Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51results about How to "Short doubling time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Formula of Chaetoceros muelleri medium and white plastic barrel aerated culture method
InactiveCN102851215AShort doubling timeIncrease productionUnicellular algaeMicroorganism based processesMarine diatomChaetoceros muelleri
The invention belongs to the field of marine microalgae cultivation, and relates to a formula of a Chaetoceros muelleri medium and a white plastic barrel aerated culture method. Chaetoceros muelleri is a small marine diatom, rich in polyunsaturated fatty acid and protein, and is a high-quality bait for raising sea treasures such as prawn and abalone. At present, an f / 2 culture medium formula used to produce Chaetoceros muelleri has problems of imbalanced and incomprehensive nutrients, slow growth, long multiplication time and low yield. ccording to experiments, nitrate is substituted by urea, which is used as a nitrogen source and supplemented by NaHCO3 and growth hormone, VB12 and VB1, so that the medium gains more comprehensive and balanced nutrients, and yield of Chaetoceros muelleri is increased by 87%. In addition, cement pool and glass cylinder barrel used for culture in current production have many disadvantages: easy pollution, difficult washing, inconvenient operation and easy cutting. Study in the invention shows that a white plastic barrel aerated culture method for culturing Chaetoceros muelleri has advantages of flexible operation, no pollution, easy washing, no cutting, low cost, high yield and large-scale promotion.
Owner:LINYI UNIVERSITY
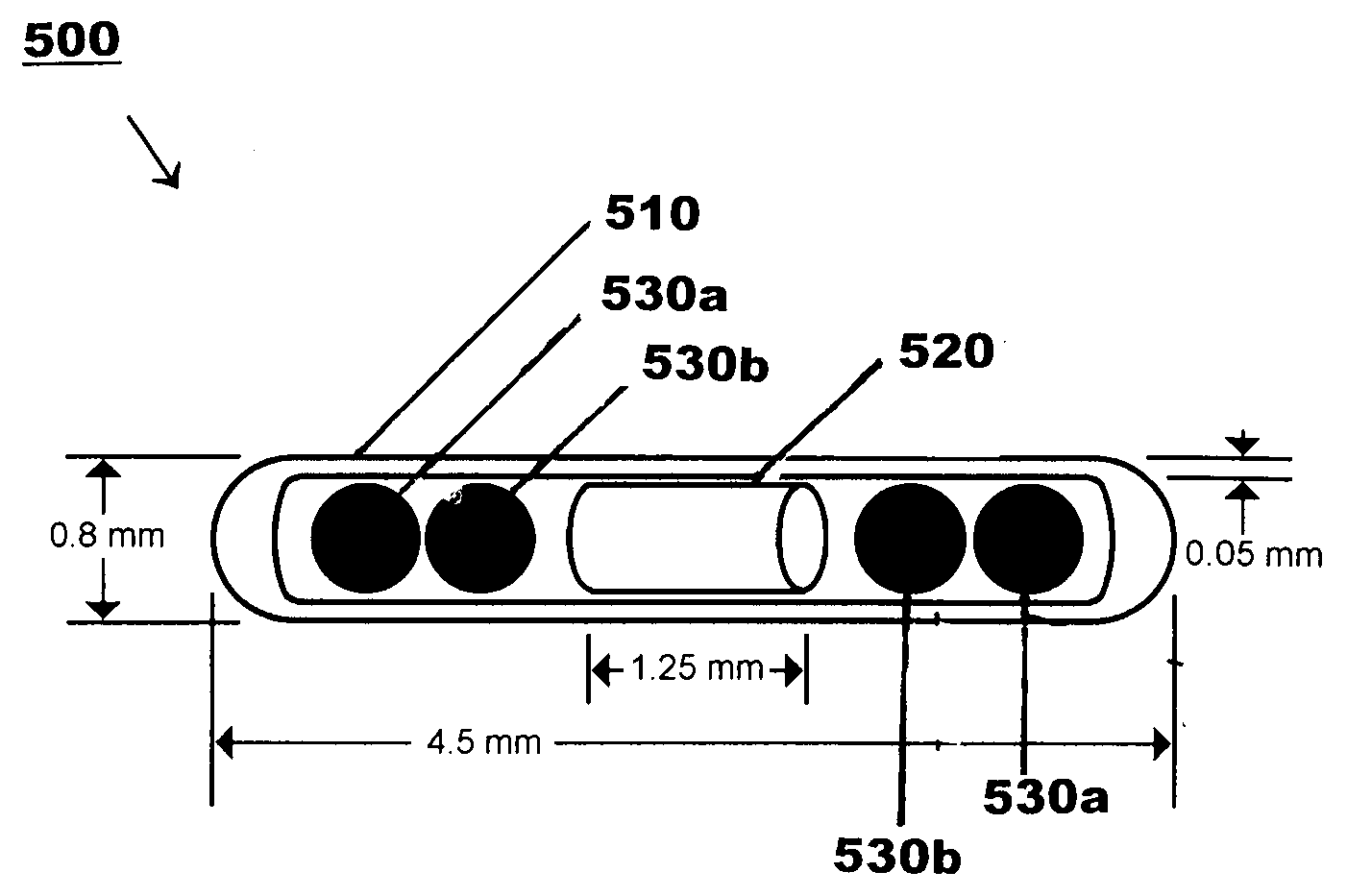
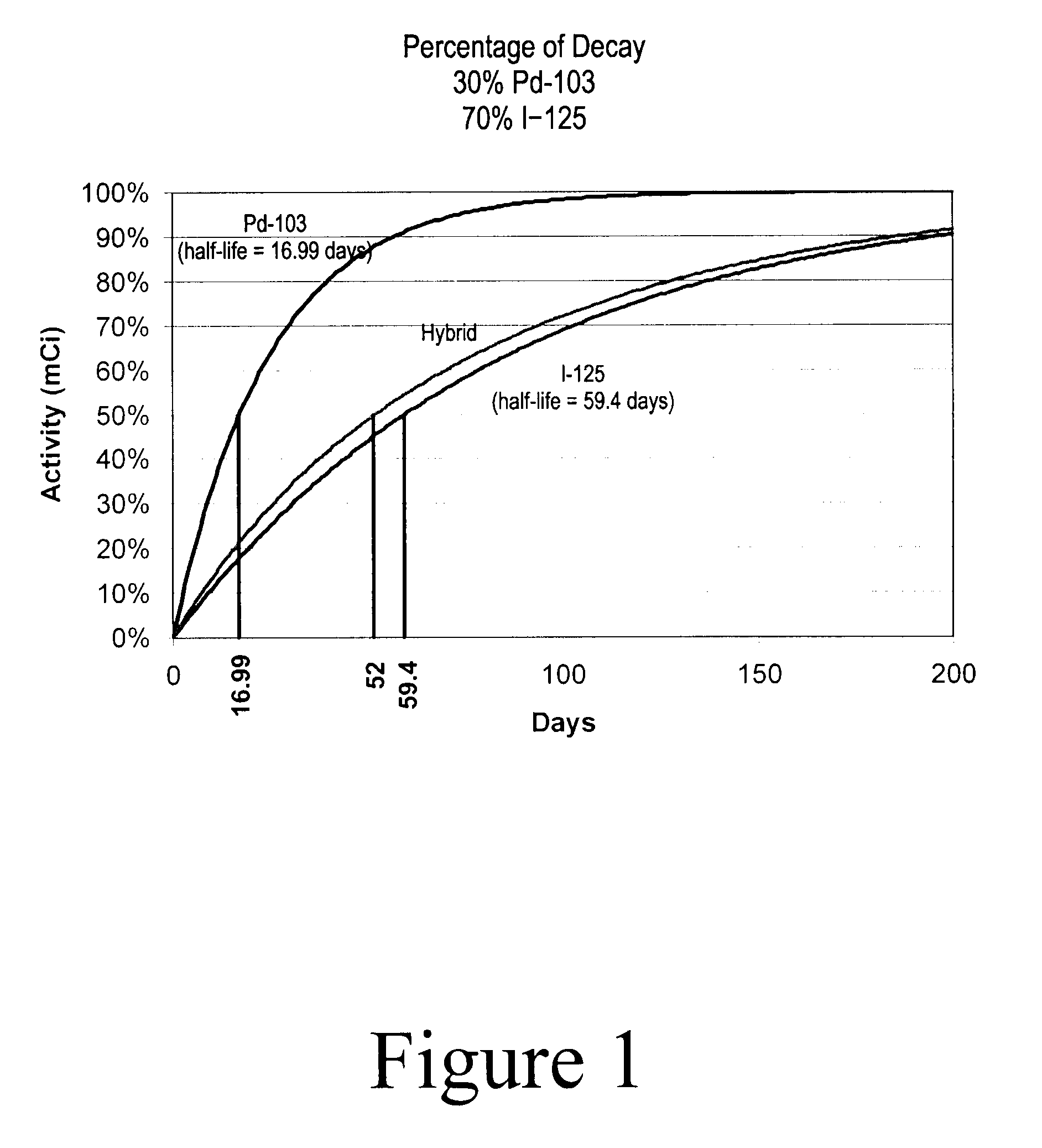
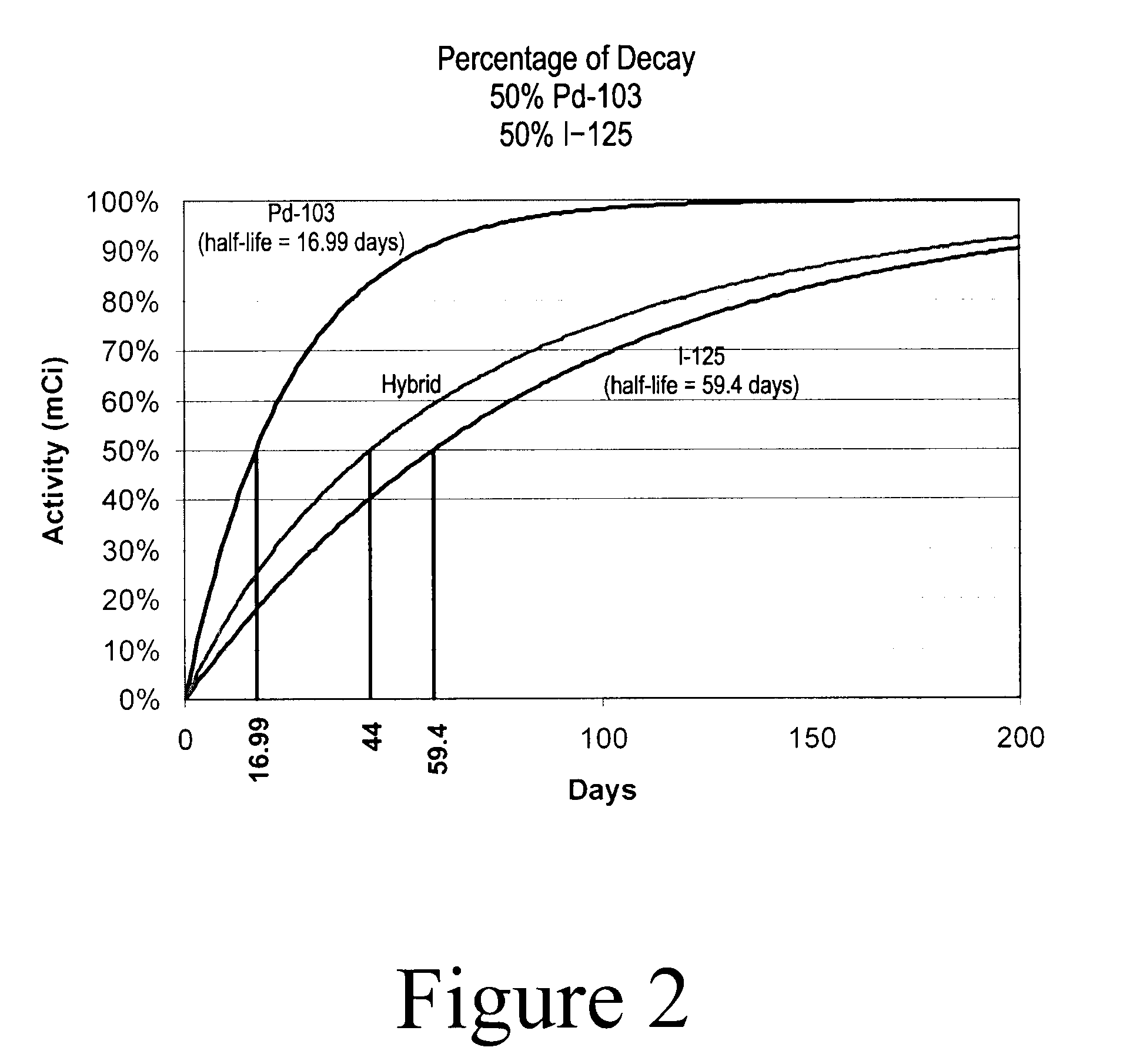
Hybrid Source Containing Multi-Radionuclides for Use in Radiation Therapy
InactiveUS20080249398A1Avoid damageGood curative effectDiagnostic recording/measuringSensorsInitial doseBrachytherapy
A hybrid multi-radionuclide sealed source for use in brachytherapy comprising a plurality of radionuclides is disclosed. The differing decay rates of the radionuclides in the hybrid multi-radionuclide sealed source combine a large initial dose of radiation followed by an extended dose of radiation contained within the single source. The sealed source may comprise a seed, a flexible strand, a rigid strand, a wire, a coil or a catheter.
Owner:HARDER GEORGE FRED +1
Method for producing glutamic acid through double-feeding fermentation optimization of corn steep liquor and glucose
ActiveCN103243132AFast growthImprove adaptabilityMicroorganism based processesFermentationBiotechnologyDouble-time
The invention discloses a method for producing glutamic acid through double-feeding fermentation optimization of corn steep liquor and glucose, which comprises the following steps of: in fermentation culture of glutamic acid, initially adding 1-10g / L of glucose to a fermentation culture medium, and initially adding 1-10g / L of corn steep liquor; after the fermentation culture starts, starting feeding the corn steep liquor and glucose at the same time, wherein 5-19g / L of corn steep liquor is fed and completely added within 5-10 hours since the fermentation starts, and the total amount of the corn steep liquor is 15-20g / L; feeding the concentrated solution of glucose until the fermentation is over; maintaining the amount of residual sugar in the fermentation culture medium at 0.05-10g / L; and controlling the pH value of the liquid ammonia in the whole process of fermentation to 6.8-7.3. The method disclosed by the invention effectively increases the growth speed of thalli and shortens the adaptation period in the initial stage of thallus fermentation and the thallus doubling time, thus shortening the overall fermentation time and reducing the overall production cost of glutamic acid.
