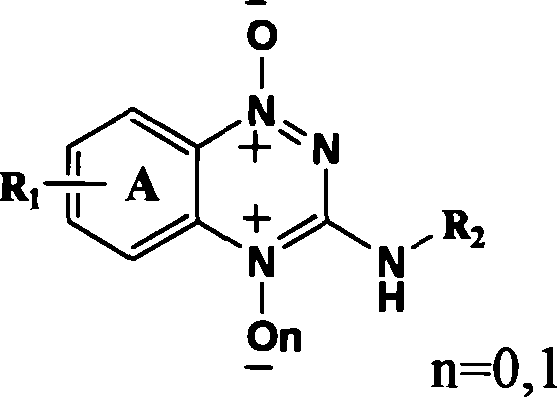
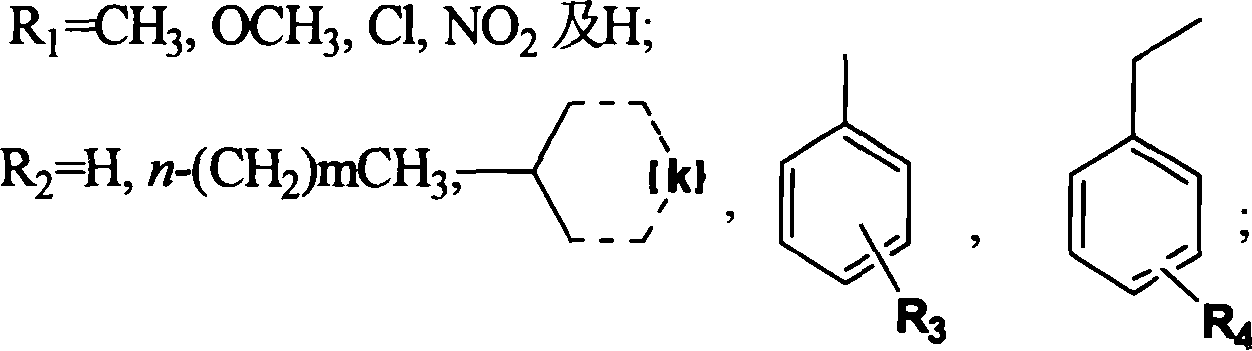
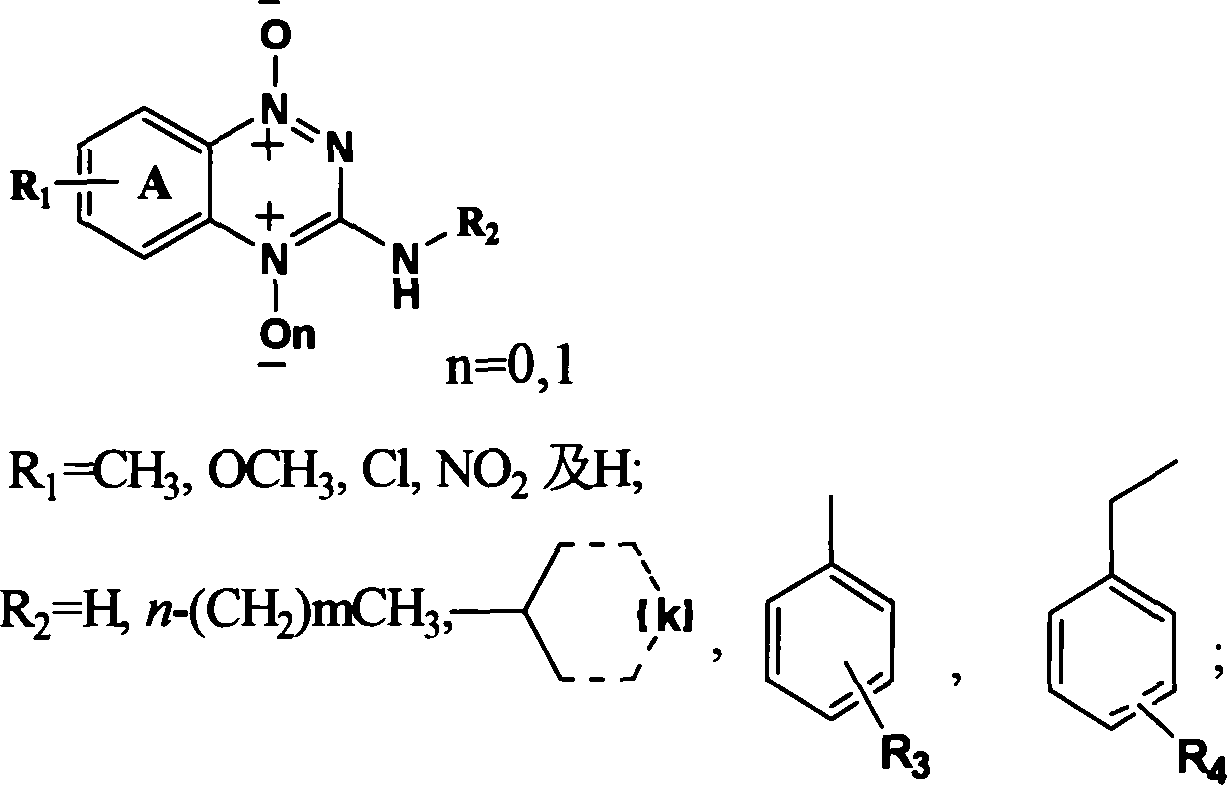
Triazinyl nitroxide and its synthesis and use
A technology of oxides and dioxides, applied in the directions of active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc., can solve the problems of limited, toxic and side effects diffusion capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The detailed steps of object compound: triphosgene 39.6g (0.13mol) is dissolved in 30ml toluene, drips under room temperature nitrogen protection containing substituted o-nitroaniline (I 1-4 ) 0.2mol and triethylamine 27.6ml (0.2mol) toluene solution, dropwise in 30min, be warming up to toluene reflux temperature, obtain o-nitroisocyanate (II 1-4 ), cooling, without separation, directly feed anhydrous ammonia for 30min, filter, wash with petroleum ether and hot water successively to obtain the substituted o-nitrourea (III 1-4 ), with 30% sodium hydroxide cyclization to obtain 3-hydroxyl-1,2,4-substituted benzotriazine-1-oxide (IV 1-4 ), 3-hydroxy-1,2,4-substituted benzotriazine-1-oxide (IV 1-4 ) and POCl 3 Substitution gives 3-Cl-1,2,4-substituted benzotriazine-1-oxides (V 1-4 ), V and C 4 -C 10 fatty amine, C 3 -C 6 Condensation of cycloalkylamines, substituted aromatic amines and substituted benzylamines to give 3-substituted amino-1,2,4-substituted benzotriazi...
