Application of hemopoietin sourced peptide in preparation of medicine for treating metabolic syndrome
A technology of erythropoietin and metabolic syndrome, which is applied in the field of biomedicine to achieve the effect of prolonging the half-life, increasing the route of administration, and reducing the frequency of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of Erythropoietin-derived Peptides
[0042] The amino acid sequence of the erythropoietin-derived peptide is as follows: GlnGluGlnLeuGluArgAlaLeuAsnSerSer, which is derived from the 58th (glutamine), 62nd (glutamic acid), 65th (glutamine), No. 69 (leucine), No. 72 (glutamic acid), No. 76 (arginine), No. 79 (alanine), No. 80 (leucine), No. 83 (day Paragine), the 84th (serine), the 85th (serine).
[0043] Erythropoietin-derived peptides were synthesized on an ARI431A solid-phase peptide synthesizer (PE, USA). Methods The standard fluorenylmethoxycarbonyl (Fmoc) protocol was used, and arginine was coupled twice. Initially select 0.125mmol p-hydroxymethylphenoxymethyl polystyrene resin (HMP resin), extend the peptide chain from the carboxyl terminal to the amino terminal one by one according to the polypeptide sequence, the amount of each amino acid is 0.5mmol, and the mole of the resin The ratio is 4:1. The α-amino acids of various amino acids are ...
Embodiment 2
[0045] Example 2 Preparation of Erythropoietin-derived Peptide Liposomes
[0046] Erythropoietin-derived peptide liposomes were prepared by reverse evaporation method. Dissolve lecithin and cholesterol in diethyl ether at a mass ratio of 1:1, distill off the organic solvent therein under reduced pressure, and form a uniform W / O emulsion in an ultrasonic water bath for 5 minutes, then add an appropriate