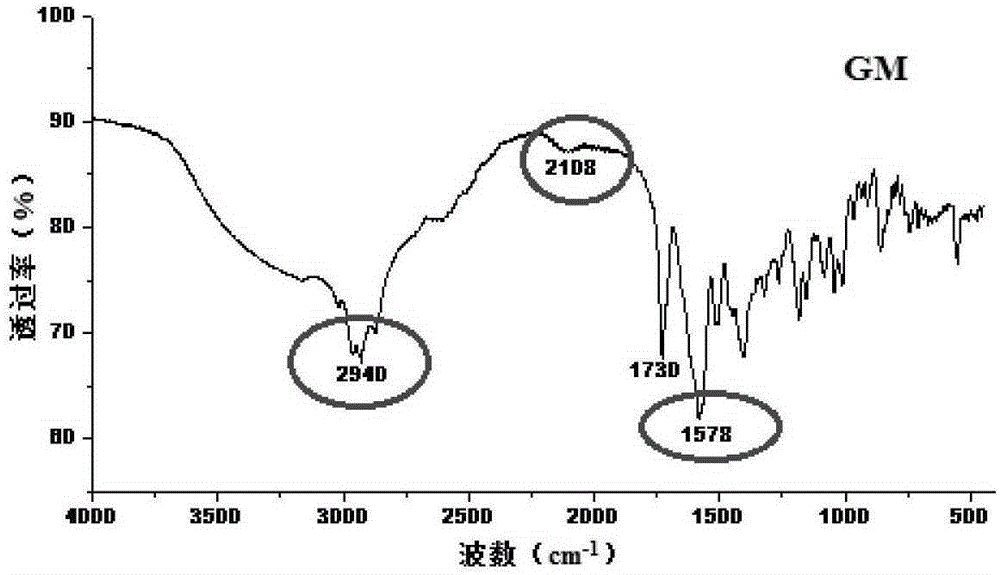
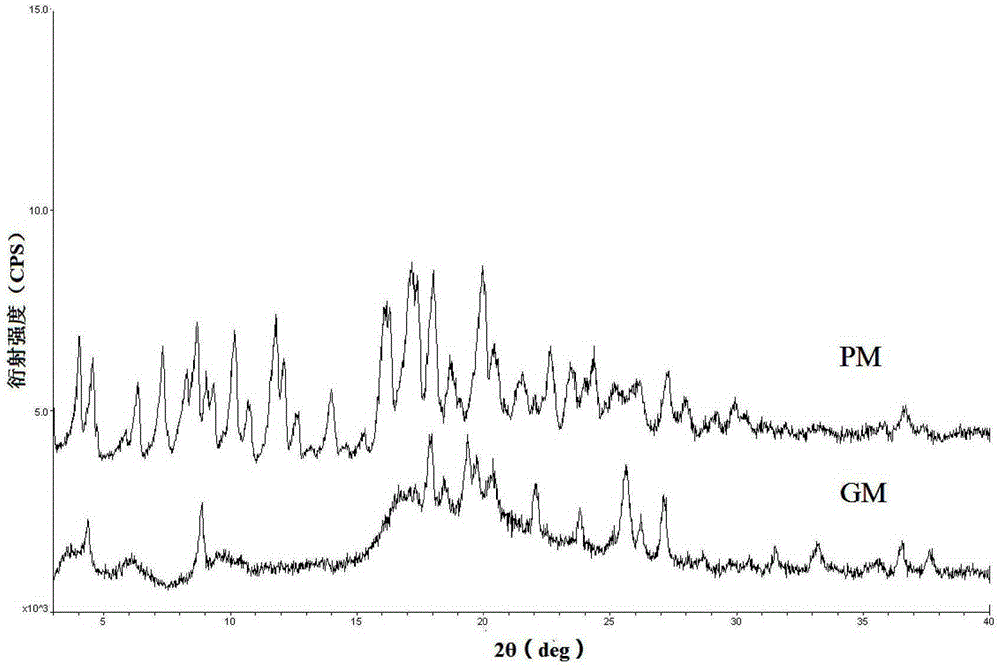
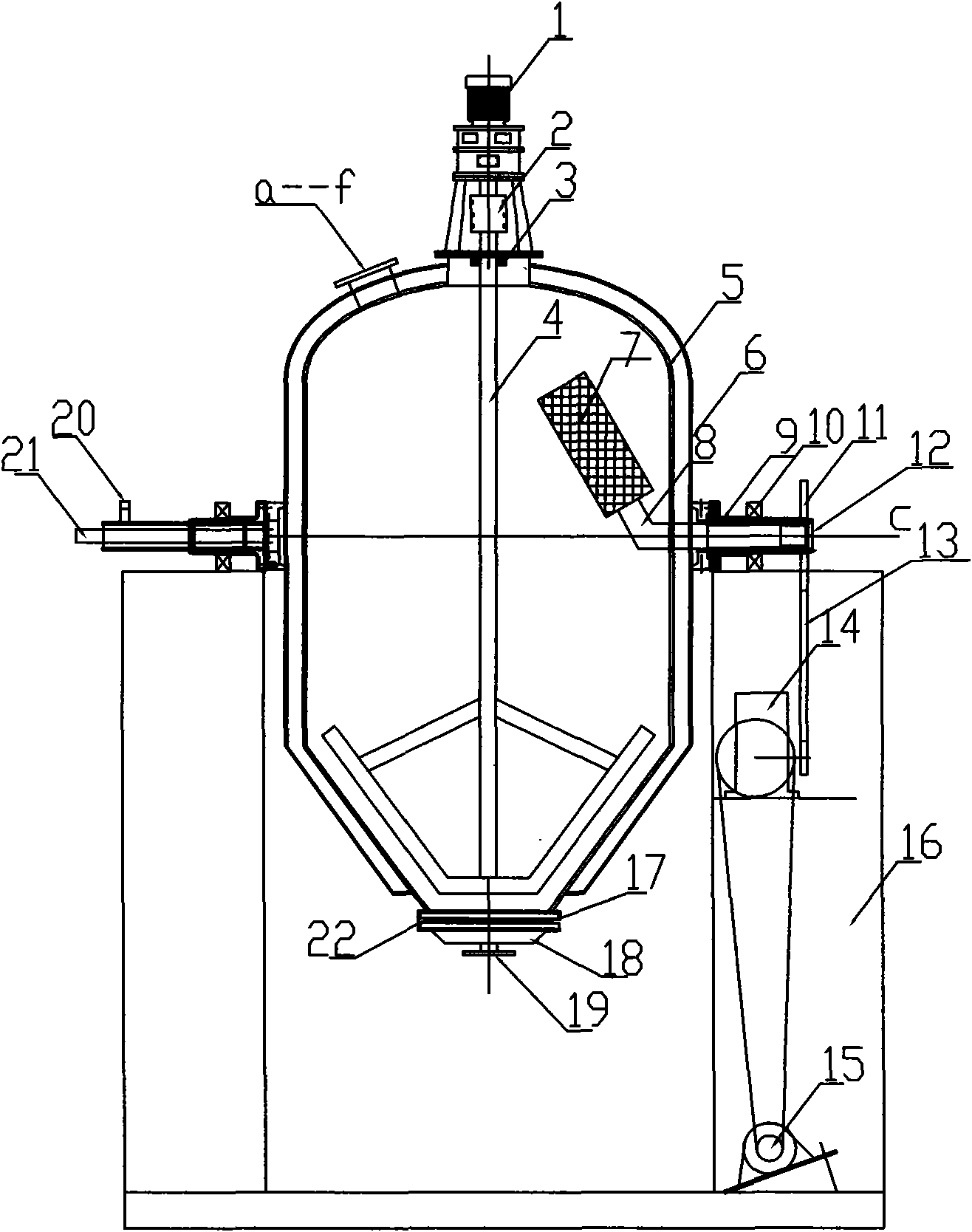
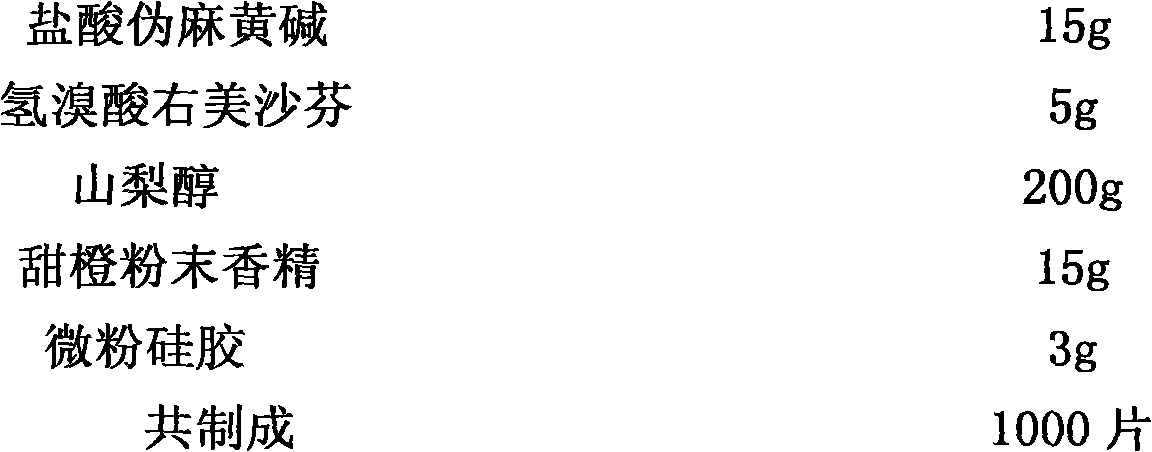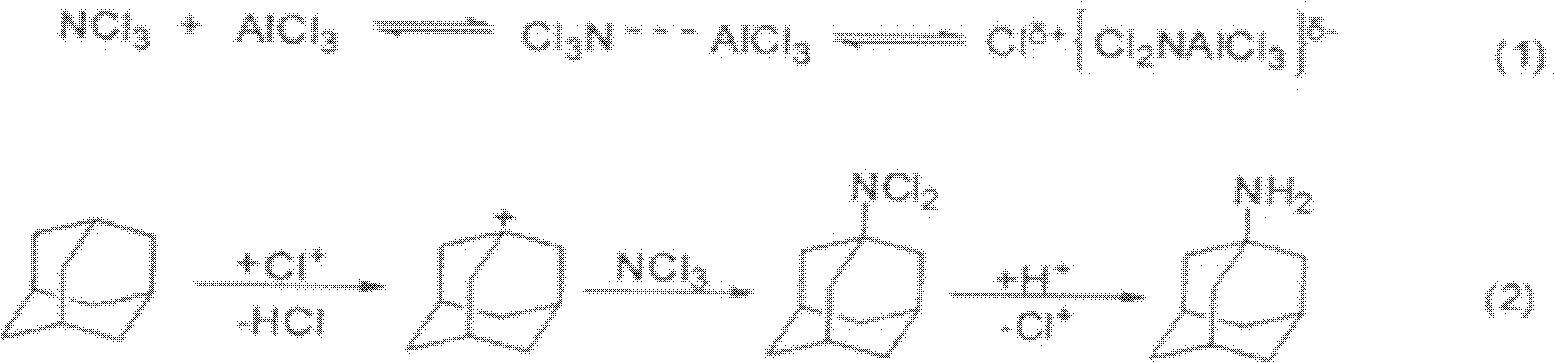Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
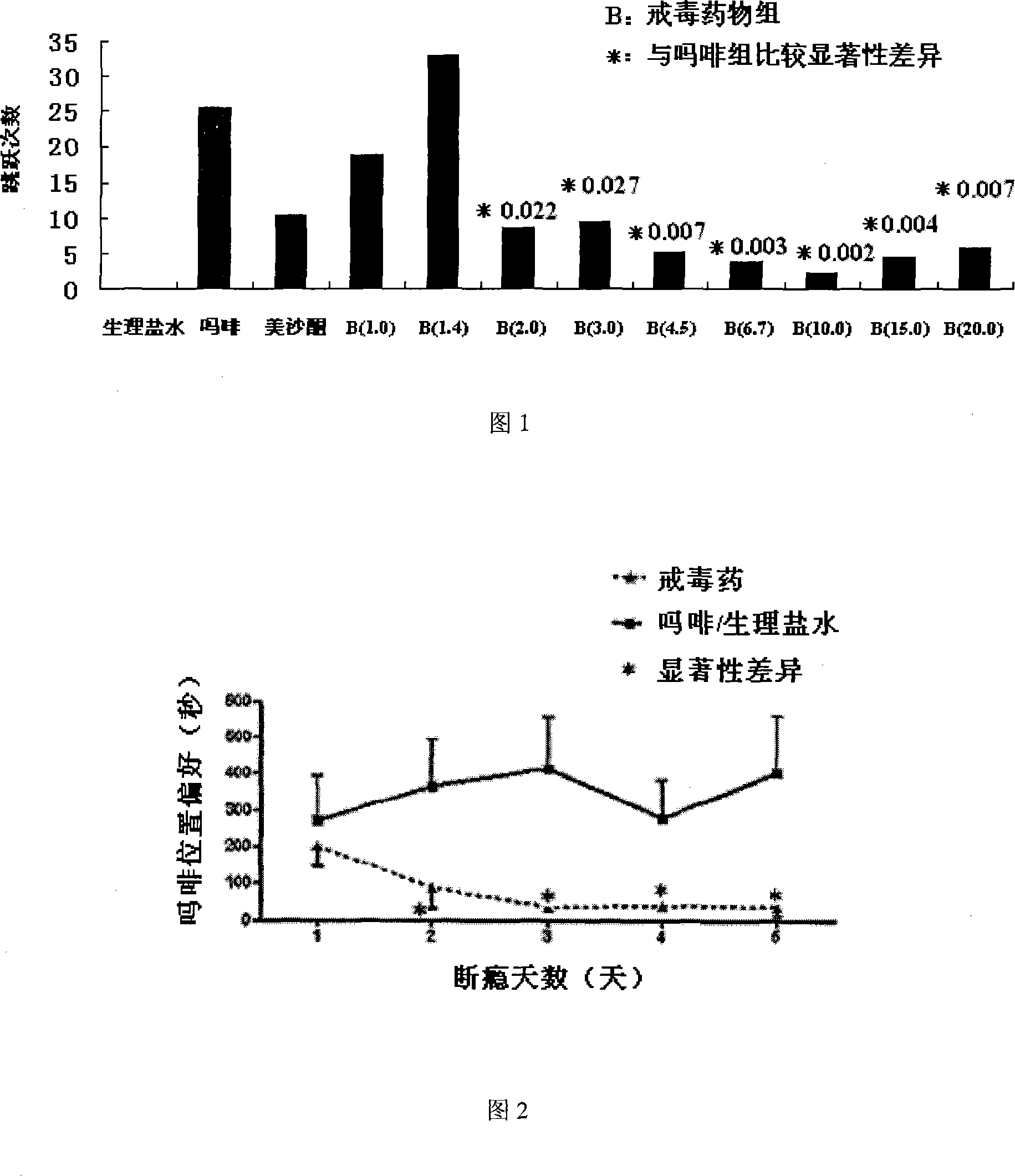
114 results about "Amantadine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The hydrochloride salt of amantadine, a synthetic tricyclic amine with antiviral, antiparkinsonian, and antihyperalgesic activities. Amantadine appears to exert its antiviral effect against the influenza A virus by interfering with the function of the transmembrane domain of the viral M2 protein, thereby preventing the release of infectious viral nucleic acids into host cells; furthermore, this agent prevents virus assembly during virus replication. Amantadine exerts its antiparkinsonian effects by stimulating the release of dopamine from striatal dopaminergic nerve terminals and inhibiting its pre-synaptic reuptake. This agent may also exert some anticholinergic effect through inhibition of N-methyl-D-aspartic acid (NMDA) receptor-mediated stimulation of acetylcholine, resulting in antihyperalgesia.
