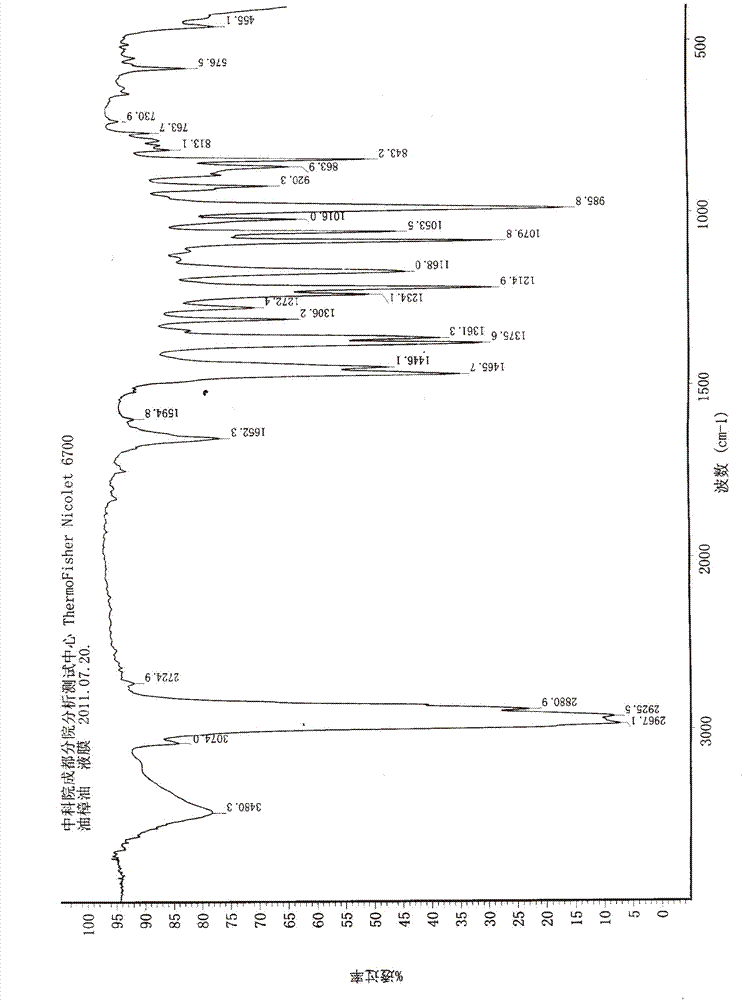
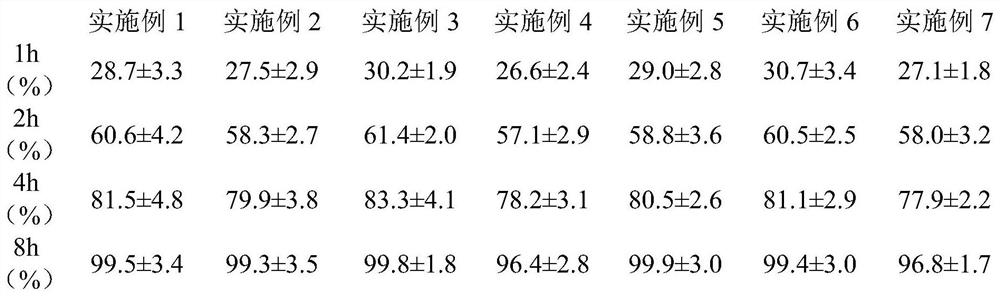
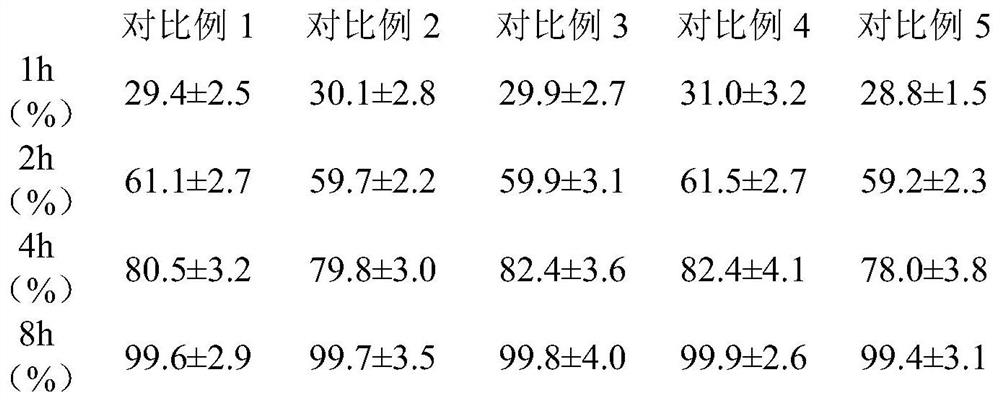
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57results about How to "Increase route of administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
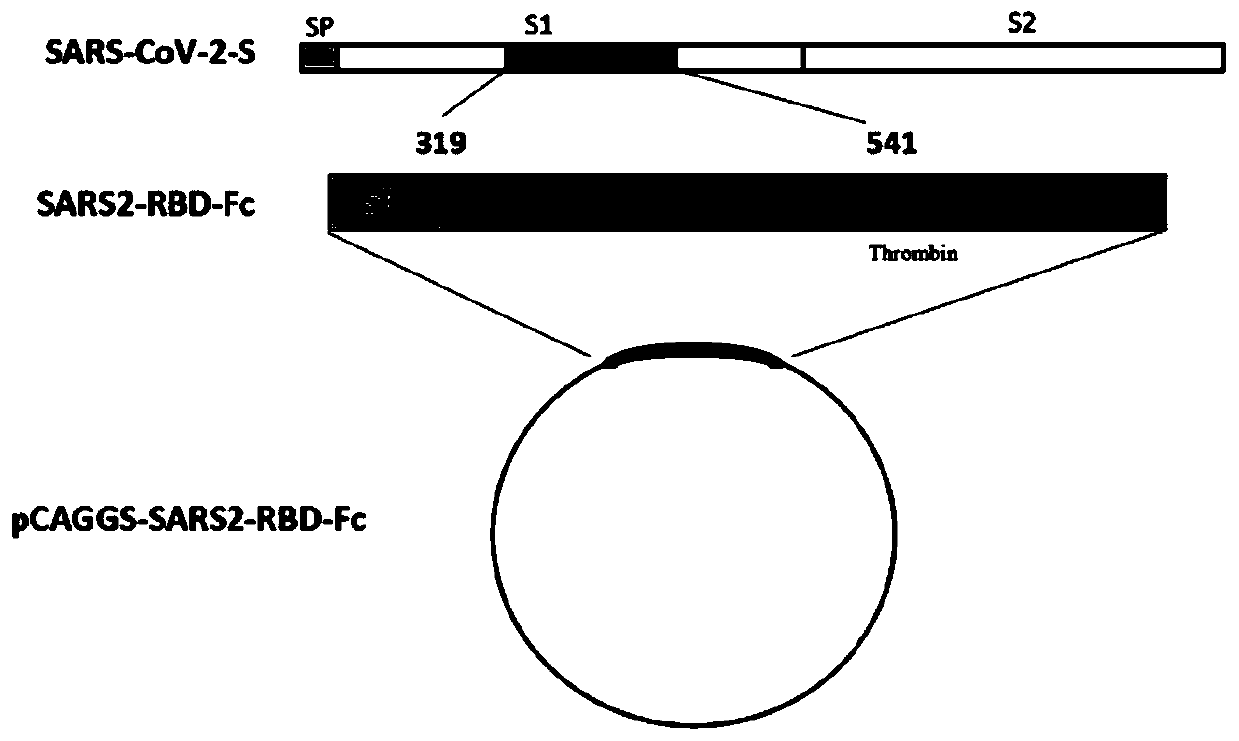
Subunit vaccine for novel coronavirus and application of subunit vaccine
InactiveCN111533809AImproving immunogenicityImprove stabilitySsRNA viruses positive-senseViral antigen ingredientsAntibody fragmentsTGE VACCINE
The invention discloses a fusion protein of a novel coronavirus envelope protein and an application of the fusion protein. The fusion protein (SARS2-RBD-Fc) is obtained by fusing an RBD structural domain of a novel coronavirus envelope protein S with an antibody Fc fragment; and as a subunit vaccine, the fusion protein can induce an organism to generate an efficient neutralizing antibody through nasal drip immunization and intramuscular injection. It indicates that the SARS2-RBD-Fc can be used as a candidate vaccine for preventing and treating new coronavirus infection.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Human monoclonal antibody
InactiveUS20050175986A1Increase concentrationReduce the amount requiredAnimal cellsSugar derivativesEpitope specificityProphylactic treatment
This invention relates to novel human monoclonal antibodies (mAbs) and to the genes encoding same. More specifically, this invention relates to human monoclonal antibodies specifically reactive with an epitope of the fusion (F) protein of Respiratory Syncytial Virus (RSV). Such antibodies are useful for the therapeutic and / or prophylactic treatment of RSV infection in human patients, particularly infants and young children.
Owner:SMIT KLINE BEECHAM +1
Arctigenin pro-drug, preparation method and use thereof
InactiveCN101284823AImprove oral bioavailabilityGood water solubilityOrganic active ingredientsOrganic chemistryGlutaric acidPhosphate
The invention relates to the following general formula compound (I) and use of the general formula compound in preparing anti-inflammatory and anti-endotoxin medicines. In formula I, R is an acid solubilizing side chain of hydroxide radical in an arctigenin molecule, and can be an acid group or side chain imported through alkylation reaction or acylating reaction, such as sulphonic acid ester, sulfuric acid ester, organic phosphate, succinate, propionic ether, butyric ester, semi-succinate, semi-glutaric acid ester, semi-tartrate, semi-phthalic acid ester, m-benzene sulfonic acid ester and so on.
Owner:YANTAI TARGET DRUG RES
Streptococcus faecium function signal molecule formulation and product thereof for reducing fat and slimming
InactiveCN101297820AEnterohepatic circulation blockadeControl levelMilk preparationPowder deliveryBacteroidesDisease
The invention relates to a streptococcus faecium function signal molecule preparation, a streptococcus faecium bacteria strain is carried out the anaerobic fermentation at 35 to 38 DEG C and the centrifugal concentration to obtain a concentrate of streptococcus faecium bacteria, the bacterial cell wall is broken by high pressure, and the freeze-drying is carried out to obtain the preparation raw powder containing the streptococcus faecium function signal molecules. As the streptococcus faecium function signal molecules can be combined with a specific receptor at the intestinal epithelial cells, the issued signal can induce the excessive cholesterol in the body to carry out the transformation, control the activity of the cholesterol synthase, reduce the excessive cholesterol generated or absorbed in the human body, and effectively control the obesity. The streptococcus faecium function signal molecule preparation is simultaneously matched with a bifidobacterium function signal molecule preparation, a lactobacillus function signal molecule preparation and a bacteroides function signal molecule preparation to be prepared into a series of products containing various probiotics function signal molecules for lipid-lowering and weight-losing, thus fundamentally reducing the risks of coronary heart disease, atherosclerosis, heart diseases, angina, myocardial infarction, obesity and other diseases during the process of being eaten by the people.
