare an intermediate 4-bromo-2'-nitrobiphenyl; 2) adding enough triethyl phosphite to the 4-bromo-2'-nitrobiphenyl to perform a ring closing reaction to the 4-bromo-2'-nitrobiphenyl and the triethyl phosphite; 3) when the ring closing reaction is finished, adding enough hydrochloric acid to damage the triethyl phosphite which is not reacted; and 4) filtering and drying a reaction product to prepare the 2-bromocarbazole.
Owner:YURUI SHANGHAI CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com