Owner:山东祥维斯生物科技股份有限公司
Medical tectorial membrane shaped radiation NiTi alloy endovascular stent
InactiveCN101161297AShort doubling timeRestore proliferative abilityStentsSurgeryTectorial membraneNiti alloy
The present invention belongs to the technique category of the medical instrument, relates to a tectorial conformal radiate NiTi alloy vascular inner rack which is implanted to the blood vessel of the human body. To settle the problems of that the existing vascular inner rack can not restrain the growth of the tumour and the thrombus is easy to form, etc., the bracket used by the invention is self-expanding NiTi alloy vascular inner rack, a layer of polycarbonate type polyurethane membrane is covered at the inner wall, the antineoplastic medicine is spraying-coated to the inner surface of the membrane, and the active particles which are used for the inner radiating of the tumour are inserted at the outer bracket of the membrane according to the conformal property. The NiTi alloy vascular inner rack applied by the invention can effectively expand and support the blood vessel; the novel film forming material polycarbonate type polyurethane has an excellent bioavailability; the active particles for the conformal radiotheraphy outside the membrane are inserted to the bracket with a simple and convenient double-buckle mode, the growth surround the blood vessel area is effectively restrained and the radioactive side injury of the normal tissue is reduced. The invention has the advantages of easy making, low cost, strong innovation and wide market prospect.
Owner:李楠
Integrated expanded granular sludge bed-membrane bioreactor whole-course autotrophy denitrification device and process thereof
ActiveCN103979683AReduce pollutionExtended duty cycleTreatment with aerobic and anaerobic processesRefluxFiltration
The invention belongs to the field of environmental engineering, which relates to an integrated expanded granular sludge bed-membrane bioreactor whole-course autotrophy denitrification device and a process thereof. The device comprises a reactor main body, an air / liquid separation tank, a three-phase separation device, a membrane assembly, an air pump, an air flow meter, a perforated aeration pipe, a water feeding pump, a reflux pump, a water discharging pump and the like, wherein the reactor main body is divided into two function regions, i.e., a lower whole-course autotrophy denitrification function region and an upper membrane filtration function region, wherein an air aeration mode is adopted by the bottom of the immersion-type membrane assembly in the upper membrane filtration function region; the upper part of the reactor is externally connected with the air / liquid separation tank; the reflux system is used for refluxing heavy-oxygen-enriched water in the tank to a water inlet in the bottom of the reactor so as to provide the necessary dissolved oxygen for the whole-course autotrophy denitrification function region; and the fed sewage is firstly denitrified by the whole-course autotrophy denitrification function region and then filtered by the membrane so as to obtain the clean discharging water. The integrated reactor is small in land occupation. The whole-course autotrophy denitrification process is simple and convenient to operate.