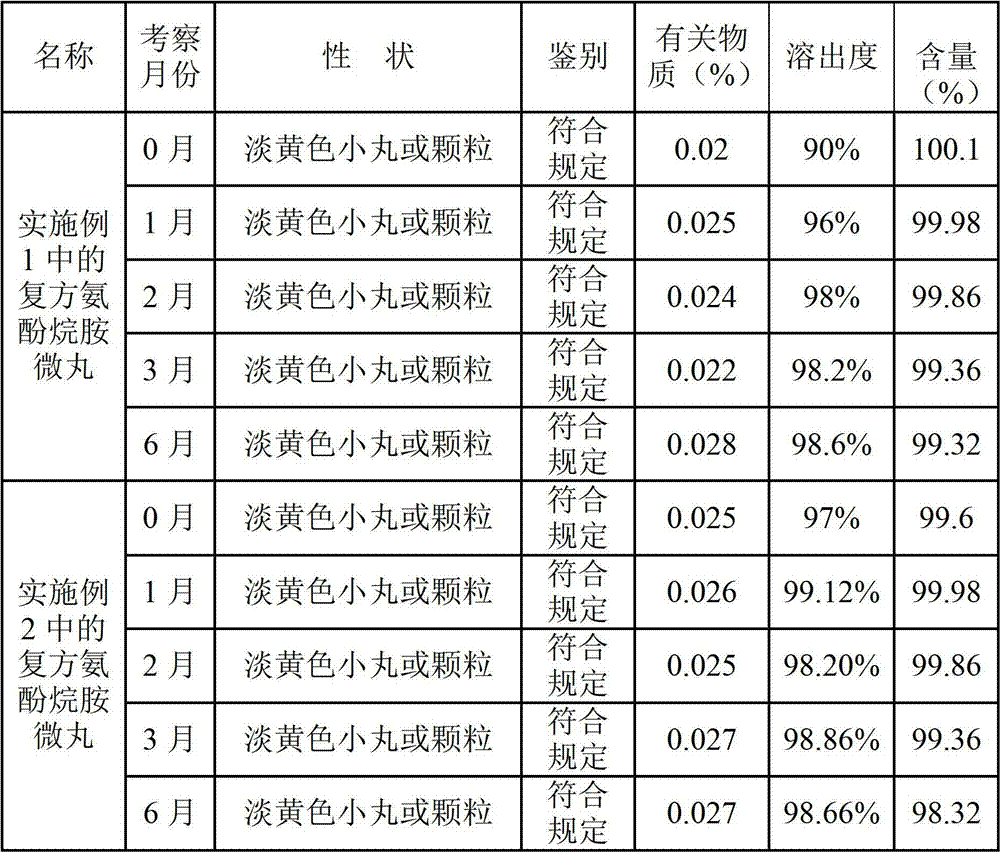
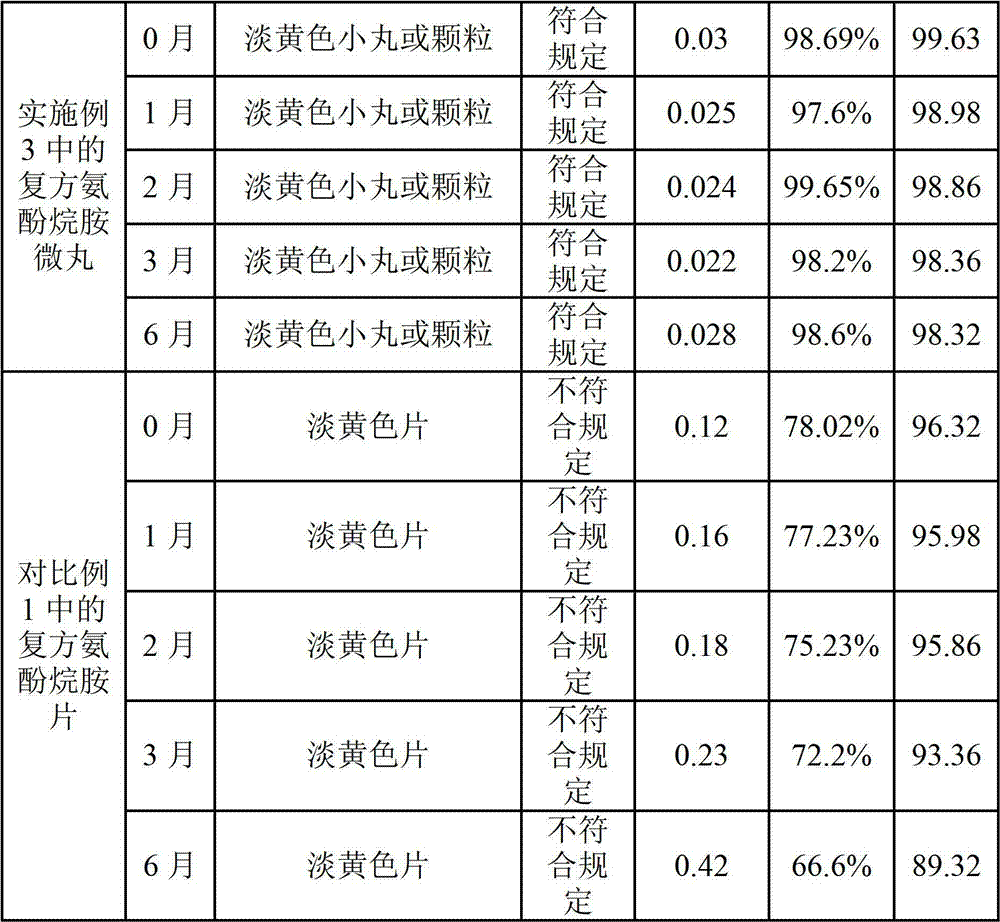
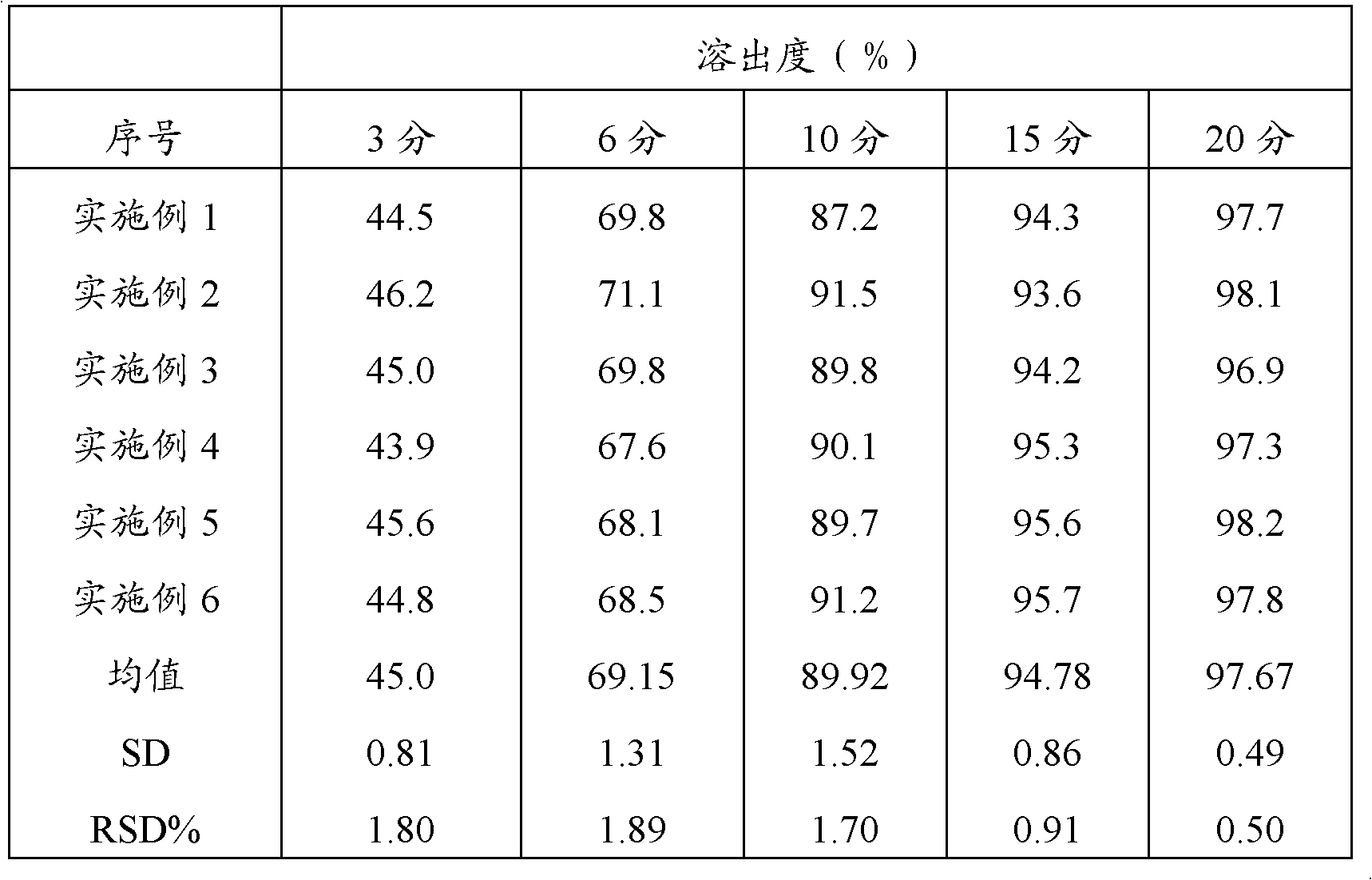
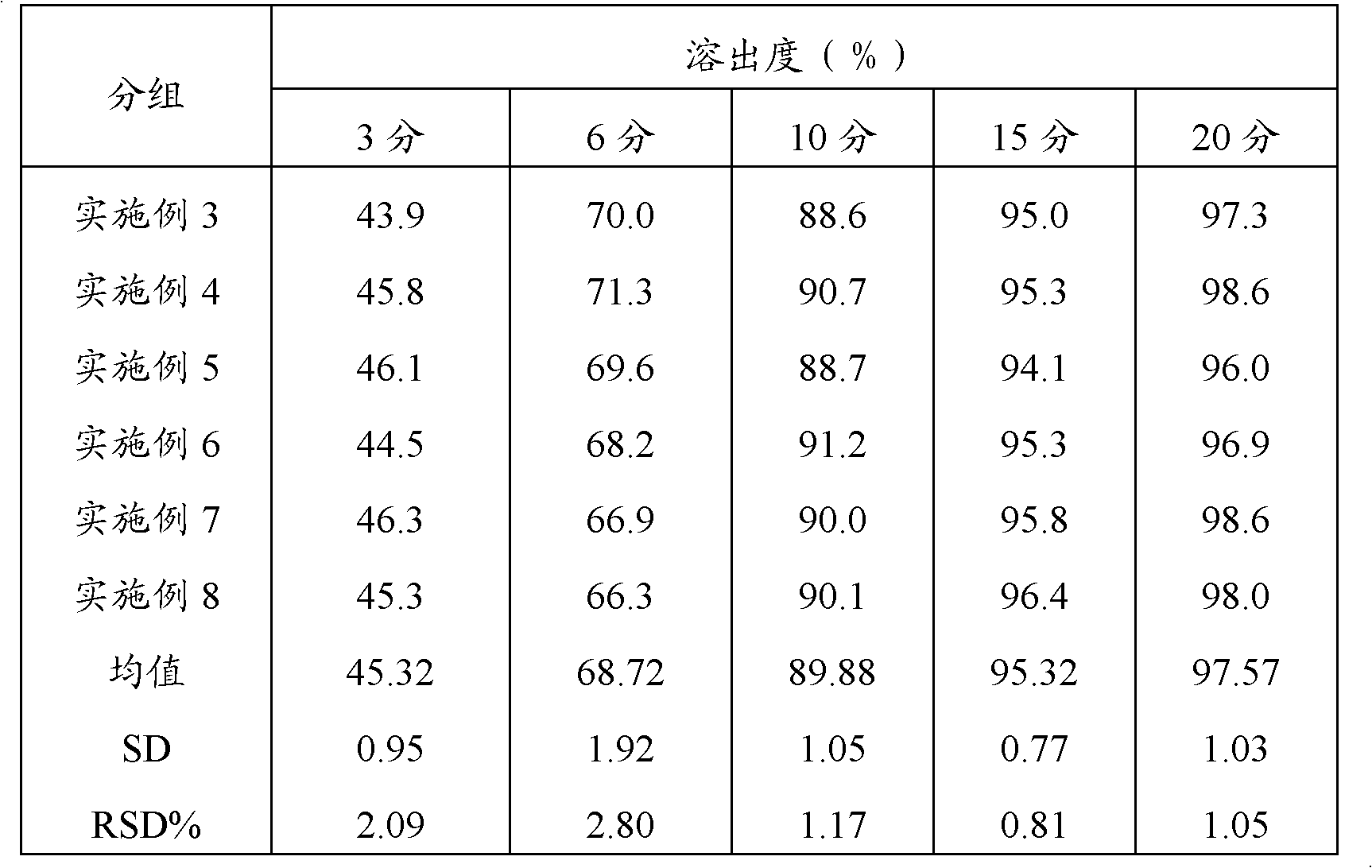
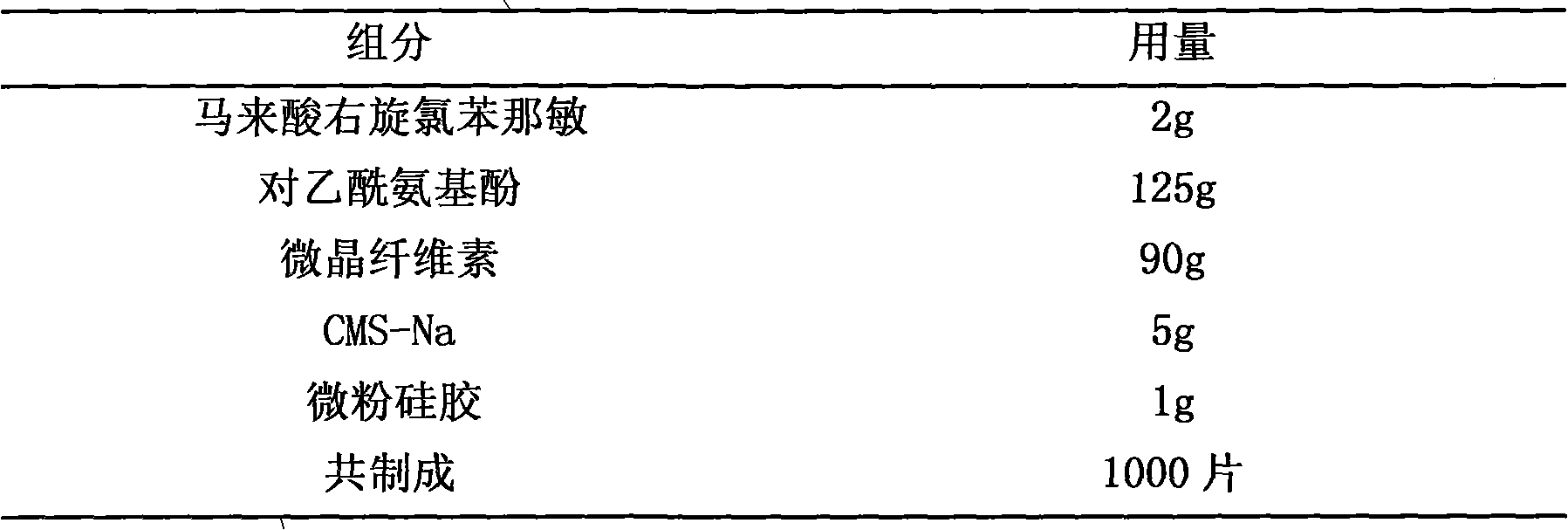
Preparation method of compound paracetamol and amantadine pellets
ActiveCN102861106AEvenly distributedEasy to fillNervous disorderAntipyreticDissolutionCalculus bovis
The invention discloses a preparation method of compound paracetamol and amantadine pellets. The compound paracetamol and amantadine pellet is mainly prepared from the following raw materials: chlorpheniramine maleate, calculus bovis factitious, caffeine, amantadine hydrochloride, acetaminophen and dextrin, and the mass ratio of the above raw materials is 1 to 5 to 7.5 to 50 to 125 to (4-5); the preparation method comprises the following steps: adding the chlorpheniramine maleate and the caffeine in the form of solution into a dextrin aqueous solution, spraying and packing the mixed solution on the mixed masterbatches consisting of the acetaminophen, the amantadine hydrochloride and the calculus bovis factitious, and then spraying the rest of dextrin and the rest of mixed powder to obtain the compound paracetamol and