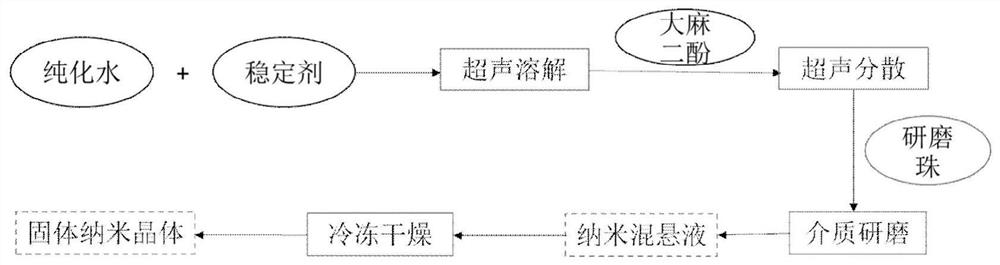
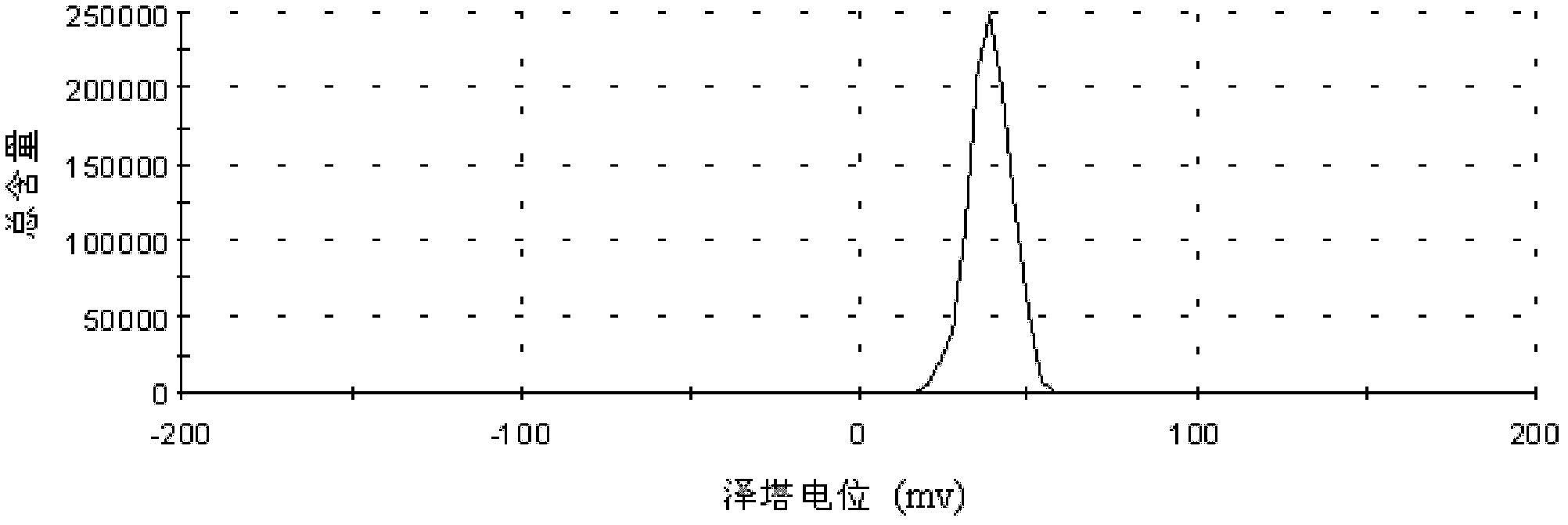
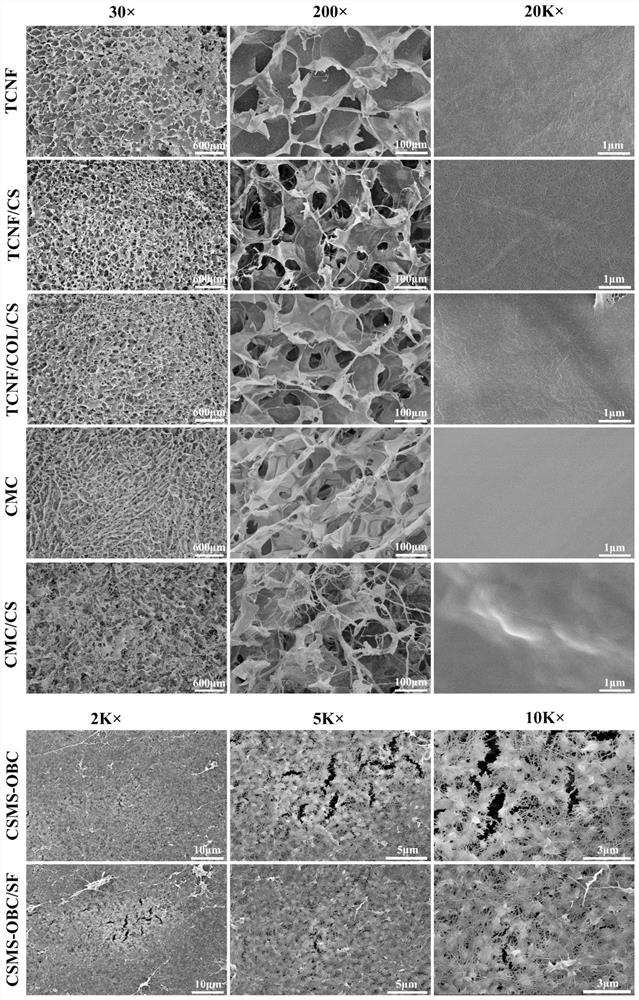
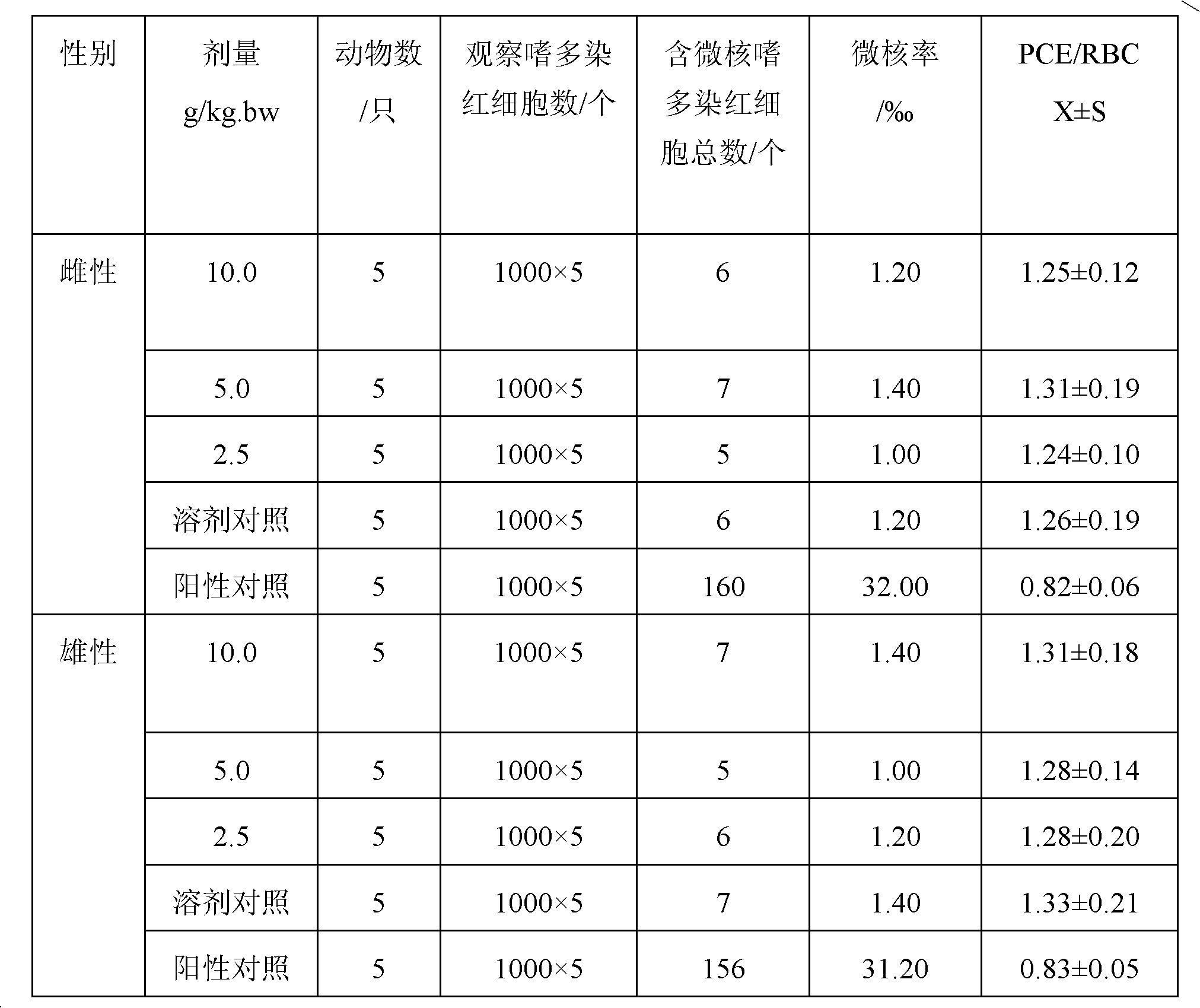
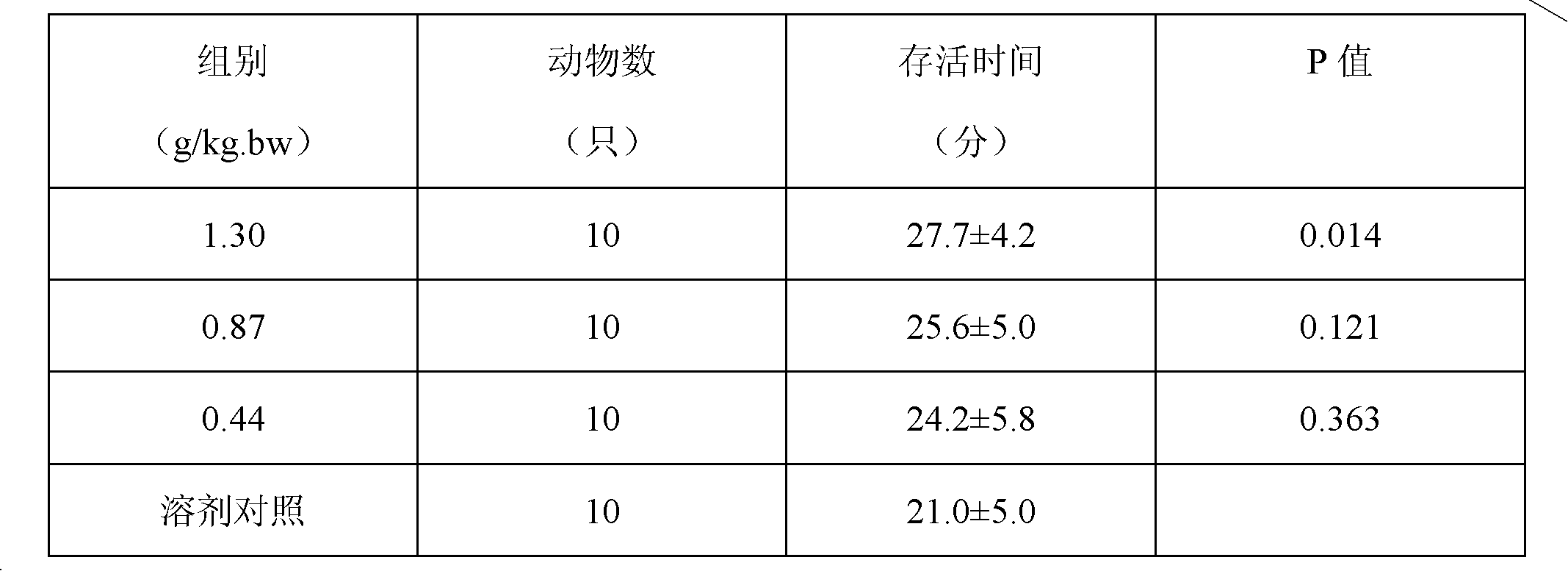
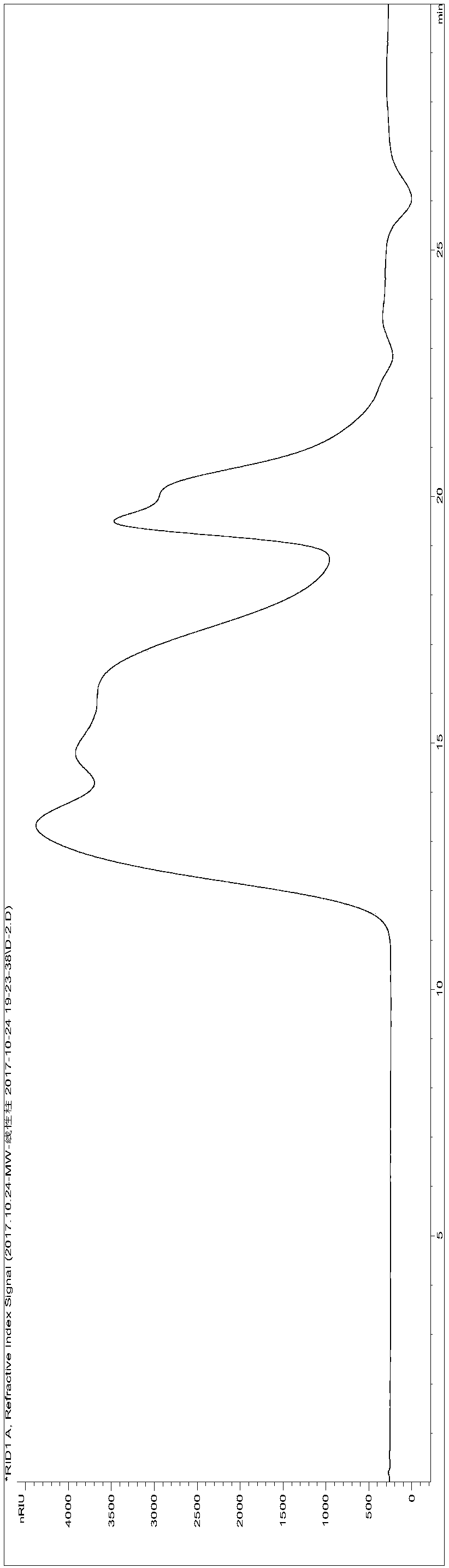
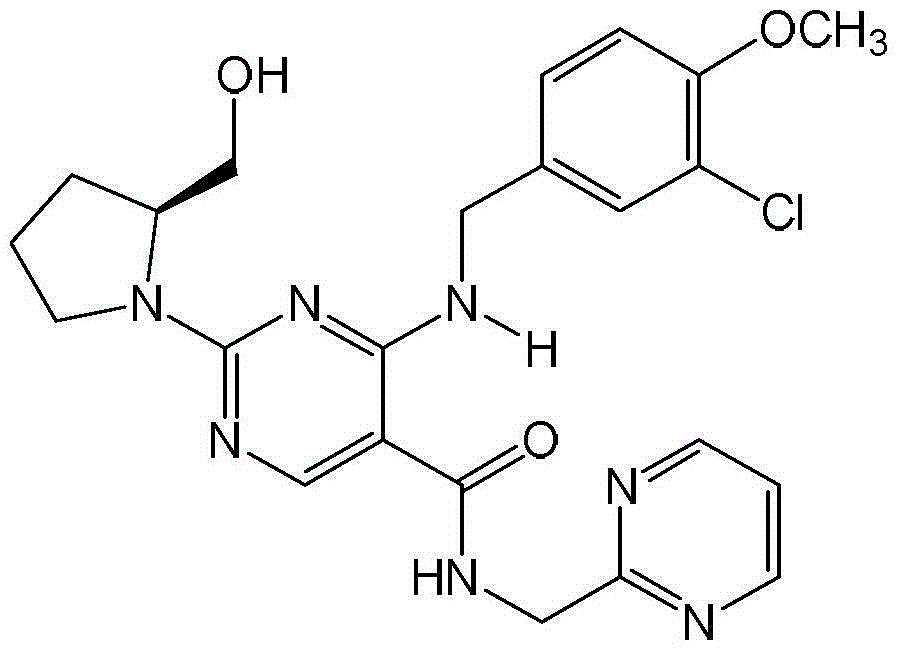
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
66results about How to "Promote absorption in the body" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Breviscapine-phosphotide compound and its preparing process
InactiveCN1359682AImprove absorption rateImprove absorptionOrganic active ingredientsUnknown materialsRefluxYolk
A breviscapine-phosphotide composition in the form of oral preparation or injection is prepared from breviscapine and soybean (or egg yolk, or synthetic) lecithine in a wt ratio of 1:(0.5-50) through heating, reflux, washing and drying. Its advantages are high stability and durability, and quickly taking its curative effect.