Embodiment 2
[0048] The synthetic steps of the target product containing nitro substitution: in 3-hydroxyl-1,2,4-benzotriazine-1-oxide (IV 1 ) 2.5g (15.34mmol), add 50ml of mixed acid dropwise, stir at room temperature for 2 hours, heat to 60°C or 100°C, heat for 4 hours, cool, add a large amount of ice water, solids are generated, filter, wash with water, and dry to obtain 3-hydroxy -1,2,4-Nitro-substituted benzotriazine-1-oxide (IV 5,6 ). 3-Hydroxy-1,2,4-6 or 7-nitro-benzotriazine-1-oxide (IV 5,6 )0.5g (2.40mmol) by adding 5ml POCl 3 , heated to reflux for 6h, evaporated to remove unreacted POCl 3 , add ice water, dichloromethane extraction, petroleum ether / ethyl acetate=4:1 is the eluent column chromatography to obtain the product 3-chloro-1,2,4-6 or 7-nitro-benzotriazine -1-Oxide (V 5,6 ). Take 0.5g (2.21mol) 3-chloro-1,2,4-7-nitro substituted benzotriazine-1-oxide (V 6 ) and C 4 -C 10 fatty amine, C 3 -C 6 Cycloalkylamine, substituted arylamine and substituted benzylamine a...
Embodiment 3
[0050] 3-Hydroxy-1,2,4-benzotriazine-1-oxide (IV 1 ): Prepared by referring to literature method (F.J.Wolf et al, J.Amer.Chem.Soc., 1954, 4611-4613).
[0051] MS(EI): 164(M+1); 1 H NMR (DMSO, 400MHz): δ8.08(d, 1H, J=8.4Hz), 7.78(t, 1H, J=7.8Hz), 7.28~7.34(m, 2H), 3.34(s, 1H); IR (KBr): 3421, 2814, 1681, 1615, 1483, 1419, 1344, 1309, 1137, 778.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com