amantadine pellet. The method is simple to operate, liable to control and suitable for industrial production, and the obtained pellet has the advantages of low related substances, good content uniformity and dissolution rate and the like.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
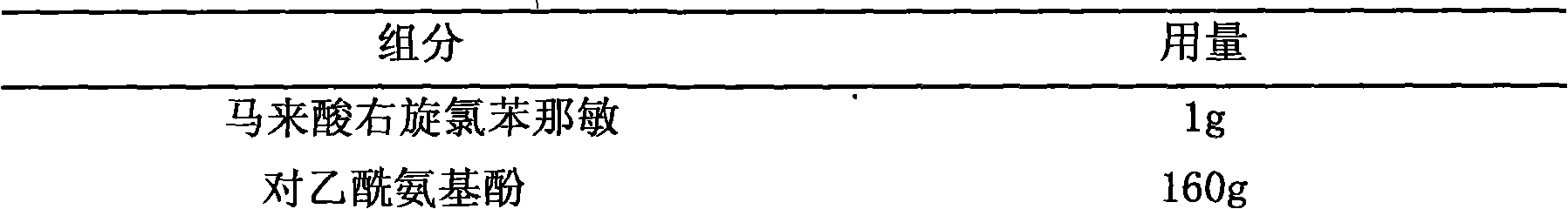
Method for preparing compound paracetamol and amantadine hydrochloride tablet
InactiveCN102488711AHigh dissolution rateAntiviralsUnknown materialsQuality standardAmantadine Hydrochloride
The invention particularly relates to a method for preparing a compound paracetamol and amantadine hydrochloride tablet, which belongs to the field of medicines. According to the method, submicron powder and ordinary fine powder which has been sieved with a 100-mesh sieve are mixed in proportion, a proper amount of accessories are added, and a prepared finished product is enabled to have better dissolvability, more than 90% on average. After six-month accelerated normal temperature stability experiments, the dissolvability of the finished product prepared by using the method is about 90%, and all the other indexes of the finished product accord with quality standard requirements.
