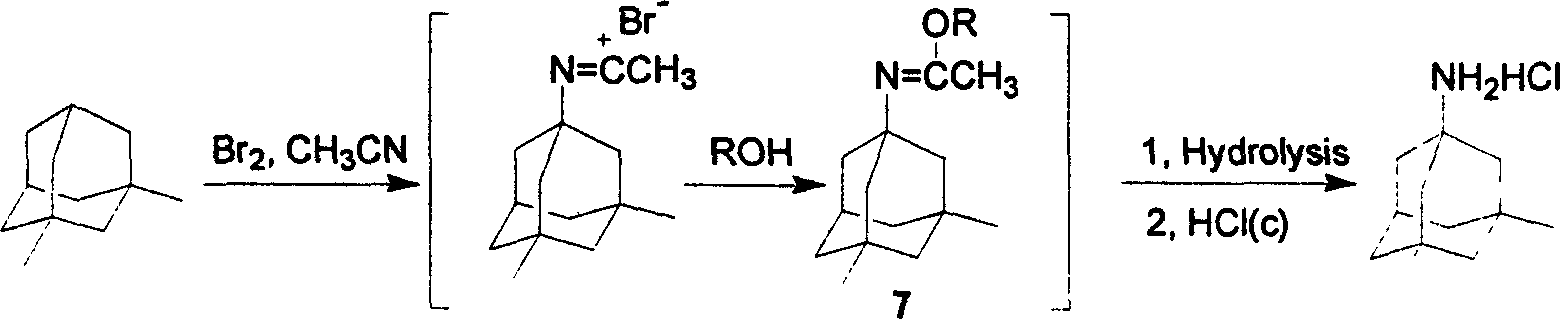
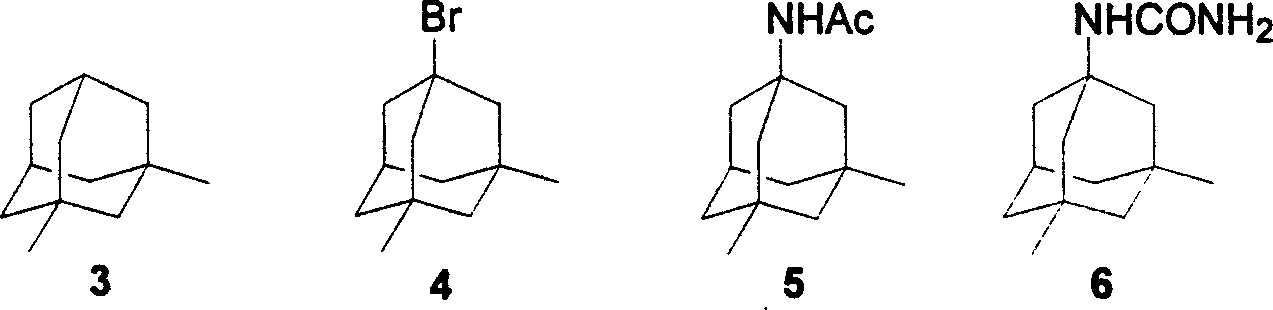Method of synthesizing amantadine hydrochloride
A technology for memantine hydrochloride and dimethyladamantane, which is applied in the field of synthesizing memantine hydrochloride, can solve problems such as large limitations and difficult to satisfy, and achieve the effects of less toxic and side effects and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0019] Embodiment: As above experimental procedure: the hydrolysis temperature of alkaline hydrolysis is preferably between room temperature and 50° C.; generally controlled at 30-50° C., and the hydrolysis time is 30 minutes to 90 minutes. When the temperature is low, the hydrolysis time is significantly prolonged.
[0020] Embodiment: as above-mentioned experimental procedure: the choice of the alkali that alkaline hydrolysis is used is very much: sodium hydroxide, potassium hydroxide are the most common, also can use calcium hydroxide, barium hydroxide and comprise lithium hydroxide, lithium carbonate, carbonic acid Sodium, potassium carbonate, cesium carbonate and other carbonates.
[0021] Example: As above experimental procedure: free memantine is generated after hydrolysis, and extracted from the water layer with a suitable aromatic hydrocarbon solvent, the solvent includes toluene, o-xylene, p-xylene, mixed xylene, etc. The combined organic layers were washed with wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com