Owner:DALIAN UNIV OF TECH
Non-serum non-animal-origin-additive insect cell culture medium
The invention relates to the field of cell culture medium, and in particular to a non-serum non-animal-origin-additive insect cell culture medium. The medium comprises mainly the following components: basic culture medium, glucose, inorganic salt, vitamins, L-arginine, L-agedoite, L-glycocoll, L-histidine, L-isoleucine, L-leucine, L-lysine, L-methionine, L-threonine, L-tryptophan, L-valine, L-proline, L-glutamine, yeast extracts, recombulin, malic acid, allomaleic acid, cholesterol, linoleic acid, granulesten, putrescine, glutathione, glycerol and fructosan. The culture medium can promote the growth of insect cell and is suitable for the large scale breeding of insect cell and the expression of recombination protein.
Owner:严志海
Serum-free culture medium system for culture of primary human epidermic cells
InactiveCN107129967APromote growthAvoid potential hazardsEpidermal cells/skin cellsCulture processPenicillinCuticle
The invention provides a serum-free culture medium system for culture of primary human epidermic cells, relates to the technical field of biological medical materials, and discloses application of a serum-free culture medium in in-vitro culture of human epidermic cells. The serum-free culture medium system has the advantage that the cholera toxin and various anchoring factors are not added, the potential hidden hazard due to complicated components is avoided, the epidermic cells can well grow, and the requirement of scaled production and application is met. The serum-free culture medium system for culture of primary human epidermic cells comprises a basic culture medium and exogenous adding factors, wherein the basic culture medium is formed by mixing a DMEM (dulbecco's modified eagle medium) commercial culture medium and a F12 commercial culture medium according to a certain ratio, and can also adopt a commercialized cell culture medium MCDB153; the exogenous adding factors comprise L-glutamine, EGF (epidermic growth factors), BPE (bovine pituitary extract), insulin, penicillin-streptomycin, calcium chloride, transferrin, adenine, hydrocortisone, and sodium selenite.
Owner:GUANGDONG BOXI BIO TECH CO LTD
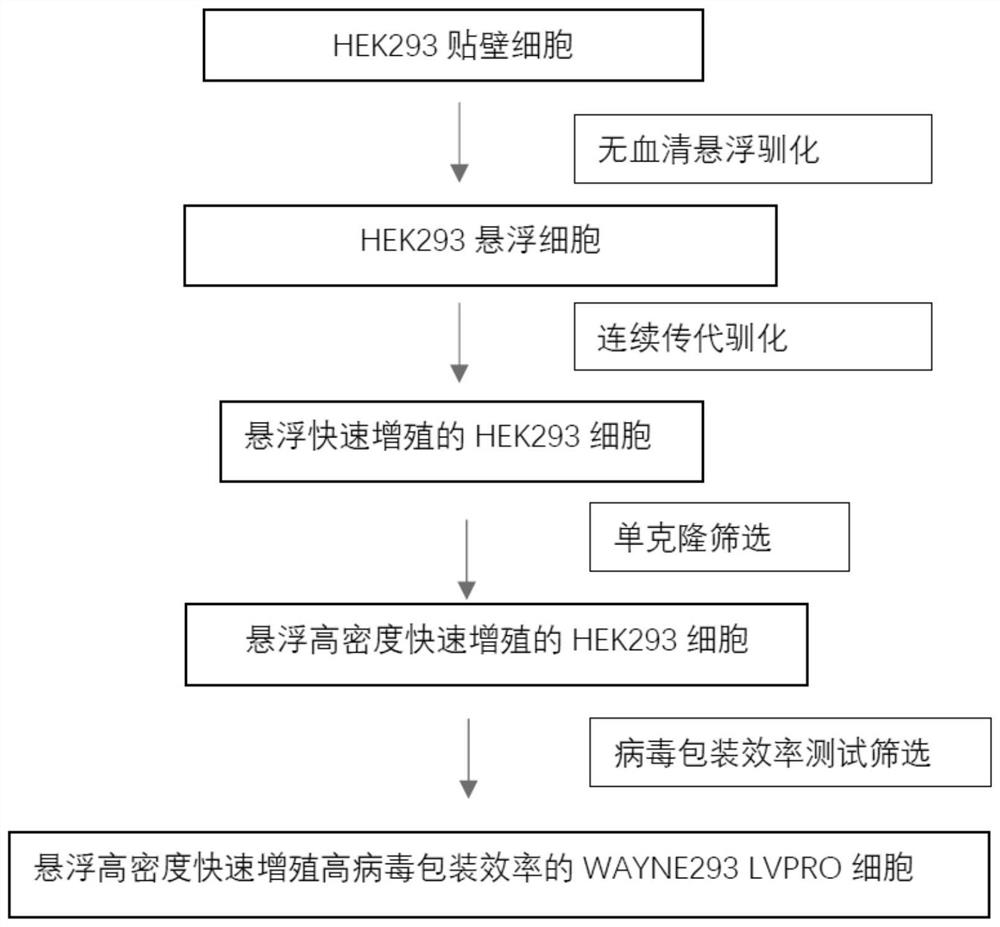
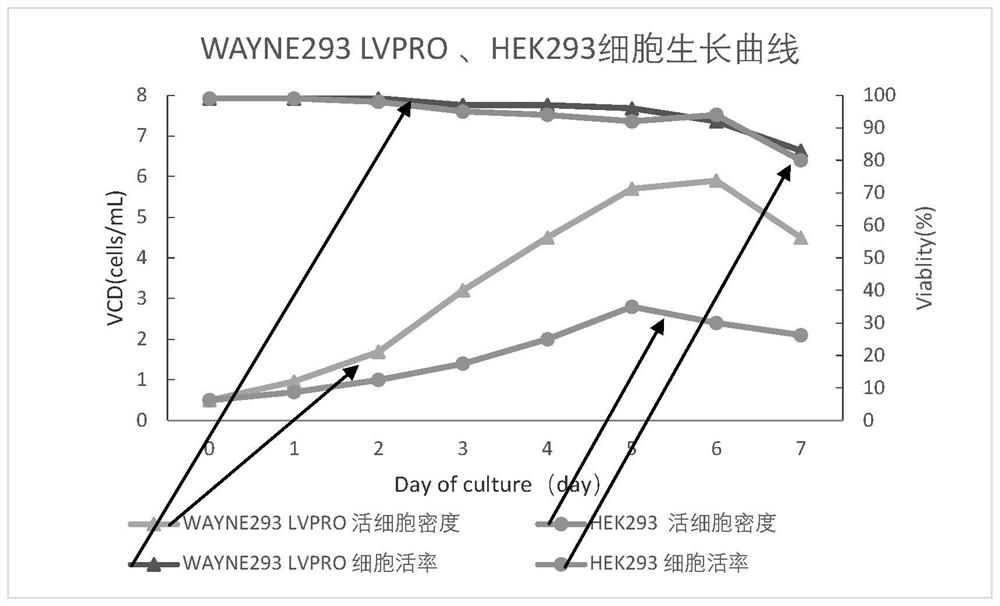
WAYNE293 LVPRO cell adapted to serum-free culture medium environment and application of WAYNE293 LVPRO cell
ActiveCN113604425AImprove packaging efficiencyShort doubling timeCulture processMicroorganism based processesLaboratory cultureSuspension culture
The invention discloses a WAYNE293LVPRO cell adapted to a serum-free culture medium environment and application of the WAYNE293LVPRO cell. The human embryonic kidney cell WAYNE 293 is preserved in China General Microbiological Culture Collection Center (CGMCC) on May 24, 2021, the address is Institute of Microbiology, Chinese Academy of Sciences, No.3, No.1 Yard, Beichen West Road, Chaoyang District, Beijing, the postal code is 100101, and the preservation number is CGMCC No.22348. The human embryonic kidney cell WAYNE 293 provided by the invention is derived from suspension culture of HEK293 cells in a serum-free culture medium without an anti-caking agent, has the characteristics of short doubling time, high growth density, high virus packaging efficiency and the like, and is an important support for industrial development in the field of cell and gene therapy.