Owner:吉林省吴太感康药业有限公司 +1
Method of synthesizing amantadine hydrochloride
InactiveCN1556094ASmall side effectsMild reaction conditionsOrganic compound preparationAmino compound preparationAlcoholBoiling point
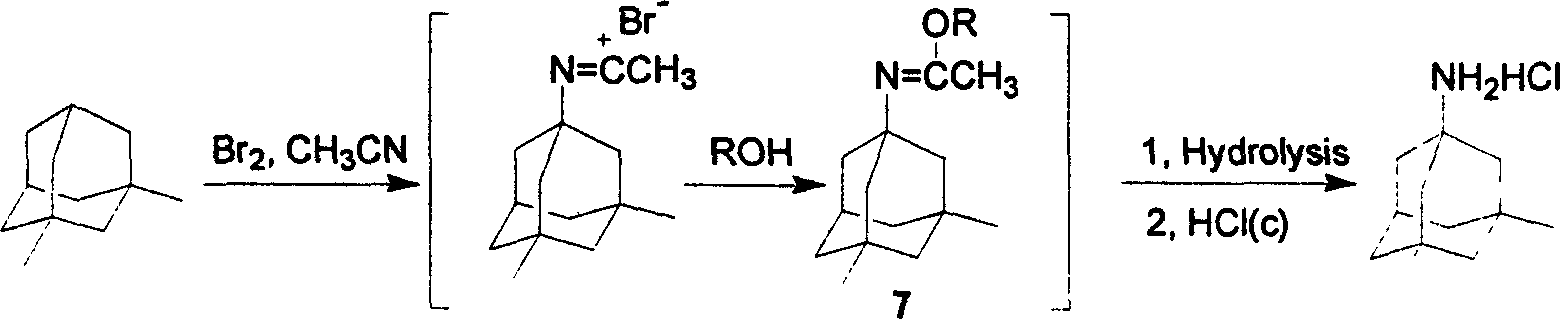
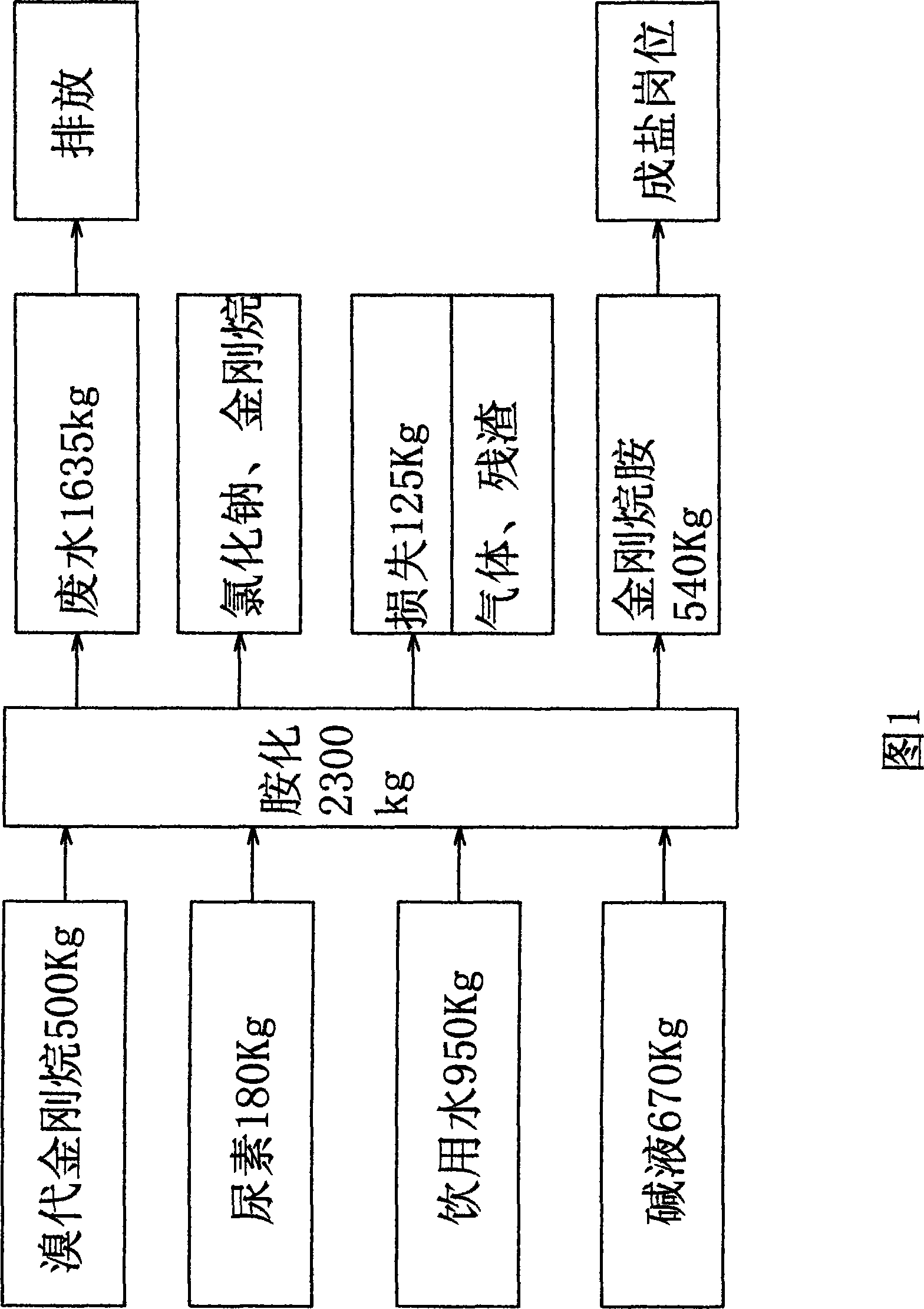
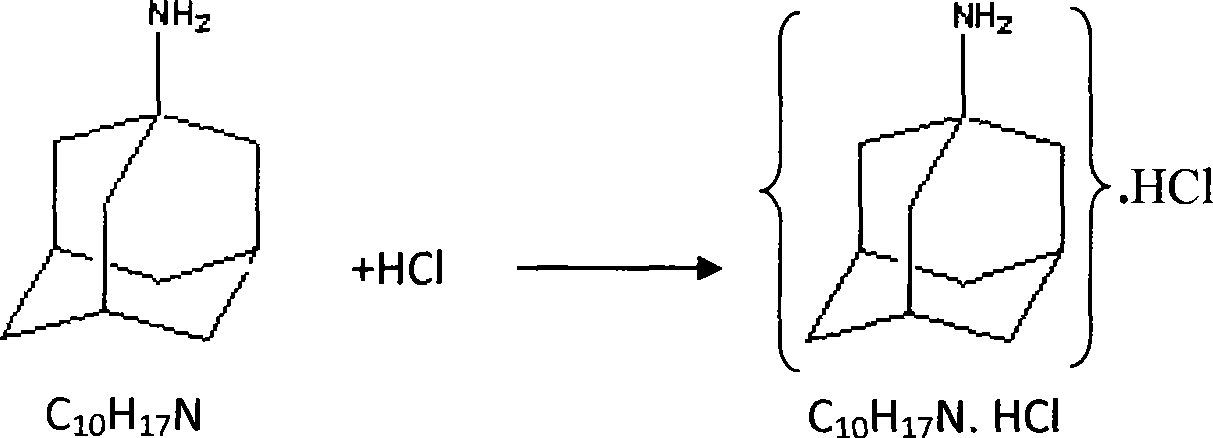
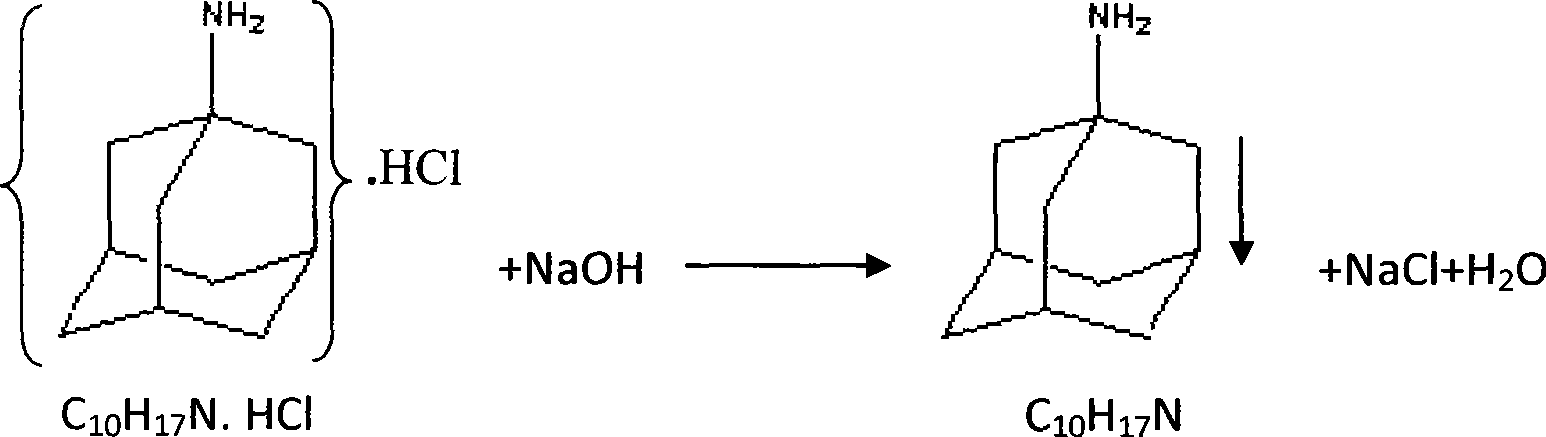
A process for synthesizing adamantanamine hydrochloride from 1,3-dimethyl adamantane include browo reaction while Ritter reaction, adding the resultant mixture in the mixture of polar proton solvent and alcohol solvent, stirring while alcoholyzing, regulating pH, alkaline hydrolyzing to generate adamantanamine, extracting in arylhydrocarbon solvent, water washing, drying, filtering, vacuum concentrating of filtrate, reacting on concentrated hydrochloric acid to obtain coarse product, and recrystallizing in the mixed solvent of alcohol and arylhydrocarbon.
Owner:NANJING UNIV
Compound paracetamol and amantadine hydrochloride dripping pills and preparation method
The present invention discloses a compound paracetamol amantadine hydrochloride dripping pills preparation and its preparation method. Said compound preparation is made up by using paracetamol, amantadine hydrochloride, chlorphenamine maleate, artificial cow-bezoar, caffeine and matrix for making dripping pills.
Owner:陈茜
Method for preparing 3-amino-1-adamantane alcohol
InactiveCN101798270AReduce dosageReduce processing costsOrganic compound preparationAmino-hyroxy compound preparationWater bathsIce water
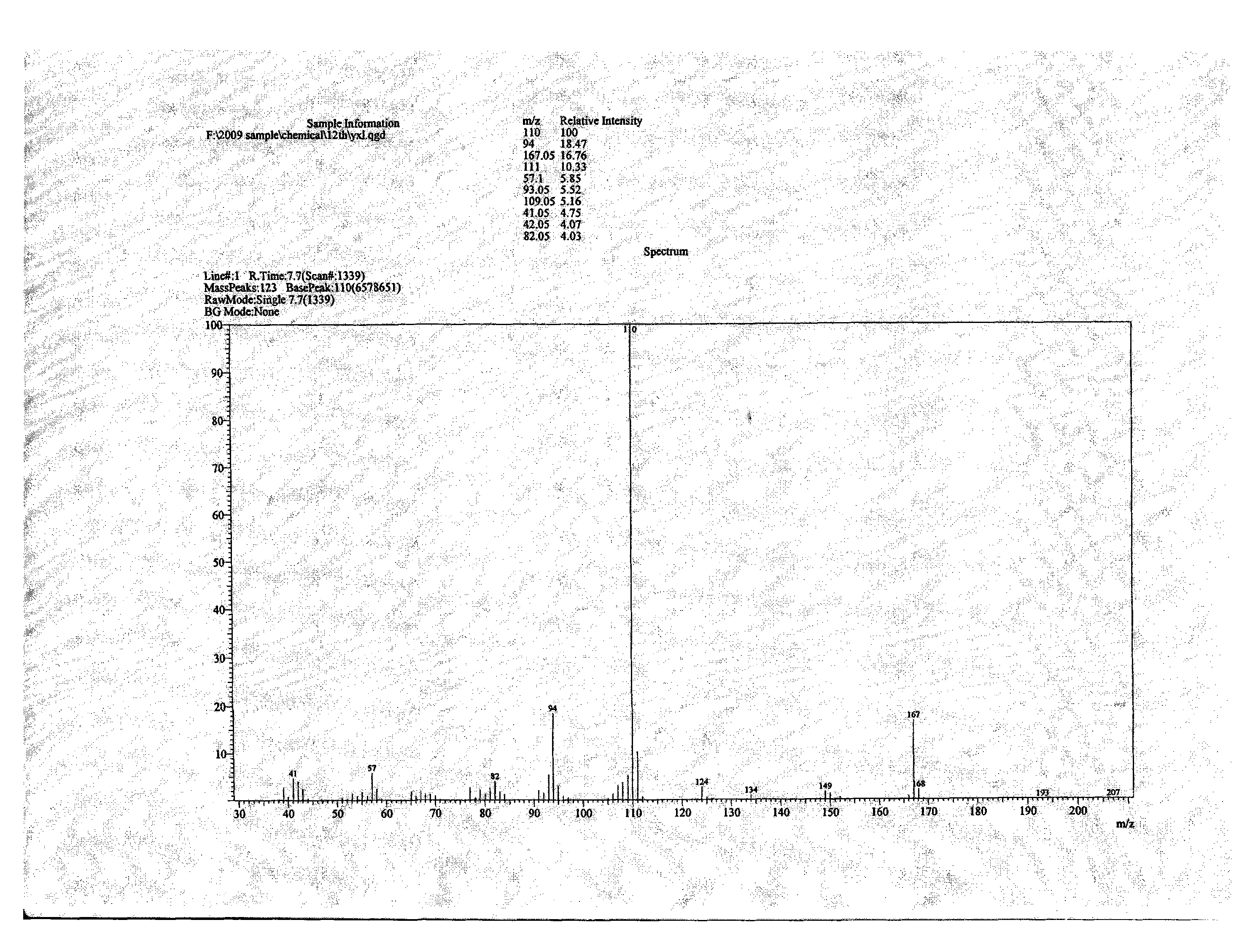
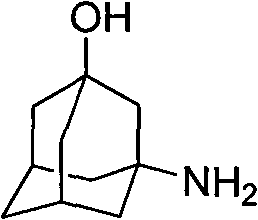
The invention relates to a method for preparing 3-amino-1-adamantane alcohol. The method comprises the following steps of: adding amantadine hydrochloride into a nitrating agent in batches, performing reaction for 1 to 2 hours in an ice-water bath and performing reaction for 1 to 30 hours at room temperature to obtain yellowish liquid; pouring the yellowish liquid into ice, continuously reacting for 0.5 to 2 hours with stirring to obtain blue-green liquid; and adding solid base into solution obtained by the step 2 with stirring, keeping temperature below 80 DEG C, regulating pH to be between 10 and 12, performing reaction for 30 minutes with stirring, leaching, extracting reaction liquid by using dichloromethane, drying the obtained product with anhydrous sodium sulfate, steaming off the dichloromethane and performing recrystallization by using ethyl acetate to obtain white solid. The preparation method has the advantages of readily available starting raw materials, simple reaction operation, short route, environmental friendliness, easy industrial production and good application prospect, and also reduces cost for the synthesis of Vildagliptin serving as a medicament for treatingdiabetes; and the yield of products reaches 75 percent.
