Synthesis method of substituted nitroaniline
A technology of nitroaniline and synthesis method, which is applied in the formation/introduction of nitro/nitroso groups, amino compound preparation, chemical instruments and methods, etc. It can solve the problems of unsafe production process, environmental pollution, high cost, etc., and achieve Low environmental pollution, simple post-treatment, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
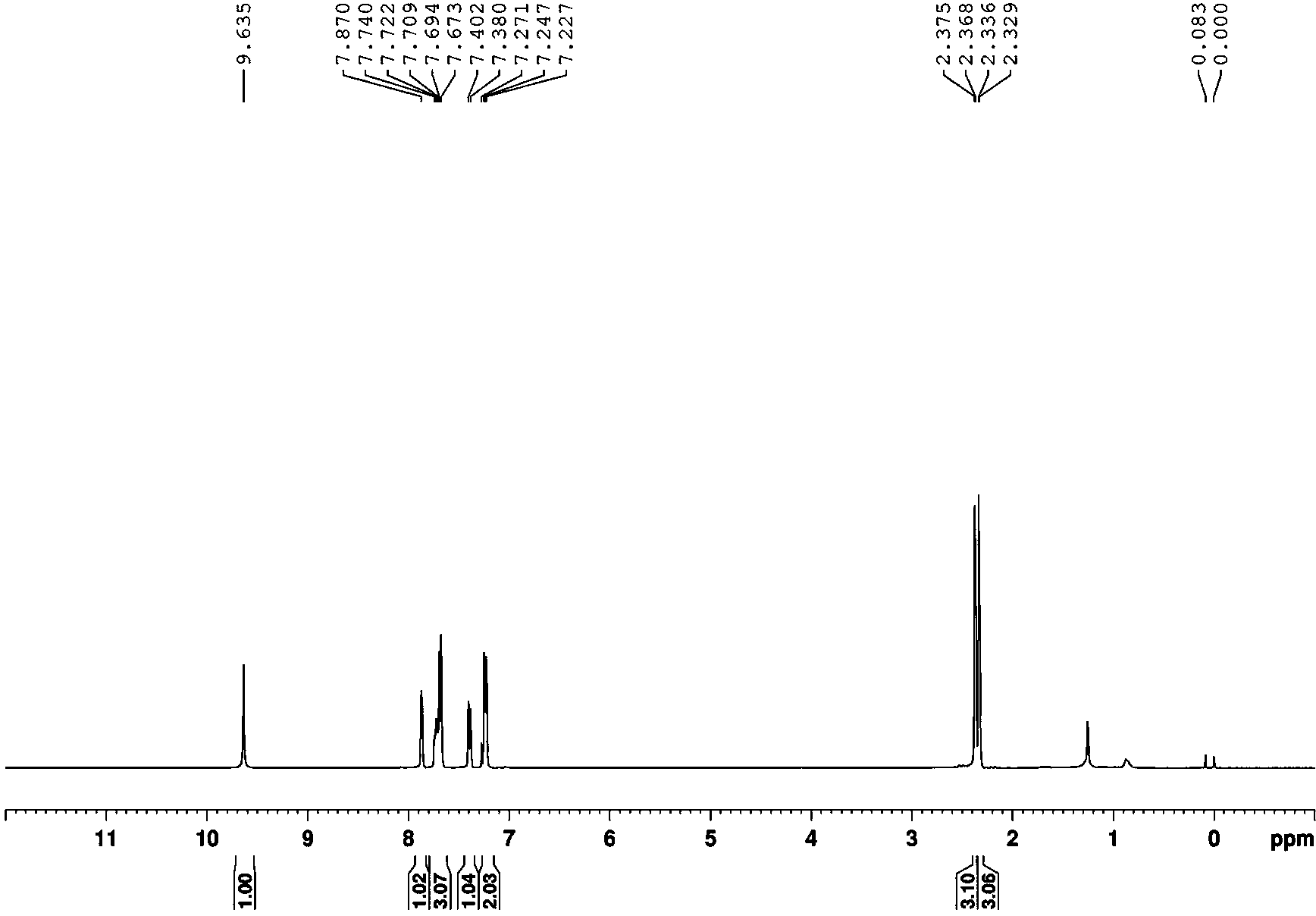
Embodiment 1
[0031] , Ts is p-toluenesulfonyl.