Owner:SENBAIAO SCI & TECH UNIV DALIAN
Beta-cyclodextrin inclusion compound of huperzine A, and preparation method and preparation thereof
InactiveCN101991859AReduce lossGood content uniformityOrganic active ingredientsNervous disorderOrganic solventWater insoluble
The invention discloses a beta-cyclodextrin inclusion compound of huperzine A, and a preparation method and preparations thereof. The beta-cyclodextrin inclusion compound of the huperzine A consists of 2.5 to 9.5 weight percent of huperzine A and 97.5 to 90.5 weight percent of beta-cyclodextrin. The preparation method comprises the following steps of: a) dissolving the huperzine A in an pharmaceutically acceptable organic solvent to prepare solution A, and heating and dissolving the beta-cyclodextrin in water to prepare solution B; and b) slowly adding the solution A into the solution B with stirring, and continuing to perform stirring until the mixed solution is uniform to prepare the beta-cyclodextrin inclusion compound of the huperzine A. The beta-cyclodextrin inclusion compound of the huperzine A further can be mixed with water-soluble carriers with viscosity, such as starch and the like, and then is pelletized, tabletted or filled into capsules with substrates. In the method, an extremely small amount of water-insoluble huperzine A is fully, effectively and uniformly dispersed, so the dissolution rate of the huperzine A is increased and the stability of the huperzine A is improved. The provided beta-cyclodextrin inclusion compound of the huperzine A can be used for preparing solid preparations and liquid preparations, operating steps are reduced and the production cost is remarkably reduced.
Owner:上海复旦复华药业有限公司 +1
Thymus gland pentapeptide oral intestine-dissolved formulated product and method of preparing the same and use thereof
ActiveCN101108246ASolve the problem of oral drug deliveryImprove complianceOrganic active ingredientsPeptide/protein ingredientsIntestinal structureAdditive ingredient
The invention provides a Thymopentin Oral Enteric-coated Agent, which takes thymopentin of effective dosage as the active ingredient and enteric-coated agent as its accessories. Each dosage contains 5mg to 150 mg thymopentin. The invention also provides the preparation method and usage of the enteric-coated agent. The medicine effect tests prove that the medicine has the same indication and efficacy as the injections and can overcome that the gastrointestinal enzyme will easily degrade the thumopentin into amino acid and small peptide so as to lose the activity when orally taking the Thymopentin; the thumopentin can not easily penetrate the gastrointestinal mucosa, resulting in low bioavailability; and the liver has the First-pass effect on the thymopentin. The invention opens a new way to apply thumopentin and increases the patients' compliance.
Owner:CHENGDU DIAO JIUHONG PHARMA FAB
Sodium alginate microspheres blood vessel suppository containing etoposide and preparation method and uses thereof
InactiveCN101385696ASolve the problem of water solubilityAddress reactivityOrganic active ingredientsSurgerySmall-cell carcinomaAtherion elymus
The invention belongs to the field of medical embolization devices, relates to a sodium alginate microsphere targeted vascular embolization agent containing an antineoplastic drug and a preparation method thereof. Alginic acid is taken as a pharmaceutical carrier, the antineoplastic drug etoposide is a pharmaceutical active ingredient, divalent metal cation or calcium ion solution is taken as a solidifying agent, and the sodium alginate encapsulates the etoposide to prepare ideal particle size-controllable sodium alginate microspheres comprising the etoposide, thus avoiding toxic side effect of traditional etoposide administration such as anaphylaxis and inconvenience. The vascular embolization agent changes the dosage form and route of administration way of the antineoplastic drug etoposide, has high efficacy and low toxicity, and is safely and effectively applied to clinical application. The vascular embolization agent has the advantages of mild preparation condition and simple and convenient operation, and is fit for large-scale production. The vascular embolization agent can be used for vascular embolization, local targeted tumor treatment, and treating small-cell carcinoma of the lung, oophoroma, carcinoma of testis, gastric cancer and liver cancer by administering the vascular embolization agent during operations.