Owner:SHENYANG PHARMA UNIVERSITY
Dutasteride liquid soft capsules
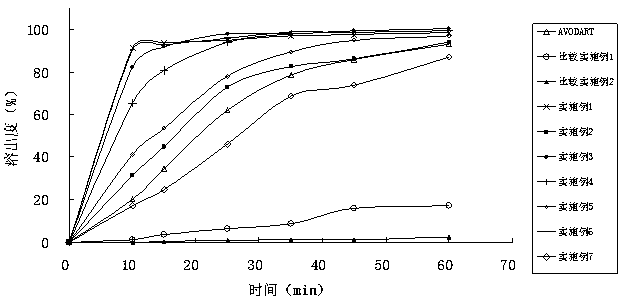
InactiveCN103830201APromote absorption in the bodyImprove the dissolution rate of dutasterideOrganic active ingredientsOrganic non-active ingredientsSoftgelGlycerol
The present invention relates to dutasteride liquid soft capsules and a preparation method thereof. The soft capsules comprise a capsule shell and contents, wherein the contents mainly comprise, by weight, 0.05-0.3% of dutasteride, 19.8-94.8% of medium chain triglyceride, and 5-80% of mono-caprylin glycerate. The soft capsules have characteristics of rapid dissolution, simple preparation process and the like.
Owner:CHONGQING PHARMA RES INST +1
Loaded aprepitant nanocrystal lipid microcapsule and preparation method thereof
ActiveCN105456228AImprove stabilityMild preparation conditionsOrganic active ingredientsNervous disorderPhospholipidHigh pressure
The invention relates to a loaded aprepitant nanocrystal lipid microcapsule and a preparation method thereof, and belongs to the technical field of a medicine. The microcapsule is prepared by the method comprising the following steps: preparing an aprepitant nanocrystal from aprepitant by virtue of a grinding process, high-speed air jet pulverization or high-pressure homogenization, then mixing and emulsifying the aprepitant nanocrystal with phospholipid, removing a liquid phase and drying so as to obtain the aprepitant nanocrystal lipid microcapsule. The prepared loaded aprepitant nanocrystal lipid microcapsule can improve the stability of the aprepitant nanocrystal and the lipid loaded nanocrystal can increase the speed and the amount of being absorbed by gastrointestinal mucosa of a body, so that the effects of relatively high bioavailability and accelerated development of efficacy are achieved; and the preparation method of the loaded aprepitant nanocrystal lipid microcapsule is mild in condition, simple and controllable, low in preparation cost and is suitable for large-scale production.
Owner:FUREN PHARMA GROUP +2
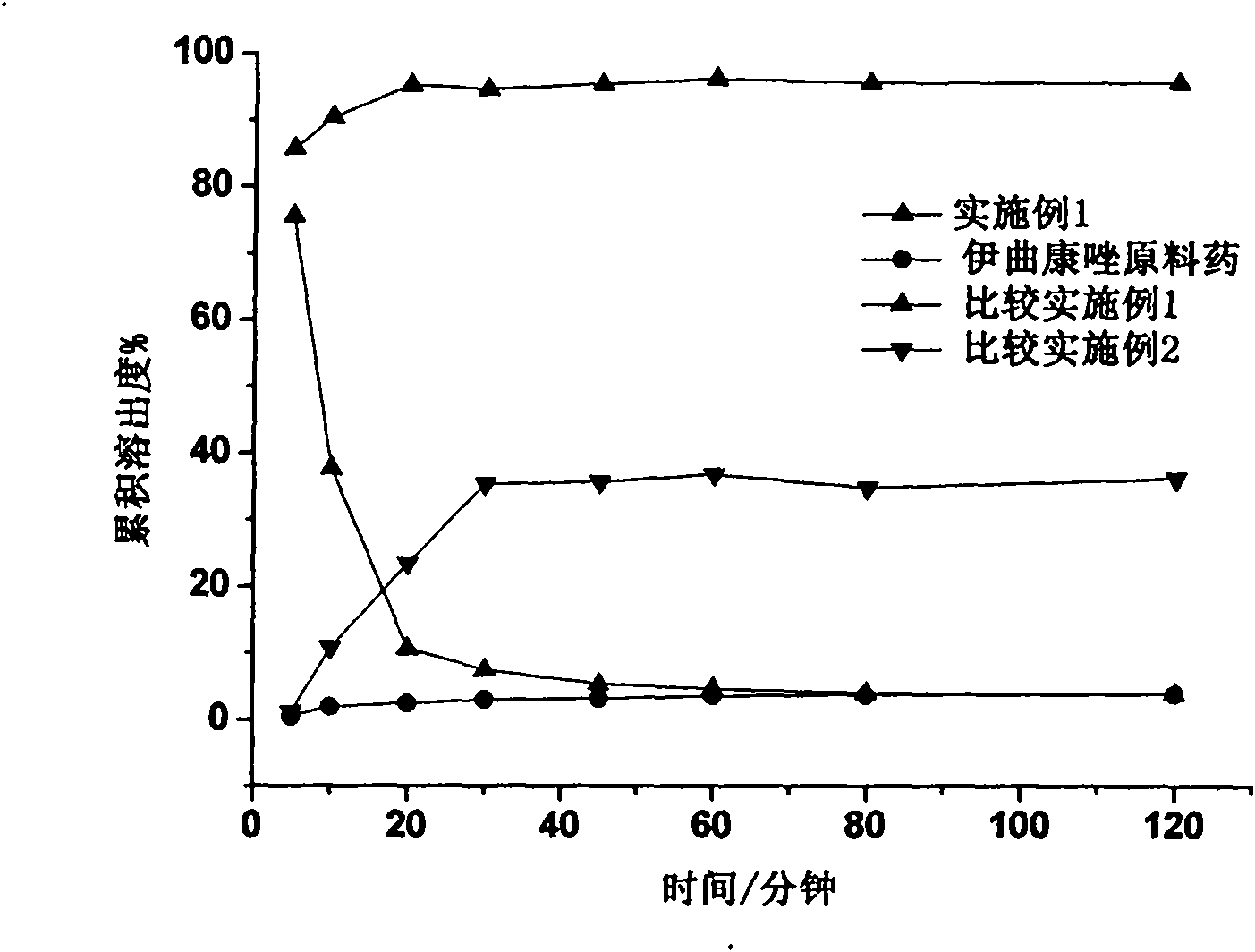
Itraconazole nanocrystals and preparation method and application thereof
ActiveCN101961313AImprove bioavailabilitySmall particle sizePowder deliveryOrganic active ingredientsSolubilityNanoparticle
The invention provides itraconazole nanocrystals and a preparation method and application thereof. Acid phase in which itraconazole is dissolved is dropwise added into alkaline phase to over-saturate the itraconazole; the itraconazole molecules are aggregated into amorphous nanocrystalline particles in the presence of a high molecular stabilizer to form a stable structure with itraconazole nanocrystals as a core and the high molecular stabilizer as a shell; and both the solubility and the dissolution rate of the itraconazole nanocrystals are greatly improved compared with those of normal itraconazole nanoparticle suspension. A hydrophilic fragment of the high molecular stabilizer, which is extended into the water, improves the hydrophobicity of the surfaces of the itraconazole nanoparticles, so that the high molecular stabilizer is more easily moistened and dissolved; and the high molecular stabilizer serving as the shell enhances the adhesion between the medicament particles and gastrointestinal mucous membrane, is favorable for improving the bioavailability and reduces the influence of a diet state on the bioavailability. The method of the invention has the advantages of no use of toxic solvent, simple preparation process, low cost and easy industrialization.
Owner:HUAZHONG UNIV OF SCI & TECH
Oral ciclosporin A sustained-release agent and preparation method thereof
ActiveCN102166201AStable drug releaseImprove bioavailabilityPharmaceutical delivery mechanismCyclic peptide ingredientsCiclosporinSolubility
The invention provides an oral ciclosporin A sustained-release agent. The sustained-release agent comprises the following components in part by mass: 1 part of ciclosporin A, 2.5 to 7.5 parts of polyvidone-K30, 0.15 to 4.82 parts of hydroxypropyl methyl cellulose 4000cPa.s, 0.1 to 3.94 parts of microcrystalline cellulose, 0 to 2.57 parts of hydroxypropyl methyl cellulose 15000cPa.s and 0.1 to 1.0part of phospholipid. By combining a solid dispersing technology and a sustained-release hydrophilic gel matrix technology and based on the 'double release' principle of fast release first and slow release second, the method prepares the insoluble medicament of ciclosporin A sustained-release agent; and the method has the obvious advantages that the solubility of ciclosporin A is improved, and the oral agent can quickly play the effect first and then smoothly and slowly release drug. The ciclosporin A sustained-release tablets reduce the lowered blood concentration peak-valley phenomenon and lower the drug-taking frequency. The invention also discloses a method for preparing the oral ciclosporin A sustained-release agent.
