Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
98results about "Aluminium nitrates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
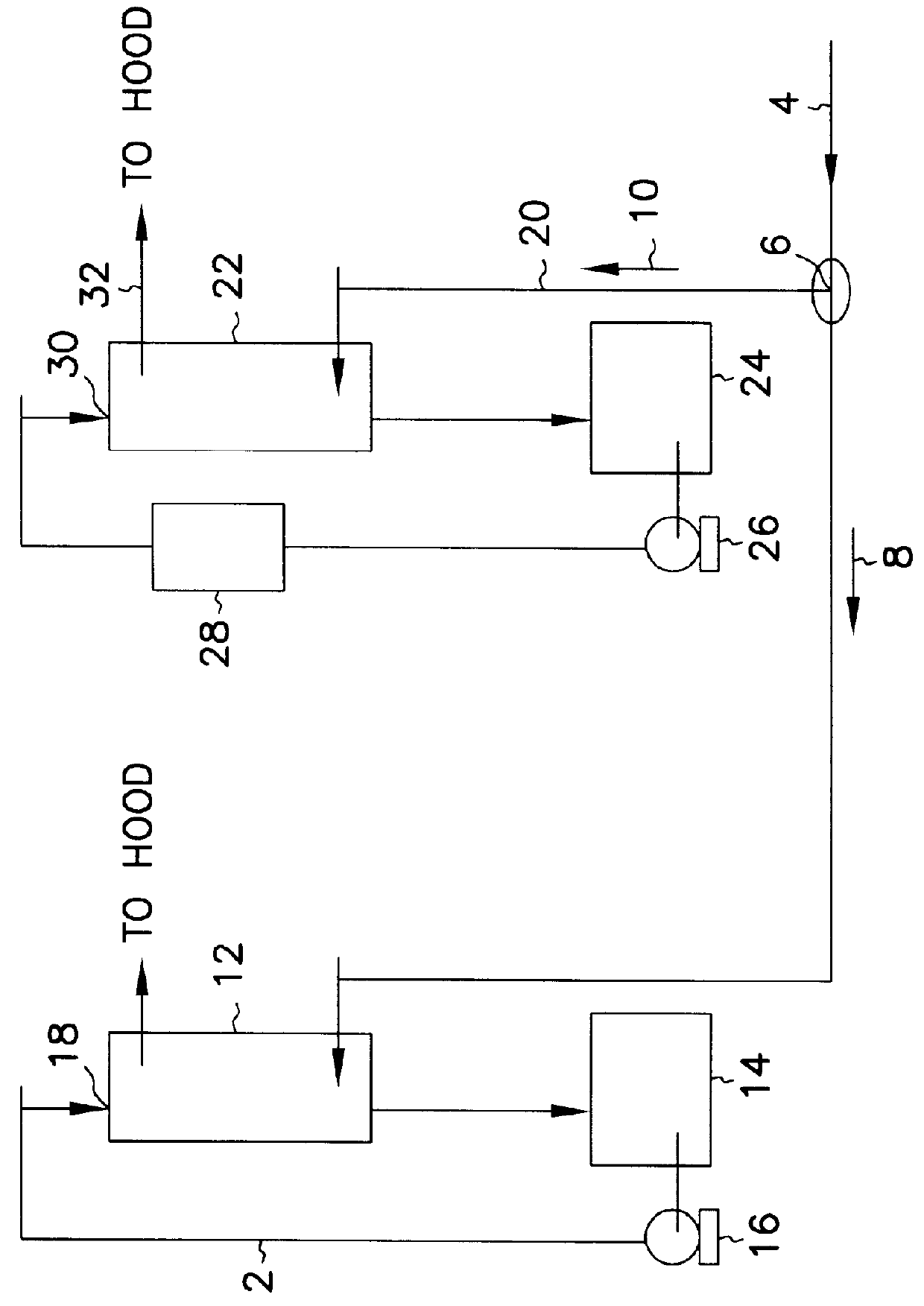
Process and equipment for nitrogen oxide waste conversion to fertilizer
The present invention describes a process for converting vapor streams from sources containing at least one nitrogen-containing oxidizing agent therein to a liquid fertilizer composition comprising the steps of: a) directing a vapor stream containing at least one nitrogen-containing oxidizing agent to a first contact zone, b) contacting said vapor stream with water to form nitrogen oxide(s) from said at least one nitrogen-containing oxidizing agent, c) directing said acid(s) as a second stream to a second contact zone, d) exposing said second stream to hydrogen peroxide which is present within said second contact zone in a relative amount of at least 0.1% by weight of said second stream within said second contact zone to convert at least some of any nitrogen oxide species or ions other than in the nitrate form present within said second stream to nitrate ion, e) sampling said stream within said second contact zone to determine the relative amount of hydrogen peroxide within said second contact zone, f) adding hydrogen peroxide to said second contact zone when a level of hydrogen peroxide less than 0.1 % by weight in said second stream is determined by said sampling, g) adding a solution comprising potassium hydroxide to said second stream to maintain a pH between 6.0 and 11.0 within said second stream within said second contact zone to form a solution of potassium nitrate, and h) removing said solution of potassium nitrate from said second contact zone.