Owner:BEIJING SHENGYIYAO SCI & TECH DEV
Emulsifiable natural oil coating agent applied to granulated compound feed
InactiveCN109965089APassivated adhesion spreading retardationAdhesion spreading delay time optimizationAnimal feeding stuffAccessory food factorsVegetable oilAntioxidant
The invention discloses an emulsifiable natural oil coating agent applied to a granulated compound feed. The emulsifiable natural oil coating agent comprises natural animal and vegetable oil, concentrated soybean lecithin oil, an emulsifying agent, an antioxidant, an anti-mildew preservative, a polar solvent, vitamins, amino acid chelated trace elements, probiotics and Chinese herbal medicine superfine powder. After mixing, the raw materials are stirred, dispersed and homogenized to obtain sticky paste; 5-10 times of clear water is added into the coating agent to form slurry in appropriate glutinousness, the coating agent and auxiliary agents thereof can be adopted for preparing the slurry, and the slurry can be mixed with water to be diluted for livestock and poultry breeding objects to drink and can also be mixed with the granulated compound feed to be used for feeding the breeding objects; when the mixed granulated compound feed is used for feeding aquaculture objects, dust is evidently reduced, and by hydrophilic and lipophilic characteristics of liposome and a phospholipid complex dissolved in aquaculture water, surface tension of water in a feeding area can be reduced, so that feeding stress response of the aquaculture objects is relieved, and implementation effects of a healthcare prevention and treatment scheme in aquaculture production are improved.
Owner:江苏欧克动物药业有限公司
Morusin anti-tumor application
InactiveCN104800204AImprove targetingEasy to operateOrganic active ingredientsAntineoplastic agentsSide effectApoptosis
The invention provides an application of morusin or its pharmaceutically acceptable derivative in preparation of medicines for resisting malignant tumor. The invention also provides an application of morusin or its pharmaceutically acceptable salt in preparation of anti-glioma medicines. It is found through researches that morusin shows a good activity of resisting malignant tumor (such as glioma) in vitro and in vivo. In addition, it shows through experiments that morusin has a good effect of suppressing tumor growth and inducing transversal differentiation and apoptosis of tumor cells and tumor stem cells, and has little toxic and side effect on normal cells. The morusin has a good application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
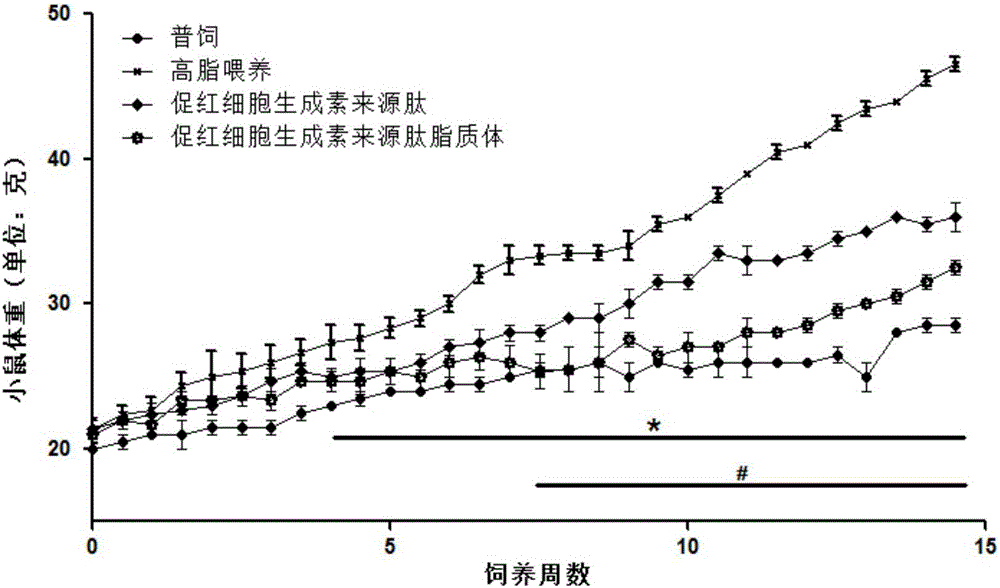
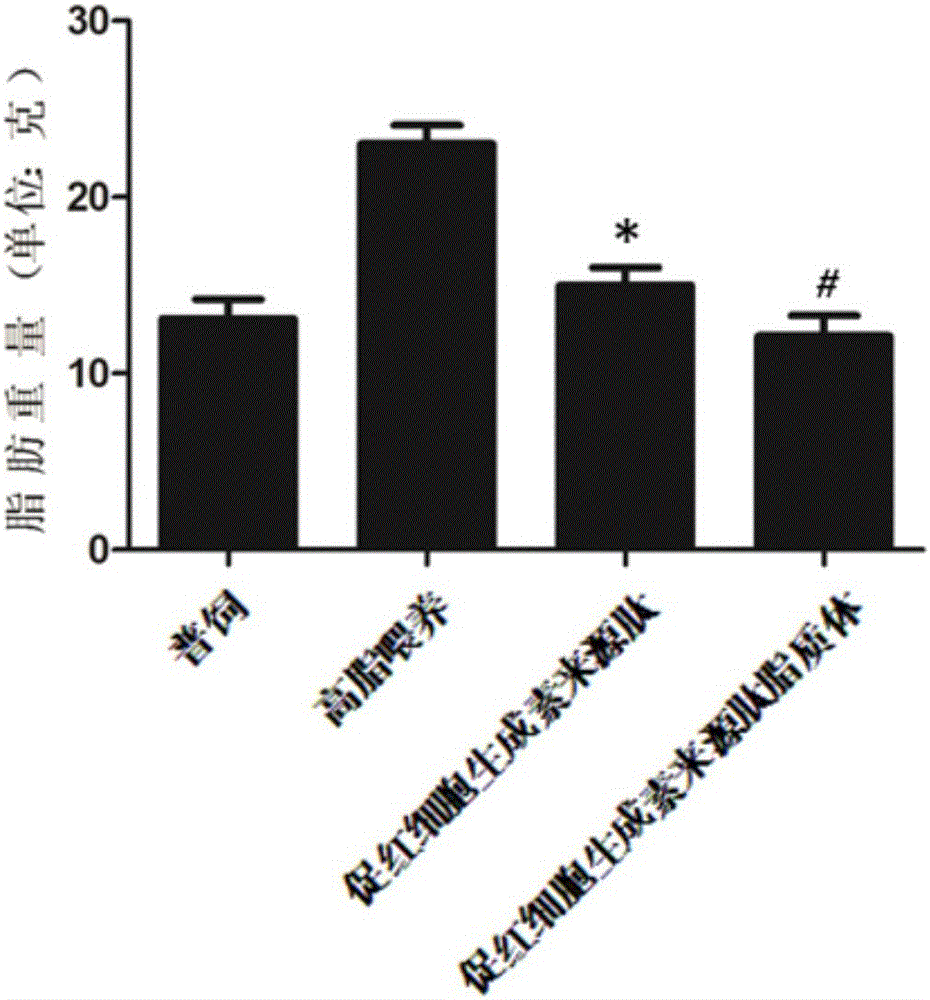
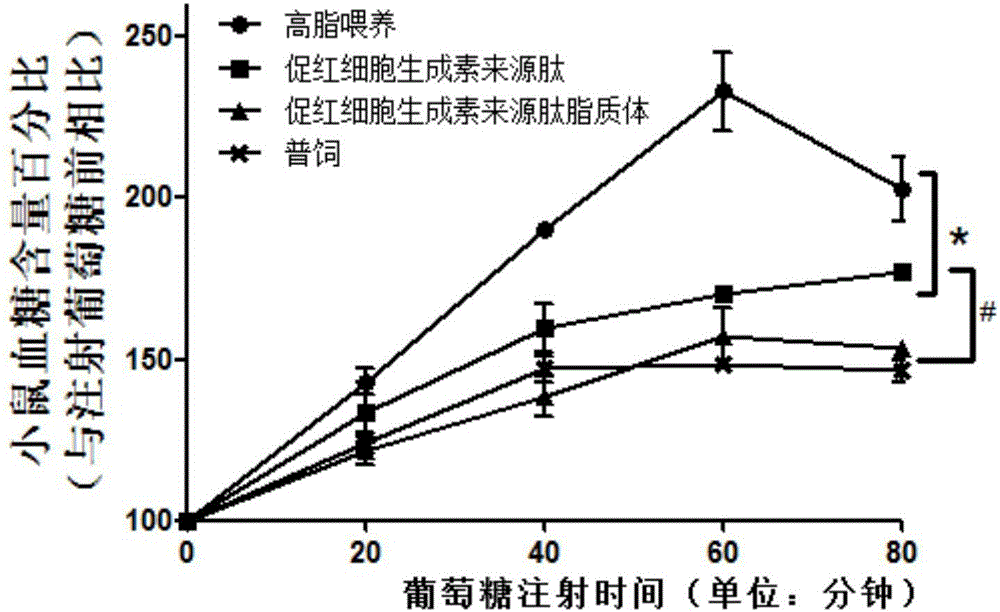
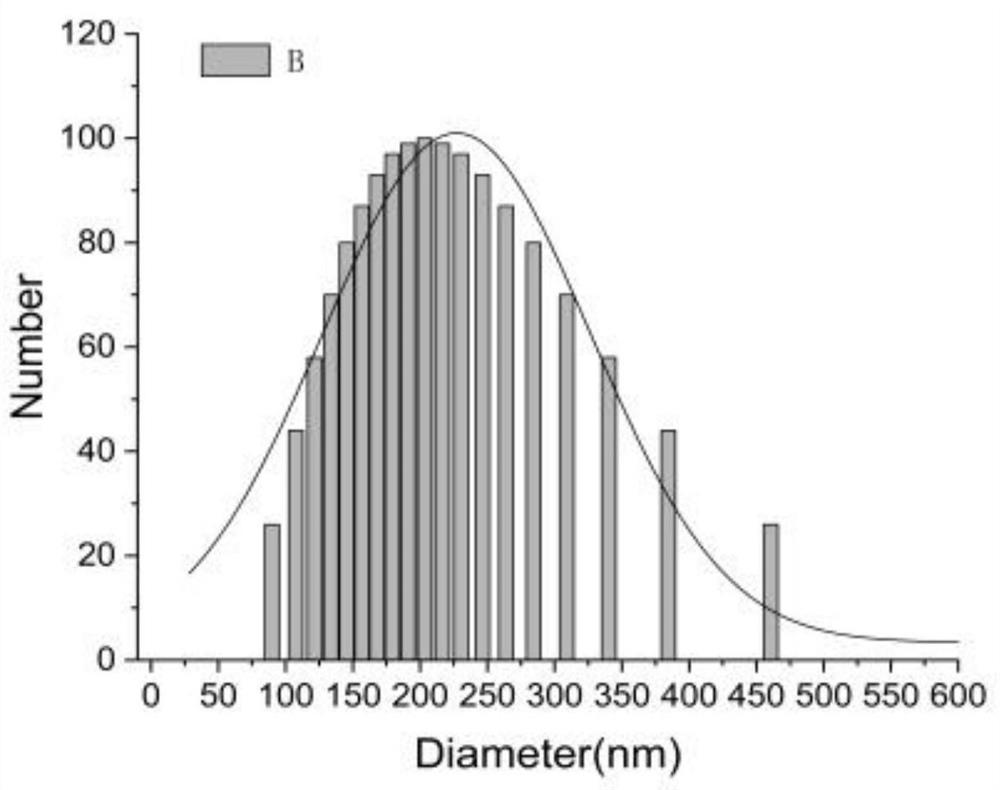
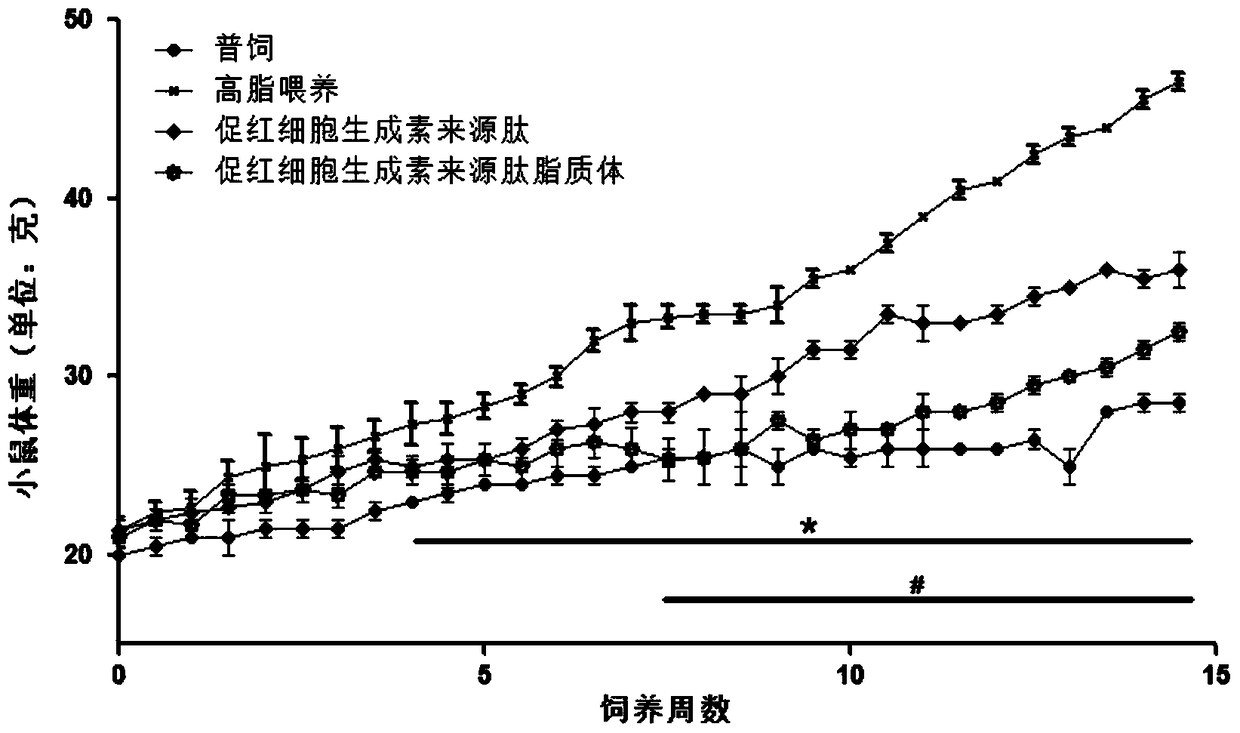
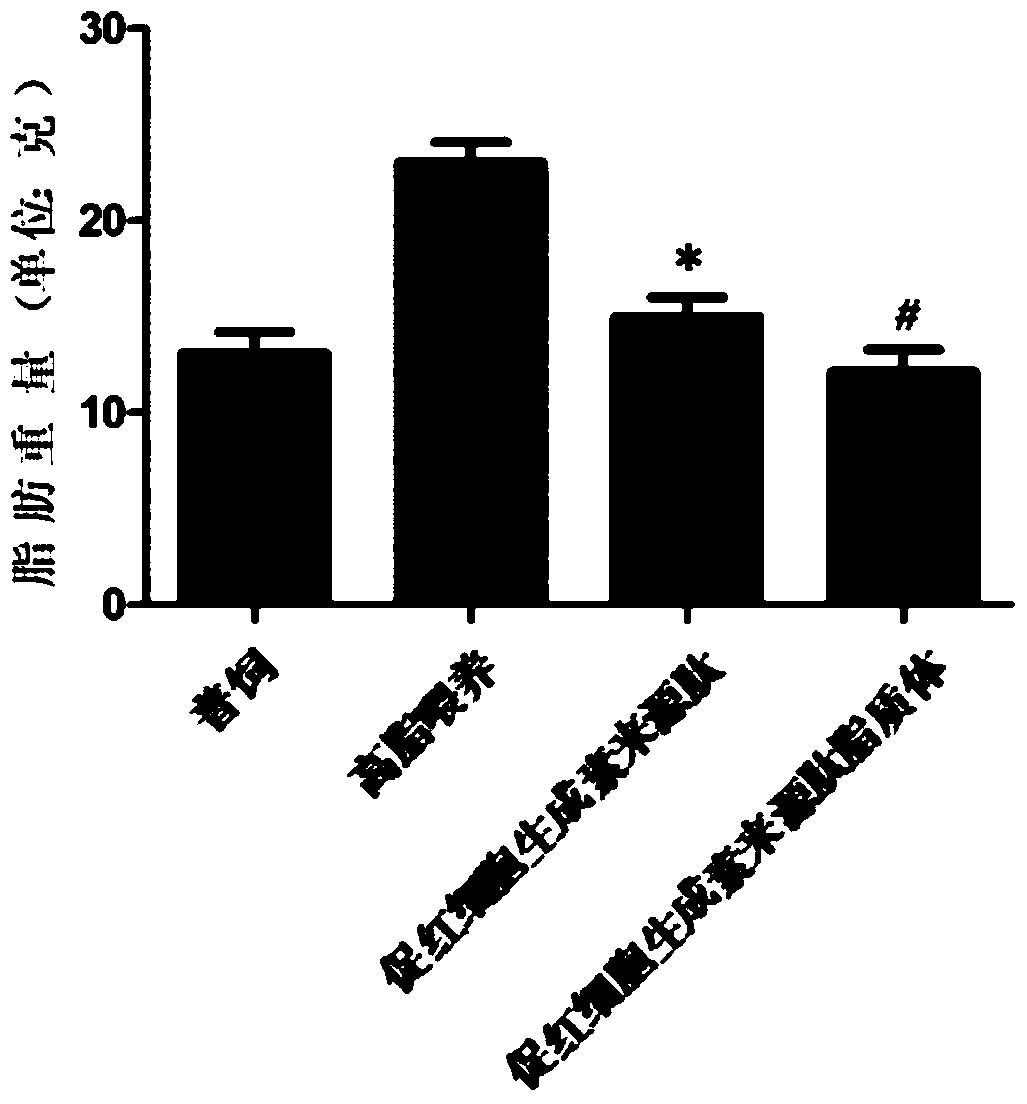
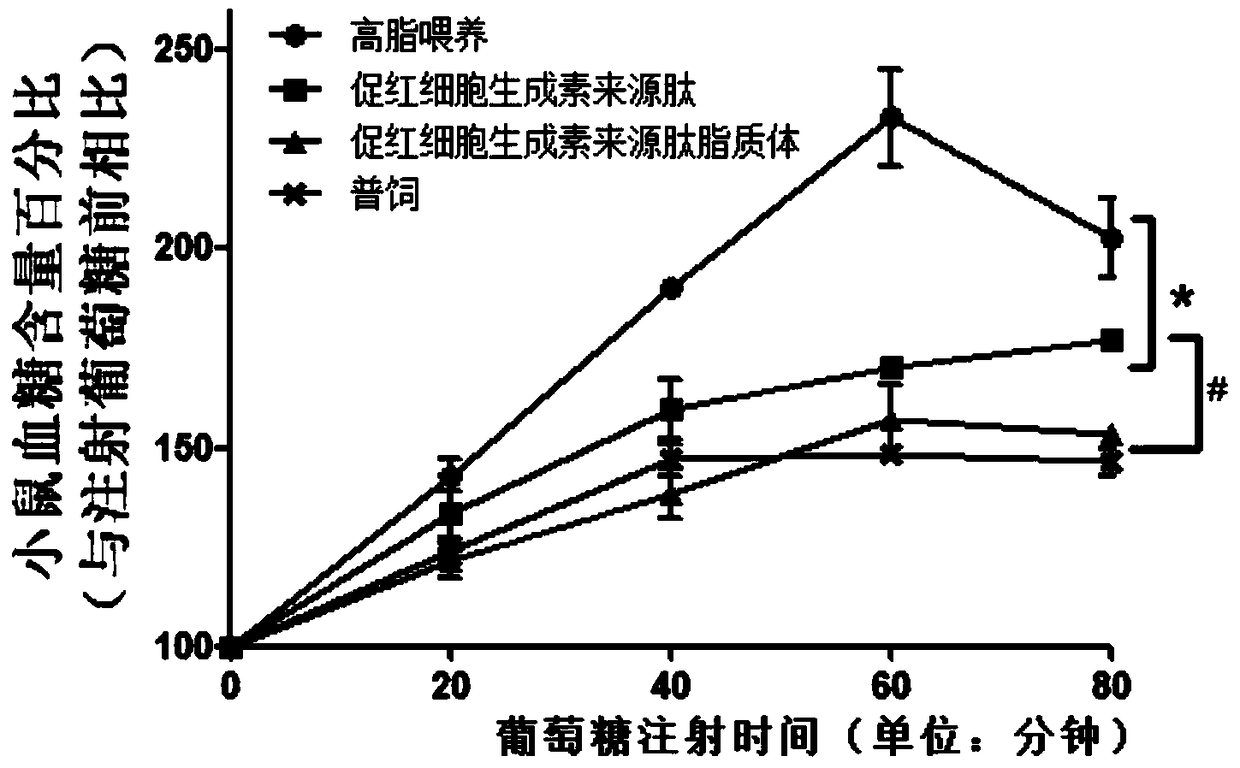
Application of hemopoietin sourced peptide in preparation of medicine for treating metabolic syndrome
ActiveCN105148257AProlong the action timeIncrease fat solubilityPeptide/protein ingredientsMetabolism disorderSolubilitySide effect
The invention belongs to the field of bio-medicines, and particularly relates to an application of a hemopoietin sourced peptide in preparation of a medicine for treating the metabolic syndrome. The application includes an application of preparation of medicines for treating obesity, diabetes mellitus and hyperlipemia, The hemopoietin sourced peptide can be prepared into lipidosome, and the metabolic syndrome can be treated by the hemopoietin sourced peptide and the hemopoietin sourced peptide lipidosome, the syndromes of the hemopoietin sourced peptide are remarkably relieved after treatment, and the effect of the hemopoietin sourced peptide lipidosome is superior to that of the hemopoietin sourced peptide, and the administration frequency can be reduced. The application of the hemopoietin sourced peptide lipidosome to medicines can be used for prolonging the medicine acting period, increasing the lipid solubility and stability of the medicines and reducing toxic and side effects. Therefore, compared with an independent application of micro-molecule polypeptide, lipidosome coated micro-molecule polypeptide has the advantages of prolonging half-life period, improving stability and lipid solubility and increasing administration routes. The hemopoietin sourced peptide has an important clinical application value.
