Beta-cyclodextrin inclusion compound of huperzine A, and preparation method and preparation thereof
A technology of cyclodextrin inclusion compound and huperzine A, which is applied in the field of β-cyclodextrin inclusion compound of huperzine A and its preparation, can solve the problem of high cost, unguaranteed product yield and quality, The preparation steps are cumbersome and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
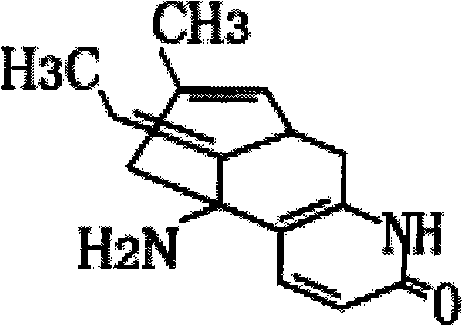
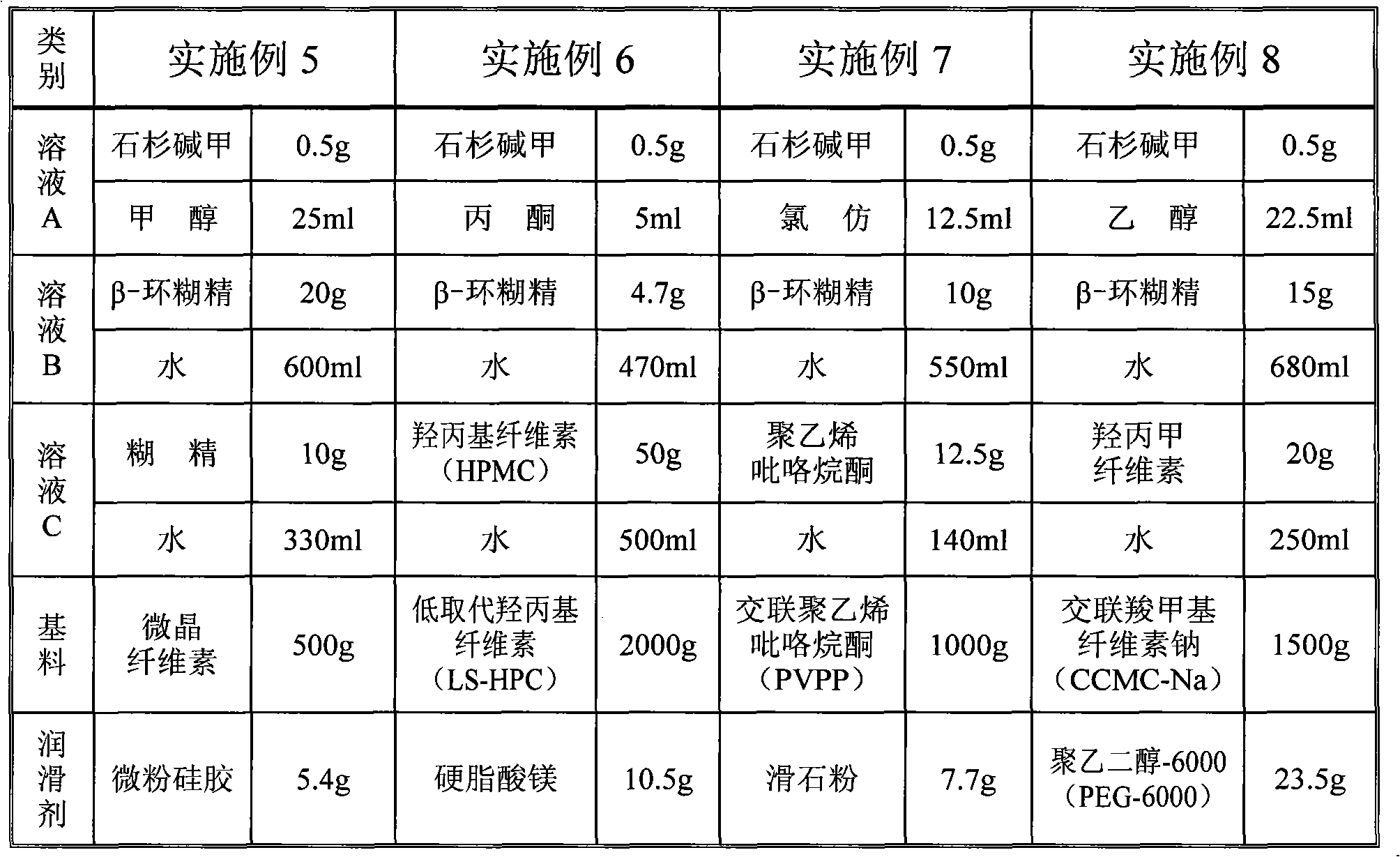
[0045] Take 0.5g of Huperzine A and dissolve it in 20.0ml of 95% medicinal ethanol. Take another 10.0g of β-cyclodextrin, add 50ml of purified water, stir and heat to 80°C to dissolve all, cool to 40°C, slowly add the above-mentioned Huperzine A / ethanol solution within 15 minutes under stirring, Continue stirring for 30 minutes, cool to 0°C, refrigerate for 12 hours, filter with a filter cloth, and dry to obtain 9.85 g of β-cyclodextrin inclusion compound of Huperzine A. The average yield is based on the weight of β-cyclodextrin. Is 98.5%. The average recovery rate of Huperzine A was 98%.
[0046] Calculation formula:
[0047] Average yield = weight of inclusion compound ÷ weight of β-cyclodextrin feed × 100%.
[0048] Average recovery rate = weight of the inclusion compound × weight percentage of Huperzine A in the inclusion compound ÷ feed weight of Huperzine A × 100%.
