Thymus gland pentapeptide oral intestine-dissolved formulated product and method of preparing the same and use thereof
A technology of enteric-coated preparations and thymus, which is applied in pill delivery, pharmaceutical formulations, peptide/protein components, etc., can solve the problems of specific indications, uncertainty of drug efficacy, and no route of thymopentin administration, so as to increase compliance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
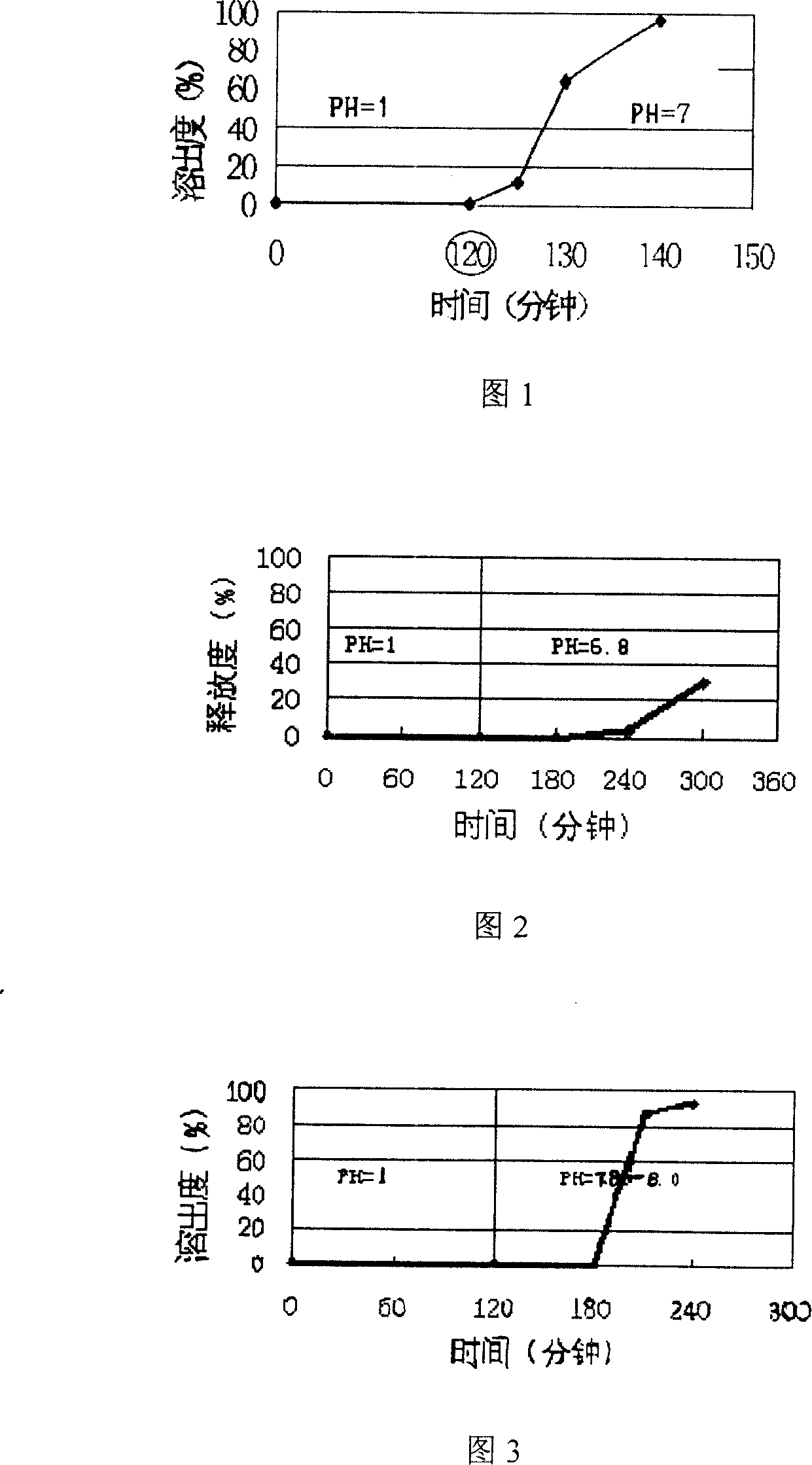
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 Thymopentin enteric-coated tablet of the present invention
[0044] (1) Prescription of plain tablets (dosage per thousand tablets):
[0045] Thymopentin 5g, sodium deoxycholate 10g, glyceryl stearate 30g, polyacrylic acid cross-linked polymer 10g, aprotinin 7g, microcrystalline cellulose 100g, sodium carboxymethyl starch 10g, micropowder silica gel 10g, stearin Magnesium acid 10g.
[0046] (2) Coating agent formula:
[0047] Talcum powder 20g, propylene glycol 20g, acrylic resin 60g.
[0048] (3) Technology for the preparation of thymopentin enteric-coated tablets
[0049] a. Get Thymopentin and auxiliary materials in the following weight ratio:
[0050] Thymopentin 5g, sodium deoxycholate 10g, glyceryl stearate 30g, polyacrylic acid cross-linked polymer 10g, aprotinin 7g, microcrystalline cellulose 100g, sodium carboxymethyl starch 10g, micropowder silica gel 10g, stearin Magnesium acid 10g, pass No. 3 pharmacopoeia sieve respectiv...
Embodiment 2
[0053] The preparation of embodiment 2 Thymopentin enteric-coated tablets of the present invention
[0054] (1) Prescription of plain tablets (dosage per thousand tablets):
[0055] Thymopentin 15g, sodium cholate 10g, glyceryl stearate 15g, polyacrylic acid cross-linked polymer 10g, pepsin inhibitor 15g, lactose 300g, sodium alginate 20g, micropowder silica gel 10g, magnesium stearate 10g.
[0056] (2) Coating agent formula:
[0057] Talcum powder 20g, propylene glycol 20g, acrylic resin 60g.
[0058] (3) Process for preparing thymopentin enteric-coated tablets: refer to Example 1.
Embodiment 3
[0059] The preparation of embodiment 3 thymopentin enteric-coated tablets of the present invention
[0060] (1) Prescription of plain tablets (dosage per thousand tablets):
[0061] Thymopentin 50g, sodium deoxycholate 20g, glyceryl stearate 30g, polyacrylic acid cross-linked polymer 10g, sodium glycocholate 20g, mannitol 700g, low-substituted hydroxypropyl cellulose 50g, micropowder silica gel 10g, hard Magnesium fatty acid 10g.
[0062] (2) Coating agent formula:
[0063] Talcum powder 20g, propylene glycol 20g, acrylic resin 60g.
[0064] (3) Process for preparing thymopentin enteric-coated tablets: refer to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com