Owner:NAT AERONAUTICS & SPACE ADMINISTATION U S GOVERNMENT AS REPRESENTED BY THE ADMINISTATOR
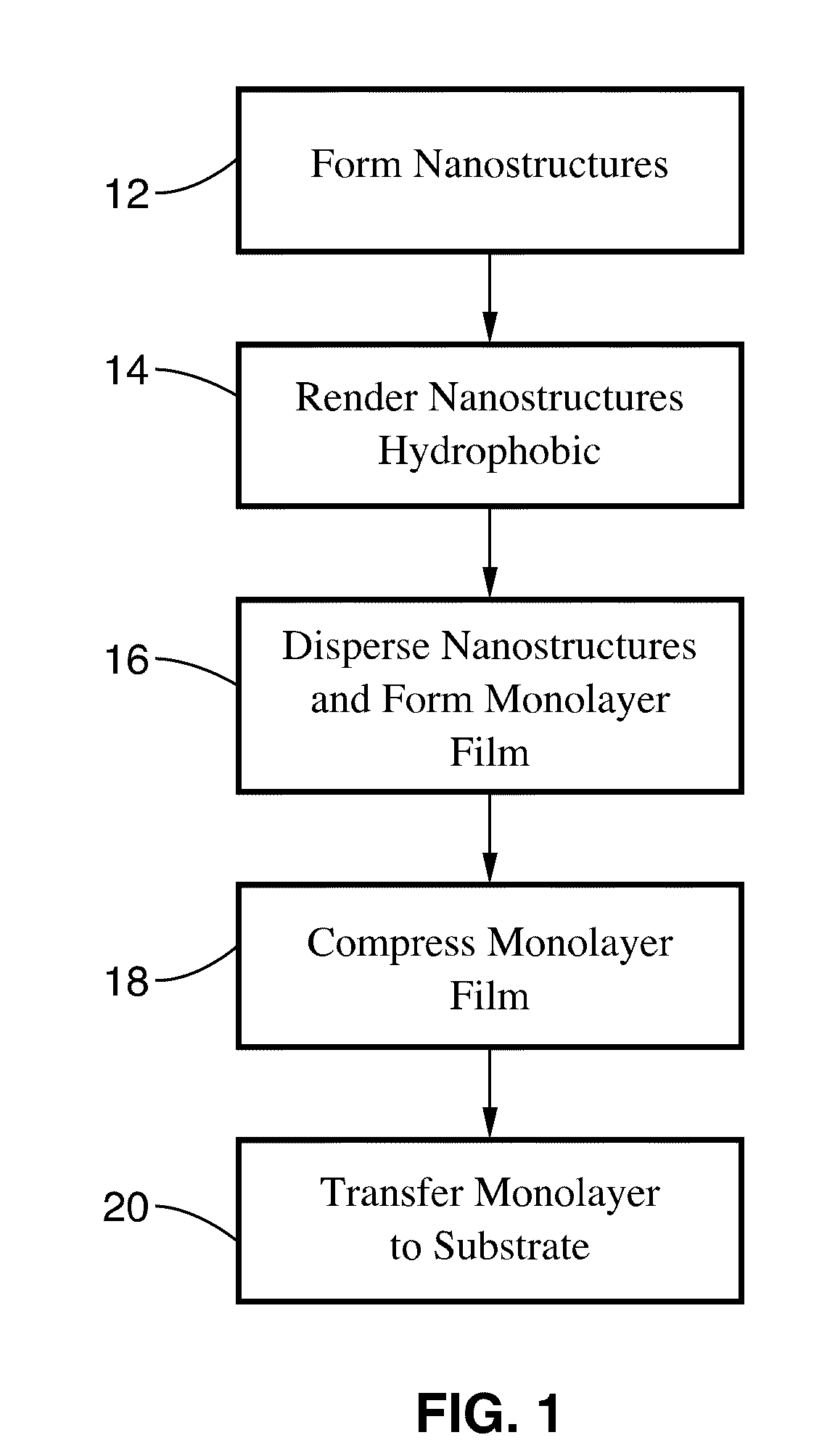
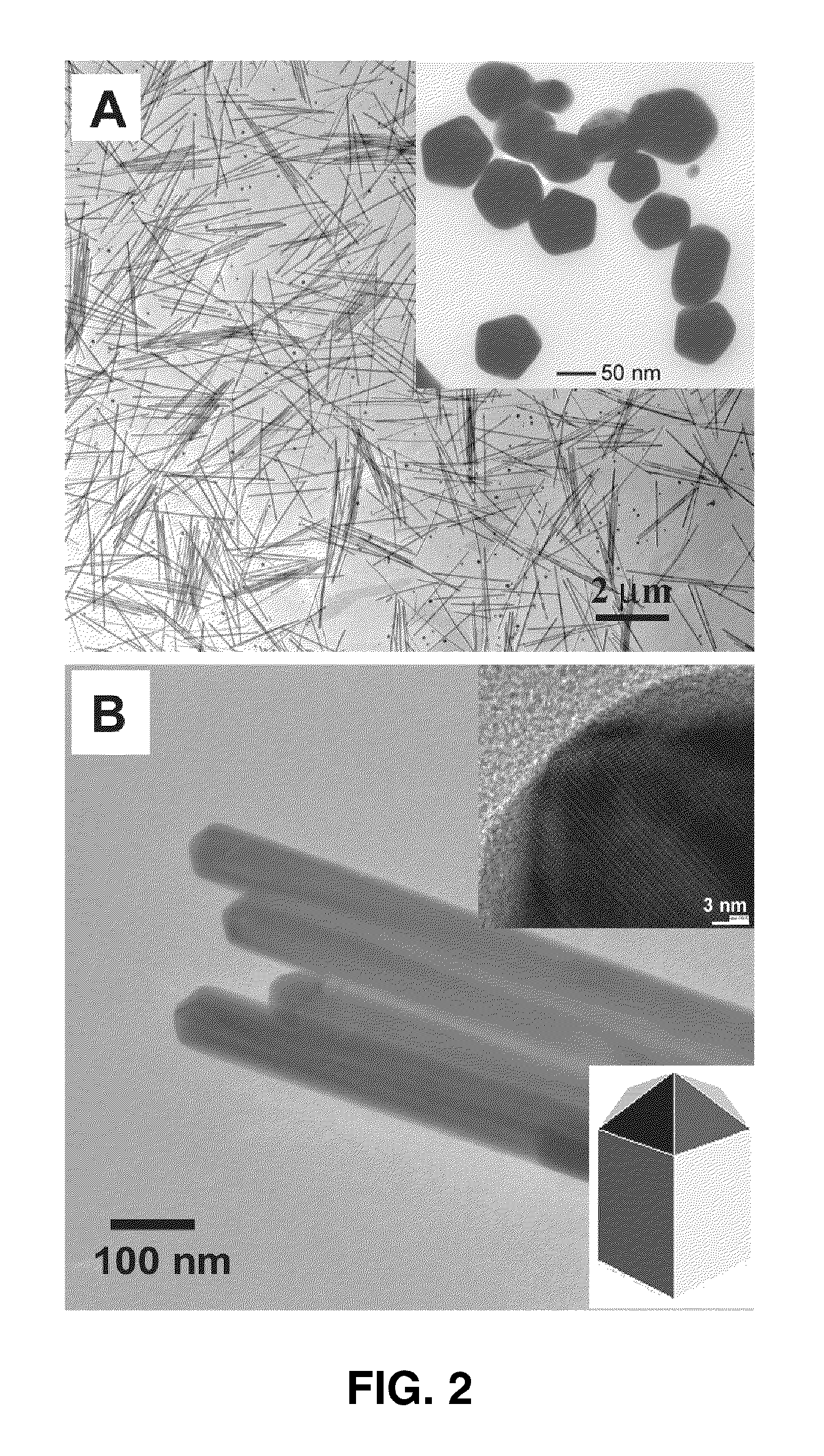
Langmuir-blodgett nanostructure monolayers
InactiveUS20090169807A1Easy to useLarge field enhancementLiquid surface applicatorsNanostructure manufactureNanoparticleEngineering
Methods for assembly of monolayers of nanoparticles using the Langmuir-Blodgett technique, as well as monolayers, assemblies, and devices are described. The surface properties of these monolayers are highly reproducible and well-defined as compared to other systems. These monolayers can readily be used for molecular detection in either an air-borne or a solution environment, and sensors using the monolayer could have significant implications in chemical and biological warfare detection, national and global security, as well as in medical detection applications.
