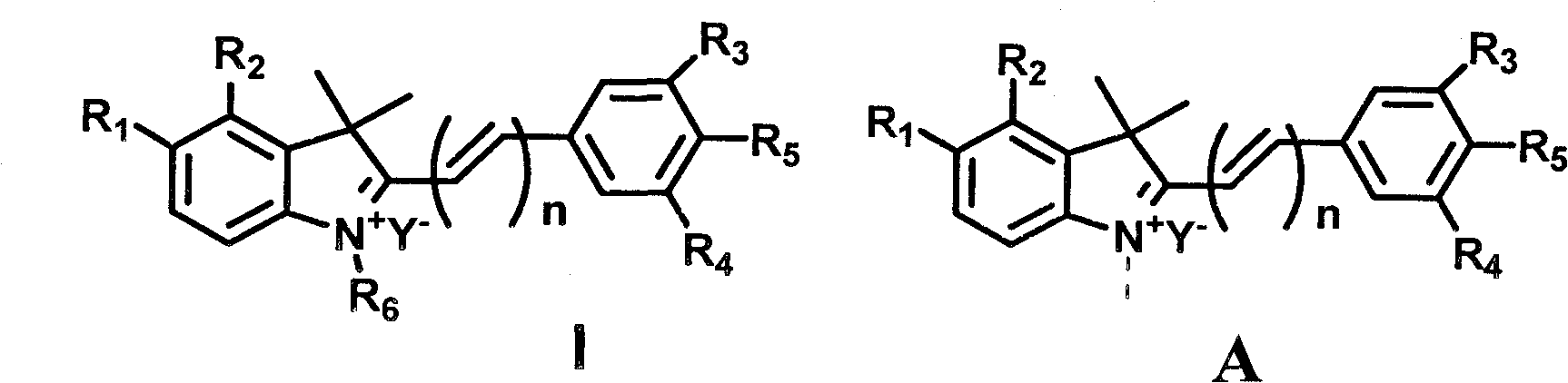
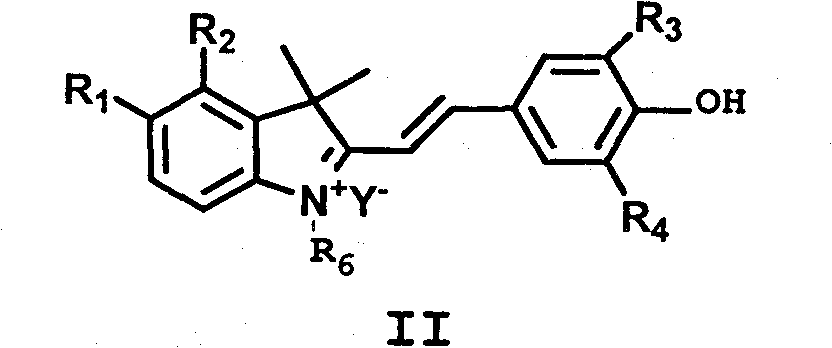
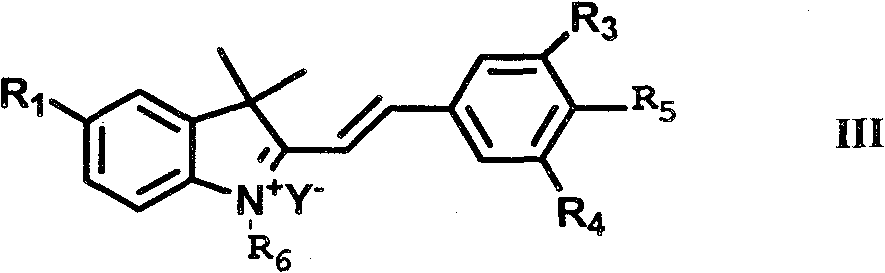
Synthesis and application of indole hemicyanine dye
A technology for indole and cyanine dyes, applied in the synthesis and application of indole semicyanine dyes, can solve problems such as hindering application, increasing the water solubility of dyes, reducing cell membrane permeability, etc. Fluorescence background interference, the effect of wide emission wavelength range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Synthesis of dye intermediate 1-methyl-5-nitro-2,3,3-trimethyl-3H indole quaternary ammonium salt:
[0098] Add 20mmol of 5-nitro-2,3,3-trimethyl-3Hindole and 40mmol of iodomethane into a 100ml round-bottomed flask containing 20ml of toluene under argon protection. The reaction was heated to reflux for 20 h and then stopped. After the mixture was cooled, the precipitate was filtered and the filter cake was washed with ether. After drying, a light yellow solid powder was obtained with a crude yield of 85%.
Embodiment 2
[0100] Preparation of Compound A:
[0101]
[0102] Add 5mmol 1-methyl-5-nitro-2,3,3-trimethyl-3H indole quaternary ammonium salt and 6mmol p-hydroxybenzaldehyde into a 25ml round bottom flask containing 10ml ethanol, nitrogen protection, room temperature After stirring for 24 h, the reaction solution was placed at -20 °C overnight. The precipitated solid was collected by filtration, rinsed with ethyl acetate, and then dried in vacuo to obtain an orange-yellow solid powder with a yield of 85%.
[0103] 1H NMR (400MHz, DMSO-d6, ppm): 1.87(s, 6H, C(CH3)2), 4.12(s, 3H, CH3-N+), 7.00(d, 2H, J=8.4, Ar-H) , 7.52(d, 1H, J=16, CH=CH), 8.06(d, 1H, J=8.8, Ar-H), 8.23(d, 2H, J=8.4, Ar-H), 8.50(d, 1H, J=8.8, Ar-H), 8.56(d, 1H, J=16, CH=CH), 8.83(s, 1H, Ar-H), 11.09(s, 1H, OH).13C NMR (100MHz , DMSO-d6, ppm): 25.45, 34.44, 52.02, 109.29, 115.36, 116.67, 118.63, 125.14, 126.09, 134.65, 144.20, 146.59, 147.00, 156.89, 154.39, 184.64. HRMS [M-I-]+323.1390, found 323.1394.
Embodiment 3
[0105] Preparation of Compound B:
[0106]
[0107] 5mmol 1-propargyl-2,3,3-trimethyl-3H indole quaternary ammonium salt and 5mmol 3-Br-4-hydroxybenzaldehyde were added to a 25ml round-bottomed flask containing 10ml methanol, nitrogen protection, After heating to reflux for 4 h, the reaction solution was placed at -20°C overnight. The precipitated solid was collected by filtration, rinsed with ethyl acetate, and then dried in vacuo to obtain an orange solid powder with a yield of 88%.
[0108]1H NMR (400MHz, CDCl3, ppm): 1.73(s, 6H, C(CH3)2), 3.31(t, 1H, J=2.38, Alkyne-H) 5.41(s, 2H, CH2-N+), 6.82( d, 2H, J=8.8, Ar-H), 7.19(d, 1H, J=15.6, CH=CH), 7.46(t, 1H, J=7.2, Ar-H), 7.54(t, 1H, J =7.6, Ar-H), 7.67 (d, 1H, J=8.0, Ar-H), 7.75 (d, 1H, J=7.2, Ar-H), 7.99 (d, 1H, J=8.8, Ar- H), 8.19 (d, 1H, J=15.6, CH=CH), 8.45 (s, 1H, Ar-H).13C NMR (100MHz, DMSO-d6, ppm): 26.09, 33.92, 51.16, 72.24, 81.35 , 113.8, 114.68, 119.45, 123.05, 125.24, 127.79, 129.14, 133.73, 137.06, 142.66,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com