Owner:JIANGSU UNIV +1
Method for preparing capsaicin-loaded pH sensitive gel microsphere, and gel microsphere prepared by same
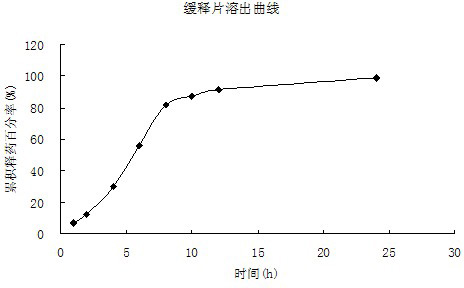
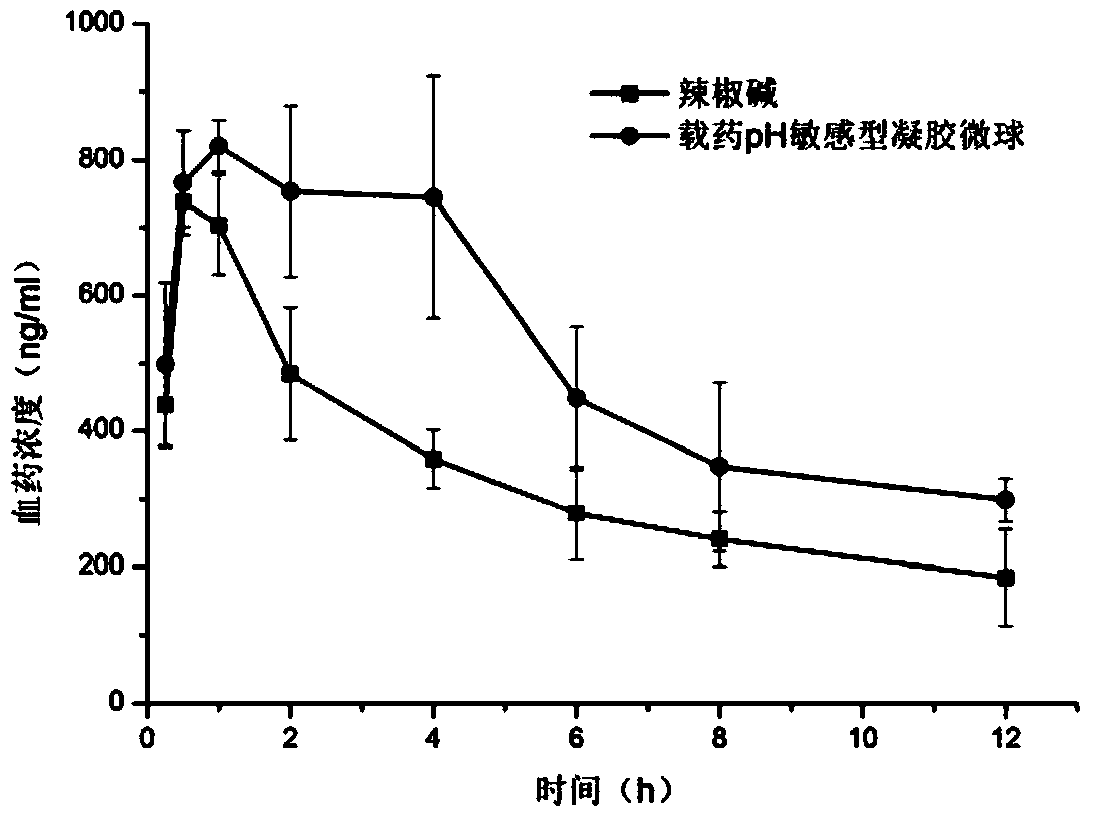
ActiveCN104042571APromote absorption in the bodyImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsDrugIrritation
The invention discloses a method preparing a capsaicin-loaded pH sensitive gel microsphere, and the gel microsphere prepared by the same. According to the method, the capsaicin-loaded pH sensitive gel microsphere is prepared by regulating the proportion and concentrations of capsaicin taken as a raw material medicine and carboxymethyl chitosan and sodium alginate with excellent biodegradability, biocompatibility and low toxicity as a carrier material. According to the gel microsphere, the drug loading capacity can be (18.06+ / -0.26) percent, the encapsulation efficiency is (97+ / -3.66) percent, the release of the gel microsphere in a medium with the pH value of 7.8 is remarkably superior to those in environments with pH values of 1.2, pH 4.8 and pH 6.8, and the gel microsphere has remarkable pH sensitivity and potential colonic targeting trend. Furthermore, after the gel microsphere is administrated in an oral manner, drugs can be multiply transferred step by step to the optimal absorption part along with changes of the pH value of gastrointestinal tracts, and certain characteristics of delayed release and sustained release can be reflected, so that in-vivo absorption of capsaicin can be improved, the bioavailability can be improved, and irritation of the capsaicin to gastric mucosa can be reduced.
Owner:JIANGSU UNIV
Self-microemulsion nutrient composition containing coenzyme Q10 and preparation method and application
ActiveCN106619588AEasy to addInhibit devitrificationNervous disorderKetone active ingredientsEnvironmental resistanceAntioxidant
The invention discloses a self-microemulsion nutrient composition containing coenzyme Q10 and a preparation method and application. The self-microemulsion nutrient composition comprises the following components in parts by weight: 1-15 parts of coenzyme Q10, 1-15 parts of curcumin, 10-50 parts of carrier oil, 15-75 parts of a nonionic surfactant, 1-5 parts of a cationic gemini surfactant, 5-30 parts of a cosurfactant and 0-2 parts of an antioxidant, wherein the components are prepared into a uniform, stable, clear and transparent solution. The self-microemulsion nutrient composition has good stability, crystallization of coenzyme Q10 is remarkably inhibited, and the product shelf life is prolonged. In the application process, the composition can be simply and conveniently added into systems of water-phase food, medicines and the like according to demand amounts, and is safe and effective. Meanwhile, the whole preparation process is green, environmental-friendly, low in energy consumption and easy in industrial production.
Owner:XIAMEN KINGDOMWAY BIOTECH CO LTD +1
Suspension containing andrographolide solid lipid nanoparticles as well as preparation method and application of suspension
ActiveCN102716080AGrowth inhibitory effectImprove tumor inhibition rateOrganic active ingredientsSolution deliveryEmulsionIn vivo absorption
The invention relates to suspension containing andrographolide solid lipid nanoparticles as well as a preparation method and an application of the suspension, wherein the andrographolide solid lipid nanoparticles are dispersed in emulsion formed by purified water and emulsifying agents and consists of andrographolide and lipid materials, the weight ratio of the andrographolide to the lipid materials to the emulsifying agents is (1-15): (35-70):(20-50), and the consumption of the purified water is metered according to the principle that 1ml of purified water is added into 5 to 20mg of emulsifying agents. The suspension has the advantages that the andrographolide is covered and sealed in lipid inner cores of the solid lipid nanoparticles, the in-vivo absorption of the medicine is improved, the bioavailability is improved, meanwhile, and the slow release performance and the targeting performance are realized, so the toxic or side effect is reduced, and the anti-tumor curative effect is improved.