Owner:RGT UNIV OF CALIFORNIA
Process for removal of pollutants
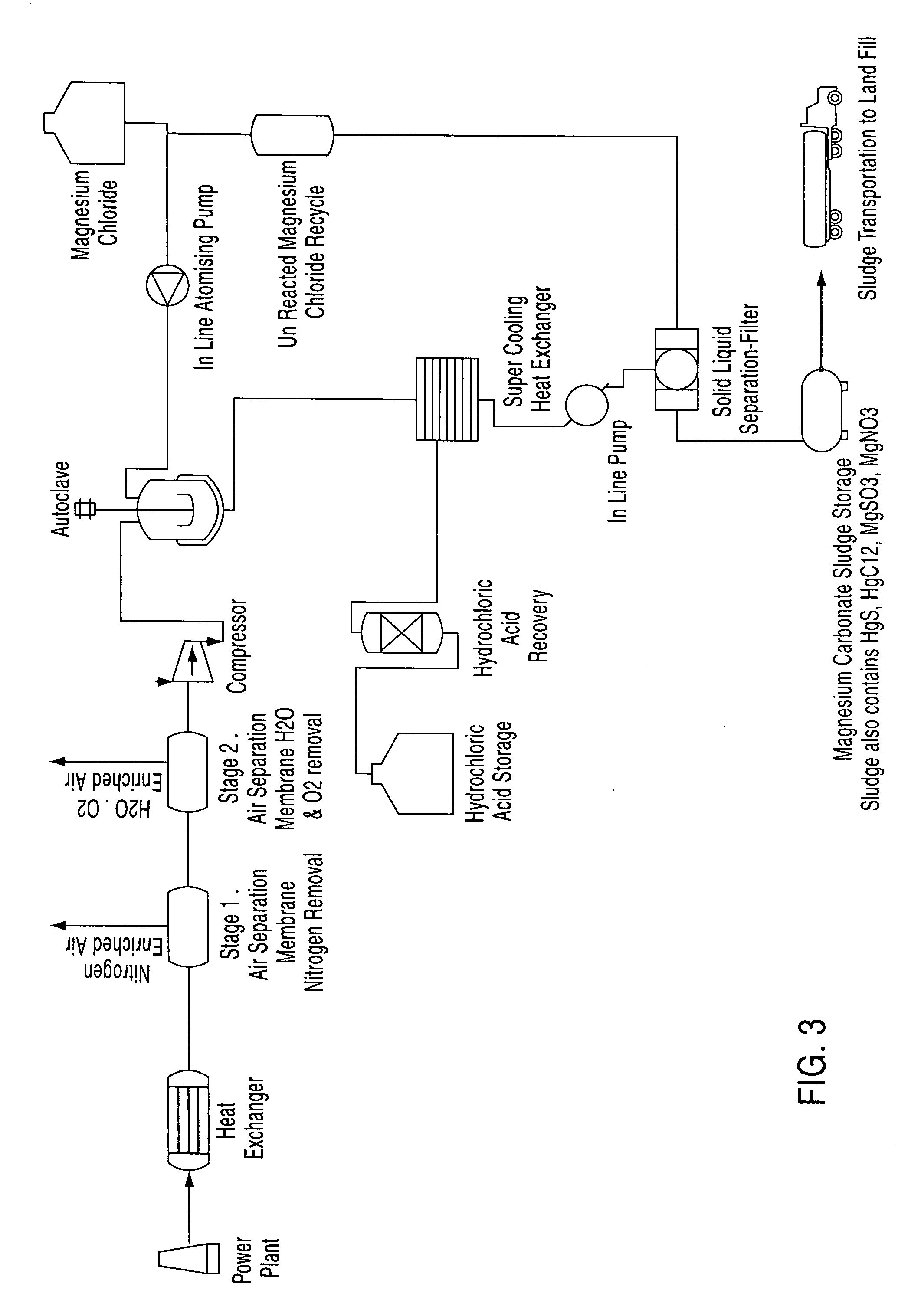
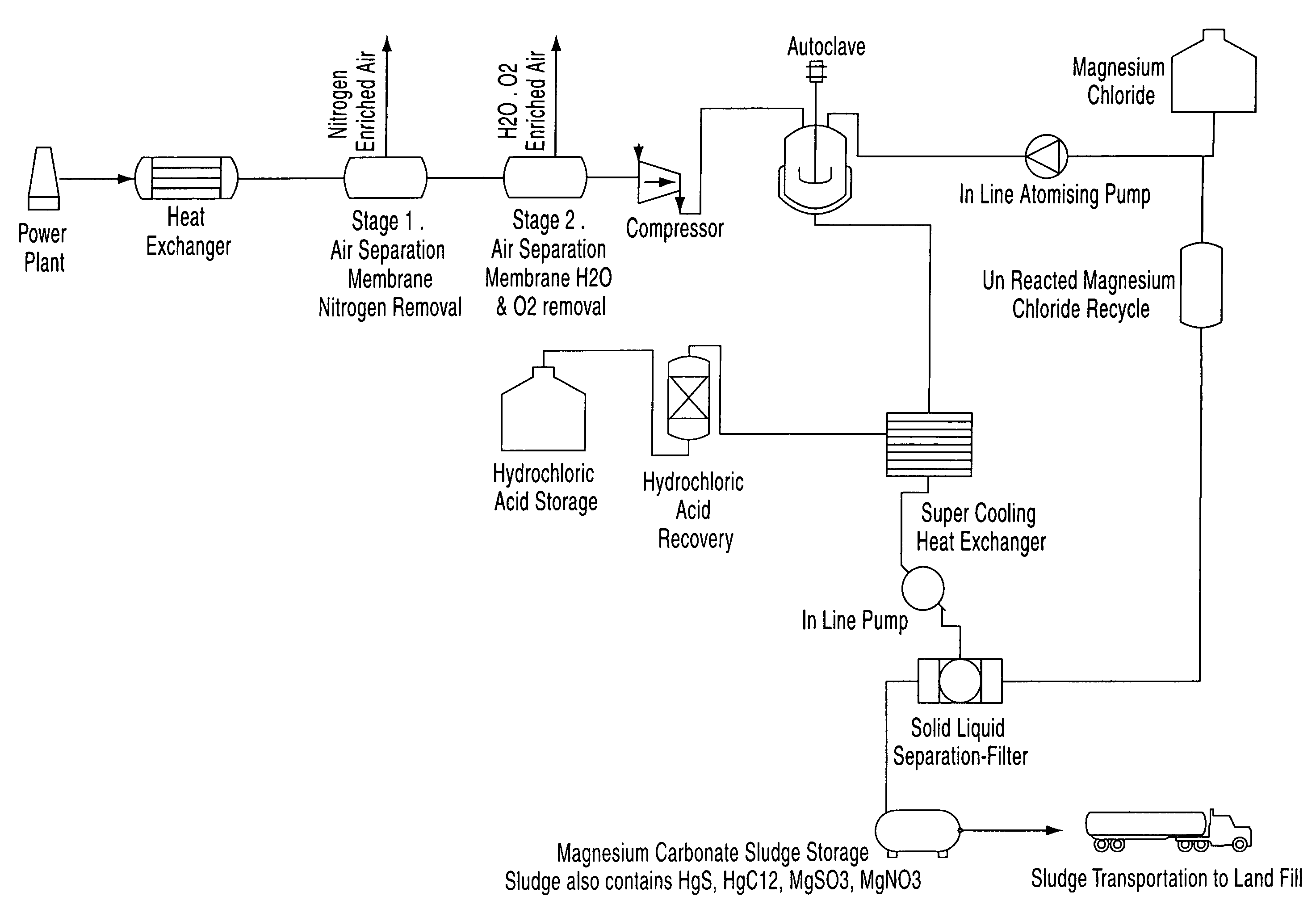
A process for the removal of pollutants from a combustion process and, more particularly, a process for removing pollutants such as carbon dioxide, mercury, sulphur dioxide, nitrogen compounds and oxygen compounds from a combustion process. The process includes the removal of pollutants from a combustion process that produces an emission comprising: cooling the emission to a temperature of about 200° C.; removing nitrogen, water and oxygen from the emission to produce a gas containing a concentration of pollutants; contacting the gas with an aqueous magnesium chloride solution, wherein a slurry mixture is formed; and cooling the gas and the slurry mixture, wherein hydrochloric acid vapour and a sludge are formed.