Owner:QUACELL BIOTECHNOLOGY CO LTD
Serum-free culture fluid for enhancing immunosuppression function of animal mesenchymal stem cells
InactiveCN106282103AImprove immunosuppressionAvoid quality changesSkeletal/connective tissue cellsSerum freeCulture fluid
The invention provides cell culture fluid of serum-free cultivation for enhancing immunosuppression function of animal mesenchymal stem cells. Multiplication factors and specific additives are added into a basic nutrient solution for serum-free cultivation of animal mesenchymal stem cells, in order to promote healthy growth and proliferation of animal mesenchymal stem cells, and provide functions for maintaining and enhancing immunosuppression of animal mesenchymal stem cells. The fluid has the function for enhancing function of animal mesenchymal stem cells. Experiments prove that the serum-free culture fluid for enhancing immunosuppression function of animal mesenchymal stem cells can be used for serum-free cultivation of animal mesenchymal stem cells, and can be applied to clinic research and application in the aspect of immunosuppression application research of mesenchymal stem cells.
Owner:周宇璠 +1
Hyaluronic acid method for promoting proliferation of human amniotic membrane stem cells and application thereof
ActiveCN105861425ADoes not affect potentialWide variety of sourcesArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsMedicinePharmaceutical drug
The invention discloses a hyaluronic acid method for promoting proliferation of human amniotic membrane stem cells and application thereof. The present invention applies hyaluronic acid in drugs for promoting proliferation of human amniotic stem cells. Through a series of tests, it is fully proved that hyaluronic acid can significantly reduce the doubling time of human amniotic stem cells (hASCs) and promote hASCs proliferation, has safety, and does not affect hASCs multi-differentiation potential of stem cells. Hyaluronic acid can be used alone, or a composition containing hyaluronic acid can be used for promoting the hASCs proliferation or manufacturing pharmaceuticals for the same purpose. The present invention has the advantages of wide range of sources, low cost, and side effects.
Owner:AFFILIATED HOSPITAL OF ZUNYI UNIV
Method for extracting mesenchymal stem cells from human umbilical cord
InactiveCN104673748ALow costShort doubling timeSkeletal/connective tissue cellsStem like cellBiophysics
The invention relates to the fields of stem cells and tissue engineering, in particular to a method for extracting mesenchymal stem cells from the human umbilical cord. To solve the problems and defects in the conventional separation and culturing technology for human umbilical cord mesenchymal stem cells, the method adopts the technical scheme that the separation method for the human umbilical cord mesenchymal stem cells comprises the following steps: 1. preparation of an umbilical cord tissue block; 2. digestion of the tissue block; 3. filtration of a digestion solution, and centrifugal cell collection; 4. combination of cell sediments, centrifugation, and cell collection; 5. subculturing; 6. cell cryopreservation through a programmable cooler, and cryopreservation in liquid nitrogen. The method provided by the invention can lower the cost, achieve rapid obtaining of the mesenchymal stem cells, reduce cell damages, prolong the cryopreservation period, improve the cell activity, shorten the operational process, reduce pollution, and improve the cell purity.
Owner:河南中科干细胞基因工程有限公司
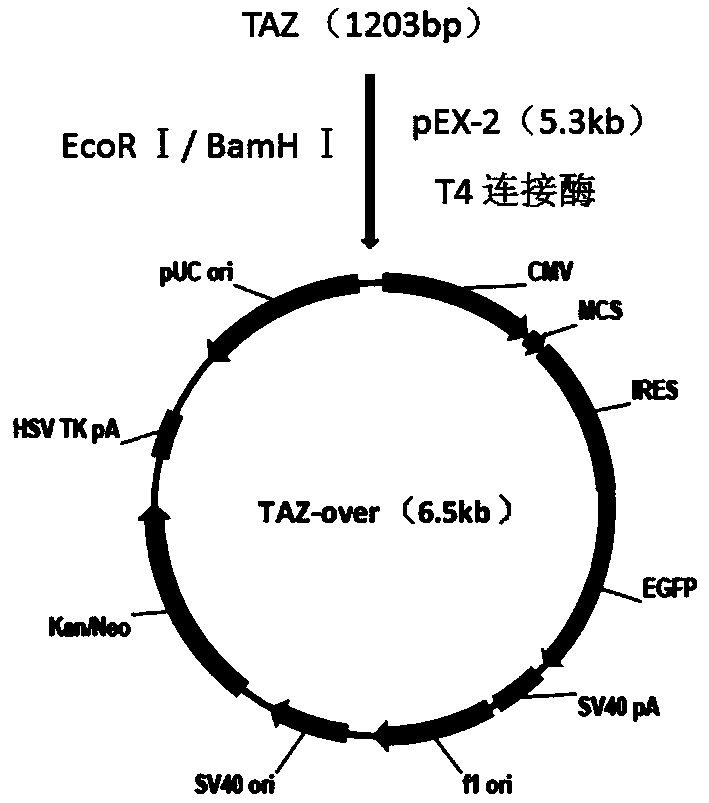
PC9 cell strain knocked down or over-expressed by TAZ as well as construction method and application of PC9 cell strain
InactiveCN103849620APromote growthLong doubling timeVector-based foreign material introductionForeign genetic material cellsFluorescenceWestern blot
The invention discloses an shRNA (Short Hairpin Ribonucleic Acid) molecule of a PC9 cell strain knocked down by a TAZ factor, a recombinant vector of the shRNA molecule, the recombinant vector of the PC9 cell strain over-expressed by the TAZ factor, and an establishment method of the PC9 cell strain knocked down and over-expressed by the TAZ. The method comprises the following steps: establishing the PC9 cell strain knocked down by the TAZ by transfecting PC9 cells via TAZ-shRNA by adopting an shRNA technology; establishing the PC9 cell strain over-expressed by the TAZ by transfecting the PC9 cells via a TAZ expression vector; detecting the TAZ in the cell strain by adopting an immune cell fluorescence technology, a RT-PCR (Reverse Transcription-Polymerase Chain Reaction) technology and a Western blot technology so as to prove the successful establishment of the cell strain; and measuring a cell growth curve and the cell doubling time. Thus, the powerful experimental material for researching the functions of the TAZ in the targeted therapy sensitivity of lung cancers is provided on the basis of the establishment of the PC9 cell strain knocked down and over-expressed by the TAZ.
Owner:JIANGSU PROVINCE HOSPITAL
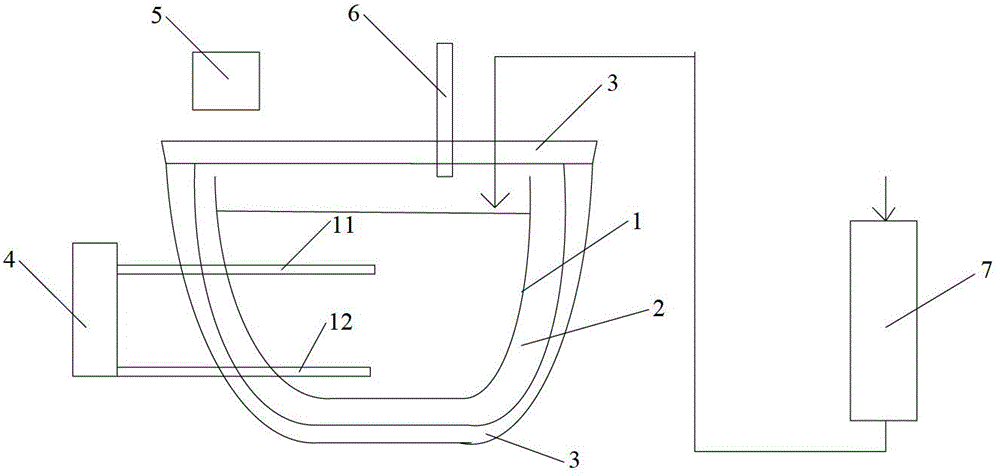
Device for fast screening and culturing anaerobic ammonia oxidation microbial communities
ActiveCN102864068AFast growthShort doubling timeBioreactor/fermenter combinationsBiological substance pretreatmentsDouble-timeUltrafiltration
The invention relates to the technical field of the wastewater biological treatment, and in particular relates to a device for fast screening and culturing anaerobic ammonia oxidation (Anammox) microbial communities. The device comprises an anaerobic reactor, an inner pressure tube type ultrafiltration membrane, a water inlet controlling device, an automatic pH regulating device, an equilibrium reagent-supplementing device, a pneumatic pressure regulation device and an electronic control automatic thermostatic device, wherein the anaerobic reactor is arranged in the electronic control automatic thermostatic device and connected with the inner pressure tube type ultrafiltration membrane, the automatic pH regulating device, the equilibrium reagent-supplementing device, the water inlet controlling device and the pneumatic pressure regulation device respectively. Compared with the prior art, the device for fast screening and culturing Anammox microbial communities can provide an environment for the rapid proliferation of Anammox, thus shortening the doubling time and increasing the growth rate of Anammox.