Owner:DONGHUA UNIV
Preparation method of compound paracetamol and amantadine hydrochloride capsule
The invention relates to the technical field of the pharmacy, and especially relates to a preparation method of a compound paracetamol and amantadine hydrochloride capsule. The method is characterized in that smaller amounts of components comprising dextrin, calculus bovis factitious, chlorphenamine maleate and caffeine in a prescription are uniformly mixed to obtain mixed powder I, and larger amounts of compounds comprising viregyt hydrochloride and paracetamol in the prescription are uniformly mixed with the mixed powder I to realize uniform mixing and the uniformity of the content of the chlorphenamine maleate in the whole capsule. Double-tapered rotary vacuum drying is adopted, the physical and chemical properties of a material are not changed after the drying of the material, and the content of relevant substances in a finally finished product is low.
Owner:HAINAN ASIA PHARM CO LTD
Method for detecting Jingan capsule
ActiveCN101797277AIncrease assayImprove quality controlComponent separationCapsule deliveryChlorphenamine maleateMedicine
The invention discloses a method for detecting a capsule for treating cold. The capsule is prepared from honeysuckle, common andrographis herb, isatis root, dandelion, acetaminophen, amantadinehydrochloride and chlorphenamine maleate. The invention increases the content detection of the amantadinehydrochloride, the honeysuckle and the dandelion on the basis of the traditional quality standard, improves a content detecting method of the acetaminophen, can carry out more effective control on main medicament components of a Jingan capsule, and enables the quality monitoring level of the Jingan capsule to be greatly improved. The application of the invention is more beneficial to manufacturers and the supervisory management department to monitor the product quality and can provide better guarantee for the medical department and the treatment of patients.
Owner:GUIZHOU BAILING GRP PARMACEUTIAL CO LTD
Preparation process of amantadine hydrochloride
InactiveCN107445848AImproved responseHigh yieldAmino compound purification/separationOrganic compound preparationAcetonitrileSide reaction
The invention provides a preparation process of amantadine hydrochloride. The preparation process comprises the following steps: A, adding fuming sulfuric acid into a reactor, cooling to 8 to 10 DEG C, adding adamantine, dropwise adding acetonitrile at the speed of 0.1 to 0.2 mL / s, heating the solution in the reactor to 15 to 20 DEG C and preserving heat for 1 to 2 hours, then heating to 25 to 30 DEG C and preserving heating for 1 to 2 hours, AND finally heating to 32 to 35 DEG C and preserving heating for 1 to 2 hours to complete the reaction; B, carrying out a hydrolysis reaction of the reaction liquid after the reaction in the step A in water, and separating out 1-acetamidoadamantane; C, carrying out a hydrolysis reaction of the 1-acetamidoadamantane in hydrochloric acid, cooling and separating out the amantadine hydrochloride. By the preparation process, the generation of side reaction is reduced by the modes of heating by stages, adding the raw materials slowly and dropwise, mixing at low temperature and the like, and the yield of the amantadine hydrochloride is increased.
Owner:SHANDONG HOLLY PHARM CO LTD
Compound isatis-root injection for animals and the preparing method thereof
InactiveCN101062066ASignificant effectReasonable compositionAntiviralsSolution deliveryMoroxydineMedicine
The invention discloses a compound isatic root injection for animals and preparation technology, which is characterized by the following: allocating main medicine, adjunct and dissolvent; choosing the main medicine from isatic root, linbevelin, moroxydine and amantadine hydrochloride; choosing the adjunct from anti-oxidant, metal chelating agent and dissolvent for injection. This invention possesses advanced productive technology and long shelf life.
Owner:江西百思技术咨询有限公司
Vildagliptin preparation method
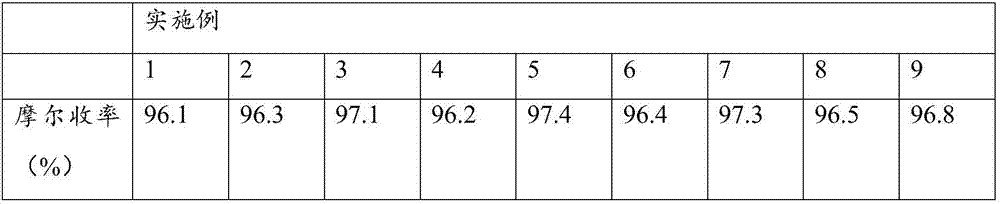
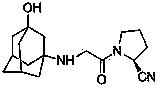
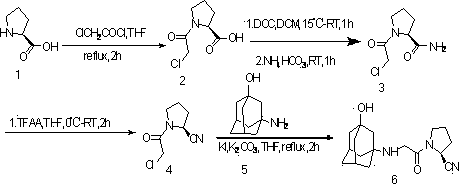
The invention provides a vildagliptin preparation method, and relates to a drug compound preparation method. According to the preparation method, L-proline is adopted as a raw material, and is subjected to reactions such as N-chloroacetylation, carbonyl amination and nitrile formation through amide dehydration to obtain an intermediate (S)-1-(2-chloroacetyl)pyrrolidine-2-formonitrile; and an amantadine hydrochloride is adopted as a raw material, and is subjected to mixing acid nitration and alkali hydrolysis to obtain the other intermediate 3-amino-adamantanol, and the two key intermediates are subjected to condensation to obtain the target product vildagliptin. The preparation method has characteristics of simpleness, easy performing, simple reaction operation, convenient time and environmental protection, and is suitable for industrial production.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Pharmaceutical composition containing dexchlorpheniramine and preparation thereof
The invention discloses a combination containing r-chlorpheniramine, which is formed by the r-chlorpheniramine and the medicine salt and the other or a plurality of active ingredients or pharmaceutical carriers selected from non-steroidal anti-inflammatory drug, ephedrine alkaloids, coffeine, dextromethorphan hydrobromide, carbetapentane citrate, glyceryl guaiacolate, bromhexine hydrochloride, artificial cow-bezoar, amantadine hydrochloride, aminophylline and zinc gluconate. The orally taken preparation developed from the combination comprises granule, tablet, capsule, dispersible tablet, chewable tablet, effervescent tablet, orally disintegrating tablet, buccal tablet, dry suspension and certain solid formulation.
Owner:FUKANGREN BIO PHARMA
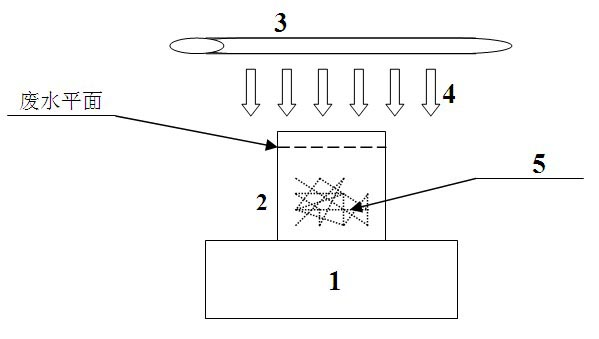
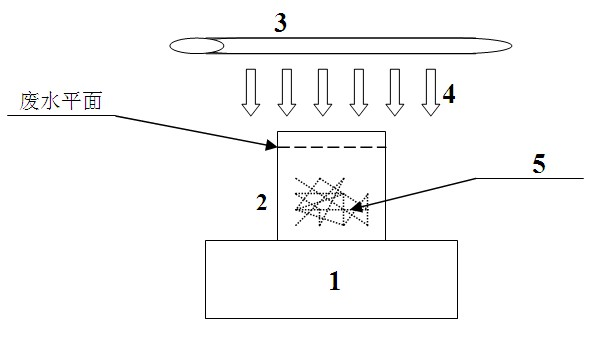
Method for treating amantadine hydrochloride-containing wastewater by using titanium-containing blast furnace slag as photocatalyst
InactiveCN102442712ATake advantage ofReasonable useWater/sewage treatment by irradiationWater/sewage treatment by oxidationSlagSample water
The invention belongs to the technical field of sewage treatment and relates to a method of treating amantadine hydrochloride-containing wastewater by using titanium-containing blast furnace slag as a photocatalyst. The method is used to solve high COD and hardly-biodegraded problems of amantadine hydrochloride-containing wastewater. The main technical scheme comprises the following steps of: placing an amantadine hydrochloride-containing wastewater sample with the concentration of 6000mg / L into a photocatalytic oxidation reactor with the volume of 100mL, adjusting the pH value of wastewater to 10-12, using the titanium-containing blast furnace slag as a photocatalyst while its addition concentration is controlled within 0.6-1.2g / L, the ultraviolet radiation time is controlled within 12-16h and the highest water surface distance from the ultraviolet radiation to simulation wastewater is controlled within 1-3cm, reacting and performing timing-sampling. It is proved through tests that titanium-containing blast furnace slag, as a photocatalyst, has a certain effect of treating amantadine hydrochloride-containing wastewater. The amantadine hydrochloride-containing wastewater is effectively degraded and simultaneously a new approach to reutilizing titanium-containing blast furnace slag is also provided so as to achieve the purpose of reutilizing solid waste and treating polluted wastewater.
Owner:SHENYANG JIANZHU UNIVERSITY
Medicine for giving up drug
InactiveCN101147741AGood inhibitory effectLow costNervous disorderPeptide/protein ingredientsGamma-Aminobutyric acidBENSERAZIDE HYDROCHLORIDE
The present invention relates to a new-type drug-stopping medicine. Its raw material composition includes (by wt%) 0.01%-30% of amantadine hydrochloride or trihexyphenidyl hydrochloride or benserazide hydrochloride or memantine bydrochloride, 99.009%-65% of gamma-aminobutyric acid A receptor agonist raw material medicine or / and gamma-aminobutyric acid B receptor agonist raw material medicine, and the rest is auxiliary material. Said drug-stopping medicine can be made into various conventional dosage forms of tablet, capsule, granules, power, dripping pills and others.
Owner:袁才尉
Jinganmin medicinal preparation and its preparation method
InactiveCN1823868ARaise pain thresholdGood analgesic effectAntibacterial agentsAntiviralsAspirinChlorogenic acid
A medicine Jinganmin with higher curative effect is prepared from aspirin, verigyt hydrochloride, coffin, chlorpheniramine, artificial bezoar and the extracts of honeysuckle flower and isatis leaf. Its preparing process is also disclosed.