Owner:ARMY MEDICAL UNIV
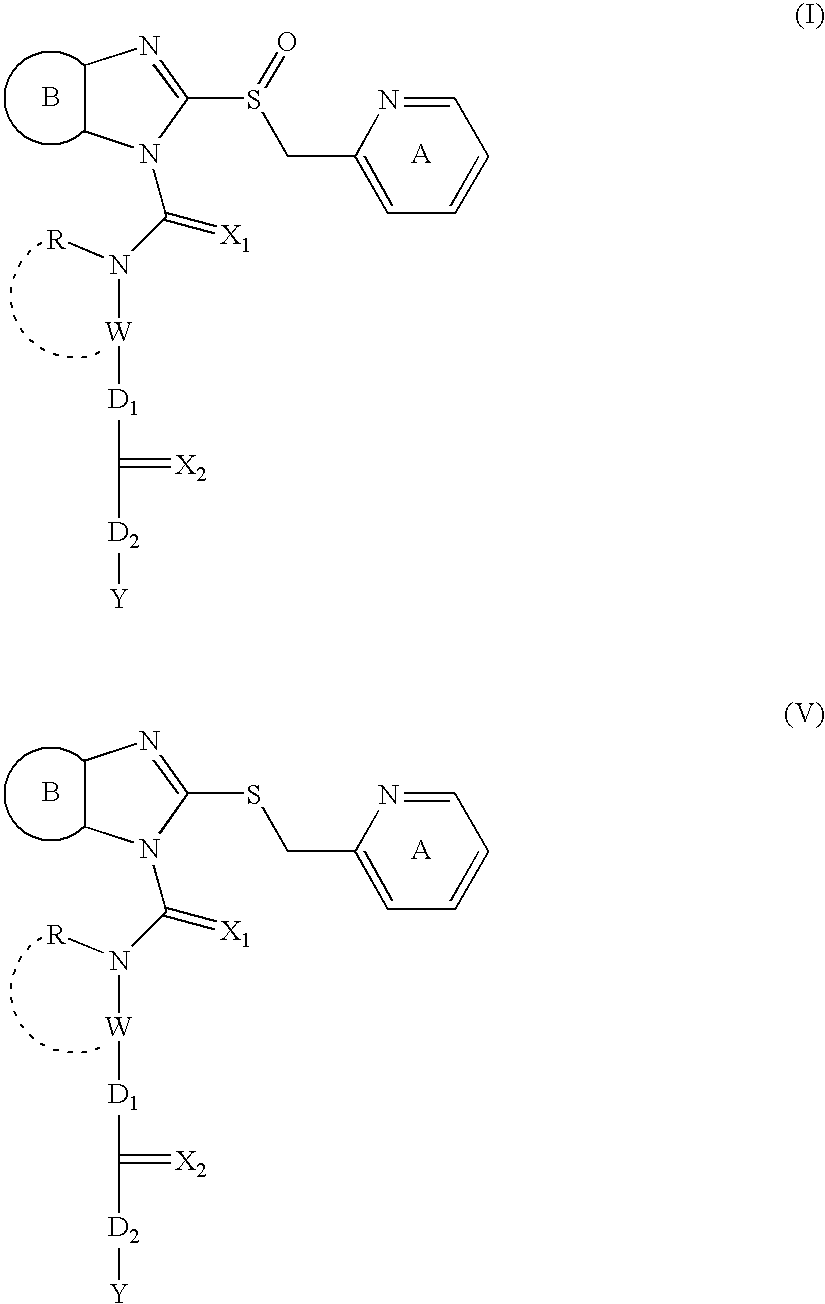
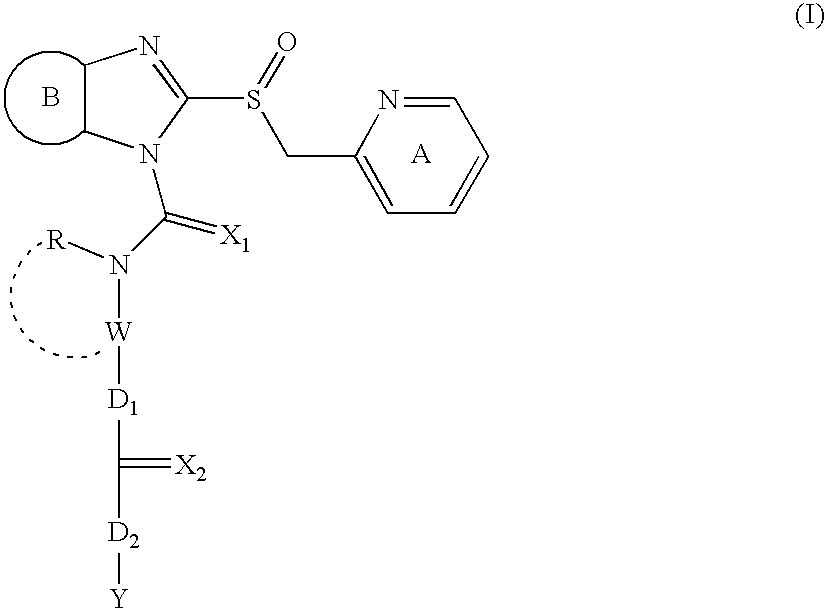
Prodrugs of imidazole derivatives, for use as proton pump inhibitors in the treatment of e.g. peptic ulcers
InactiveUS20050222210A1Easy to swallowRelieve symptomsAntibacterial agentsBiocideAnti-Helicobacter pylori IgGHelicobacter pylori gastritis
An imidazole compound represented by the formula (I), a salt thereof and a compound of the formula (V), which is one of the intermediates thereof. wherein each symbol is as defined in the present specification. The compound of the present invention shows a superior anti-ulcer activity, a gastric acid secretion inhibitory action, a mucosa-protecting action, an anti-Helicobacter pylori action and the like. Since it shows low toxicity, the compound is useful as a pharmaceutical product.
Owner:TAKEDA PHARMACEUTICALS CO LTD
Complex salt or composition medicine of breviscapine and basic amino acid for treating cardiac and cerebral vascular diseases
InactiveCN1857295AImprove hydrophilicityImprove solubilityOrganic active ingredientsPill deliverySolubilityVascular disease
The present invention relates to complex salt or composition medicine of breviscapine and basic amino acid for treating cardiac and cerebral vascular diseases. The composition medicine consists of breviscapine 1 weight portions, and basic amino acid 0.01-100 weight portions, and proper amount of supplementary material. It may be prepared into powder for injection, injection, oral liquid, capsule and other suitable clinical preparation forms. The composition medicine of the present invention has obviously raised dissolubility and bioavailability of breviscapine, high medicine effect, obvious synergistic effect and wide clinical application foreground.
Owner:SICHUAN GUOKANG PHARMA
Cucurbitacin medicinal composition and pharmaceutical application thereof
ActiveCN103735555ALow toxicityGood curative effectOrganic active ingredientsDigestive systemAntioxidantHepatitis
The invention provides a cucurbitacin medicinal composition and pharmaceutical application thereof. The cucurbitacin medicinal composition comprises cucurbitacin, a solubilizer, an antioxidant and a pH regulator, wherein the using amount of the pH regulator is enough for regulating the pH value of the medicinal composition to 3.5-7.5; the weight ratio of the cucurbitacin to the solubilizer is (1:600)-(1:3,150). The medicinal composition can be used for preparing medicaments for lowering the toxicity of cucurbitacin as well as anti-hepatitis, jaundice-removing and anti-cancer medicaments.