Owner:上海同济环境工程科技有限公司
Domesticated CHO-S cell system and culture method and application thereof
ActiveCN106282090AShort doubling timeEasy to shapeMicroorganism based processesArtificial cell constructsMicrobiologyCulture mediums
The invention relates to a domesticated CHO-S cell system and a culture method and application thereof. The domesticated CHO-S cell system is obtained by passing CHO-S cells for at least 5 generations through a specially formed XH001 culture medium by a gradual domestication method. The invention also discloses a domestication method and application of the domesticated CHO-S cell system. The domesticated CHO-S cell system has the characteristics of high growth speed, short multiplication time, good cell form, uniform shape and the like. When the domesticated CHO-S cell system is used for recombinant antibody expression, the expression quantity can be obviously improved.
Owner:QILU PHARMA CO LTD
Preparing method for serum-free and animal-source-free culture medium additive suitable for growth of insect cells
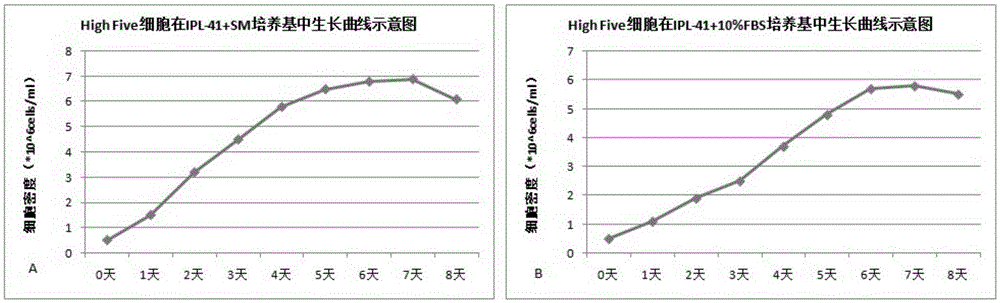
The invention relates to the technical field of biological pharmacy, in particular to development of a serum-free and animal-source-free culture medium additive suitable for insect cells mainly including Sf9 and High Five cells. A traditional culture medium Grace or IPL-41 serves as a basis, the additive beneficial for the growth of the insect cells is added, in the insect cell culture process, the additive has a function of replacing serum, price is low, the insect cells can grow for 240 h continuously, and cell activity can keep over 95%.
Owner:内蒙古金源康生物工程股份有限公司
Platelet lysate supported micro-sphere preparation method
ActiveCN108715833AGood biocompatibilityEffective proliferationSkeletal/connective tissue cellsBlood/immune system cellsDouble-timeMicrosphere
The invention relates to a platelet lysate supported micro-sphere preparation method, and aims to solve the problem of large platelet lysate consumption or poor micro-carrier adsorption effect in existing cell culture. The preparation method includes the steps of platelet lysate preparation, micro-sphere component preparation and platelet factor and micro-capsule supported micro-sphere preparation. A prepared micro-sphere is quite strong in osteoblast carrying capacity, most of cells are fitted on the micro-sphere under the condition that the ratio of osteoblasts to the micro-sphere is 500:1,and only few individual cells are scattered in culture solution. Mesenchymal stem cells on the micro-sphere are good in growth condition, cell viability exceeds 95%, doubling time is short, positive expression rate exceeds 95% in terms of purity, and the preparation method is applied to the technical field of biology.
Owner:天晴干细胞股份有限公司
Amniotic membrane mesenchymal stem cell release molecule (ASRM) as well as preparation method and application of ASRM
InactiveCN107744526AHigh purityLow immunogenicityPowder deliveryCosmetic preparationsHuman plateletAging skins
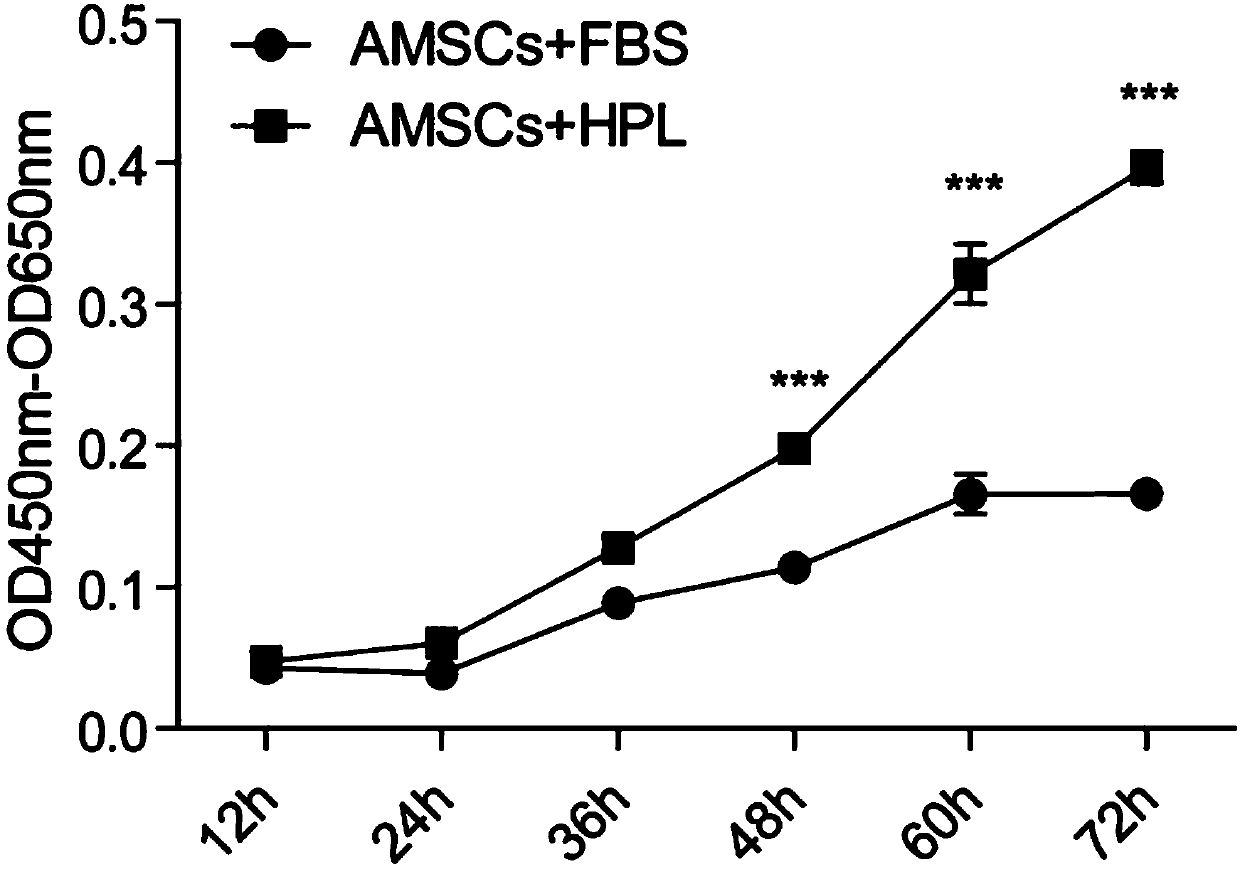
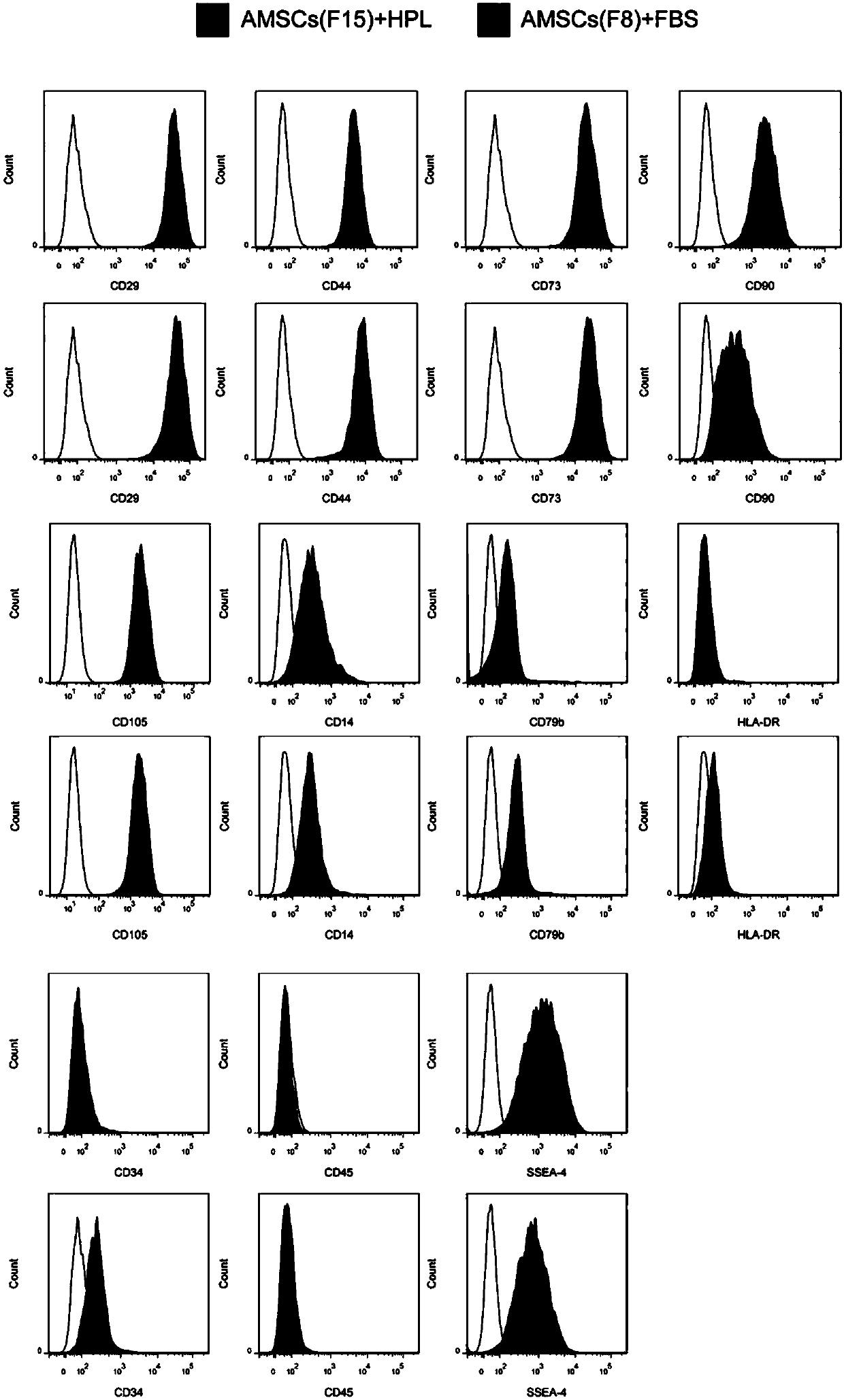
The invention discloses a sheep stem extract and its preparation method and application, and discloses a preparation method of its preparation freeze-dried powder. The sheep stem extract is obtained by separating and culturing amniotic mesenchymal stem cells from human placenta amniotic tissue, and using human platelets The lysate is cultured and expanded, and then the culture supernatant of human amniotic mesenchymal stem cells is collected, concentrated and collected, which solves the problems of long doubling time and poor multi-differentiation potential of AMSCs, and can qualitatively and Quantitative analysis can improve the active ingredients in sheep stems, such as antioxidants and various growth factors, and prevent the active ingredients from degrading. The results of animal experiments and clinical trials show that the sheep dry extract prepared by this technology can be used to delay aging, prevent and treat osteoporosis and skin aging, and promote skin wound healing.