Owner:博安兄弟制药(中国)有限公司
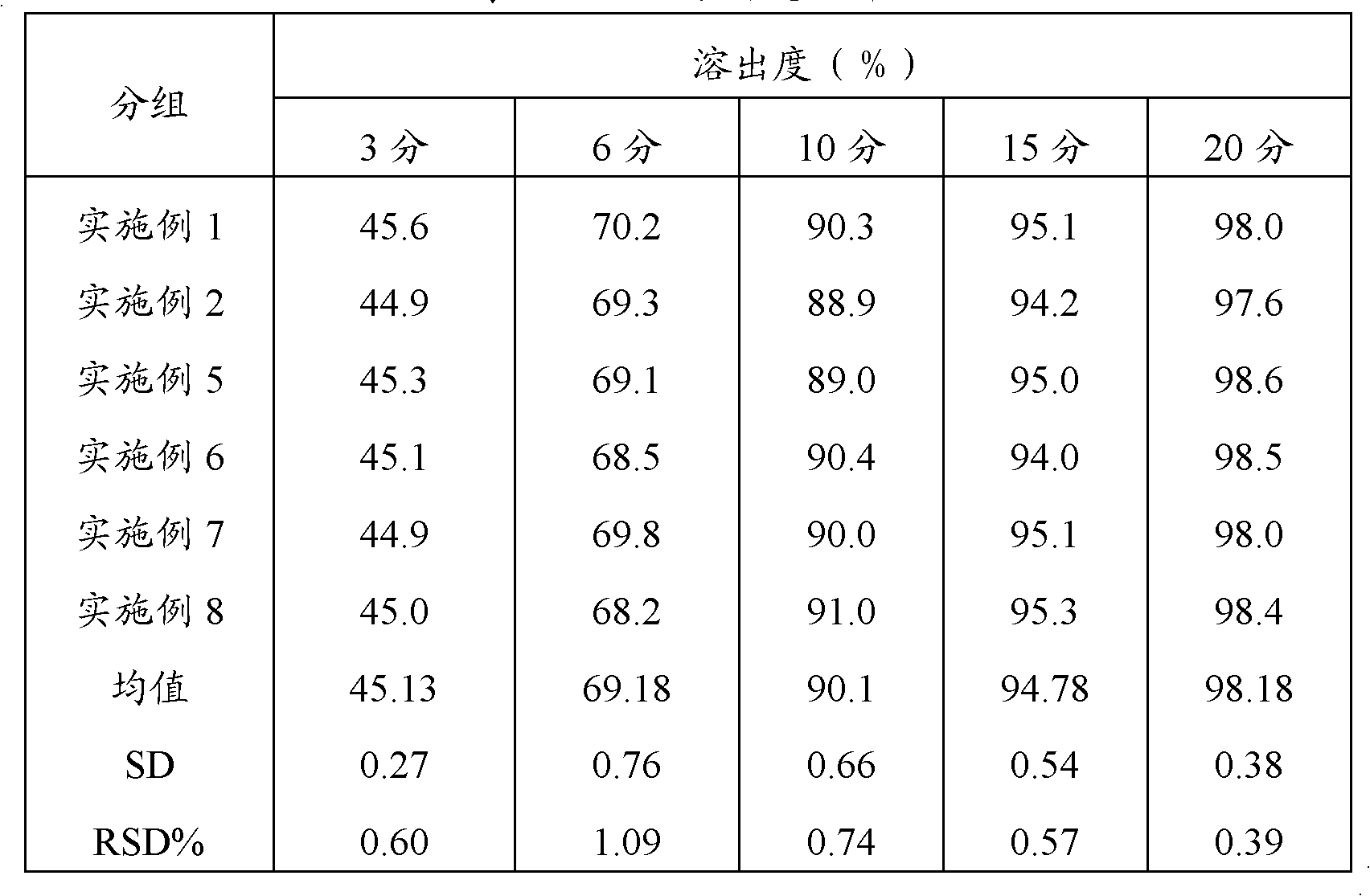
Method for detecting releasing rate of amantadine hydrochloride sustained release tablets
InactiveCN104237407AOvercoming Hard-to-Detect ShortcomingsHigh detection sensitivityComponent separationMemantine HydrochloridePhotochemistry
The invention discloses a method for detecting the releasing rate of amantadine hydrochloride sustained release tablets. The method comprises the steps of under chromatographic conditions that a high performance liquid chromatograph-evaporative light-scattering detector is adopted and methyl alcohol-acetonitrile-isopropanol-aqueous solution (in a ratio of 50:20:20:10) is taken as a flowing phase, processing six units of amantadine hydrochloride sustained release tablets with 100ml of 0.1mol / L hydrochloric acid solution as a releasing medium at a rotating speed of 75r / min according to a dissolution rate measurement method, taking 5ml of solution after one hour, two hours, four hours and eight hours respectively, filtering the extracted solution while supplementing the solution by 5ml in time, and measuring subsequent filtrate to obtain a test solution; adding water into a contrast to prepare a 0.1mg / ml solution serving as a contrast solution; and recording chromatogram maps of the two solutions, wherein the volume of each solution is 20 microliter. According to the releasing rate detection method, the defect that memantine hydrochloride with a relatively stable structure cannot be effectively detected as being free of absorption in an ultraviolet region is overcome; under a simple flowing phase condition, the dissolving rate of memantine hydrochloride can be accurately detected; the releasing rate detection method has the advantages of simplicity, quickness and accuracy.
Owner:SHANDONG INST OF PHARMA IND
Synthesis method for Amantadine Hydrochloride
ActiveCN102050744AAvoid it happening againReduce the severity of the reactionOrganic compound preparationAmino compound preparationIsomerizationSynthesis methods
The invention discloses a synthesis method for Amantadine Hydrochloride, which comprises the steps of hydrogenation, isomerization and amination, hydrolysis, refining, etc. to get Amantadine Hydrochloride finished product. Compared with the prior art, Trichloro ethane is adopted as solvent in the isomerization and amination steps, and bromination is cancelled, so that atmospheric pollution is lessened remarkably, and environmental pollution in the traditional process in Amantadine Hydrochloride synthesis is effectively solved. The invention has the advantages of no pollution, environmental friendliness, and better market application prospect.
Owner:GUIZHOU MAQIKA PHARMA
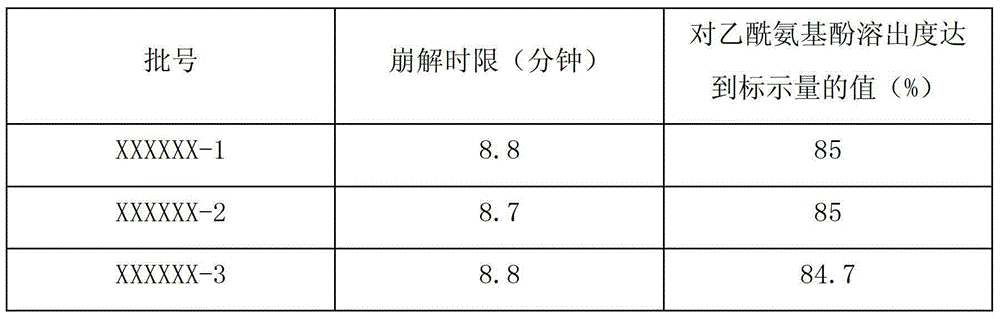
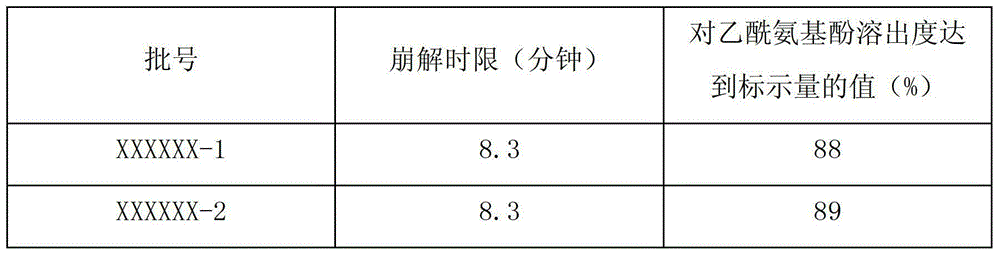
Quality testing method for compound paracetamol and amantadine hydrochloride granules
InactiveCN102879517AQuality improvementGuaranteed clinical efficacyComponent separationClinical efficacyDissolution
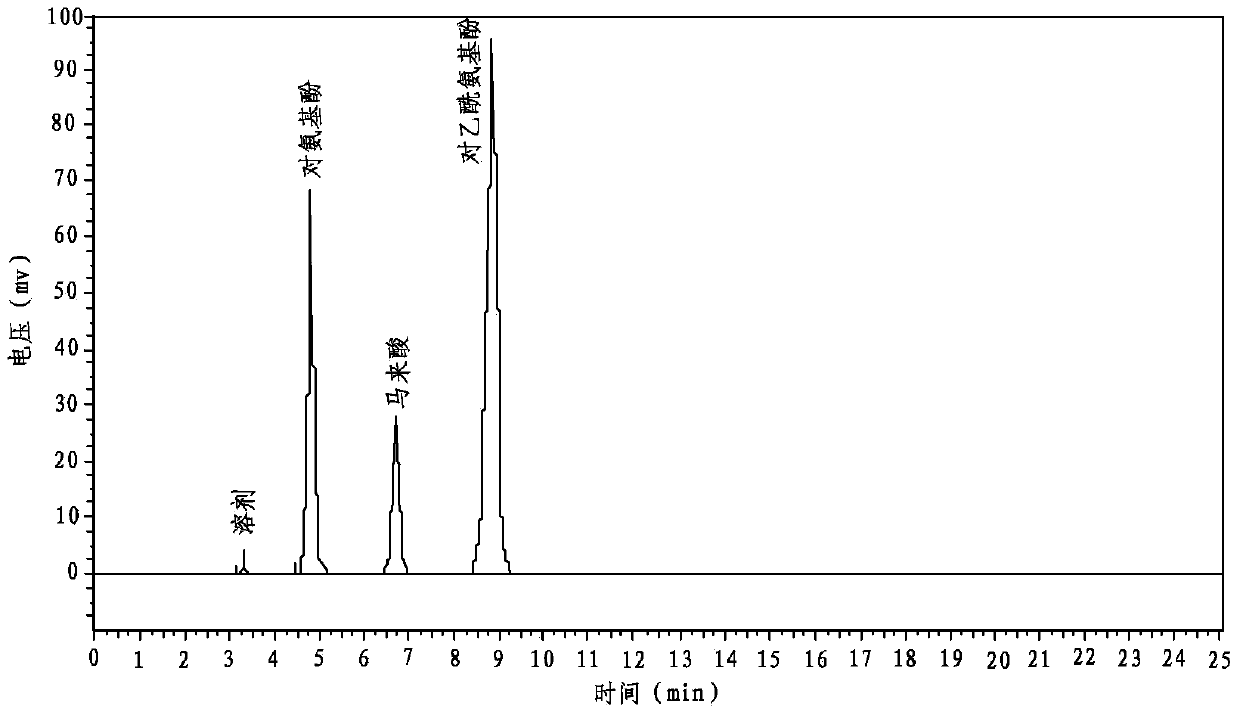
The invention discloses a quality testing method for compound paracetamol and amantadine hydrochloride granules. The method mainly comprises the steps of appraising the quality of the compound paracetamol and amantadine hydrochloride granules by using a thin layer chromatography method, performing content uniformity measuring and content measuring for acetaminophen, amantadine hydrochloride, caffeine, chlorpheniramine maleate and artificial bezoar by using a liquid chromatography method, measuring the dissolution rate, and measuring the content of bilirubin to achieve the artificial bezoar content measuring. The quality testing method of micro samples of the compound paracetamol and amantadine hydrochloride granules is built, researches on appraisal, content measuring and content uniformity measuring of the compound paracetamol and amantadine hydrochloride granules are achieved, the accuracy is high, good in reproducibility, and the quality of the compound paracetamol and amantadine hydrochloride granules can be controlled comprehensively and effectively, so that clinical effects of the preparation are guaranteed.
Owner:葵花药业集团(衡水)得菲尔有限公司
Method for preparing 'youkadan' granule
InactiveCN101057865AGuaranteed content uniformityAvoid adverse reactionsAntipyreticAnalgesicsCommon coldGallstones
The invention discloses a process for preparing pediatric paracetamol and amantadine hydrochloride granules for treating children's common cold, wherein each unit of the preparation comprises Paracetamol 100mg, amantadine hydrochloride 40mg, artificial ox gallstone 4mg, caffeine 6mg and chlorpheniramine maleate 0. 8mg.
Owner:杨文龙
An effervescence tablet of cold-treating preparation and preparation method thereof
InactiveCN1883507AGood molding effectGood effervescent effectUnknown materialsAntiinfectivesCommon coldEffervescent tablet
The invention relates to effervescent tablet for treating common cold which has good taste and rapid release speed, as well as the process for preparation, wherein the tablet comprises active constituents and effervescent materials, the active constituents include acetaminophen panadol, amantadine hydrochloride, caffeine, artificial ox gallstone and chlorpheniramine.