[0032] Compound a (0.3mmol), 0.6mmol NaNO 2 and 0.2mmol potassium persulfate into the reaction vessel, add 3ml of nitromethane, and stir for 2 hours at 50°C; Volume ratio 1:10) was used as the eluent to pass through a silica gel column to obtain the product a' with a yield (98%).
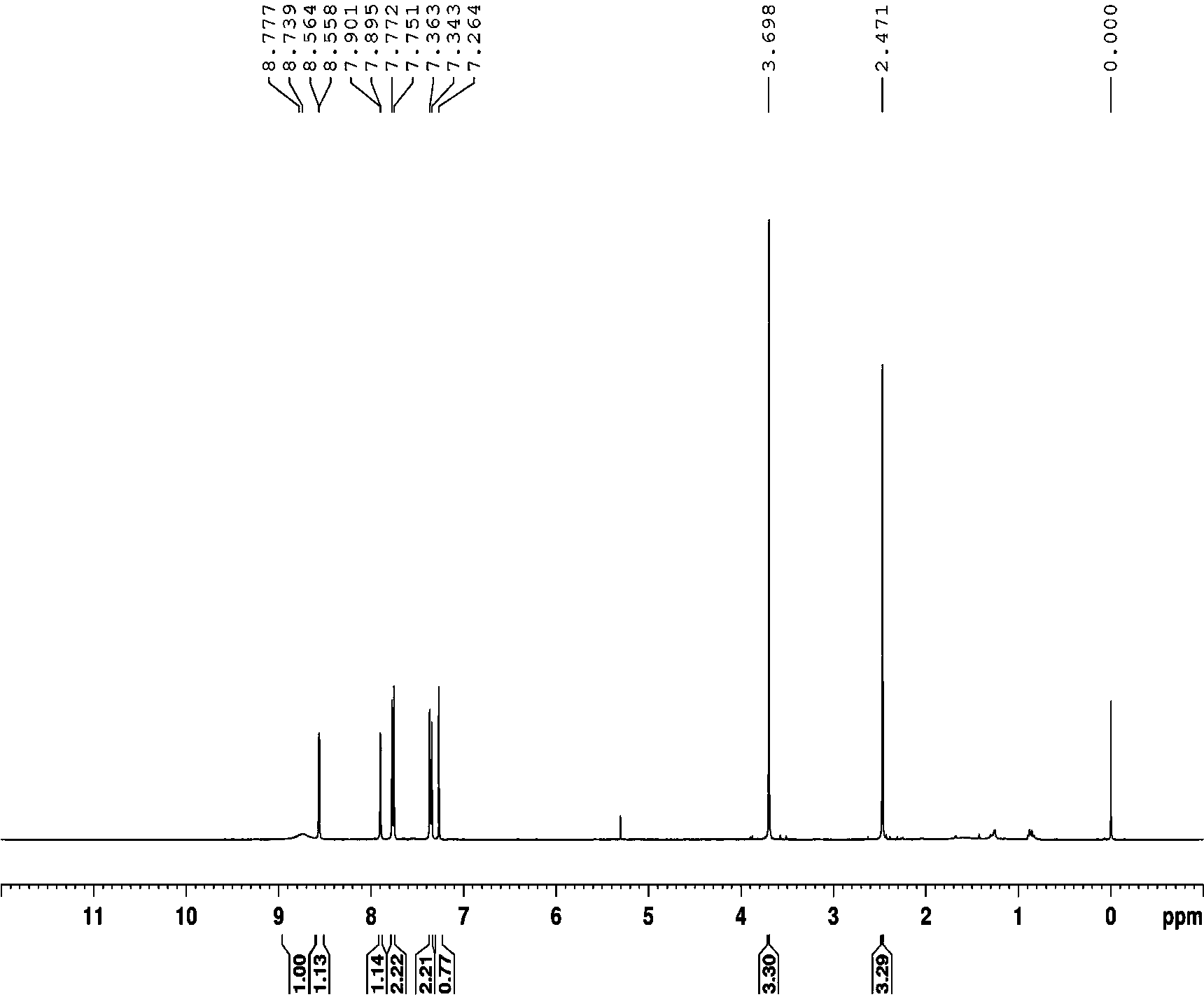
Embodiment 2
[0034]
[0035] Compound b (0.3mmol), 0.6mmol of NaNO 2 and 0.2 mmol of potassium hydrogen persulfate were added to the reaction vessel, 3ml of nitromethane was added, and stirred at 50°C for 3 hours; (volume ratio 1:8) was used as the eluent to pass through a silica gel column to obtain products b' (50% yield) and b'' (32% yield).