Owner:NANJING MEDICAL UNIV
Method for inducing and differentiating human embryonic stem cells into mesenchymal stem cells
ActiveCN111849885AEasy to operateReduce manufacturing costCulture processSkeletal/connective tissue cellsExtracellularMesenchymal stem cell
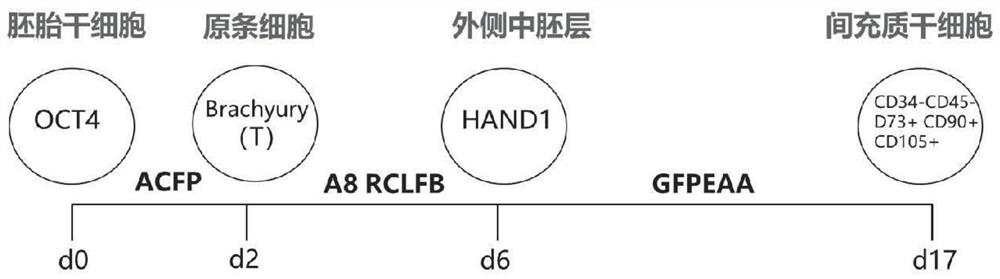
The invention provides a method for inducing and differentiating human embryonic stem cells (hESCs) into mesenchymal stem cells (MSCs). The method mainly relates to three stages, including induction of primitive streak (PS) cells, differentiation to outer mesoderm cells and differentiation of the MSCs. According to the method, a staged differentiation method is adopted, the whole differentiation process can be tracked and subjected to quality control, the cost is low, and operation is easy. An intermediate product of hESCs induced and differentiated into MSCs is obtained and comprises PS cells, outer mesoderm cells and MSCs, more MSCs can be efficiently generated, the multiplication time is shorter, the MSCs can be repeated, and mature MSCs can be obtained in 17 days at the shortest time,wherein the differentiation induction culture media in the first two stages are serum-free culture media with clear components, so that differentiated MSCs are prevented from being polluted by exogenous substances, and the uniformity and clinical applicability of the differentiated MSCs are guaranteed.
Owner:ZHEJIANG UNIV
Method for promoting in-vitro expansion of human amniotic epithelial cells and use thereof
ActiveCN106834217AEasy to operateGood securityEpidermal cells/skin cellsCulture processNutritional compositionGrowth cell
The invention relates to a method for promoting in-vitro expansion of human amniotic epithelial cells. The method comprises adding a nutritional composition during human amniotic epithelial cell culture, wherein the nutritional composition mainly comprises hyaluronic acid, an epidermal cell growth factor, vitamin C, a GlutaMAX-I additive, beta-mercaptoethanol, glycine, L-alanine, L-aspartic acid, L-asparagine, L-glutamic acid, L-proline and L-serine. The method can significantly promote human amniotic epithelial cell proliferation, reduce the doubling time, maintain biological characteristics such as human amniotic epithelial cell surface marker expression, multi-directional differentiation potential and immune tolerance, and significantly enhance the expression level of a human amniotic epithelial cell related gene and an immunosuppressive factor. Therefore, the nutritional composition as a human amniotic epithelial cell proliferation regulator has obvious advantages and has a great clinical significance to alleviate the shortage of seed cells in the field of regenerative medicine.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Bioactive substance composition, serum-free culture medium containing composition and application of serum-free culture medium
PendingCN113692282AHigh activityAchieve primary culturePeptide/protein ingredientsCulture processOrgan damageMotor system
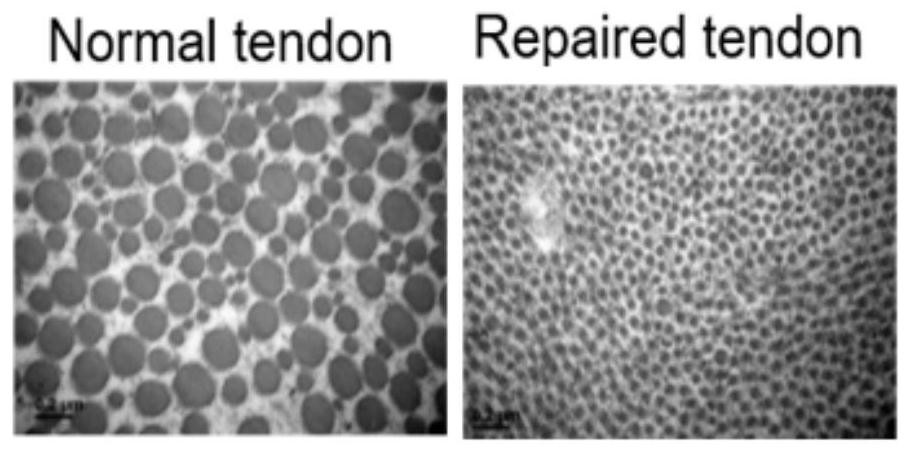
The invention provides a bioactive substance composition, a serum-free culture medium containing the composition and application of the serum-free culture medium. The bioactive substance composition is used for a serum-free culture medium and / or a composition and preparation thereof; the serum-free culture medium and / or composition can be used for primary culture and subculture of cells and / or tissues. The cells are selected from any one or more of tendon and / or ligament source cells, chondrocytes, meniscus stem cells, mesenchymal stem cells, skeletal stem cells and muscle stem cells. The tissue is a source tissue of a motor system. The bioactive substance composition and / or the serum-free culture medium and / or the composition can be used for preparing a medicine for treating tissue and / or organ injury; the tissue or organ injury is selected from movement system tissue or organ injury.