[0049] The "inclusion compound" mentioned in these two formulas refers to the "β-cyclodextrin inclusion compou...
Embodiment 2
[0051] Take 0.5g of Huperzine A and dissolve it in 20.0ml of 95% medicinal ethanol. Take another 10.0g of β-cyclodextrin, add 50ml of purified water, stir and heat to 80°C to dissolve it completely, cool to 40°C, and slowly add the above-mentioned Huperzine A / ethanol solution within 20 minutes under stirring. Continue stirring for 30 minutes. Take another 20g starch, add 230g water to disperse, heat to 80°C and stir to form a slurry, cool to 40°C, mix with Huperzine A's β-cyclodextrin solution under stirring, and spray dry to obtain Huperzine A The β-cyclodextrin inclusion compound / starch mixture 29.55g. The average yield (based on the weight of the sum of β-cyclodextrin and starch) was 98.5%. The average recovery rate of Huperzine A was 98.5%.
[0052] Calculation formula:
[0053] Average yield=mixture weight÷(β-cyclodextrin weight+starch weight)×100%.
[0054] Average recovery rate = weight of mixture x weight percentage of Huperzine A in the mixture ÷ feeding amount of Huper...
Embodiment 3
[0057] Prescription: Huperzine A for medicinal use 0.5g
[0058] 95% ethanol medicinal 20.0ml
[0059] β-cyclodextrin medicinal 10.0g
[0060] 8% starch slurry medicinal 250g (starch 20g)
[0061] Starch medicinal 379.5g
[0062] Lactose medicinal 580.0g
[0063] Magnesium stearate medicinal 10.0g
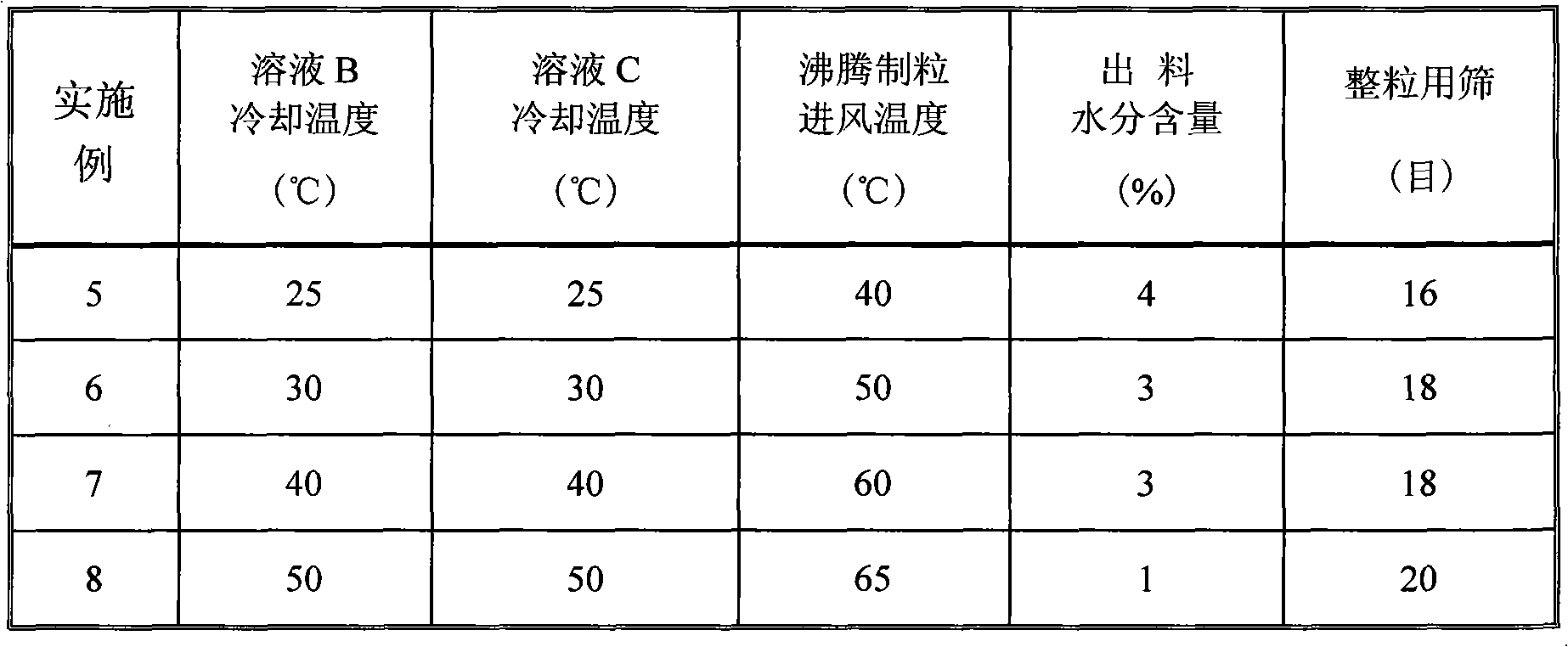
[0064] Process: According to the prescription, take Huperzine A and dissolve it with 95% medicinal ethanol. Take another β-cyclodextrin, add 50ml purified water, stir and heat to 80℃ to dissolve it completely, cool to 40℃, add Huperzine A / ethanol solution under stirring, continue stirring for 30 minutes to obtain Huperzine A The β-cyclodextrin inclusion compound solution. Take another 20g starch, add 230g purified water to disperse, heat to 80°C and stir to form a slurry, cool to 40°C, and mix with Huperzine A's β-cyclodextrin inclusion compound solution under stirring to obtain slurry. Boiling granulation, controlling the inlet air temperature to 60°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com