Owner:SUNSTONE TANGSHAN PHARM CO LTD
Process for high-yield preparation of amantadine hydrochloride
InactiveCN105523942AReduce harmEnvironment-friendlyOrganic compound preparationCarboxylic acid amides preparationTwo stepAmmonia
The invention discloses a process for high-yield preparation of amantadine hydrochloride; the process includes two steps of (1) preparation of 1-acetamidodamantane and (2) preparation of amantadine hydrochloride. Amantadine hydrochloride can be prepared in one reaction kettle, so the operation steps are simplified; in addition, by using a phase transfer catalyst, the reaction effect is promoted; with a selected solvent system after screening, the reaction conversion rate and yield are increased, and costs, energy consumption and environmental pollution are reduced.
Owner:天津民祥生物医药股份有限公司
Synthesis method of SSZ-13 molecular sieve
InactiveCN109534354AEasy to handleImprove processing qualityMolecular sieve catalystsMolecular sieve catalystAluminium hydroxideSynthesis methods
The invention relates to a synthesis method of a SSZ-13 molecular sieve. The synthesis method includes: 1) adding an inorganic alkali solution to an aluminum source solution to prepare an Al(OH)3 precipitate, and press-filtering and washing the Al(OH)3 precipitate to prepare an Al(OH)3 precursor; 2) adding the Al(OH)3 precursor into pure water to make slurry, ultrasonically dispersing the slurry,adding a template agent, amantadine hydrochloride, and continuously performing ultrasonic dispersion to form an Al(OH)3 colloid, concentration being 0.1-0.7 mol / L; 3) under intensive stirring, addingan inorganic alkali solution, a template agent N,N,N-trimethyl-1-adamantyl amine hydroxide, a SSZ-13 molecular sieve seed crystal, and a silicon source, performing intensive stirring to uniform mixing, thereby preparing sol, solid content being 20-40%; 4) crystallizing the sol at 150-200 DEG C for 20-60 h; 5) washing, drying and roasting the product. The synthesis method achieves controllability on particle size and appearance of the SSZ-13 molecular sieve.
Owner:SHANDONG SINOCERA FUNCTIONAL MATERIAL CO LTD
Process for preparing amantadine hydrochloride with high yield
InactiveCN105348111AReduce harmEnvironment-friendlyOrganic compound preparationCarboxylic acid amides preparationAmmoniaPhase-transfer catalyst
The invention discloses a process for preparing amantadine hydrochloride with high yield. The process comprises the steps of (1) preparing 1-acetamidoadamantane; and (2) preparing amantadine hydrochloride. By using the process, amantadine hydrochloride is prepared in one reaction kettle, so that the operation step is simplified; in addition, the reaction effect is promoted due to the adoption of a phase-transfer catalyst, the conversion rate and yield of the reaction can be increased due to the adoption of a solvent system selected after screening, and the cost, energy consumption and environment pollution are reduced.
Owner:天津民祥生物医药股份有限公司
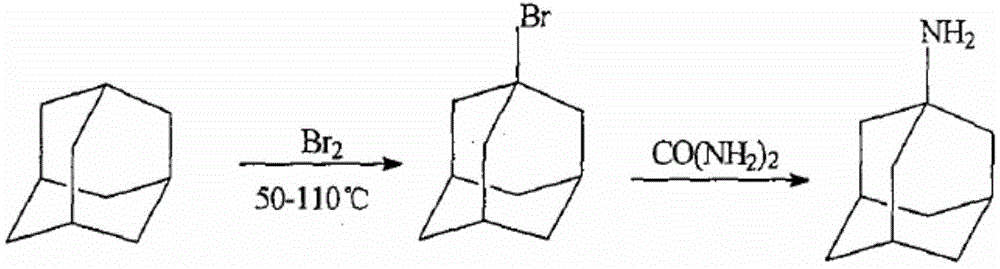
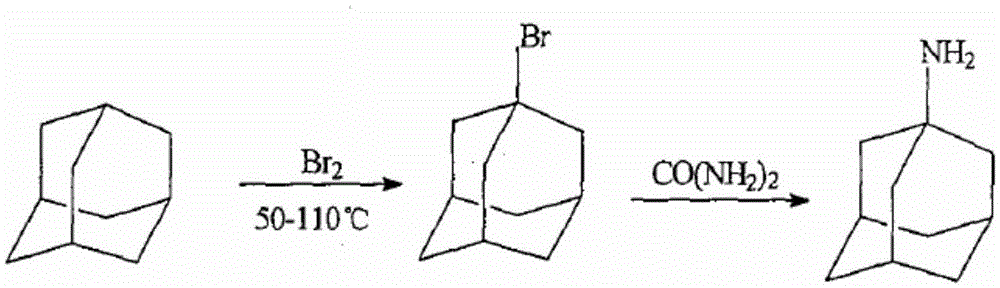
Method for extracting admantadine
InactiveCN101429129AHigh yieldHigh purityAmino preparation by functional substitutionWater insolubleWater soluble
The invention discloses a method for extracting amantadine, which aims to provide a method for extracting amantadine by a chemical means. The method has the advantages of simple technological process, little needed equipment and energy consumption, high yield, high purity of obtained amantadine and good quality. The invention adopts a technical proposal that after bromoadamantane and urea react to form water-insoluble amantadine, the amantadine is extracted according to the following steps: A. excessive hydrochloric acid is added to resultant, and the amantadine reacts with the hydrochloric acid to form water-soluble amantadine hydrochloride; and B. water-insoluble impurities are filtered and removed; excessive sodium hydroxide solution is added to residual solution; the amantadine hydrochloride and sodium hydroxide react to form the water-insoluble amantadine again; and the amantadine is filtered out. Due to the technical proposal of the invention, the amantadine yield of the method is higher than that of the prior process by more than 8 percent, and the purity of the obtained amantadine is more than 60 percent.
Owner:庞明
Pravastatin sodium pharmaceutical co-crystal and preparation method and application thereof
ActiveCN105175263AIncrease spawn rateEasy to moveUrea derivatives preparationAmino compound purification/separationMoisture absorptionLysine
The invention belongs to the technical field of organic pharmaceutical co-crystals, and particularly relates to a pravastatin sodium pharmaceutical co-crystal and a preparation method and application thereof. The pravastatin sodium pharmaceutical co-crystal comprises co-crystal formations and active components pravastatin sodium. The co-crystal formations are amino acid, urea, adamantine derivatives or other small molecule compounds. The result shows that before and after the co-crystal is formed, the moisture absorption performance of medicine is remarkably changed, for example, the co-crystal hygroscopicity of lysine and urea is increased, and a co-crystal formed by amantadine hydrochloride basically does not absorb moisture at the humidity of 70% or lower.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Manufacturing technique of amantadine hydrochloride and special equipment thereof
InactiveCN101560157AAvoid pollutionReduce in quantityAmino compound purification/separationChemical/physical/physico-chemical stationary reactorsBiochemical engineeringCoupling
The invention relates to a manufacturing technique of amantadine hydrochloride, which comprises: after being processed by decarburization and filtered, amantadine hydrochloride water solution is added into a tank to be concentrated; after the material in the tank is added with water and cooled, the remained water in the material is pressed out by a filter board; acetone is added into the tank to stand still for soaking the material; the tank is pressurized, and the acetone is pressed out by compressed air; a jacket enters a steam rotary tank and is dried; and the amantadine hydrochloride in the tank is discharged out. Special equipment of the amantadine hydrochloride comprise: a stirring motor arranged on the tank, an agitating vane arranged inside the tank, and a shaft coupling connected with the upper end of the agitating vane; wherein, axle mechanical seal is adopted between the agitating vane and the tank; the upper part of the tank is provided with a man-hole opening, a viewing mirror hole, a temperature measuring hole, a stirring hole and a feeding hole; the lower end of the tank is provided with a sintering filter board, and silica gel ring seal is adopted between the tank and the filter board as well as a cover board. The technique leads the whole process flow of reaction, concentration or crystallization, washing, filtering and drying to be completed in a device in a sealing way, thus improving the safety of production and avoiding the pollution of materials.