amount as shown in SEQ ID NO: 1 The indicated erythropoietin-derived peptides were repeatedly frozen and thawed in a 42°C water bath and dry ice, and passed through a film extruder with a pore size of 100 nm several times. Utilizing that the volume of the erythropoietin-derived peptide shown in SEQ ID NO: 1 becomes larger after being bound to the liposome, the erythropoietin-derived peptide not bound to the liposome is separated with an agarose gel CL-4B column. The mass ratio of lecithin, cholesterol and small molecule polypeptide in the liposome is 50:50:1. Then the morpholo...
Embodiment 3
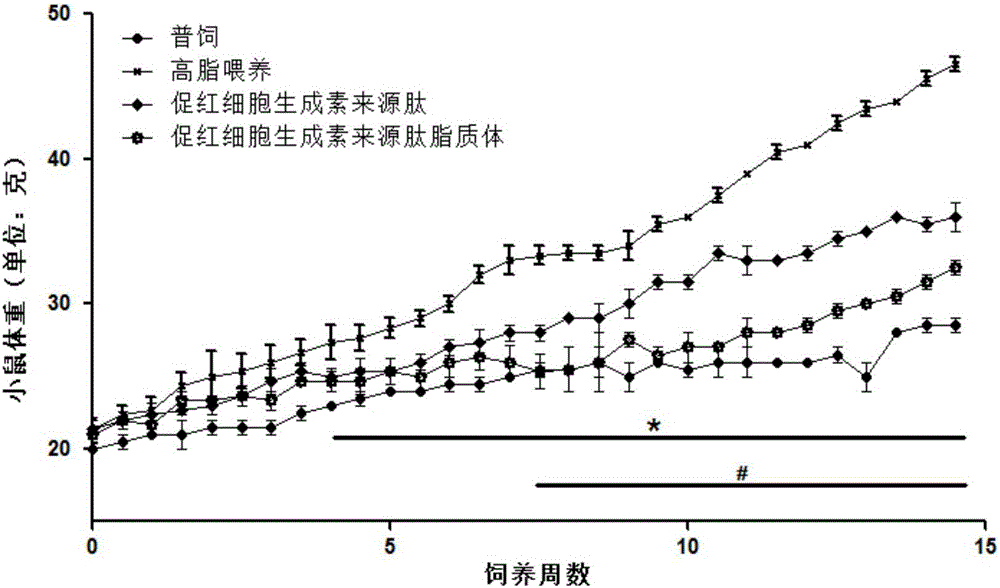
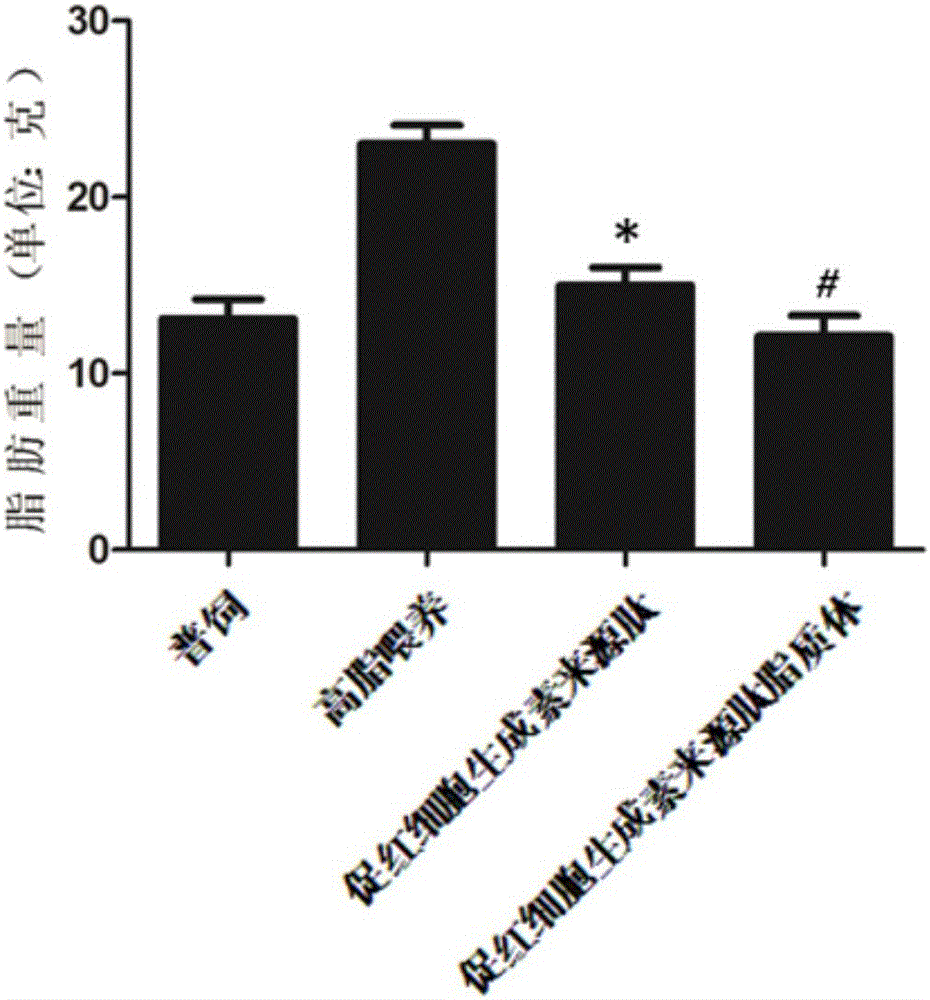
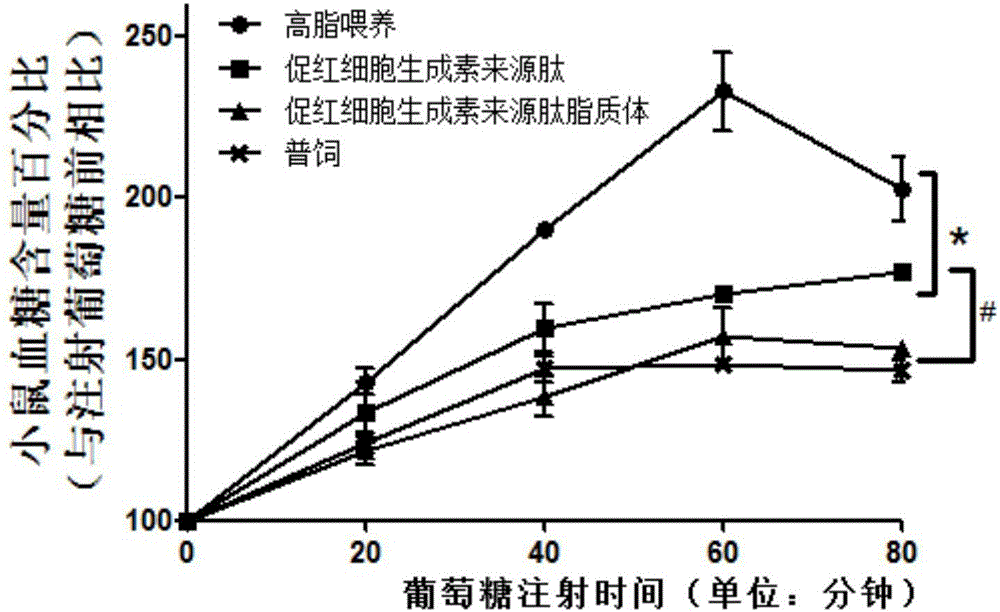
[0047] Example 3 The effect of erythropoietin-derived peptide and liposomes on obesity, diabetes and hyperlipidemia
[0048] 1. Test animals:
[0049] Wild-type C57 mice, male, 5-6 weeks old, were purchased from the Experimental Animal Center of Daping Hospital, Third Military Medical University, and were normally kept in a clean animal room.
[0050] 2. Drugs and grouping:
[0051] Erythropoietin-derived peptide treatment group: intraperitoneal injection of 30 μg / kg body weight of erythropoietin-derived peptide shown in SEQ ID NO: 1, a total of 18 animals, injected once every other day;
[0052] Erythropoietin-derived peptide liposome treatment group: intraperitoneal injection of 30 μg / kg body weight of erythropoietin-derived peptide liposome, a total of 18, injected once every other day;
[0053] High-fat feeding group: give the same volume of PBS as the treatment group for intraperitoneal injection, a total of 18 animals, and inject once every other day;
[0054] General...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com