Owner:HENAN UNIVERSITY
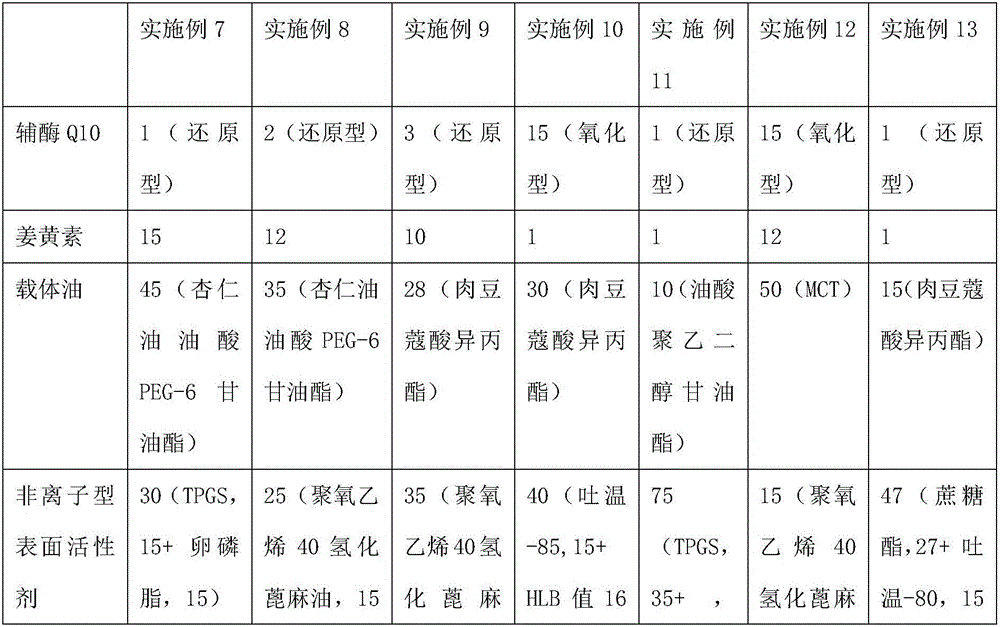
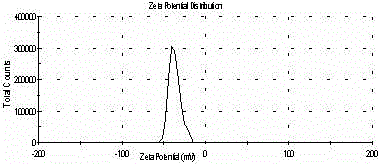
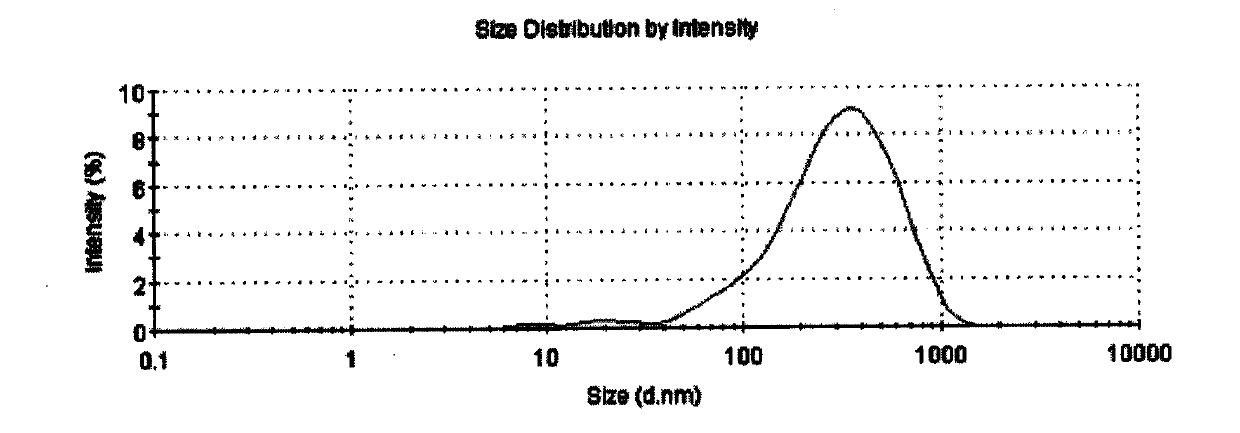
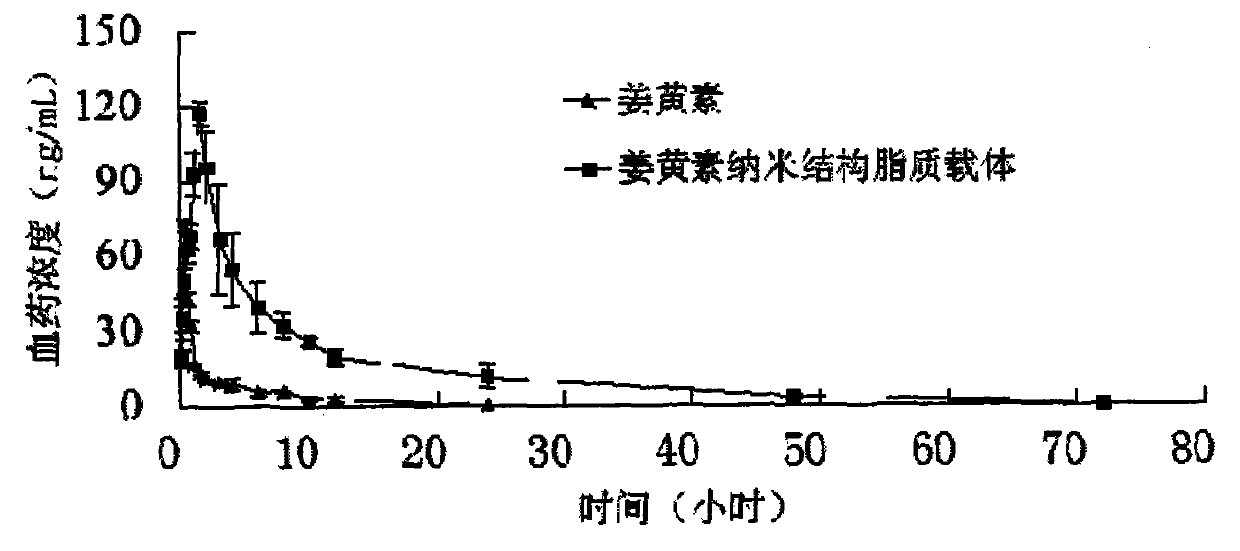
Lipid carrier of curcumin in nano structure and preparation method of lipid carrier
ActiveCN103989659ALow encapsulation efficiencyIncrease inclusivenessAntibacterial agentsMetabolism disorderLipid formationNano structuring
The invention belongs to the field of medicine preparations and relates to a formula of a lipid carrier of curcumin in a nano structure and a preparation method of the lipid carrier. The lipid carrier of curcumin in the nano structure extremely overcomes the deficiencies that curcumin is difficult to be dissolved in water, low in oral bioavailability, fast in metabolism and the like, provides a novel selectable transfer system for curcumin and has important meaning.
Owner:CHONGQING MEDICAL UNIVERSITY
Cyclosporine A fast release solid dosage forms and preparation thereof
InactiveCN101239048AImprove apparent solubilityGood stabilityInorganic non-active ingredientsCyclic peptide ingredientsSolubilitySide effect
Owner:SHANGHAI JIAO TONG UNIV
Dobby polyethylene glycol and its active derivative
InactiveCN107200838AReduce polydispersityImprove solubilityOrganic active ingredientsAerosol deliveryAlcoholEthylene oxide
The invention belongs to the technical field of a macromolecular function material, and particularly relates to a novel narrow-distributed / high-purity dobby polyethylene glycol formed by using polyhydric alcohol glyceryl ether as initiator to polymerize ethylene oxide, and its active derivative. The dobby polyethylene glycol has a structure of a general formula II, wherein B is polyhydric alcohol group and n is integer from 3 to 22; PEG is the same or different -(OCH2CH2)m-, the mean value of m is integer ranged from 3 to 250; number-average molecular weight of the dobby polyethylene glycol is 1500-80000. The invention further provides gel formed by active derivative of the narrow-distributed / high-purity dobby polyethylene glycol, a drug composition formed by the gel and the drug molecule and its application in drug preparation.
Owner:JENKEM TECH
Solid nano-powder composition containing nanoscale cannabidiol and preparation method and application of composition
ActiveCN111991355AEquilibrium Solubility ImprovementImprove physical stabilityPowder deliveryCosmetic preparationsBioavailabilityCannabidiol
The present technology relates to a cannabidiol nano powder, a composition containing the cannabidiol nano powder, a solution of the composition in an aqueous solvent, and application of the composition. The invention also relates to a preparation method of the cannabidiol nano powder. According to the composition containing cannabidiol nanocrystals, the dissolution rate of cannabidiol is improved, and the bioavailability of the cannabidiol in vivo is remarkably improved.
Owner:YUNNAN HEMPMON PHARMA CO LTD
Substituted amine derivative and medicinal composition comprising same as the active ingredient
InactiveCN102216316ANot easy to hydrolyzePhysicochemically stableOrganic active ingredientsAntipyreticDouble bondBULK ACTIVE INGREDIENT
A compound represented by general formula (I) [wherein --- represents a single bond or a double bond; L represents -NH- or the like; X1 represents N or C, provided that in the case where X1 is N, X2 represents C-R and in the case where X1 is C, X2 represents N-R1 or the like; Y's may be either the same or different and represent CH2 or the like; A represents a non-natural sugar residue represented by general formula (II) [wherein m represents 0 to 1; --- represents a single bond or a double bond; X represents 0 or the like; and R3 to R9 may be either the same or different and represent a hydroxyl group or the like]; and B represents a group represented by general formula (III) [wherein n and k represent 0 to 5; --- represents a single bond or a double bond; Z1 to Z16 may be either the same or different and represent CH or the like; R10 and R11 may be either the same or different and represent a lower alkoxy group having 1 to 5 carbon atoms or the like; and R15 represents a hydrogen atom or the like]], a pharmaceutically acceptable salt thereof, a prodrug thereof, a hydrate thereof or a solvate thereof. Because of having an excellent CNT2 inhibitory activity and showing a high in vivo stability and stable physicochemical properties, the aforesaid compound is useful as a remedy for hyperuricemia.
Owner:KOTOBUKI PHARMA CO LTD
Antitumor compound used as AXL inhibitor and application of antitumor compound
InactiveCN110330479AGood choiceSignificant AXL inhibitory activityOrganic active ingredientsOrganic chemistryDiseaseDisease injury
The invention discloses a compound shown in a general formula (I) or pharmaceutically acceptable salt of the compound and preparation methods of the compound and the salt, and further discloses pharmaceutical composition containing the compound and an application of the compound and the pharmaceutical composition in preparation of an AXL inhibitory drug. The AXL inhibitory drug is used for treating tumor, nephropathy, immune system disease or circulatory system disease.
