High-efficient oral silibinin sustained-release preparation and preparation method thereof
A technology for oral administration of silibinin and silibinin, which is applied in the field of preparation of high-efficiency oral sustained-release preparations of poorly soluble drugs, can solve the problems of reducing the number of drug administrations, not providing in vivo measurement results, etc., and achieves faster release speed and faster release rate. Constant, solubility-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
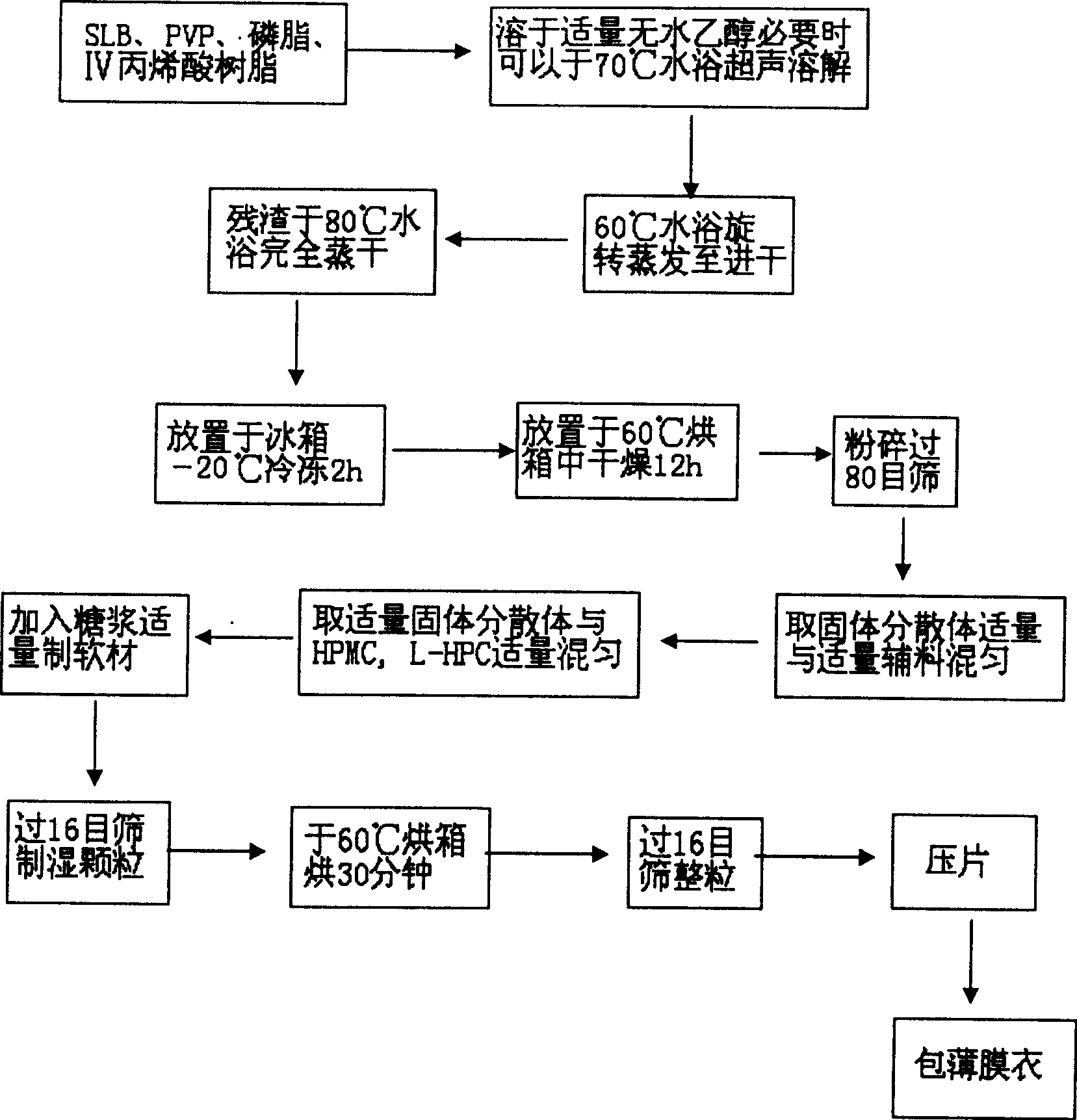
[0032] 1. Weigh 2g of SLB, 304g of PVP-K, 1g of phospholipid, 0.6g of IV acrylic resin, add 20ml of absolute ethanol to dissolve (if necessary, put it in a 70°C water bath to accelerate the dissolution), put it in a 60°C water bath, 90rpm rotary evaporation to nearly dry , in a 70°C water bath to completely evaporate the solvent, put it in a -20°C refrigerator for 2 hours, put it in a 60°C oven for 12 hours, pulverize it, and pass through a 80-mesh sieve to obtain a solid dispersion, which is set aside.
[0033] 2. Take 7g of solid dispersion, mix it with HPMC4000cPa·s0.5g and L-HPC1g, and then add an appropriate amount of 70% syrup to prepare a soft material. Pass through a 16-mesh sieve to obtain wet granules, bake at 60°C for 30 minutes, and then take them out. Sieve through a 16-mesh sieve for granulation, press into tablets, and control the pressure at 40-60N to obtain 34 bare sustained-release tablets.
[0034] 3. Put 0.058g of IV acrylic resin into a beaker, add 1.0ml o...
Embodiment 2
[0036] 1. Weigh 2g of SLB, 304g of PVP-K, 1g of phospholipid, 0.6g of IV acrylic resin, add 20ml of absolute ethanol to dissolve (if necessary, put it in a 70°C water bath to accelerate the dissolution), put it in a 60°C water bath, 90rpm rotary evaporation to nearly dry , in a 70°C water bath to completely evaporate the solvent, put it in a -20°C refrigerator for 2 hours, put it in a 60°C oven for 12 hours, pulverize it, and pass through a 80-mesh sieve to obtain a solid dispersion, which is set aside.
[0037] 2. Take 7g of solid dispersion, mix with HPMC4000cPa·s2g, L-HPC3g, after mixing, add an appropriate amount of 70% syrup to prepare soft material, pass through a 16-mesh sieve to obtain wet granules, bake at 60°C for 30 minutes, take out, pass through 16 Mesh sieve granulation, tabletting, the pressure is controlled at 40-60N, to obtain 48 bare sustained-release tablets.
[0038] 3. Put 0.07g of IV acrylic resin in a beaker, add 1.5ml of absolute ethanol to dissolve it,...
Embodiment 3
[0040] 1. Weigh 2g of SLB, 303g of PVP-K, 0.8g of phospholipid, 0.4g of IV acrylic resin, add 20ml of absolute ethanol to dissolve (if necessary, put it in a water bath at 70°C to accelerate the dissolution), put it in a water bath at 60°C, and evaporate it at 90rpm to nearly Dry, completely evaporate the solvent in a water bath at 70°C, place in a refrigerator at -20°C for 2 hours, then place in an oven at 60°C for 12 hours, pulverize, pass through an 80-mesh sieve to obtain a solid dispersion, and set aside.
[0041] 2. Take 6.3g of solid dispersion, mix with HPMC4000cPa·s0.9g, L-HPC1.35g, after mixing, add an appropriate amount of 70% syrup to prepare soft material, pass through a 16-mesh sieve to obtain wet granules, and bake at 60°C for 30 minutes Finally, take it out, pass through a 16-mesh sieve for granulation, and press into tablets with a pressure of 40-60N to obtain 34 bare sustained-release tablets.
[0042] 3. Put 0.054g of IV acrylic resin into a beaker, add 1.0m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com