Owner:DELI WEI BEIJING BIOLOGICAL TECH
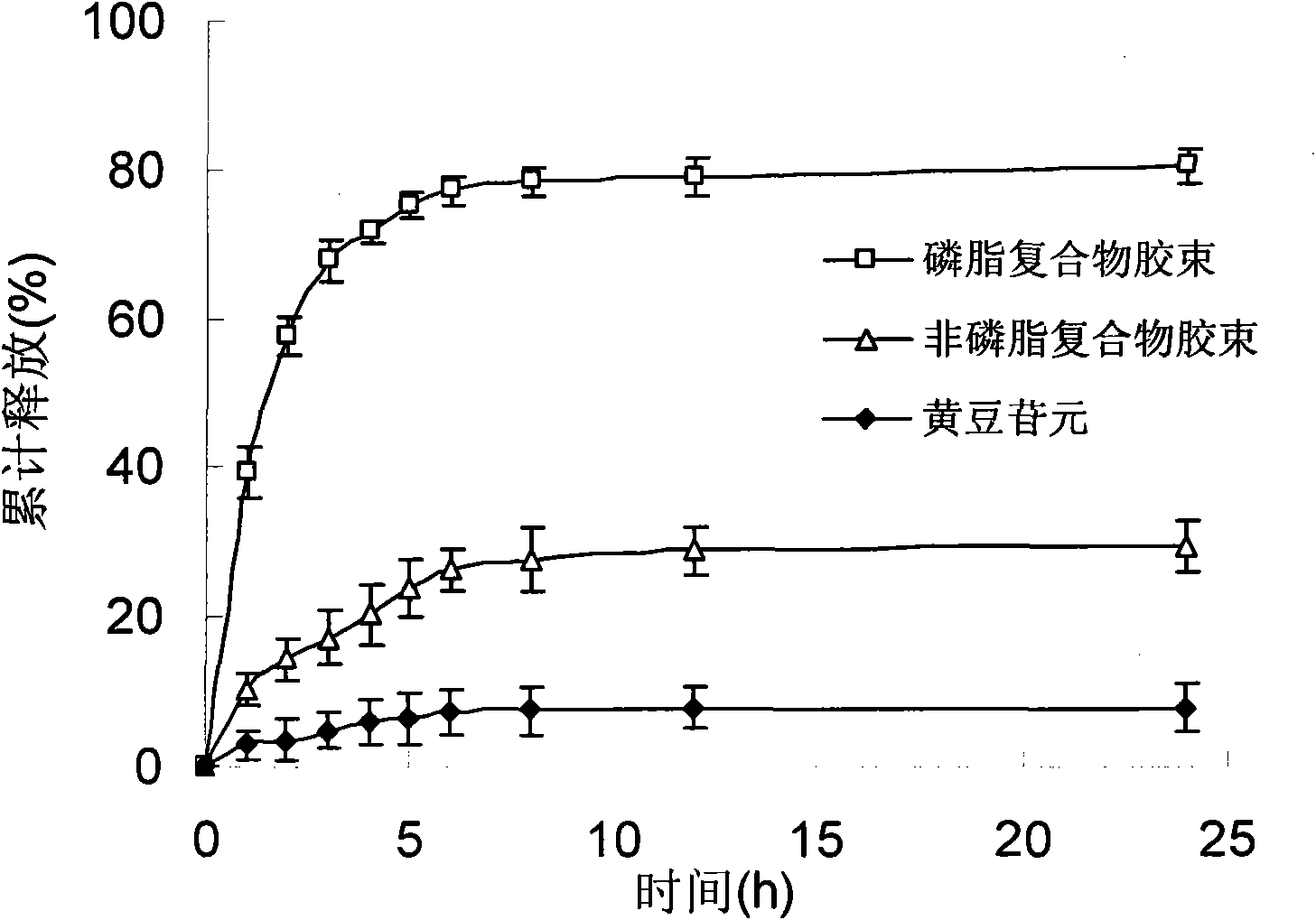
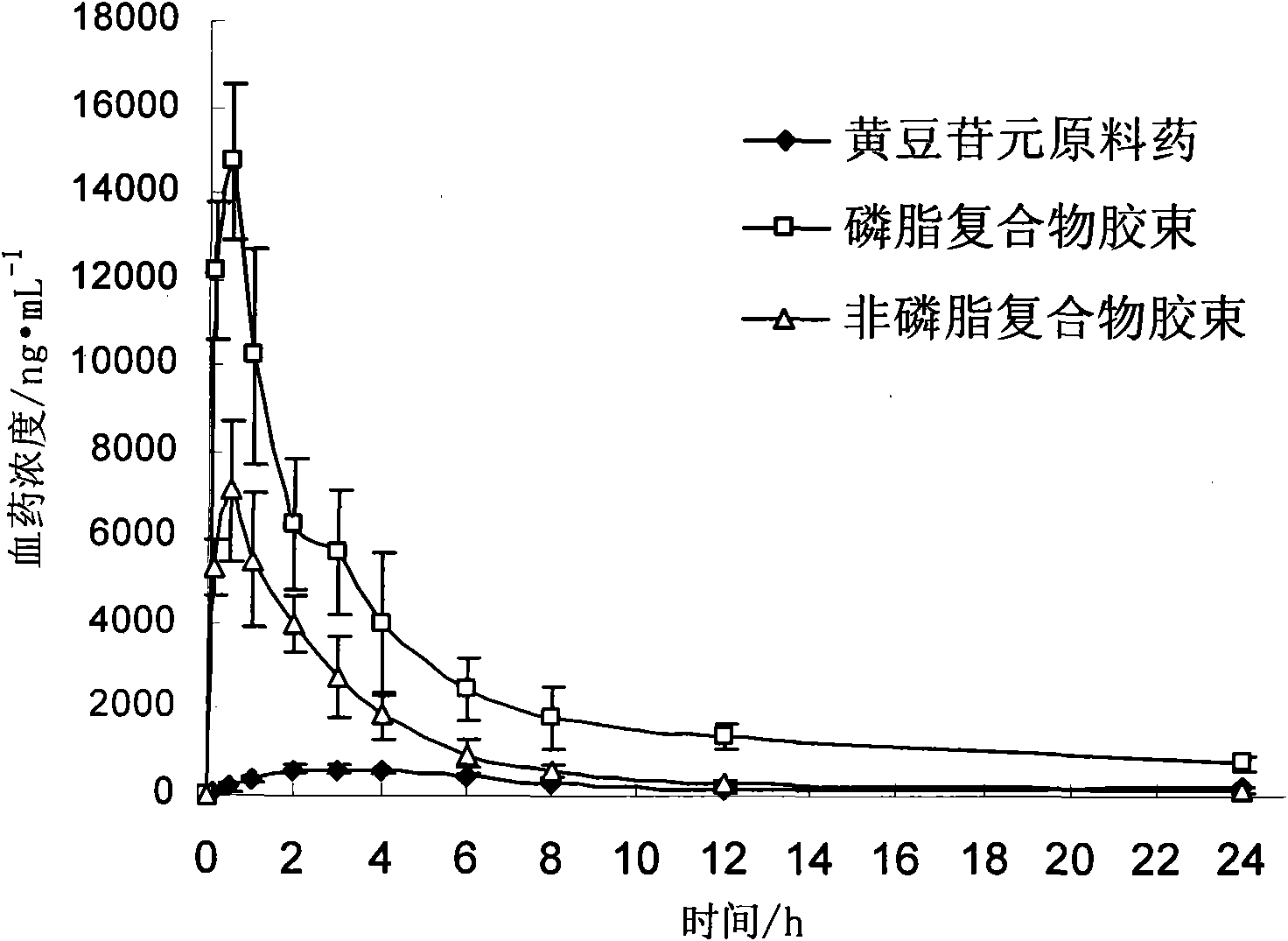
Daidzein micelles and preparation method thereof
InactiveCN102058528AImprove bioavailabilityIncrease route of administrationOrganic active ingredientsPowder deliveryFreeze-dryingDaidzein
The invention relates to daidzein micelles and a preparation method thereof. The daidzein micelles contain 1 part of daidzein, 12 to 30 parts of phospholipid and 1 to 25 parts of additive. The preparation method comprises: preparing a daidzein and phospholipid composite, namely, adding daidzein and 50 to 95 percent of phospholipid into an organic solvent, heating the solution to 40 to 60 DEG C, refluxing under reduced pressure, keeping temperature and stirring for 2 to 10 hours, recovering the organic solvent, drying and crushing to obtain the daidzein and phospholipid composite; and preparing daidzein micelles from the phospholipid composite, namely, dissolving the daidzein and phospholipid composite, the rest phospholipid and the additive in an organic solvent, subjecting the solution to rotary evaporation to form a film, and hydrating at 40 to 60 DEG C to obtain daidzein micelle suspension with opalescence. The average particle size of the daidzein micelles is less than 50 nanometers. The daidzein micelles can be further prepared into oral or injection preparations including capsules, oral mixed suspension, oral liquid, injection, injection freeze-dried powder injection.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Derivative of fibrauretine, and preparation method
InactiveCN1958584AMeet different needsGood water solubilityAntibacterial agentsOrganic active ingredientsSolubilityClinical efficacy
This invention discloses a method for preparing fibrauretine derivative with antibacterial, anti-inflammatory, cardiomyocyte protective and anti-arrhythmia functions. The method comprises: dissolving fibrauretine in 0-100 deg.C water, adding the same mol amount of alkali or salt containing Na+ or K+, reacting under stirring and normal pressure to obtain transparent liquid with the color from orange to bordeaux and a pH value of 7.0-12.5, sterilizing, filtering, and heating or vacuum-drying at low temperatures to obtain solid fibrauretine derivative with the color from light yellow to dark brown. The fibrauretine derivative has improved water solubility, absorbency, bioavailability and therapeutic effects, newly developed administration method and dosage form, and widened clinical applications.
Owner:吴晓枫
A traditional Chinese medicine preparation for preventing diarrhea in weaned piglets and its preparation method
ActiveCN104886345BPrevent diarrheaImprove immunityAntibacterial agentsDigestive systemHouttuyniaGlucose polymers
The invention belongs to the technical field of traditional Chinese medicine preparations for beasts, and particularly relates to a traditional Chinese medicine preparation for preventing weaned piglets from suffering diarrhea, and a preparation method of the traditional Chinese medicine preparation. The traditional Chinese medicine preparation is mainly prepared from the following raw materials in parts by weight: plant extracts and glucose, wherein the plant extracts comprise dandelion extracts, astragalus membranaceus extracts, herba andrographitis extracts, and cordate houttuynia extracts. The preparation method of the medicine preparation comprises the following steps: sieving the plant extracts with a sieve of 50-70 meshes, uniformly mixing the sieved plant extracts, adding the glucose sieved by a sieve of 70-100 meshes in the mixed plant extracts, and uniformly mixing the sieved glucose with the mixed plant extracts so as to obtain the traditional Chinese medicine preparation. The traditional Chinese medicine preparation for preventing weaned piglets from suffering, disclosed by the invention, has significant effects of resisting bacteria and resisting viruses, and can significantly reinforce the immunity of weaned piglets; the traditional Chinese medicine preparation is rich in nutritional substances, so that the nutritional needs of weaned piglets can be met; the traditional Chinese medicine preparation has the advantages that the gastroenteric functions of weaned piglets can be regulated, the absorption of nutritional substances can be promoted, the daily gain of weaned piglets can be increased, and the feed converting rate can be increased, so that the phenomenon that the weaned piglets are liable to get diarrhea is effectively avoided.
Owner:GUANGDONG GALLOPER VETERINARY PHARMA
Resveratrol external use gel and preparation method thereof
InactiveCN106265488AIncrease route of administrationImprove bioavailabilityHydroxy compound active ingredientsAerosol deliveryChemistryMethyl hydroxybenzoate
The invention discloses resveratrol external use gel and a preparation method thereof. The resveratrol external use gel is prepared from the following materials: 0.01 percent to 5 percent of resveratrol, 0.2 percent to 1.5 percent of carbomer, 5 percent to 15 percent of propanediol, 0.01 percent to 0.1 percent of sodium pyrosulfite, 0.5 percent to 2.0 percent of triethanolamine, 0.05 percent to 1 percent of methyl p-hydroxybenzoate, 0.02 percent to 2 percent of laurocapram and 73.4 percent to 94.21 percent of pure water. A modern preparation technology is used; the resveratrol external use gel is prepared by taking the carbomer as a gel substrate;; the medication route of the resveratrol is expanded; the bioavailability of the medicine for treating body surface skin diseases is improved. The resveratrol external use gel is suitable for human skin and mucosa local medication. Compared with oral medication, the resveratrol external use gel has the advantages that the effective blood concentration of local affected parts is greatly improved; the medicine bioavailability is improved.
