Oral ciclosporin A sustained-release agent and preparation method thereof
A technology for sustained-release preparations and cyclosporine, which is applied in the directions of cyclic peptide components, pharmaceutical formulations, medical preparations with inactive ingredients, etc. Slow drug release, high bioavailability and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
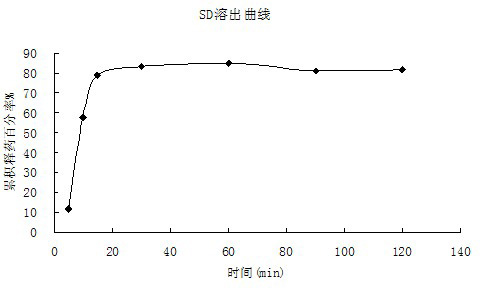
[0038] 1. Accurately weigh 1 g of cyclosporine A, 2.5 g of povidone-K30, 0.1 g of phospholipids, and 1.0 g of poloxamer 188, add 20 ml of absolute ethanol to dissolve, put in a water bath at 60 ° C, and 90 rpm rotary evaporation until nearly dry, The solvent was completely evaporated in a water bath at 70°C, placed in a -20°C refrigerator for 2 hours, then placed in a vacuum oven at 60°C for 12 hours, pulverized, and passed through an 80-mesh sieve to obtain a solid dispersion for future use.
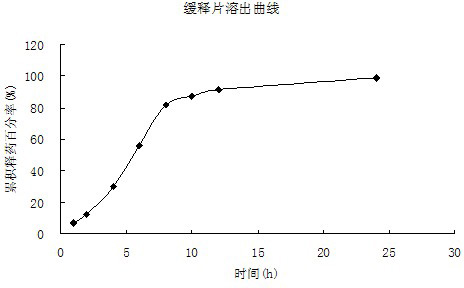
[0039] 2. Weigh 2.6g of solid dispersion, mix with hypromellose 4000cPa.s2.41g, microcrystalline cellulose 1g, add 70% syrup to prepare soft material, pass through a 16-mesh sieve to obtain wet granules, and store at 60°C After baking in an oven for 30 minutes, take it out, pass through a 16-mesh sieve for granulation, and press into tablets with a pressure of 4 to 5 kg to obtain about 20 sustained-release tablets.
[0040] 3. The experimental results are as follows: Water, artificial g...
Embodiment 2
[0043] 1. Accurately weigh 1g of cyclosporine A, 3.5g of povidone-K30, 0.35g of phospholipid, and 0.45g of poloxamer 188, add 20ml of absolute ethanol to dissolve, put in a 60°C water bath, 90rmp rotary evaporation to nearly dryness, The solvent was completely evaporated in a water bath at 70°C, placed in a -20°C refrigerator for 2 hours, then placed in a vacuum oven at 60°C for 12 hours, pulverized, and passed through an 80-mesh sieve to obtain a solid dispersion for future use.
[0044] 2. Weigh 2.65g of solid dispersion, mix with hypromellose 4000cPa.s1g, hypromellose 15000cPa.s1.285g, microcrystalline cellulose 1.97g and mix evenly, add 70% syrup to prepare soft material, pass Wet granules were obtained through a 16-mesh sieve, dried in an oven at 60°C for 30 minutes, taken out, passed through a 16-mesh sieve for sizing, and compressed into tablets with a pressure controlled at 4 to 5 kg to obtain about 19 sustained-release tablets. The obtained sustained-release tablet ha...
Embodiment 3
[0046] 1. Accurately weigh 1g of cyclosporin A, 4.5g of povidone-K30, 0.55g of phospholipids, and 0.55g of poloxamer 188, add 20ml of absolute ethanol to dissolve, put in a water bath at 60°C, 90rmp rotary evaporation until nearly dry, The solvent was completely evaporated in a water bath at 70°C, placed in a -20°C refrigerator for 2 hours, then placed in a vacuum oven at 60°C for 12 hours, pulverized, and passed through an 80-mesh sieve to obtain a solid dispersion for future use.
[0047] 2. Weigh 3.3g of solid dispersion, mix with hypromellose 4000cPa.s1.5g, hypromellose 15000cPa.s0.75g, microcrystalline cellulose 1.5g, add 70% syrup to prepare soft material , passed through a 16-mesh sieve to obtain wet granules, baked in an oven at 60°C for 30 minutes, took them out, passed through a 16-mesh sieve for sizing, and pressed into tablets with a pressure controlled at 4-5kg to obtain about 19 sustained-release tablets.
[0048] 3. The solubility and in vitro drug release perfo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com