Owner:王彦明
Method for preparing Jingan capsules
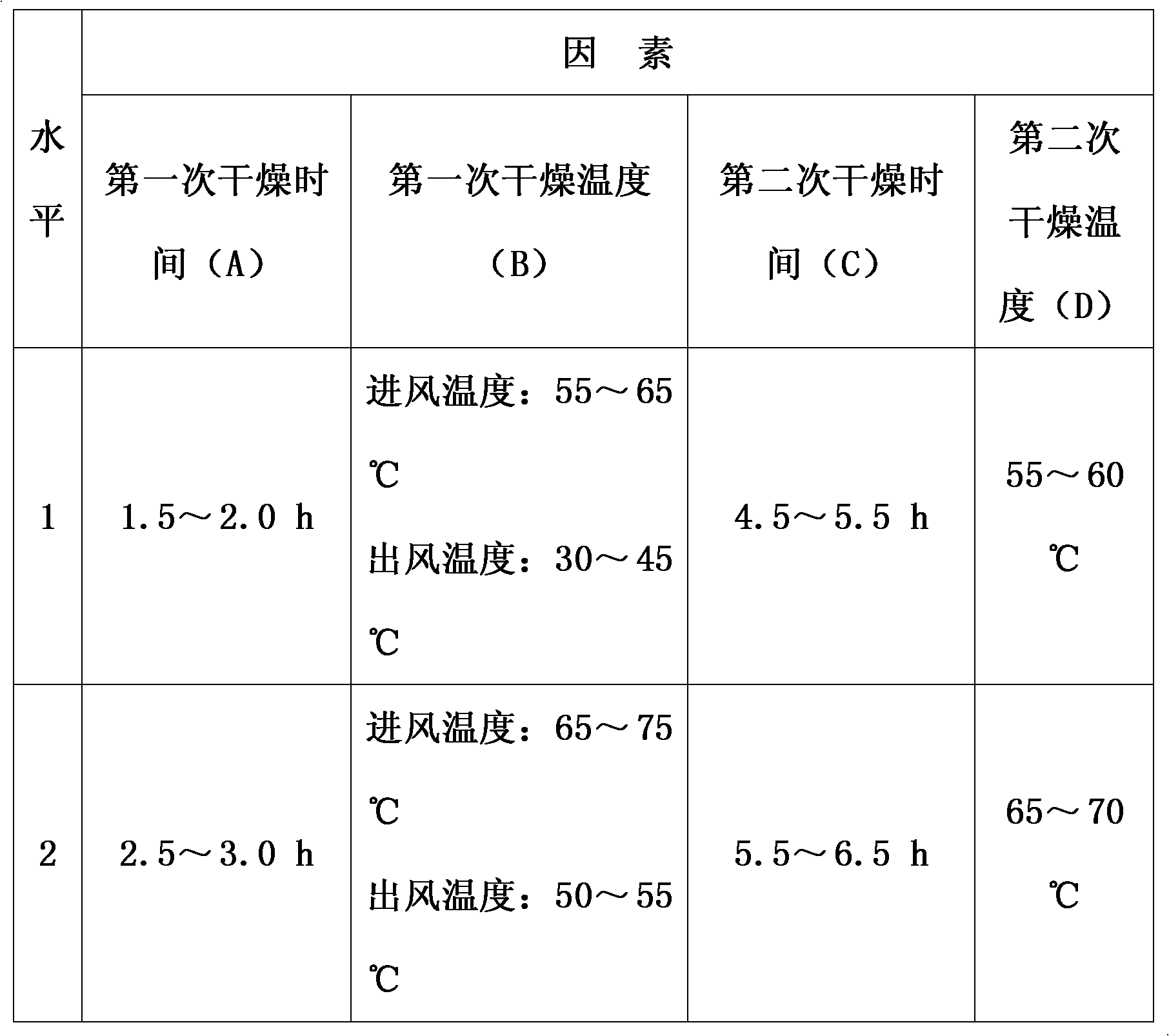
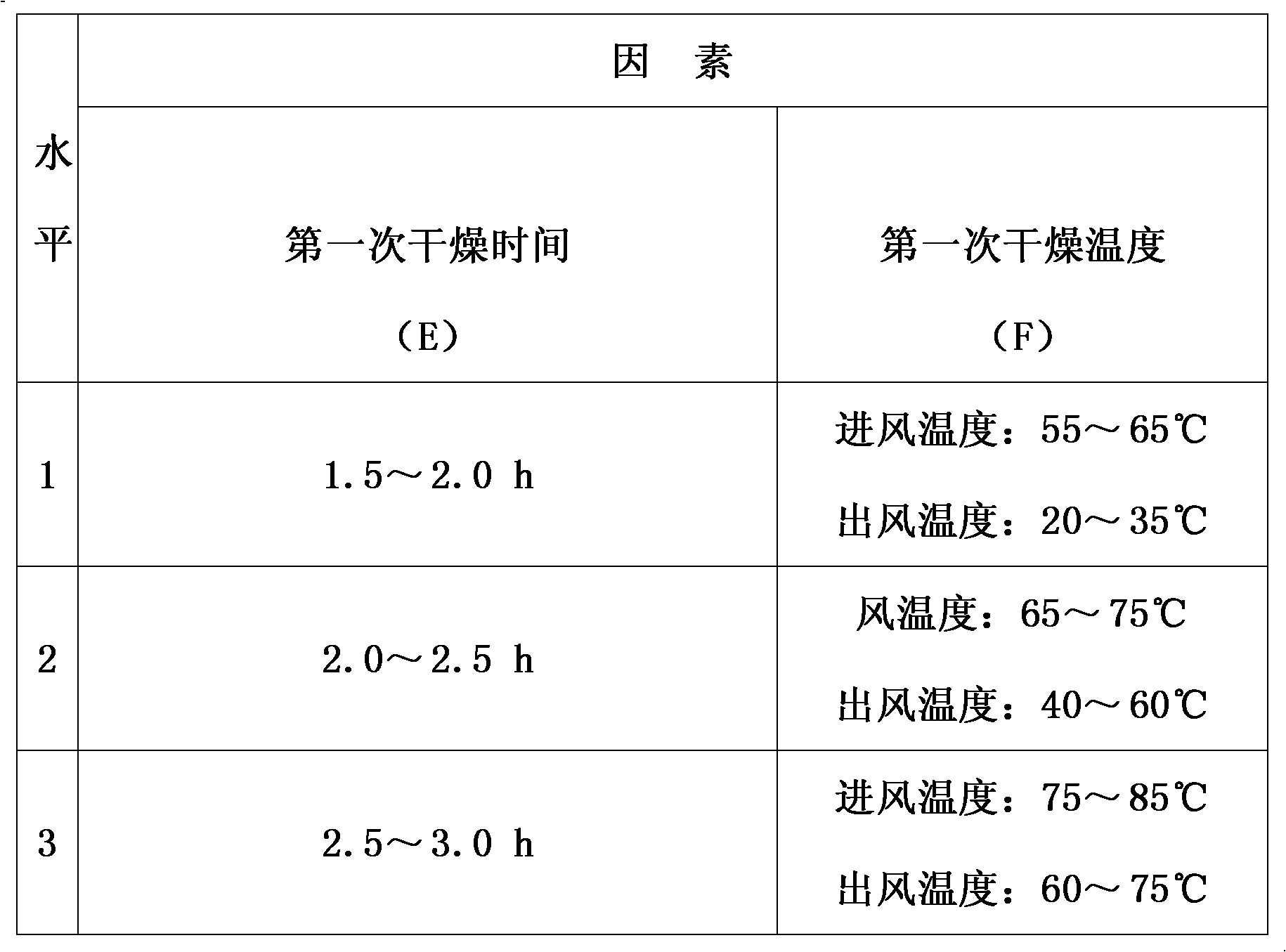
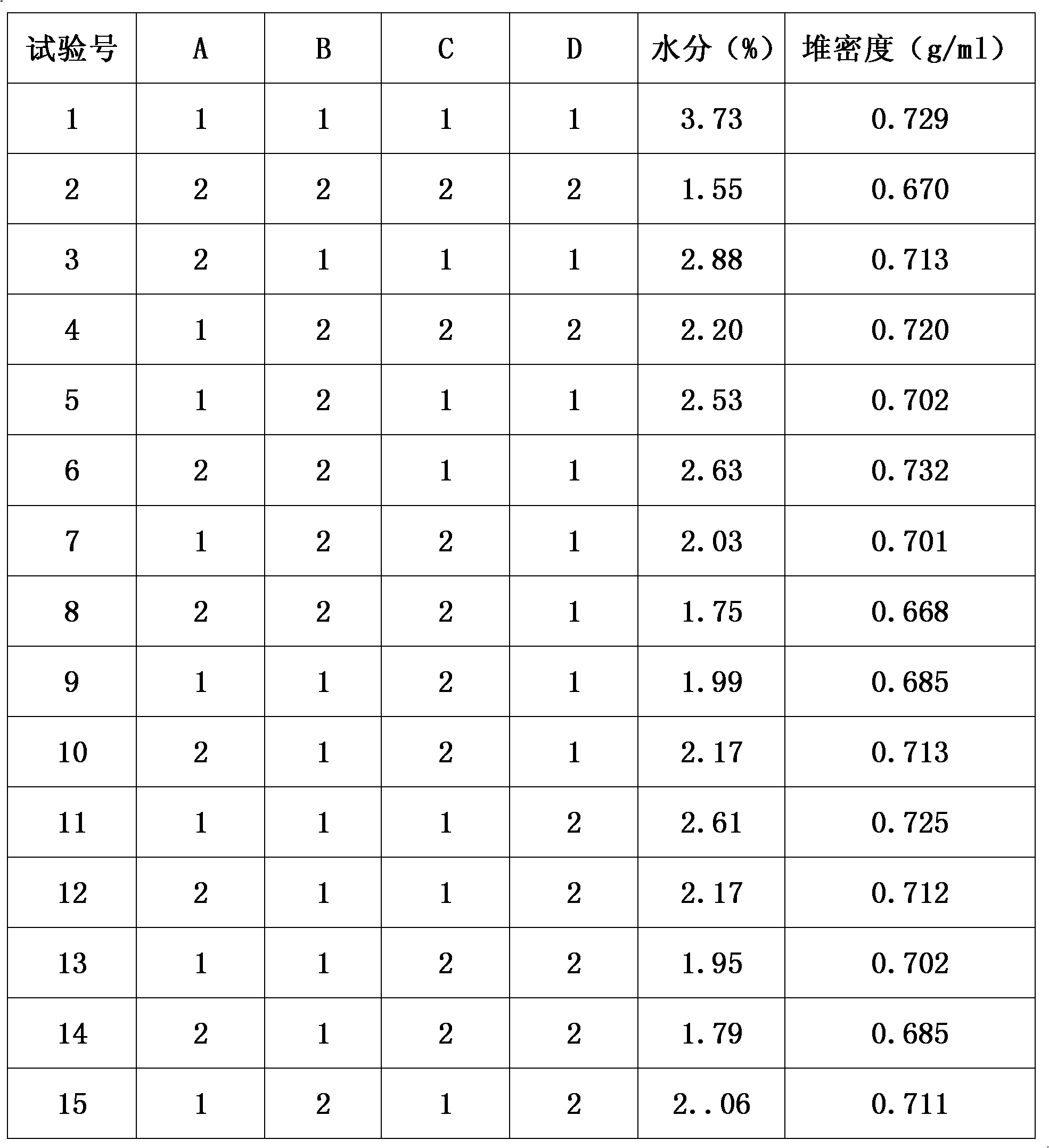
The invention discloses a method for preparing Jingan capsules. The Jingan capsules are prepared from honeysuckle, common andrographis herb, indigowoad root, dandelion, acetaminophen, amantadinehydrochloride and chlorphenamine maleate. Adopting a secondary drying method during preparation, the method completes drying operation quickly at a low temperature that does not causes component change, makes the particles uniformly dried and the water content reach a standard, makes the particles reach required standards in both properties and various quantized indexes under the condition of ensuring high compactness of the particles of the capsules and meet the requirements of a subsequent process procedure, and ensures the overall quality of the products.
Owner:GUIZHOU BAILING GRP PARMACEUTIAL CO LTD
Soluble hydrofluoride pesticide comprehensive formula
InactiveCN104273171ASignificantly restorative growthNo drug resistanceBiocideDisinfectantsPlant hormone1-adamantanamine
The invention belongs to a pesticide preparation and particularly relates to a soluble hydrofluoride pesticide comprehensive formula. The soluble hydrofluoride pesticide comprehensive formula is composed of rimantadine hydrochloride, 1-adamantanamine hydrochloride, plant hormones and soluble hydrofluoride. According to the soluble hydrofluoride pesticide comprehensive formula, rimantadine hydrochloride, 1-adamantanamine hydrochloride, cytokinin and soluble hydrofluoride are dissolved into clean water until the concentration of rimantadine hydrochloride is 0.00667-0.667 gram per 15 kilograms, the concentration of 1-adamantanamine hydrochloride is 0.00667-0.667 gram per 15 kilograms, and the concentration of fluorine ions is 0.00093mol-0.156mol per liter of solution or the concentration of the fluorine ions is 0.0071mol-1.19mol per liter of solution; and the plant hormones are of 1ppm-20ppm of cytokinin or 5ppm-30ppm of gibberellin or 0.1ppm-10ppm of brassica oleracea hormone lactone. A soluble hydrofluoride pesticide has no drug resistance, can be used for multiple times, has less use amount, strong compatibility, middle toxicity and no carcinogenicity, and is safe to use, economical and convenient, convenient to process and low in cost.
Owner:陈本建
Method for using amantadine hydrochloride drug to culture lily under virus-free state
InactiveCN102440188AHigh detoxification efficiencyShorten the timeMicrobiological testing/measurementHorticulture methodsConcentration gradientBud
The invention discloses a method for using an amantadine hydrochloride drug to culture lily under a virus-free state and belongs to the fields of plant tissue culturing fast propagation technique and biologic technique. The method comprises the following steps of: inspecting lily mother bulb virus, preparing a culture medium and reserving, sterilizing and inoculating explants, culturing the explants, inspecting virus of tissue culturing seedlings, and the like, thereby quickly obtaining lots of tissue culturing seedlings. According to an orthogonal method, a formula of the best induced culture medium of buds and root-less tissue culturing seedlings directly differentiated from lily is obtained; an amantadine hydrochloride antiviral drug with a series of concentration gradients is prepared and an optimum detoxifying concentration is selected, so that the detoxifying efficiency can reach 87%; a period of induced culturing is shortened, and meanwhile, the detoxifying efficiency is high and the enhancement factor is high; and a technical basis for researching on the culturing for lily virus-free plants and the popularization for virus-free culturing is provided.
Owner:杨凯 +2
Preparation method of quickly-releasing compounded paracetamol and amantadine hydrochloride tablet
InactiveCN104415054APromotes quick releaseGood dispersionUnknown materialsPill deliveryMedicineDissolution
The invention provides a preparation method of a quickly-releasing compounded paracetamol and amantadine hydrochloride tablet. The preparation method mainly comprises following steps: pulverization, mixing, granulation, granule drying, granule shaping, totally blending and tablet pressing. In the steps of the pulverization, the mixing and the granulation, a preparation technology of a solid dispersing body is employed, wherein a carrier is an indissolvable hydrophilic material. After pulverizing the raw materials and the auxiliary materials into a certain degree, the raw materials and the auxiliary materials are dispersed with the carrier uniformly to prepare the solid dispersing body, and then the solid dispersing body is subjected to other processes and finally is subjected to the tablet pressing to obtain the tablet, which can achieve a good quickly-releasing effect with a dissolution rate being not less than 82% which is indicated. The invention has significant clinical significance in treatment of cold patients.
Owner:哈药集团人民同泰医药股份有限公司
SSZ-13 molecular sieve and preparation method thereof
The invention relates to a preparation method of an SSZ-13 molecular sieve. The preparation method comprises the following steps: 1) dissolving template agents, namely, amantadine hydrochloride and barium hydroxide in water, and adding the template agent N, N, N-trimethyl-1-ammonium adamantane for stirring and mixing, adding a strong alkali solution for stirring and mixing, and adding silica sol for stirring and mixing to obtain a mixed solution A; 2) dissolving an aluminum source in water to prepare an aluminum source solution, adding the aluminum source solution to the mixed solution A prepared in the step 1), and performing stirring to prepare a mixed solution B; 3) crystallizing the mixed solution B, and performing suction filtering, washing, drying and roasting to obtain a molecular sieve sample; 4) adding the molecular sieve sample to an ion exchanger aqueous solution for ion exchange, performing suction filtration, washing and drying, and repeating ion exchange, suction filtration, washing, drying and roasting to obtain the SSZ-13 molecular sieve. The SSZ-13 molecular sieve is synthesized with a composite template agent method, nontoxic amantadine hydrochloride is used as asecond template agent, and the preparation method is safe and environmentally friendly.
Owner:REZEL CATALYSTS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com