Owner:NANJING HUAWE MEDICINE TECH DEV
Tripterine nanostructure lipid carrier modified by lentiviral vector and appliance for preparing and treating prostatic cancer, lung cancer and breast cancer drug
ActiveCN102670510AImprove physical stabilityGood chemical stabilityOrganic active ingredientsMacromolecular non-active ingredientsTherapeutic effectNanostructure
The invention discloses a tripterine nanostructure lipid carrier modified by lentiviral vector and preparation method and application thereof, wherein teh tripterine nanostructure lipid carrier modified by the lentiviral vector consists of tripterine and a lipid carrier, various components comprise 1 part by weight of tripterine, 0.5 to 10 parts by weight of lentiviral vector, 5 to 100 parts by weight of lipid blends, 0.5 to 20 parts by weight of emulsifier and 5 to 100 parts by weight of stabilizer. The tripterine nanostructure lipid carrier modified by the lentiviral vector can increase nanoparticles encapsulating rate to 78-90%. The tripterine nanostructure lipid carrier modified by the lentiviral vector uses oral administration, thereby promoting absorbing of the tripterine in a body,improving bioavailability, reducing administration dosage, reducing accumulation of the tripterine in the body at last, reducing toxic reaction by combining sustained release of a nanostructure lipidcarrier, and improving clinical therapeutic effect of the tripterine at last.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Atorvastatin calcium and phospholipid compound and preparation method thereof
InactiveCN106692979AChange solubilityImprove solubilityMetabolism disorderPharmaceutical non-active ingredientsMolecular levelPhosphatidic acid
The invention discloses an atorvastatin calcium and phospholipid compound. The atorvastatin calcium and phospholipid compound is prepared from atorvastatin calcium and phospholipid, wherein the mol ratio of the atorvastatin calcium to the phospholipid is (1-4) to (4-1); the concentration of reactants is 0.1mg / ml-10mg / ml; and the phospholipid is selected from at least one of natural soybean phospholipid, natural lecithin or artificially synthetic phosphatidylcholine, phosphatidylglycerol, phosphatidylethanolamine, phosphatidylserine and phosphatidic acid. According to the atorvastatin calcium and phospholipid compound and a preparation method, disclosed by the invention, structural features of insoluble drugs are improved from a molecular level, so that the aims of improving the permeability and increasing the oral bioavailability are realized.
Owner:GUANGDONG PHARMA UNIV
Processing method for increasing solubility and bioavailability of fat-soluble active component
InactiveCN104473168AImprove qualityImprove sustained release efficacyNatural extract food ingredientsFood preparationSolubilityActive component
The invention discloses a processing method for increasing the solubility and the bioavailability of a fat-soluble active component and belongs to the technical field of processing of modern nutritional foods.Water-soluble starch particles are used as carriers, and a nanoscale fat-soluble active component-starch particle compound is prepared with a nano suspension technology, so that the water solubility as well as absorption and utilization effects of the fat-soluble active component are improved. The method can significantly increase the solubility and the bioavailability of insoluble food active components and can be applied to various fields of foods, medicines, daily chemicals and the like.
Owner:JIANGNAN UNIV
A kind of preparation method of Angelica dahurica decoction pieces
InactiveCN102258552AHigh speedPromote dissolutionNervous disorderAntipyreticMedicineBULK ACTIVE INGREDIENT
The invention provides a method for preparing angelica dahurica decoction pieces. The method comprises the following steps: (1) grinding a medicinal material such as angelica dahurica into coarse powder; and (2) drying the coarse powder prepared by the step (1), grinding at 12 to 16 DEG C for 20 minutes in a Bailey supermicro pulverizer BFM-100B till a particle size of 300 to 500 meshes. The invention also provides angelica dahurica decoction pieces prepared by the method. The angelica dahurica decoction pieces prepared by the method have the advantages that: after the supermicro medicinal material is ground on a cell level, the surface area is greatly increased, and active ingredients dissolve out quickly; meanwhile, the active ingredients can well contact gastrointestinal mucosa, so theabsorption is easier, the bioactive is retained and medicinal effect is enhanced; and the angelica dahurica super micro powder obtained by the method can ensure good absorption in vivo when the particle size is between 300 and 500 meshes, so the energy consumed in the supermicro grinding process of the angelica dahurica is reduced greatly.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Absorbable hemostasis composite material based on polyanionic cellulose, and preparation method of absorbable hemostasis composite material
InactiveCN112773929AHemostatic and healing effectWith acute hemostasisSurgical adhesivesPharmaceutical delivery mechanismCelluloseMicrosphere
The invention relates to an absorbable hemostasis composite material based on polyanionic cellulose, and a preparation method of the absorbable hemostasis composite material. The absorbable hemostasis composite material is obtained in a way that a network structure is established by taking the polyanionic cellulose as a base material, and then, the network structure carries out electrostatic adsorption self-assembling with chitosan or chitosan derivatives or carries out electrostatic self-assembling with the chitosan or the chitosan derivatives to load protein polypeptide, or the absorbable hemostasis composite material is obtained in a way that the network structure is established by taking the polyanionic cellulose as the base material, chitosan microspheres or chitosan microspheres and the protein polypeptide are subjected to in situ pelletizing, and then, loading is carried out on the surface and the network of the cellulose. The absorbable hemostasis composite material has the characteristics of acute hemostasis, broad-spectrum bacteria resistance, healing acceleration and in vivo absorption.
Owner:DONGHUA UNIV
Preparation method of full-ingredient active ginseng extracting solution and lyophilized product
InactiveCN107260784APromote absorptionImprove bioavailabilityPlant ingredientsClean technologyGinsenoside
The invention discloses a preparation method of a full-ingredient active ginseng lyophilized product. According to the method, a fresh ginseng is used as a main raw material; advanced technical measures such as a high-pressure bubble cleaning technology, a part-of-tissue shearing and wall breaking technology, a ginseng anaerobic endophytic bacteria bioconversion-ginseng aerobiotic endophytic fungi bioconversion-ginsenosidase conversion combined conversion technology, a centrifugal separation technology, an ultrasonic sterilization technology and a lyophilizing technology; compared with a conventional ginseng processing product preparation method such as drying, steaming and high-temperature extraction, the method has the advantages that the prepared full-ingredient active ginseng lyophilized product has the characteristics of complete retention of active ingredients of the ginseng, high rare ginsenosides content, rapid oral absorption, high biological utilization rate and the like. The product retains the cold and moistening nature of the ginseng; compared with dried and other ginseng processing products with relatively high dryness, the full-ingredient active ginseng lyophilized product has the distinct characteristic that the product is cold but not causing the dryness. A technical support can be provided for upgrading the ginseng industry in China.
Owner:李学龙
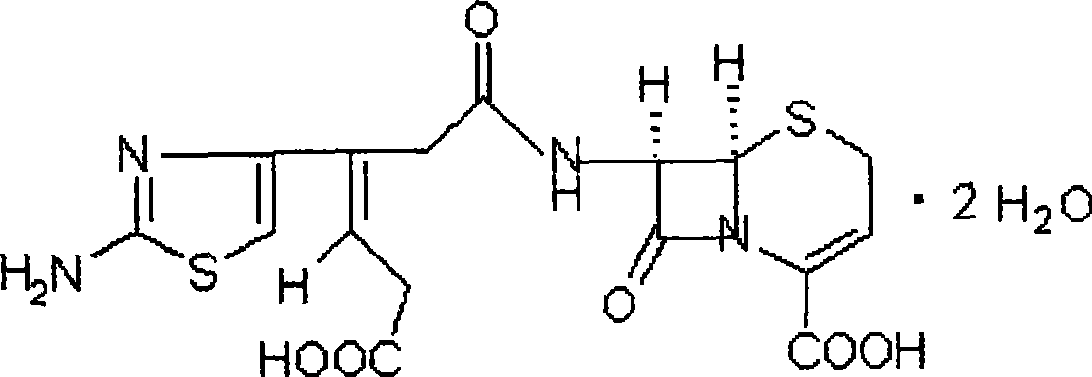
Ceftibuten dispersible tablet
InactiveCN101468017ASimple methodGood stabilityOrganic active ingredientsDigestive systemSolubilityWater soluble
The invention belongs to the novel technology field of the medicines and relates to a novel dosage form of ceftibuten for treating: upper respiratory tract infection such as pharyngitis, antiaditis, scarlet fever, paranasal sinusitis, and otitis medium; lower respiratory tract infection such as acute bronchitis, acute attack of chronic bronchitis and pneumonia; urinary tract infection; enteritis and gastroenteritis. The dosage form is characterized in that the dosage form is dispersing tablets, and can disintegrate quickly into fine granules when encountering water, and the granules uniformly disperse and can pass through size 2 sieve (24 mesh). Preparing the medicines with small dose and low water-solubility into the dispersing tablets can obviously improve absorption in vivo and improve bioavailability.