Owner:ZHEJIANG UNIV
Application of exosome in promotion of growth of mesenchymal stem cells of decidua parietalis
PendingCN113151162ABroaden the extraction conditionsImprove extraction efficiencyCell dissociation methodsCulture processCell activityMesenchymal stem cell
The invention discloses application of a human umbilical mesenchymal stem cell exosome in promotion of growth of mesenchymal stem cells of decidua parietalis and a method for promoting the growth of the mesenchymal stem cells of the decidua parietalis. According to the application and the method, after the human umbilical mesenchymal stem cell exosome and PDB-MSCs are cultured jointly, the PDB-MSCs is high in cell proliferation capability, good in cellular form and high in cellular activity, thus, the human umbilical mesenchymal stem cell exosome disclosed by the invention can be used for improving the quality of the PDB-MSCs, the cell factor VEGF and SCF secreting capability of the PDB-MSCs is improved effectively, thus, the problems of the PDB-MSCs that repeated transfer of generation is lowered in proliferation capability and poor in cell activity can be solved, and then, the large-scale culture and clinical application of the PDB-MSCs is facilitated effectively.
Owner:GUANGDONG VITALIFE BIOTECHNOLOGY CO LTD
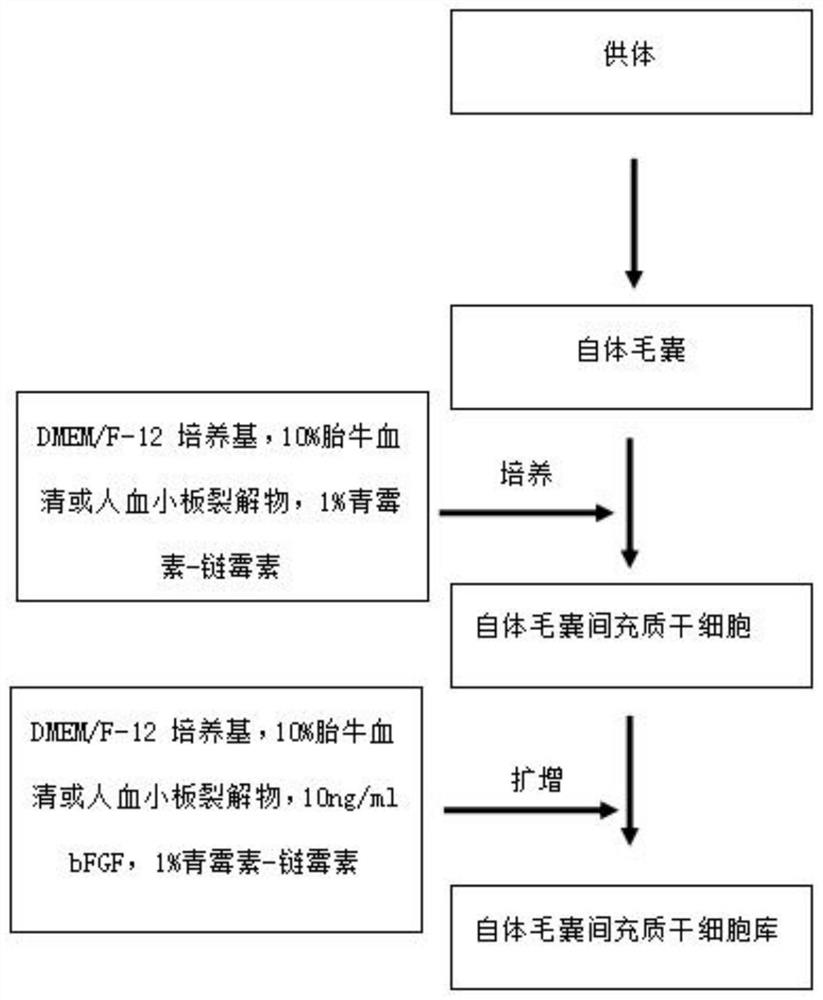
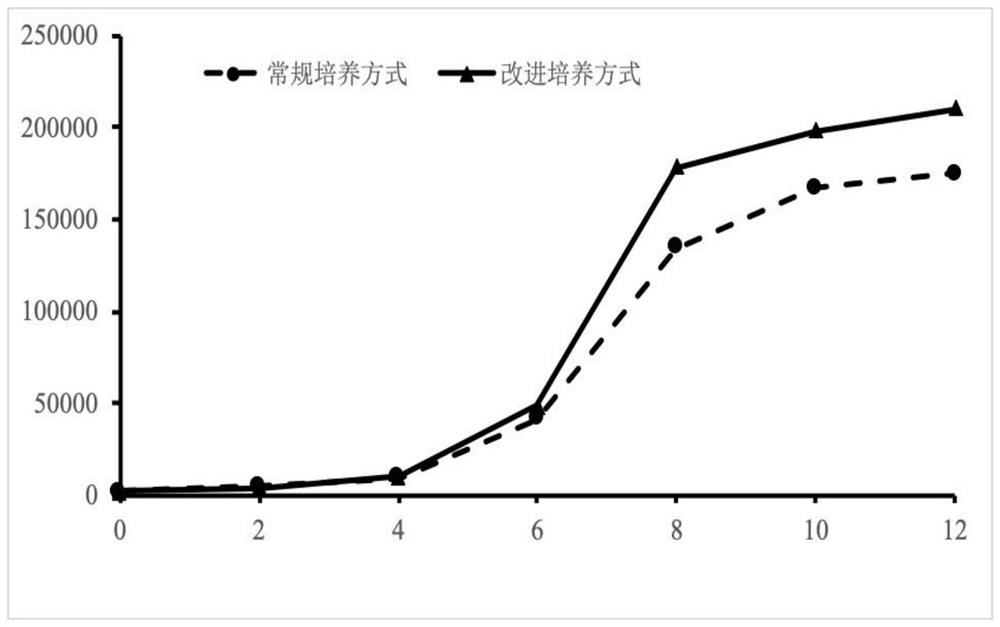
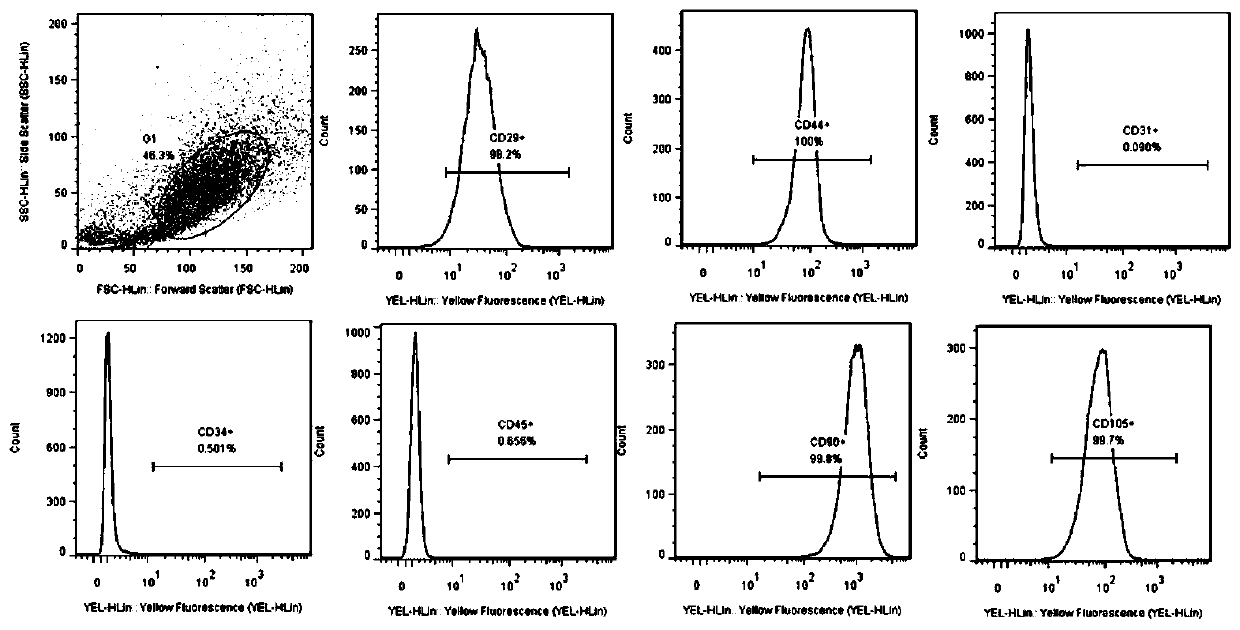
Culture medium for in-vitro amplification of autologous human hair follicle mesenchymal stem cells and culture method thereof
PendingCN111849883AIncrease proliferation rateProliferation rate is fastCulture processSkeletal/connective tissue cellsPenicillinWhole blood product
The invention provides a culture medium for in-vitro amplification of autologous human hair follicle mesenchymal stem cells and a culture method of the culture medium, and belongs to the technical field of cell culture. The culture medium for in-vitro amplification of the autologous human hair follicle mesenchymal stem cells takes DMEM / F-12 as a basic culture medium and is prepared from the following components in percentage by volume: 4 to 6 percent of autologous serum, 9 to 11ng / ml of bFGF and 0.8 to 1.2 percent of penicillin-streptomycin. According to the culture method, human autoserum isused as an additive to prepare an amplification culture medium, and in-vitro large-scale amplification of autologous human hair follicle mesenchymal stem cells is carried out. Compared with the traditional mesenchymal stem cells, the hair follicle mesenchymal stem cells cultured by the method have the advantages that other animal serum or allogeneic blood products are not required to be added, thesafety is high, the allogeneic disease propagation risk is avoided, the cell proliferation rate is high, and the characteristics of the mesenchymal stem cells can still be maintained after multiple passages.