Owner:LESHAN VOCATIONAL & TECHN COLLEGE
Ketoprofen solution and preparation method thereof
InactiveCN103432067AImprove solubilityIncrease route of administrationOrganic active ingredientsAntipyreticSodium acetateSodium acetrizoate
The invention discloses a ketoprofen solution. The ketoprofen solution comprises the following components in every 100mL: 0.1-1g of ketoprofen, 0.1-10g of sodium acetate, glacial acetic acid used for regulating the pH value to 5.5-6.5, and the balance of injection water. A preparation method of the ketoprofen solution comprises the following steps: adding the sodium acetate with the prescribed amount into 50-80mL of injection water, uniformly stirring and mixing; adding ketoprofen with the prescribed amount into the mixture, stirring under a condition of 50+ / -5 DEG C until the ketoprofen is completely dissolved; cooling the liquid medicine to room temperature, and regulating the pH value to 5.5-6.5 with glacial acetic acid; fixing the volume to 100mL by using the injection water, uniformly mixing, and filtering. According to the ketoprofen solution, the sodium acetate is used as a cosolvent of ketoprofen, so that the defect of an existing ketoprofen cosolvent can be overcome; the sodium acetate and the glacial acetic acid or ketoprofen form a buffering pair, and play an important role in stabilizing the pH value of the solution; the ketoprofen solution is simple in preparation process, good in repeatability and low in cost, is suitable for industrial large-scale production, does not contain an organic solvent, benzyl alcohol or other component, is safer in clinical application, can be produced into an oral solution and small-dose injection, as well as a preparation for venous transfusion.
Owner:SOUTHWEST UNIVERSITY
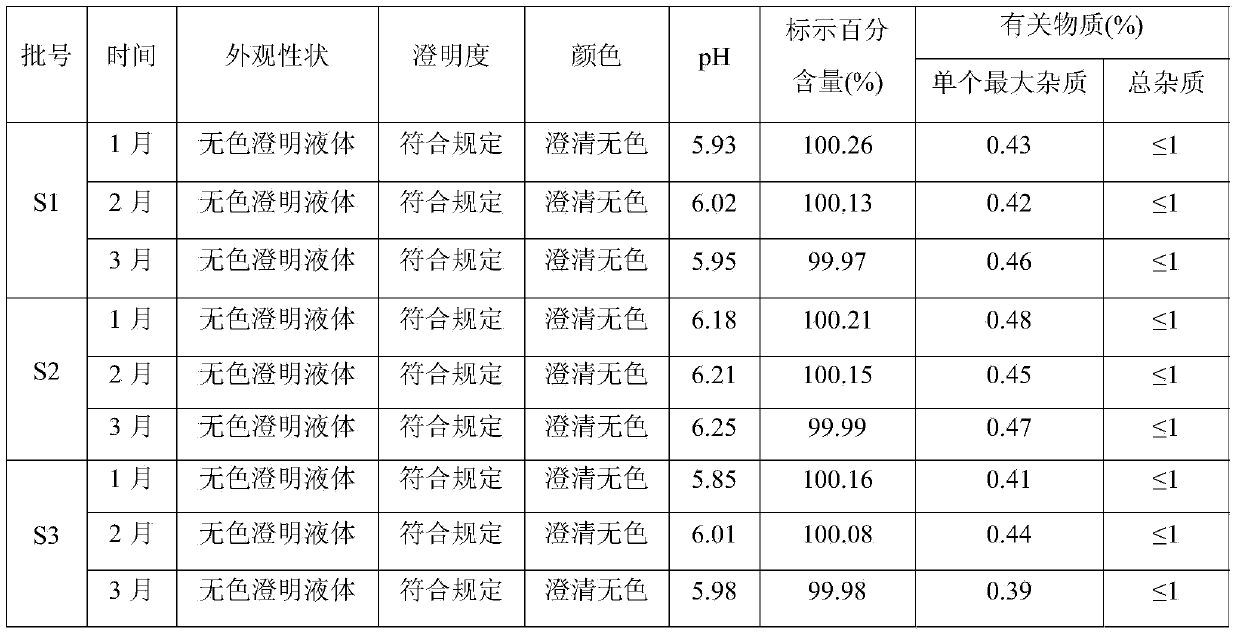
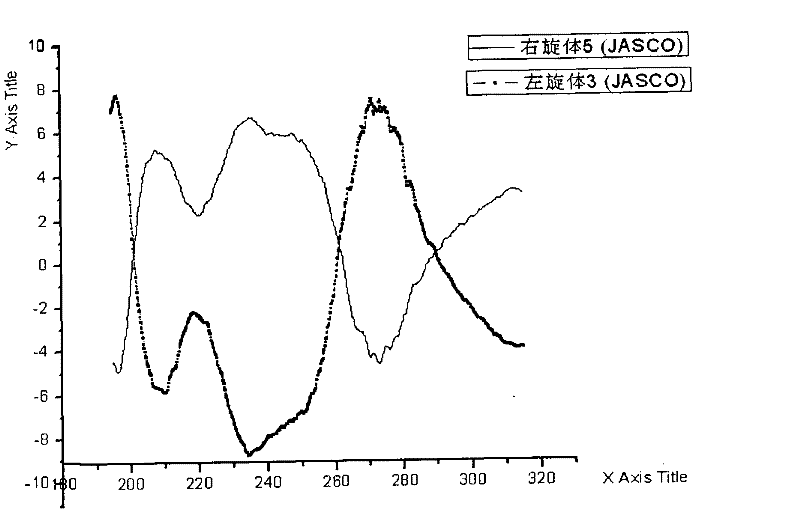
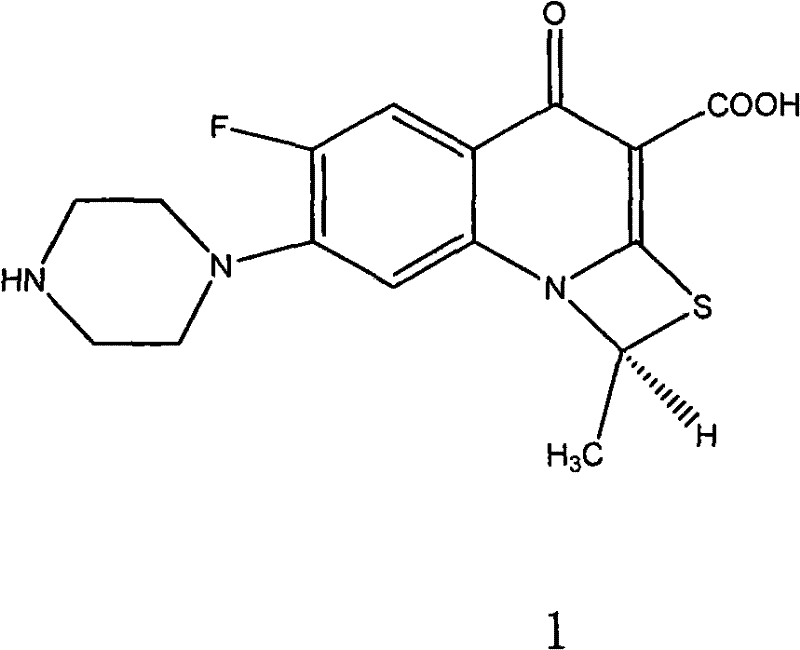
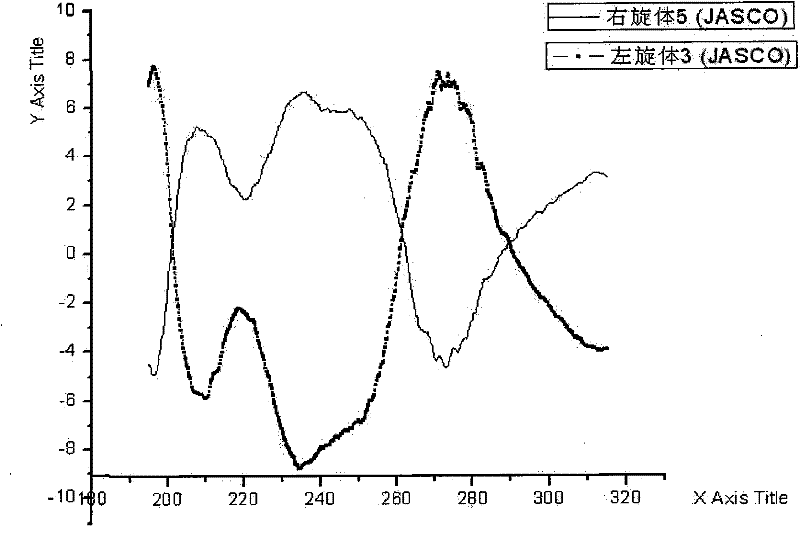
Levo-ulifloxacin injection and preparation method thereof
Levo-ulifloxacin injection and preparation method thereof, relate to pharmaceutical preparations containing fluoroquinolone antibacterial drugs, in particular to injection of prulifloxacin active body Ulifloxacin (S-(-)-Ulifloxacin) and preparation thereof method. The present invention is based on (S)-(-)-6-fluoro-1-methyl-4-oxo-7-(1-piperazinyl)-1H, 4H-[1,3]thiazetidine Alkane[3,2-a]quinoline-3-carboxylic acid (levofloxacin for short) is the active ingredient of the drug, and the rest are pharmaceutically acceptable additives for injections, which are liquid injections or solid injections Injections, wherein each injection or bottle contains 10-100 mg of active pharmaceutical ingredients. The commonly used dose in clinic may be 10-100 mg of levofloxacin, once or twice a day; 1-10 ml for intramuscular injection and 50-1000 ml for intravenous injection. The invention increases the route of administration, expands the range of application groups, and significantly reduces the cost and risk of medication for patients.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE
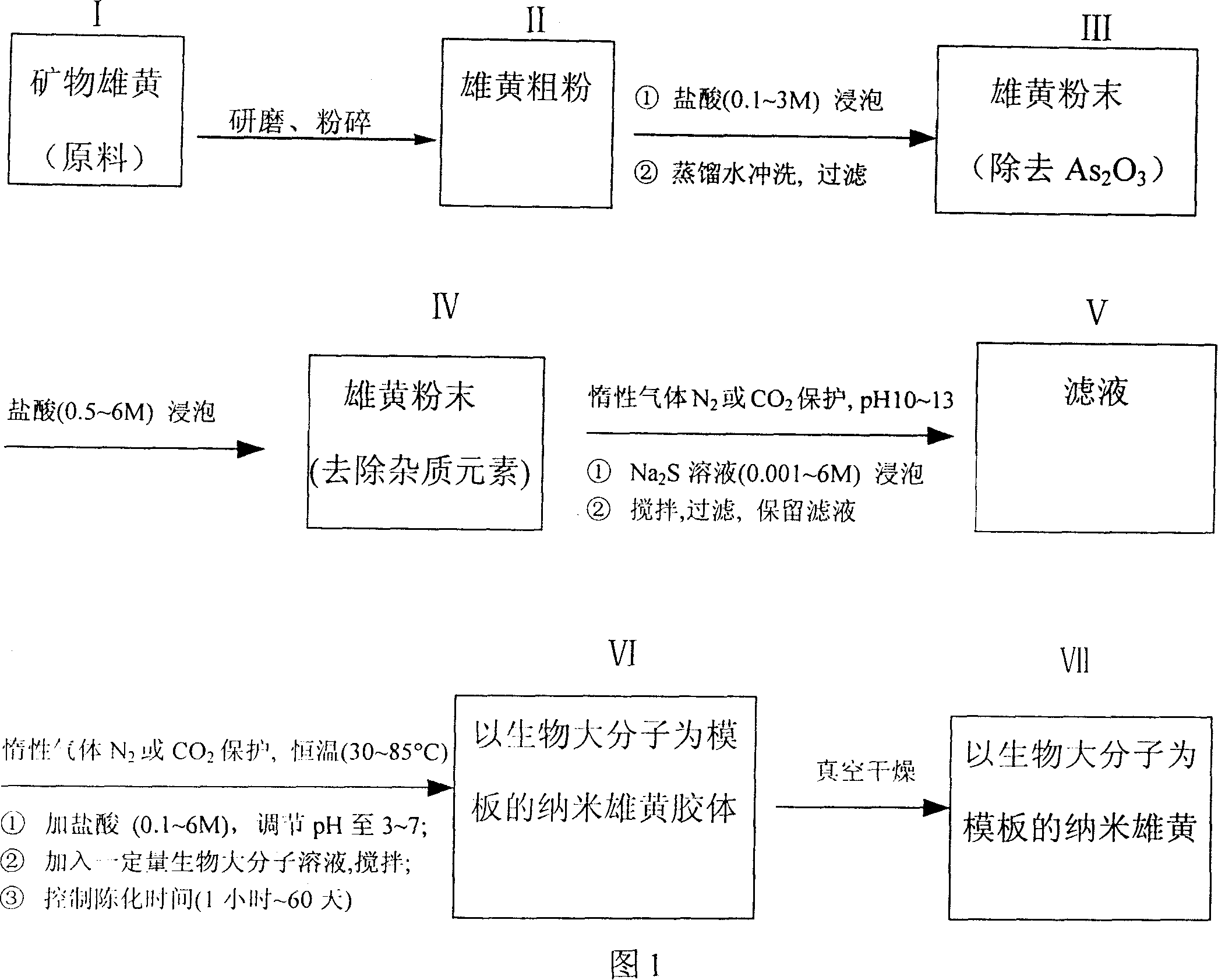
Method for preparing nanometer realgar gel and nanometer realgar with biomacromolecule template to regulate shape and particle thereof
This invention supplies a method for preparing nanorealgar colloid and nanorealgar through modulating their appearance and particle size with biology macromolecular mold. Its characteristic is: chemically remove arsenic and impurity elements from natural realgar mine, make nanorealgar colloid and nanorealgar by taking biology macromolecular as mold to modulate the appearance and particle size, so nanorealgars of various appearance and particle size can be prepared by changing mold varieties and ageing conditions. This invention has broad processing raw materials, and the yielded nanorealgar has high purity, uniform particle size, good water-solubility, it is uneasy to oxidation or reunion and has obverse synergism effect with mold molecule. It can markedly strengthen the effect of realgar antibiosis and anti-tumour, improve realgar biology availability, depress realgar toxic side effect and expand realgar route of administration.