Owner:BEIJING HOPE HUGE PHARM SCI
Medicinal composition of methanesulfonic acid arbidol oral solid preparation
ActiveCN102357093ARapid dissolutionPromote absorption in the bodyOrganic active ingredientsSulfonic acids salts preparationExcipientDispersible tablet
The invention relates to the technical field of medicinal oral solid preparations, in particular to a medicinal composition of a methanesulfonic acid arbidol oral solid preparation serving as an anti-influenza virus medicament. The composition exists in the form of film-coated tablets, dispersible tablets, chewable tablets, capsules or particles; the film-coated tablets, dispersible tablets, chewable tablets, capsules or particles consist of an effective dosage of methanesulfonic acid arbidol and excipient; and the excipient contains organic acid for enhancing the stability of the medicinal composition, and contains more than three of a diluent, a bonding agent, a disintegrating agent, a flavoring agent and a lubricant. The medicinal composition has the advantages of stable mass, reasonable formula, application of nontoxic excipient, high safety, small using amount of auxiliary materials, capability of meeting the requirement of the oral solid preparation, quick dissolving of all dosage forms, medicament dissolving rate over 85 percent within 15 minutes, contribution to in-vivo absorption of the oral solid preparation and guarantee of better curative effect.
Owner:HUBEI LIYI PHARM TECH CO LTD
Composition of tall gastrodia tuber and lecithin, and preparation method thereof
ActiveCN101972412AHas a sedative effectImprove hypoxiaOrganic active ingredientsNervous disorderGastrodia Elata ExtractYolk
The invention discloses the composition of tall gastrodia tuber and lecithin, and a preparation method thereof. The composition is prepared by mixing tall gastrodia tuber extract and the lecithin according to the mass ratio of 10:90 to 95:5, wherein the tall gastrodia tuber extract is whole tall gastrodia tuber powder or the alcohol extract of medicinal parts of the tall gastrodia tuber; the lecithin is egg yolk lecithin or soybean lecithin; and the alcohol extract is powder obtained by refluxing and extracting the whole tall gastrodia tuber powder or the alcohol extract of the medicinal parts of the tall gastrodia tuber by using 30 to 90 volume percent ethanol, collecting extract, concentrating the extract into paste and drying and crushing the paste. The composition is prepared by compounding the tall gastrodia tuber extract and the lecithin, is rational in formula, advanced in process, safe and effective, has complementary functions and the effects of invigorating the brain and tolerating hypoxia, supplements neural transmitting substances by the lecithin, and simultaneously has the central nervous system depression function of the tall gastrodia tuber extract and the functions of improving the anaerobic condition of a human body, particularly the brain, and protecting the brain from damage.
Owner:陕西医药控股医药研究院有限公司
New application of cistanche polysaccharides and pharmaceutical composition with same
InactiveCN108524535APromote absorption in the bodyHas estrogen-like effectsOrganic active ingredientsSkeletal disorderIn vivo absorptionBULK ACTIVE INGREDIENT
The invention belongs to the field of medicines, and relates to a new application of cistanche polysaccharides and a pharmaceutical composition with the same, in particular to application of cistanchepolysaccharides for preparation of a medicament for promoting in vivo absorption of phenylethanoid glycosides. The invention also relates to a pharmaceutical composition containing cistanche polysaccharides as an active ingredient, a phenylethanoid glycoside compound and / or a plant extract containing a phenylethanoid glycoside compound. Cistanche polysaccharides can effectively promote the in vivo absorption of phenylethanoid glycosides. The pharmaceutical composition disclosed by the invention has an estrogen-like action and is effective for treating and / or preventing osteoporosis.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Amorphous form of avanafil, preparation method of, application and medicine composition of amorphous form of avanafil
InactiveCN104628707AStable in natureHigh purityOrganic active ingredientsOrganic chemistryMedicineDrugs preparations
The invention relates to an amorphous form of avanafil, a preparation method, application and a medicine composition of the amorphous form of avanafil. The amorphous form of avanafil has no obvious characteristic peak in powder X-ray diffraction pattern based on Cu-K alpha radiation and shows a valuable characteristic in drug preparation; the amorphous form is high in purity and stable, and the in-vivo absorption of the amorphous form is superior to that of a crystal form.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Medicinal composition and its preparation and use
InactiveCN1582950AApparent particle size effectImprove solubilityOrganic active ingredientsMetabolism disorderMedicineSURFACTANT BLEND
A composite medicine in the form of tablet, capsule, suspension, or injection for protecting liver is prepared from oleanolic acid, surfactant and water. Its preparing process is also disclosed.
Owner:HUAZHONG UNIV OF SCI & TECH +1
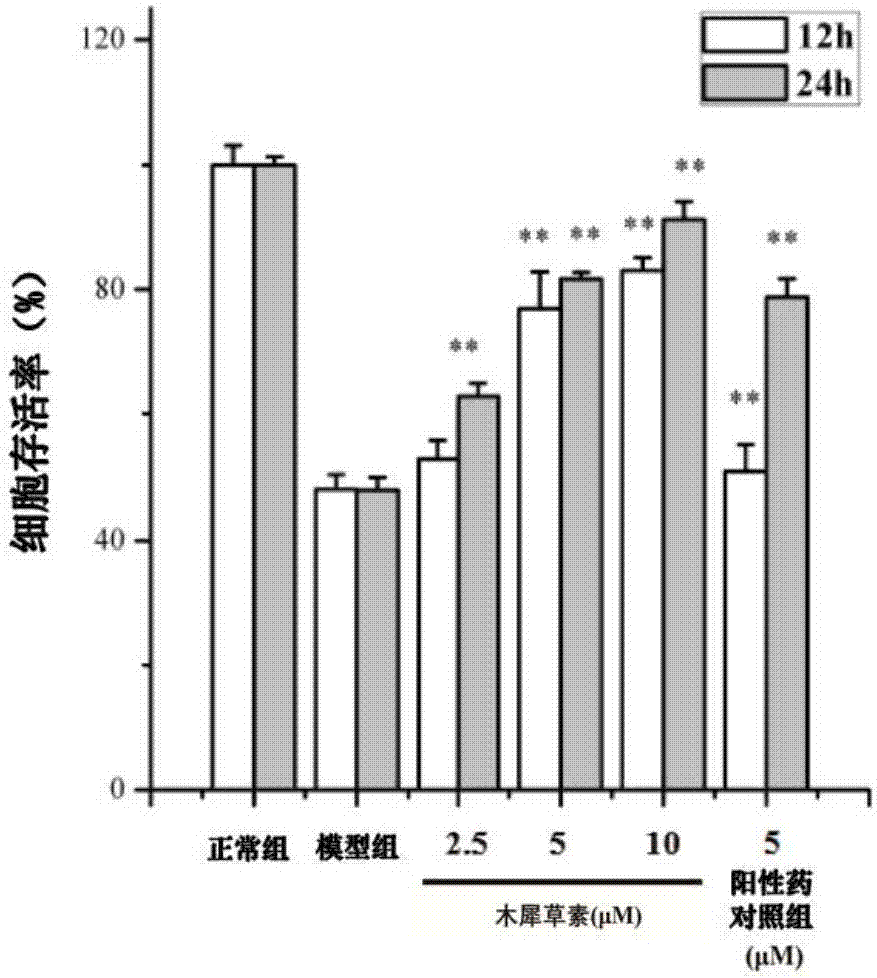
Application of luteolin to preparation of medicine for treating oxidative stress injury of testicular tissue
InactiveCN107198684AImprove protectionGood absorption in the bodyOrganic active ingredientsSexual disorderGynecologySustentacular cell
The invention provides application of luteolin to the preparation of a medicine for treating an oxidative stress injury of testicular tissue, and relates to the field of male reproduction and endocrinic medicines. An active component of a medicinal composition is the luteolin. Discovered through an experiment, the injury, which is caused by hydrogen peroxide and triptolide, of a testicular sustentacular cell can be reversed through the intervention treatment of the luteolin; the apoptosis of the testicular sustentacular cell induced by the hydrogen peroxide is inhibited; the accumulation of ROS (Reactive Oxygen Species) is inhibited; the collapse of a cellular mitochondrial membrane potential caused by the hydrogen peroxide is reversed; the expression of a downstream antioxidase gene can be activated; the autologous oxidation-resistance capacity of the sustentacular cell is improved; the injury to the testicular tissue of a mouse induced by the triptolide is effectively reversed. Besides, the luteolin can be also used for inhibiting the increment of the permeability of a blood-testis barrier, which is caused by the triptolide-induced oxidative stress injury, through regulating and controlling gap junction, occlusion junction and tight junction protein; the completeness of the blood-testis barrier is protected. Therefore, the luteolin can be applied to the preparation of the medicine for treating the oxidative stress injury of the testicular tissue.