Owner:李鹏东 +1
Culture and three-line differentiation induction methods of adipose tissue-derived mesenchymal stem cells
InactiveCN110846273AImprove proliferative abilityShort doubling timeCell dissociation methodsCulture processMedicineMesenchymal stem cell
The invention belongs to the technical field of cell biology and particularly relates to culture and three-line differentiation induction methods of adipose tissue-derived mesenchymal stem cells. Theculture method specifically includes: placing adipose tissue into a centrifugal tube, adding normal saline, pipetting with a pipet, standing, sucking out liquid on the lower layer of the tissue, adding collagenase type I / PBS digestive juice with the concentration being 0.1% to perform digestion, and placing the culture bottle into a culture tank of 37 DEG C and with 5% CO2; taking out cells when the cells grow to 80% and merge, adding TrypLE digestive juice, terminating the digestion when the cells start retracting and rounding, and performing subculture and multiplication culture. The differentiation induction method has the advantages that differentiation time is shortened, the method needs 10 days to differentiate the cells into adipose and bones and needs 14 days to differentiate the cells into cartilage, and differentiation efficiency is increased greatly as compared a common induction method which needs 14 days to differentiate cells into adipose and needs 21 days to differentiate the cells into bones and cartilage.
Owner:山东省齐鲁细胞治疗工程技术有限公司
Integrated expanded granular sludge bed-membrane bioreactor full autotrophic denitrification device and its process
ActiveCN103979683BReduce pollutionExtended duty cycleTreatment with aerobic and anaerobic processesRefluxWater discharge
Owner:DALIAN UNIV OF TECH
Protoperidinuim culture medium and method
ActiveCN106635815AShort doubling timeExtended growth timeUnicellular algaeMicroorganism based processesMicrobiologyCHITOSAN OLIGOSACCHARIDE
The invention discloses a protoperidinuim culture medium and method. The protoperidinuim culture medium is composed of, by weight, 20-100 mg of NH4NO3, 20-60 mg of CO(NH2)2, 1-10 mg of KH2PO4, 100-800 mg of NaHCO3, 15-50 g of MnCl2 4H2O, 10-30 mg of FeC6H5O7 5H2O, 1*10-5-1*10-3 mg of VB12, 50-200 mg of VB1, 0.5-5 mg of KCl, 10-15 mg of Na2EDTA, 0.2-5 g of chitosan oligosaccharide with a molecular weight of 1500-2000 Da, and 1000 mL of seawater. The protoperidinuim culture method comprises the steps of phase one, conical flask culture and phase two, mineral water barrel culture. The protoperidinuim culture medium and method provides the optimal nutritive salt formula for protoperidinuim culture, thereby improving the yield of protoperidinuim poison and reducing the culture cost; compared with traditional outdoor open cement ponds expanded culture manners, culturing protoperidinuim in mineral water barrels is lower in cost, less prone to pollution and easier to operate and can greatly reduce workload, increase the growth speed of the protoperidinuim and shorten the culture period.
Owner:QINGDAO UNIV OF SCI & TECH +1
C3A serum-free clonal cell line and methods of use
InactiveUS7390651B2Increase probabilityShort doubling timeBioreactor/fermenter combinationsBiological substance pretreatmentsSerum free mediaArtificial liver
A serum-free C3A clonal cell line and methods for generating the same are provided. The C3A cell line has a reduced doubling time in serum-free medium compared to a corresponding C3A cell line from which it is derived. Methods using the cells of the serum-free C3A clonal cell line for the production, expression and recovery of harvestable polypeptides, screening compounds for metabolic activity, studying enteric disease and for use in a bio-artificial liver device are also provided.
Owner:VITAGEN ACQUISITION
Serum-free full-suspension domestication method for Sf9 cells
ActiveCN111718889AEasy accessLower serum concentrationInvertebrate cellsCulture processBiotechnologyHigh concentration
The invention belongs to the field of biology, and discloses a serum-free full-suspension domestication method for Sf9 cells. The method comprises the following steps: step 1, resuspending the Sf9 cells in an Sf-900 III SFM culture medium added with dextran sulfate, and diluting the Sf9 cells to 0.6-1.0x10<6> cells / mL; step 2, adding a heat-inactivated fetal calf serum to the product in the step 1, and culturing the Sf9 cells; and step 3, repeating the step 1 and the step 2 when the density of the Sf9 cells in the step 2 reaches 4x10<6> cells / mL or above in the third day, wherein the amount ofthe added heat-inactivated fetal calf serum is gradually reduced every time the step 1 and the step 2 are repeated until the density of the Sf9 cells still reaches 4x10<6> cells / mL or above without adding the heat-inactivated fetal calf serum, and the final concentration of dextran sulfate in the Sf-900 III SFM culture medium in the step 1 is 20-30 mg / L. The method has the advantages of low domestication frequency, high concentration of the domesticated cells after proliferation, and high survival rate.
Owner:ZHAOQING INST OF BIOTECHNOLOGY CO LTD
Ex vivo antibody production
PendingCN106029873AShort doubling timeStable productionVectorsGenetically modified cellsAntibody productionEx vivo
The present invention provides means and methods for producing improved ex vivo B cell cultures with a short doubling time.
Owner:科凌生物医疗有限公司
Serum-free chemical component definition culture medium for EB66 cell strain suspension growth and preparation method thereof
InactiveCN110317778AHigh densityShort doubling timeCulture processCell culture active agentsDouble-timeVitamin C
The invention belongs to the field of culture mediums, and particularly relates to a serum-free chemical component definition culture medium for EB66 cell strain suspension growth. The culture mediumcomprises an additive A including, according to the concentrations of the substances in the culture medium, 1-10 mg / L of insulin, 5-10 microgram / L of IGF-1, 0.5-2 mg of putrescine, 0.5-5 mg / L of glutathione, 1-5 mg / L of neovaricaine, 10-50 micromole of beta-mercaptoethanol, 1-5 mg / L of lecithin, 10-50 mg / L of vitamin C, 50-100 mg / L of taurine, 2-5 mg / L of cholesterol and 3-6 millimole of glutamine, wherein 1000-3000 mg of an amino acid mixed component is added to per liter of the culture medium. The culture medium supports rapid proliferation of EB66 cells under the serum-free and full-suspension conditions, and the doubling time is short. The cells are high in density. Besides, the invention further provides a preparation method of the culture medium.
Owner:ZHAOQING INST OF BIOTECHNOLOGY CO LTD
A kind of low-serum medium suitable for bhk-21 cell spinner bottle culture and preparation method thereof
ActiveCN105255812BAdd lessGrow fastVertebrate cellsArtificial cell constructsCell growth rateCulture mediums
The present invention discloses a low-serum culture medium suitable for BHK-21 cell flask rotation culture, and a preparation method thereof. According to the present invention, in the flask rotation adherence culture, the serum consumption is substantially to 1% from the conventional 10%, the cell growth rate is rapid, the achieved cell density is high, and the high vitality is maintained; and during the culture medium development process, a plurality of compounds having special functions, lipid components, yeast, plant hydrolyzates and recombinant proteins are used to replace the conventionally-added animal-derived components in the culture medium, hormones, lipids, trace elements, hydrolyzates and other components are added to replace the partial functions of the serum so as to substantially reduce the serum addition amount, and reduce the serum cost while reduce the probability of the pollution caused by exogenous pathogenic bacteria carried by the serum and the animal-derived proteins in the culture medium, and the protein content in the culture medium is substantially reduced, such that the purification process at the late stage is easily achieved, and the stability of the cell product is improved.
Owner:内蒙古金源康生物工程股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com