Owner:GUANGXI NORMAL UNIV
Traditional Chinese medicine composition used for reducing lipid, discharging turbidity and clearing intestines, application thereof and preparation method therefor
InactiveCN111388551AAchieve anti-inflammatory and detoxificationRegulating fat loss and weight lossMetabolism disorderDigestive systemColonic irrigationLipid lowering
The invention relates to a traditional Chinese medicine composition, in particular to a traditional Chinese medicine composition for reducing lipid, discharging turbidity and clearing intestines, application thereof and a preparation method therefor, and overcomes the problem that such existing drugs have large toxic and side effects and a poor curative effect. The traditional Chinese medicine composition is prepared from the following traditional Chinese medicine raw materials in parts by weight: 8-10 parts of radix bupleuri, 8-12 parts of radix scutellariae, 10-20 parts of semen cassiae, 10-15 parts of fructus crataegi, 10-20 parts of semen cannabis and 10-20 parts of semen pruni. The traditional Chinese medicine composition has the effects of clearing liver and removing blood stasis, removing dampness and purging turbidity, and can be deeply retained locally by whole colon irrigation, and accordingly, the purposes of regulating intestinal floras, reducing lipid and losing weight, reducing inflammations and discharging turbidity are achieved; and the traditional Chinese medicine composition is mainly used for patients with fatty liver and patients with hyperlipemia and metabolicsyndromes, and stagnated heat in liver meridian by TCM syndrome differentiation, has a significant curative effect, and is safe and non-toxic.
Owner:西安市中医医院
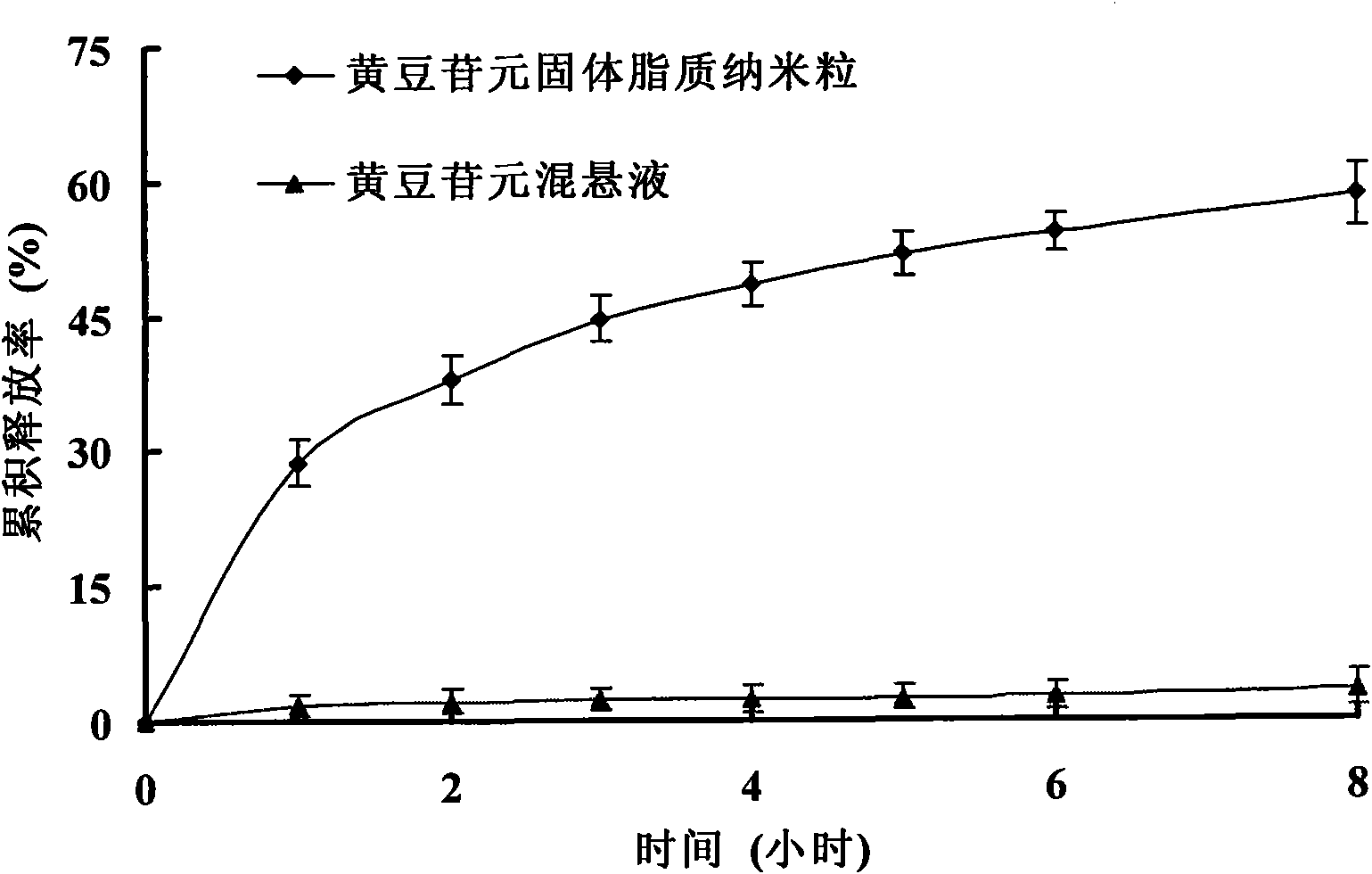
Daidzein solid lipid nanoparticles and preparation method thereof
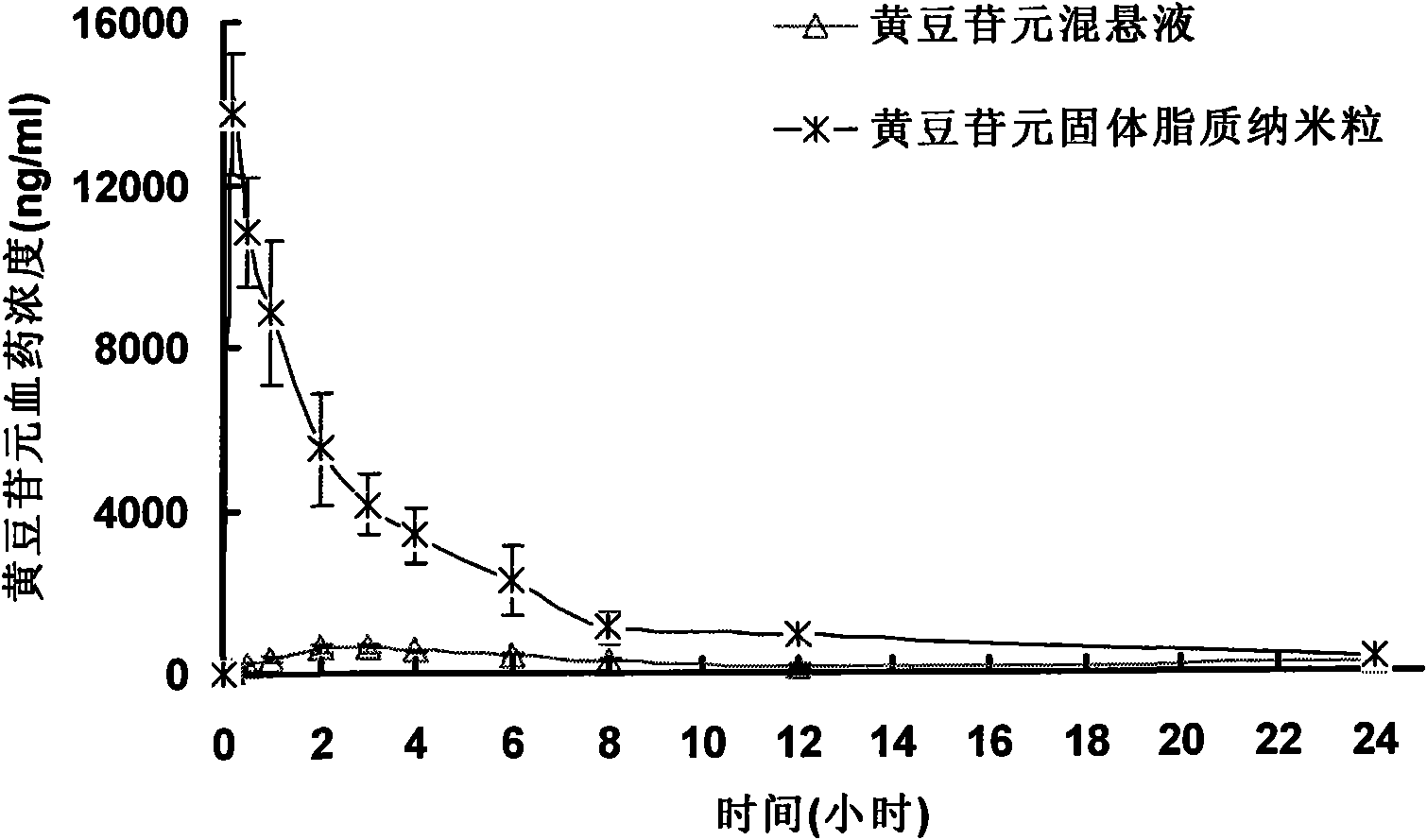
InactiveCN102258475BImprove oral bioavailabilityEasy to take medicinePowder deliveryOrganic active ingredientsSolubilityOral suspensions
The invention relates to solid lipid nanoparticles of daidzein which is a hydrophobic medicine and a preparation method thereof. The average particle size of the solid lipid nanoparticles of daidzein is less than 100 nanometers; and based on weight part, the solid lipid nanoparticles comprise 1 part of daidzein, 5 to 30 parts of phospholipid, 1 to 50 parts of surfactant and 1 to 40 parts of solidlipid material, wherein the daidzein and phospholipid form a composite which is covered by solid lipid or the solid lipid and phospholipid. The daidzein solid lipid nanoparticles are prepared by a thin-film dispersion method or hot-melt dispersion method. The preparation method comprises: forming a composite of the daidzein and phospholipid; and covering the composite with the solid lipid nanoparticles for solving the problems of low solubility, low stability and preparation difficulty in preparation process of the daidzein. The average particle size of the solid lipid nanoparticles is less than 100 nanometers, and the rate of in-vitro release in 8 hours reaches 58.4 percent, and the oral bioavailability is high; and the solid lipid nanoparticles can be further prepared into capsules, oral suspension, oral liquid and other oral preparation, and can make the medicine taken by the patient easily.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
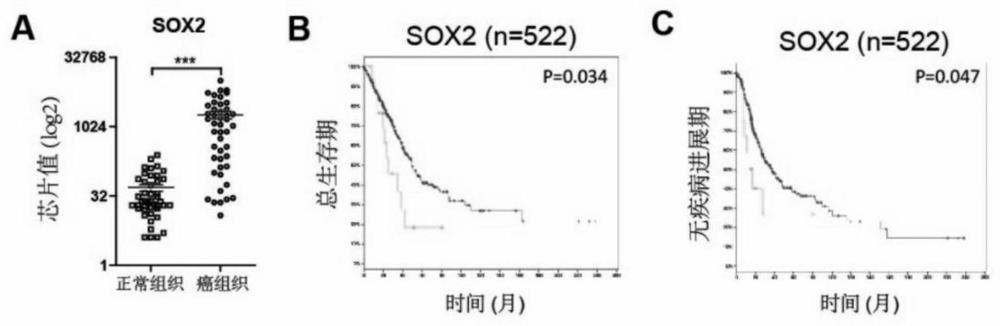
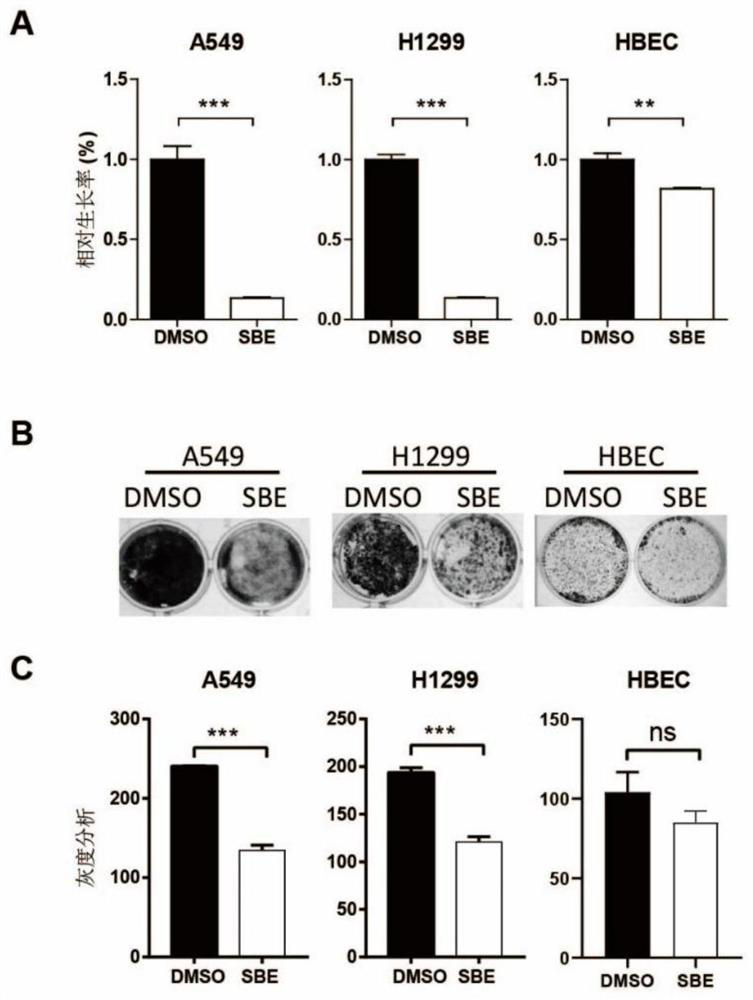
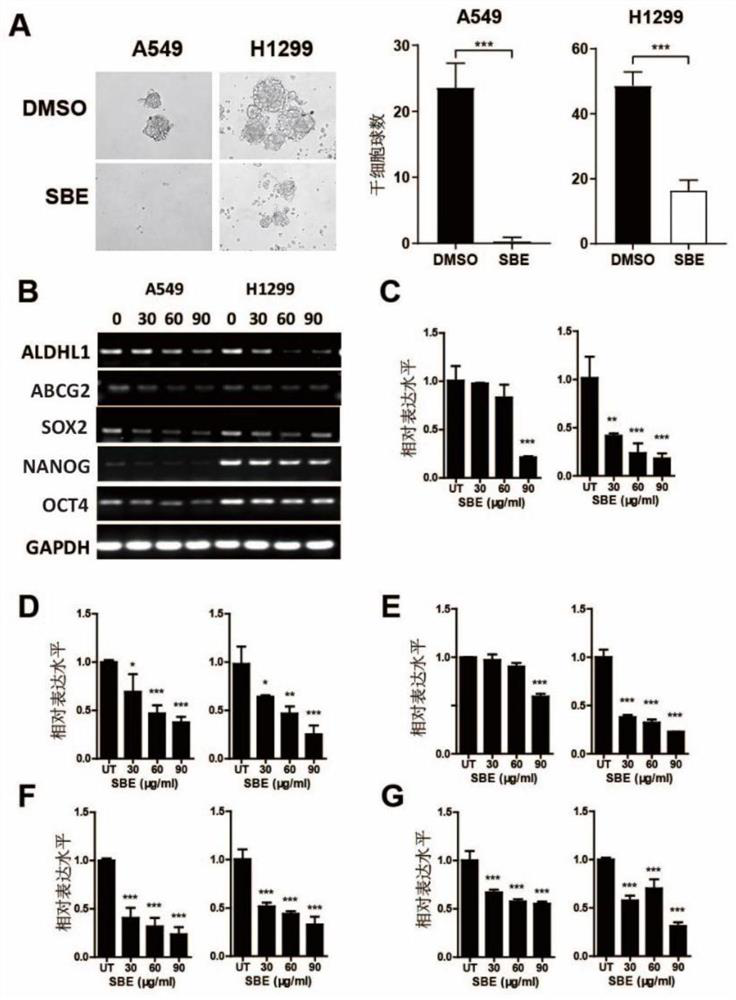
Application of SOX2 targeted drug to inhibiting proliferation of lung cancer stem cells
InactiveCN111728997AImprove targetingEasy to operateAntineoplastic agentsPlant ingredientsStem like cellSide effect
The invention provides an application of an SOX2 targeted drug to inhibiting proliferation of lung cancer stem cells. The SOX2 targeted drug is a scutellaria barbata D.Don extract; and the SOX2 targeted drug interferes with proliferation of lung cancer stem cells and multidrug resistance thereof through targeted inhibition of SOX2. The drug has toxic and side effects, and is tolerable and easy totake orally and absorb. Cell proliferation, cell apoptosis and cell cycle arrest assays prove that a polypeptide provided by the invention can effectively inhibit proliferation of tumor stem cells andthe drug has no obvious tissue damage or toxicity to mice at high doses.
Owner:滨州医学院附属医院
Preparation method of water-soluble acanthopanax powder capsule
ActiveCN113318137AGood effectBiodegradableAntinoxious agentsPharmaceutical non-active ingredientsBiologyRhizome
