Preparation method of quickly-releasing compounded paracetamol and amantadine hydrochloride tablet
A compound paracetamol tablet and immediate-release technology, which can be applied to medical preparations containing active ingredients, pharmaceutical formulations, amine active ingredients, etc., can solve problems such as limited effects, and achieve good immediate-release effects and great clinical significance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
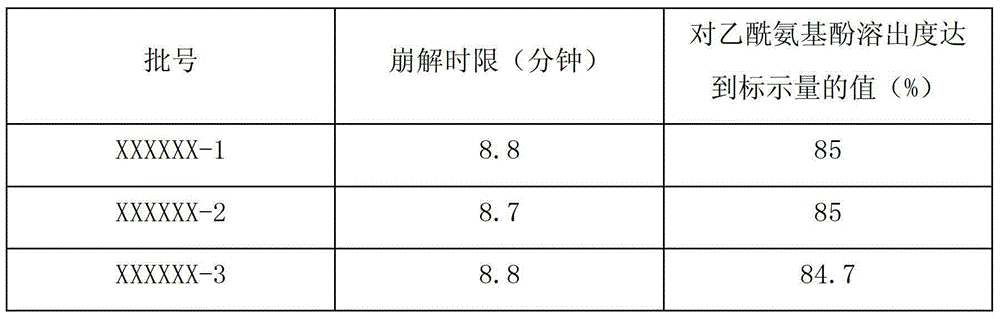
[0018] 1. Pulverize all the raw and auxiliary materials and pass through a 100-mesh sieve, in which silicon dioxide passes through a 50-mesh sieve;
[0019] 2. Mix 15 parts by weight of caffeine, 2 parts by weight of chlorpheniramine maleate and 10 parts by weight of artificial bezoar. During the mixing process, the materials need to pass through a 50-mesh sieve for 3 times;
[0020] 3. Put 100 parts by weight of acetaminophen, 250 parts by weight of amantadine hydrochloride, pre-mixed materials, 40 parts by weight of silicon dioxide and 20 parts by weight of sodium carboxymethyl starch into a wet mixer, and mix for 5 minutes , then add the binder, mix for 3 minutes, and then granulate.
[0021] 4. Move the granules into the boiling dryer, control the temperature of the material at 30-80°C, ventilate and dry for 20-60 minutes, cool the granules to room temperature, carry out sizing, and then move the granules into the mixing machine, add 3 parts by weight of carboxyl Sodium s...
example 2
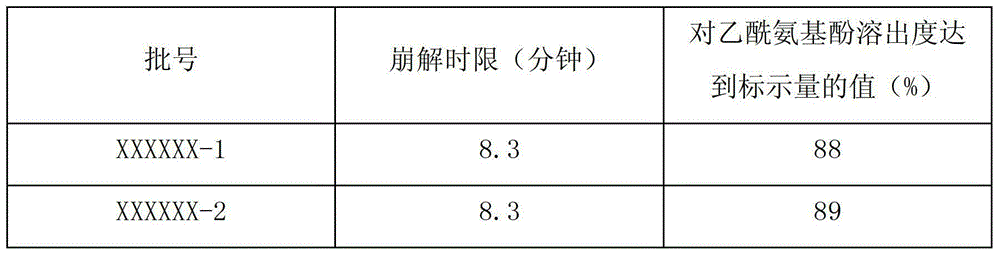
[0026] 1. Crush all the raw and auxiliary materials and pass through a 120-mesh sieve, among which calcium phosphate passes through a 80-mesh sieve.
[0027] 2. Mix 15 parts by weight of caffeine, 2 parts by weight of chlorpheniramine maleate and 10 parts by weight of artificial bezoar. During the mixing process, the materials need to pass through an 80-mesh sieve for 3 times;
[0028] 3. Put 100 parts by weight of acetaminophen, 250 parts by weight of amantadine hydrochloride, pre-mixed materials, 40 parts by weight of calcium phosphate and 30 parts by weight of sodium carboxymethyl starch into a wet mixer, and mix for 5 minutes , then add the binder, mix for 3 minutes, and then granulate.
[0029] 4. Move the granules into the boiling dryer, control the temperature of the material at 30-80°C, ventilate and dry for 20-60 minutes, cool the granules to room temperature, carry out sizing, and then move the granules into the mixing machine, add 3 parts by weight of carboxyl Sodi...
example 3
[0035] 1. Pulverize all the raw and auxiliary materials and pass through a 120-mesh sieve, in which silicon dioxide passes through a 80-mesh sieve.
[0036] 2. Mix 15 parts by weight of caffeine, 2 parts by weight of chlorpheniramine maleate and 10 parts by weight of artificial bezoar. During the mixing process, the materials need to pass through a 100-mesh sieve for 3 times;
[0037] 3. Put 100 parts by weight of acetaminophen, 250 parts by weight of amantadine hydrochloride, pre-mixed materials and 50 parts by weight of silicon dioxide and 50 parts by weight of sodium carboxymethyl starch into a wet mixer, and mix for 5 Minutes, then add the binder, mix for 3 minutes, and then granulate.
[0038] 4. Move the granules into the boiling dryer, control the temperature of the material at 70°C, ventilate and dry for 20-60 minutes, cool the granules to room temperature, carry out granulation, and then move the granules into the mixing machine, add 7 parts by weight of carboxymethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com