Owner:CLEAN WORLD STRATEGIES CORP
Method for producing iron oxyhydroxide and adsorbing material comprising iron oxyhydroxide
InactiveUS20090028770A1Improve adsorption capacityEfficient productionPhosphorus oxidesNitrogen compoundsIndustrial effluentHazardous substance
The present invention provides a method for advantageously producing an iron oxyhydroxide exhibiting excellent capability of adsorbing harmful substances, such as a phosphrous components and endocrine disrupting chemicals, which are contained in industrial wastewater, exhaust gases, etc., and an adsorbent material comprising the iron oxyhydroxide produced by the method as a main component. Specifically, the present invention provides an adsorbent material produced by a method comprising the steps of:(a) adding a base to an aqueous iron ion-containing solution, adjusting the pH of the resultant mixture to 9 or less, to form a precipitate that contains an iron oxyhydroxide;(b) drying the precipitate at a temperature of 100° C. or lower to obtain an iron oxyhydroxide;(c) contacting the resultant iron oxyhydroxide with water; and(d) subjecting the resultant iron oxyhydroxide to a heat treatment under a gas atmosphere having an inert gas concentration of 80% or greater at a temperature of 100 to 280° C.
Owner:JAPAN SCI & TECH CORP +2
Aqueous solution of chromium salt and method for producing same
InactiveUS20070086938A1Overcome disadvantagesPigmenting treatmentNitrogen compoundsOXALIC ACID DIHYDRATEChloride
Disclosed is an aqueous solution of a chromium salt, in which the oxalic acid content is 8% by weight or less relative to chromium. In the aqueous solution of the chromium salt, the total organic carbon content is 4% by weight or less relative to chromium. The chromium salt is preferably a chromium chloride, a chromium phosphate, or a chromium nitrate. The chromium chloride preferably contains a basic chromium chloride represented by the composition formula Cr(OH)xCly (wherein 0<x≦2, 1≦y<3, and x+y=3). The chromium phosphate is preferably one represented by the composition formula Cr(H3−3 / nPO4)n (wherein n is a number satisfying 2≦n≦3). The chromium nitrate is preferably a basic chromium nitrate represented by the composition formula Cr(OH)x(NO3)y (wherein 0<x≦2, 1≦y<3, and x+y=3).
Owner:NIPPON CHECMICAL IND CO LTD
Pastillation of Ammonium Sulfate Nitrate
A process is presented for the production of ammonium sulfate nitrate. The process provides for producing a highly uniform product and having a substantially uniform size. The process includes reacting ammonium sulfate and ammonium nitrate to form an FASN slurry melt. The slurry melt is continuously stirred and heated to keep the slurry melt under a shear thinned condition and at a uniform temperature until the slurry melt is extruded, cooled and solidified.
Owner:ADVANSIX RESINS & CHEM LLC
Methods of making cesium salts and other alkali metal salts
A method of making a cesium salt is described and involves reacting a cesium sulfate containing solution with lime to form 1) a solution containing at least cesium hydroxide and 2) a residue comprising calcium sulfate. The method further involves removing the residue from the solution and converting the cesium hydroxide that is present in the solution to at least one type of cesium salt. The present invention further relates to uses of the cesium salt as well as methods of making cesium hydroxide using lime. Also, methods of making alkali metal salts and alkali metal hydroxides are also described.