The invention discloses a preparation method of a water-soluble acanthopanax powder capsule, and belongs to the technical field of extraction and processing of plant rhizomes. According to the preparation method, the acanthopanax is taken as a raw material, saponin is dissolved out by adopting ethanol reflux extraction, and the water-soluble acanthopanax powder capsule is obtained through spray drying after being wrapped by chitosan oligosaccharide, so that the technological process is green, environment-friendly, simple and convenient to operate and good in dissolution property, and can be easily amplified to industrial production; a new thought is provided for development and utilization of water-soluble preparation of other Chinese herbal medicines insoluble in water, and the administration route and the use object are further widened; and in addition, the clathrate compound prepared by the invention is high in bioavailability, so that the curative effect of the clathrate compound is greatly improved, and the treatment cost is greatly reduced. Therefore, the technical scheme disclosed by the invention has great market application and popularization values.
Owner:NORTHEAST FORESTRY UNIVERSITY
Application of erythropoietin-derived peptide in preparation of medicine for treating metabolic syndrome
ActiveCN105148257BProlong the action timeIncrease fat solubilityPeptide/protein ingredientsMetabolism disorderSolubilitySide effect
Owner:ARMY MEDICAL UNIV
A compound and its application in preparation of medicine for treating rheumatoid arthritis
InactiveCN107903245BImprove targetingEasy to operateOrganic chemistrySkeletal disorderMedicineMedicine use
The invention discloses a compound shown in a formula (I) (described in the specification), wherein R is selected from two groups (described in the specification). The compound shown in the formula (I) can inhibit production of IL-12 in vitro and shows excellent activity in an SD rat collagen-induced rheumatoid arthritis model. Therefore, the compound shown in the formula (I) can be researched anddeveloped more deeply as a candidate drug for preparation of medicines used for preventing and / or treating rheumatoid arthritis.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Compound, preparation method and application thereof in preparation of medicine for preventing/curing autoimmune diseases
InactiveCN108047239AImprove targetingEasy to operateOrganic active ingredientsNervous disorderImmunologic disordersMedicine
The invention discloses a compound of formula (I) as shown in the description, wherein R is selected from formulas in the description. The compound of formula (I) can inhibit the generation of IL-12 in vitro and has an inhibiting effect on HaCat cell proliferation, and the compound has excellent activity in SD rat collagen induced rheumatoid arthritis models. The compound of formula (I) can be used for deeper development of the preparation of candidate medicine for preventing / curing autoimmune diseases.
Owner:田甜
Cinnamomum longepaniculatum leaf volatile oil inclusion complex and preparation method thereof
InactiveCN102274286BImprove bioavailabilityIncrease route of administrationAntipyreticAnalgesicsCyclodextrinStaphyloccocus aureus
Owner:SICHUAN AGRI UNIV +1
A kind of liposome preparation of topinastat and preparation method thereof
ActiveCN107375212BIncrease route of administrationImprove efficacyPowder deliveryOrganic active ingredientsPhospholipinCholesterol
The invention relates to the technical field of medicine and particularly relates to a preparation of topiroxostat liposome and a preparation method thereof. The preparation of topiroxostat liposome comprises, by weight, 1 to 3 parts of topiroxostat, 30 to 90 parts of phospholipid and 10 to 50 parts of cholesterol. The preparation of topiroxostat liposome can be processed into a freeze-dried powder injection. The freeze-dried powder injection comprises, by weight, 1 to 3 parts of topiroxostat, 30 to 90 parts of phospholipid, 10 to 50 parts of cholesterol, 5 to 20 parts of a lyoprotectant and 0 to 10 parts of a redissolution aid. The preparation of topiroxostat liposome has high encapsulation efficiency, uniform particle sizes and stable quality. The preparation method has simple processes, increases the administration path of topiroxostat and improves patient compliance. The topiroxostat liposome prolongs the action time of topiroxostat and produces unexpected technical effects.
Owner:CP PHARMA QINGDAO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com