Owner:NANJING UNIV OF TECH
Curcumin self-microemulsion and preparation method thereof
InactiveCN101869692BImprove solubilityImprove membrane permeabilityMetabolism disorderAntipyreticSolubilityMembrane permeability
The invention discloses a self-microemulsion, in particular to a curcumin self-microemulsion and a preparation method thereof. The curcumin self-microemulsion is characterized by the following components by weight percent: 0.05%-10% of curcumin, 1%-35% of oil phase, 20%-80% of surfactant, and 2%-40% of cosurfactant. The curcumin self-microemulsion and the preparation method thereof has the benefits that: a curcumin self-microemulsion release system increases the solubility of drugs, protects the drugs dissolved in oil drops, and improves the stability in a body; and the surfactant can change the flowability of cell membranes, improve membrane permeability of the drugs, enhance the absorption of the drugs in the body and increase the relative bioavailability of oral drugs.
Owner:SHANDONG LUNUO ANIMAL PHARMA
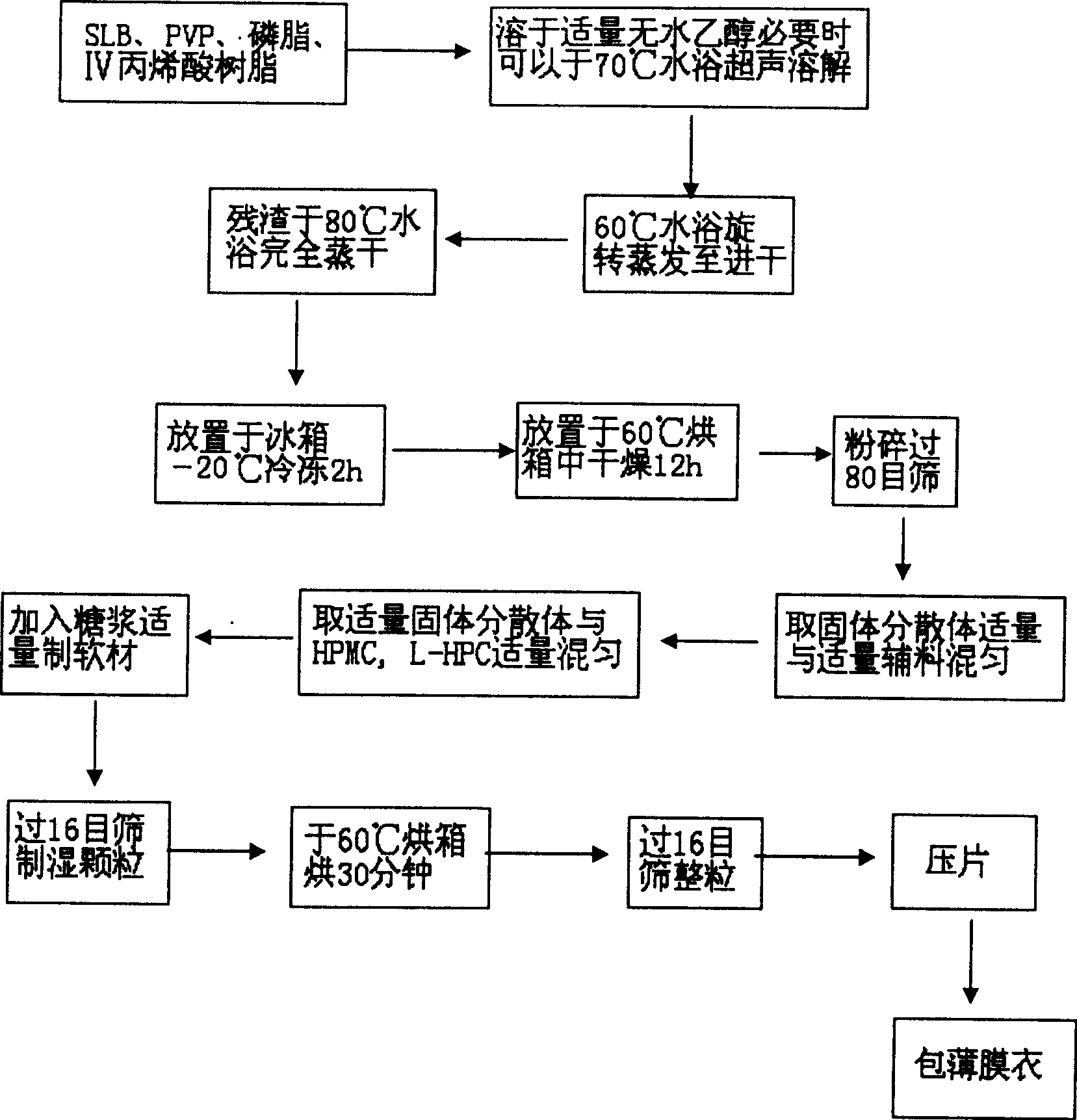
High-efficient oral silibinin sustained-release preparation and preparation method thereof
ActiveCN101164537BImprove solubilityRapid drug releaseOrganic active ingredientsDigestive systemMedicineMethyl cellulose
The present invention relates to a high-effective oral silibinin (SLB) slow-released preparation. Its composition includes (by mass component portion) 1 portion of silibinin, 1.5-2.5 portions of polyvidone-K30, 0.23-0.58 portion of hydroxypropyl methyl cellulose 4000cPa.S, 0.46-1.38 portions of low-substituted hydroxypropyl cellulose. Said invention adopts the combination of solid dispersion technique and slow-released hydrophilic gel skeleton technique to raise the dissolubility of silibinin.
Owner:JIANGSU UNIV
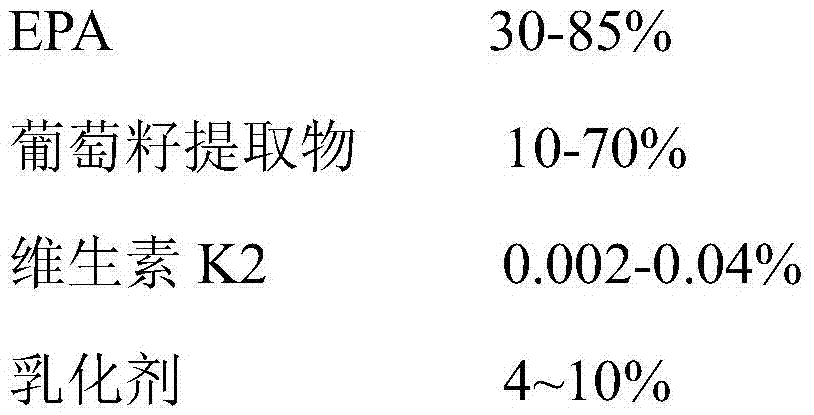
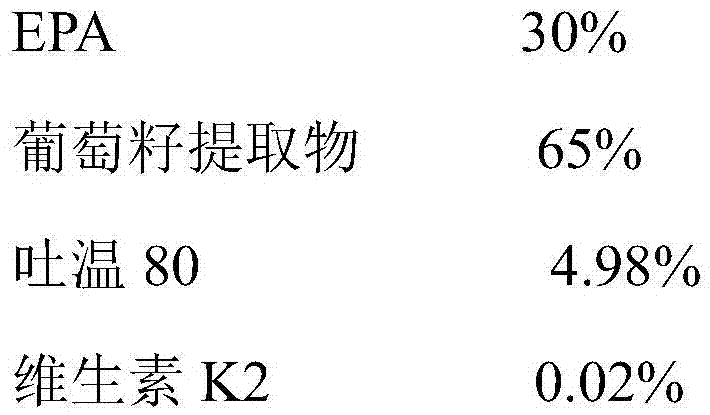
Self-emulsifying eicosapentaenoic acid nutrient composition and application thereof
InactiveCN106937987AGood treatment effectSmall side effectsOrganic active ingredientsSkeletal disorderVitamin K2Side effect
The invention provides a self-emulsifying EPA (eicosapentaenoic acid) nutrient composition and an application thereof. The self-emulsifying EPA nutrient composition comprises, by weight, 30-85% of EPA, 10-70% of grape seed extract, 0.002-0.04% of vitamin K2, and 4-10% of an emulsifier. The self-emulsifying EPA nutrient composition has a substantial arthritis treatment effect, has small toxic and side effects, can be taken for a long term without dependence, also can promote calcium absorption to make bones healthy, and has healthcare effects. The self-emulsifying EPA nutrient composition increases in-vivo absorption, and the addition of a calcium absorption promoting factor-vitamin K2 promotes the healthiness of the bones during therapy.
Owner:SINO NUTRACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com