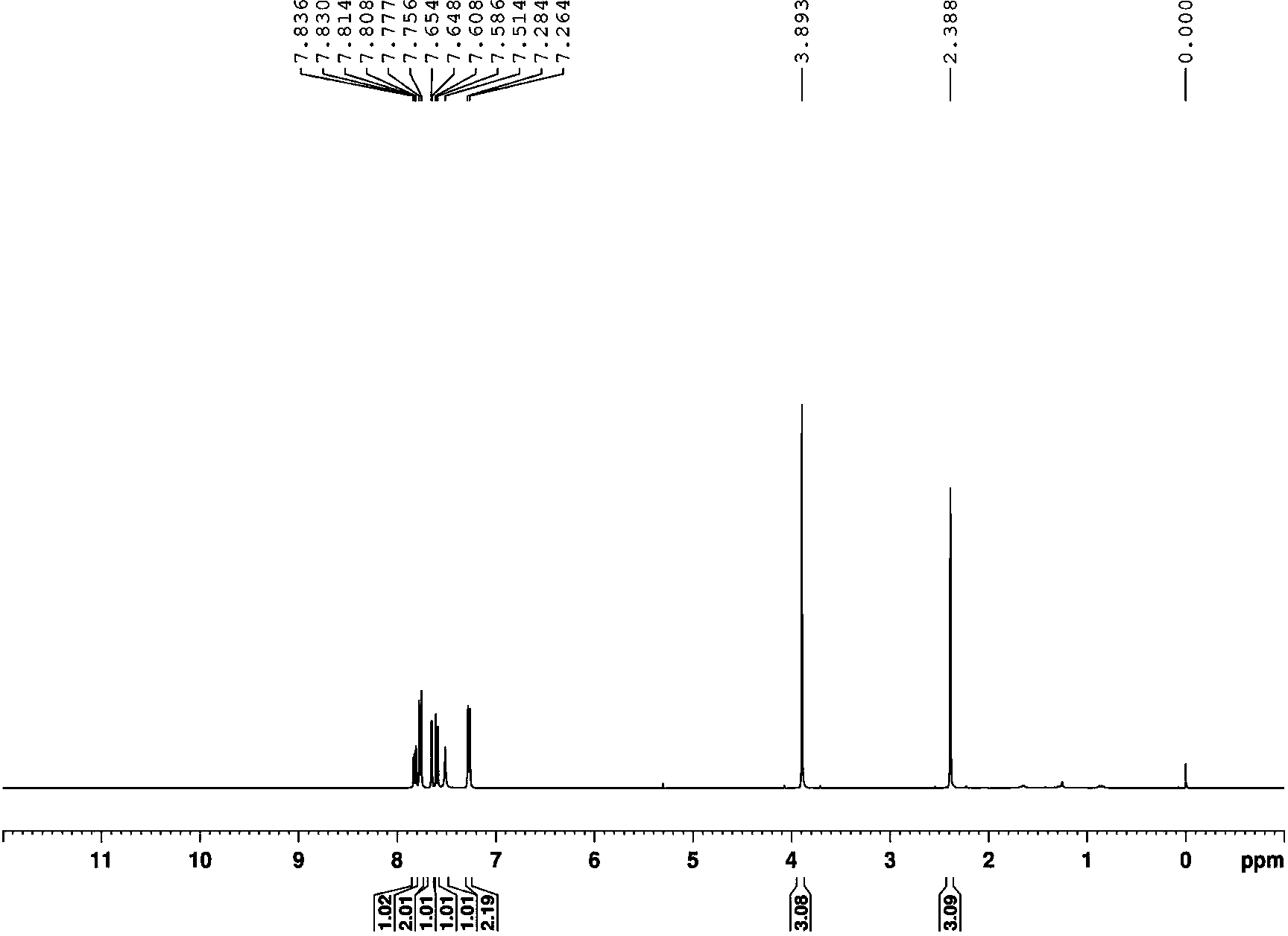
Embodiment 3
[0037]
[0038] Compound c (0.3mmol), 0.6mmol of NaNO 2 and 0.2mmol of potassium hydrogen persulfate were added to the reaction vessel, 3ml of nitromethane was added as solvent, and stirred at 50°C for 3 hours; after the detection reaction was completed, the nitromethane was removed by rotary evaporation, and the The mixed solution (volume ratio 1:8) was used as the eluent to pass through the silica gel column to obtain the product c' with a yield of 62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com