Itraconazole nanocrystals and preparation method and application thereof
A technology of itraconazole and nano-crystallization, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients. And clinical trials, low bioavailability of preparations and other issues, to achieve the effect of facilitating drug absorption, low cost, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Mix and stir 100mg itraconazole with 3ml 3mol / L hydrochloric acid solution and 2ml absolute ethanol as a co-solvent to completely dissolve and form an acid phase;
[0039] Mix the polymer stabilizer 10mg hydroxypropyl methylcellulose (50cp), 0.3g NaOH and 10ml water, stir to dissolve each component completely to form an alkali phase;
[0040] The acid phase is added dropwise to the base phase under stirring at a stirring speed of 50 r / min. After the dropwise addition is completed, the itraconazole nanocrystalline drug suspension is obtained by stirring evenly.
[0041] The effective particle size was measured at 25° C. with a laser particle size analyzer (Nano ZS90, Malvern, UK), and the effective particle size was 309.26 nm.
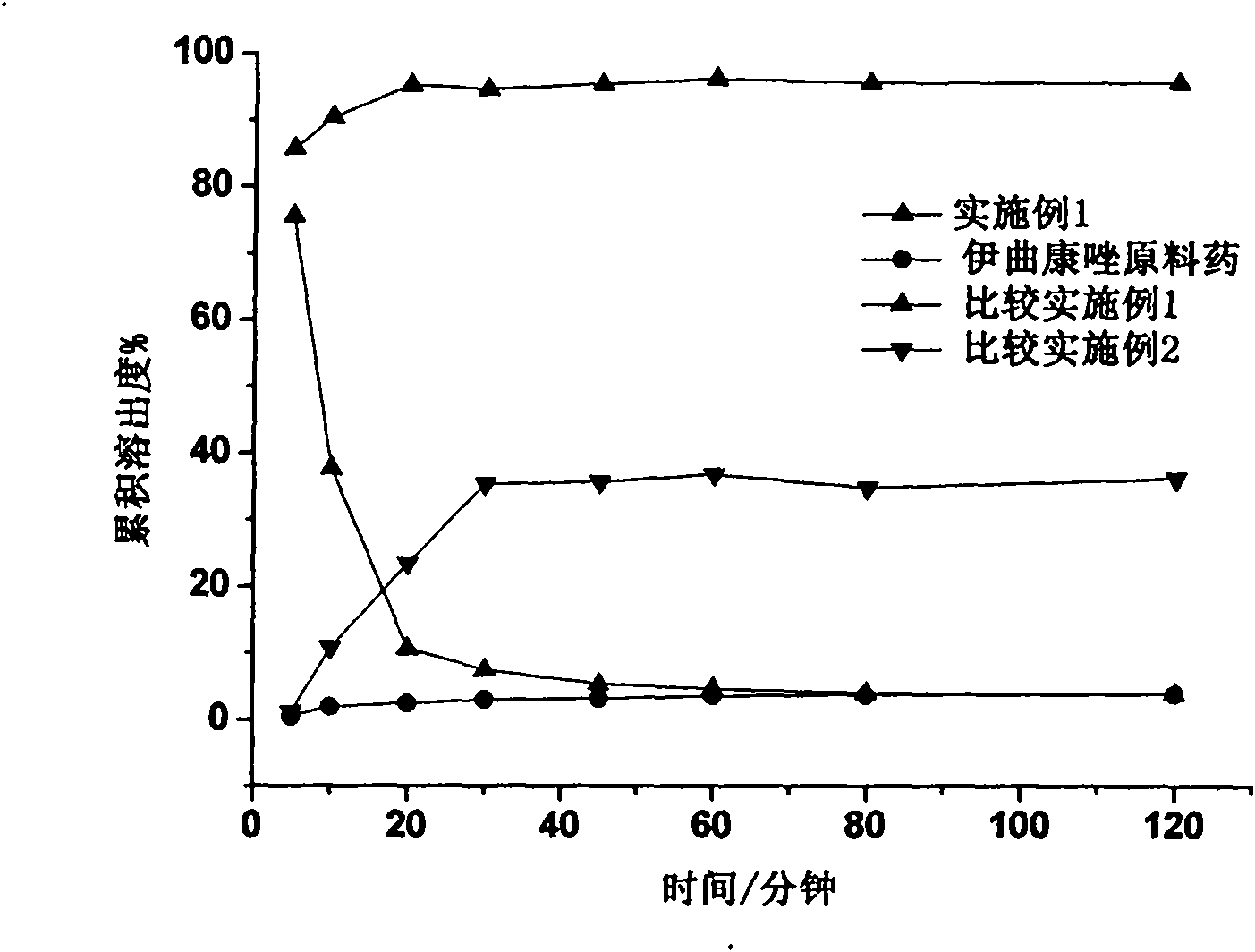
[0042] Dissolution in vitro: adopt the second method by "Chinese Pharmacopoeia" 2005 edition two appendix X C dissolution assay method, adopt rotating speed per minute 100 turns; Dissolution medium pH=1.38 hydrochloric acid solution (degassing tr...
Embodiment 2
[0045] 100mg of itraconazole, 5ml of 3mol / L hydrochloric acid solution, mixed and stirred to dissolve completely, and then the solution formed by dissolving polymer stabilizer 50mg hydroxypropyl methylcellulose (50cp) in 4ml of distilled water was mixed with it to form an acid phase;
[0046] Polymer stabilizer 50mg hydroxypropyl methylcellulose (50cp), 0.3g NaOH and 10ml water were mixed, stirred to completely dissolve each component to form an alkali phase.
[0047] The acid phase is added dropwise into the alkali phase under stirring condition, and the stirring speed is 500r / min, and the itraconazole nano crystal drug suspension is obtained.
[0048] Measure its effective particle size with a laser particle size analyzer at 25°C, and the effective particle size is 287.9nm. 45min in vitro dissolution up to 91%.
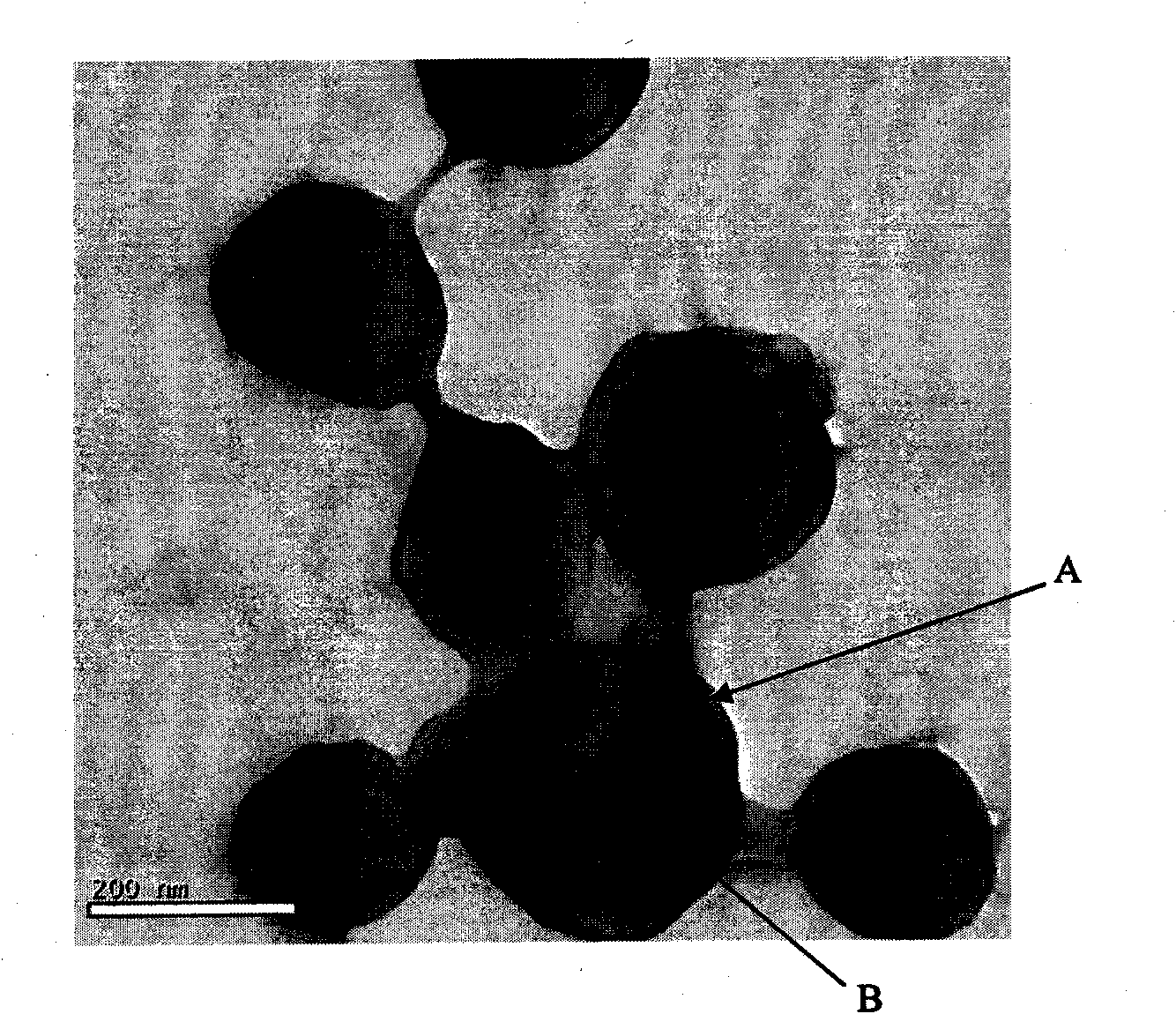
[0049] X-diffraction (X'Pert PRO Dutch Panalytical B.V.) inspection see figure 2 , the results showed that itraconazole nanocrystals were in an amorphous state. ...
Embodiment 3
[0051] 100mg itraconazole, polymer stabilizer 200mg polyvinylpyrrolidone (K30), 2ml 3mol / L hydrochloric acid solution, mixed and stirred to completely dissolve to form an acid phase;
[0052] Surfactant 10mg poloxamer188, 0.3g NaOH mixed with 10ml water, stirred to completely dissolve each component to form an alkali phase.
[0053] The acid phase is added dropwise to the alkali phase under stirring condition, and the stirring speed is 5000r / min, and the itraconazole nano crystal drug suspension is obtained.
[0054] Measure its effective particle size with a laser particle size analyzer at 25°C, and the effective particle size is 296.33nm. 45min in vitro dissolution up to 92%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com