Owner:CABOT SPECIALTY FLUIDS
Methods of making cesium salts and other alkali metal salts
A method of making a cesium salt is described and involves reacting a cesium sulfate containing solution with lime to form 1) a solution containing at least cesium hydroxide and 2) a residue comprising calcium sulfate. The method further involves removing the residue from the solution and converting the cesium hydroxide that is present in the solution to at least one type of cesium salt. The present invention further relates to uses of the cesium salt as well as methods of making cesium hydroxide using lime. Also, methods of making alkali metal salts and alkali metal hydroxides are also described.
Owner:CABOT SPECIALTY FLUIDS
Method of purifying barium nitrate aqueous solution
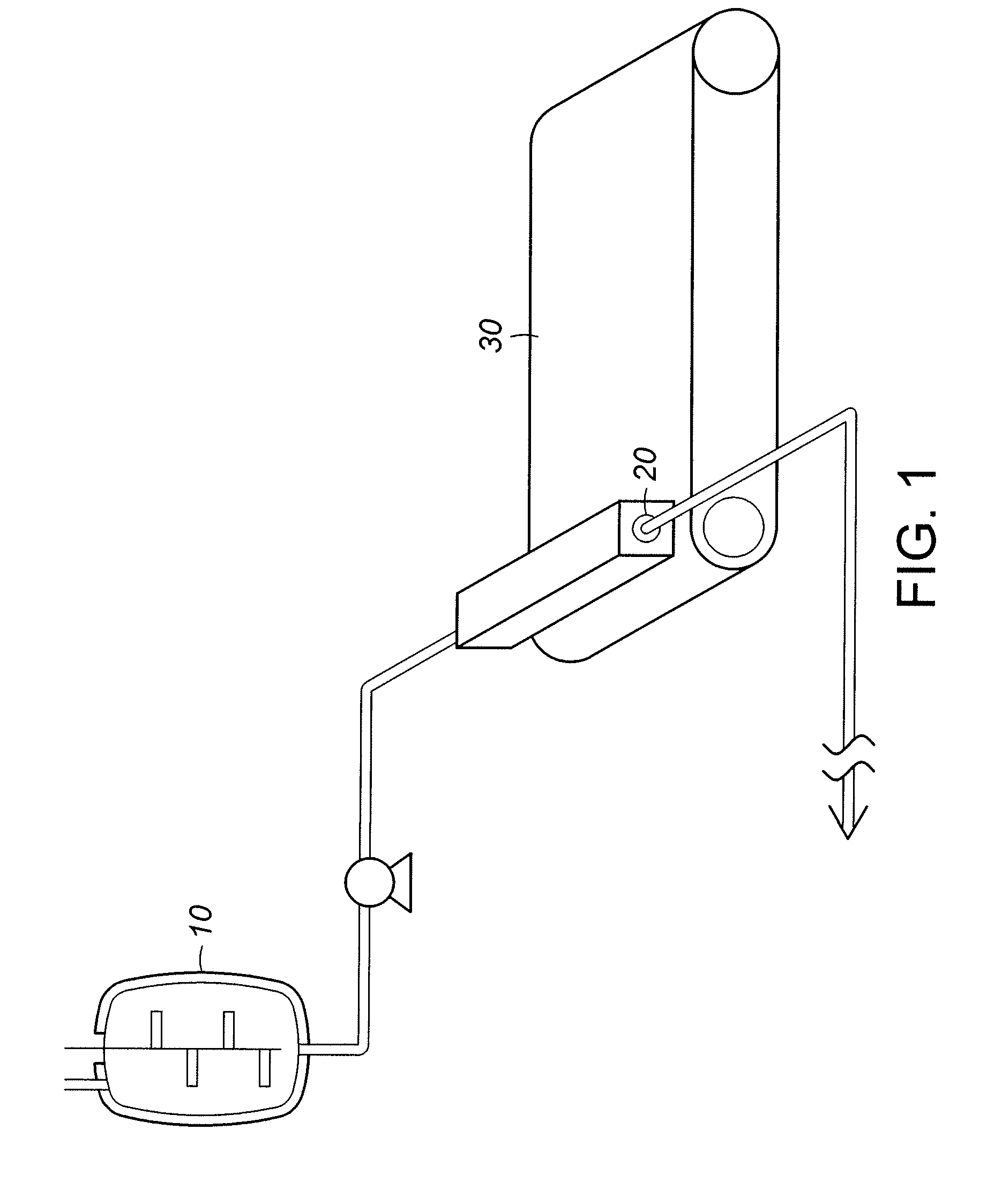
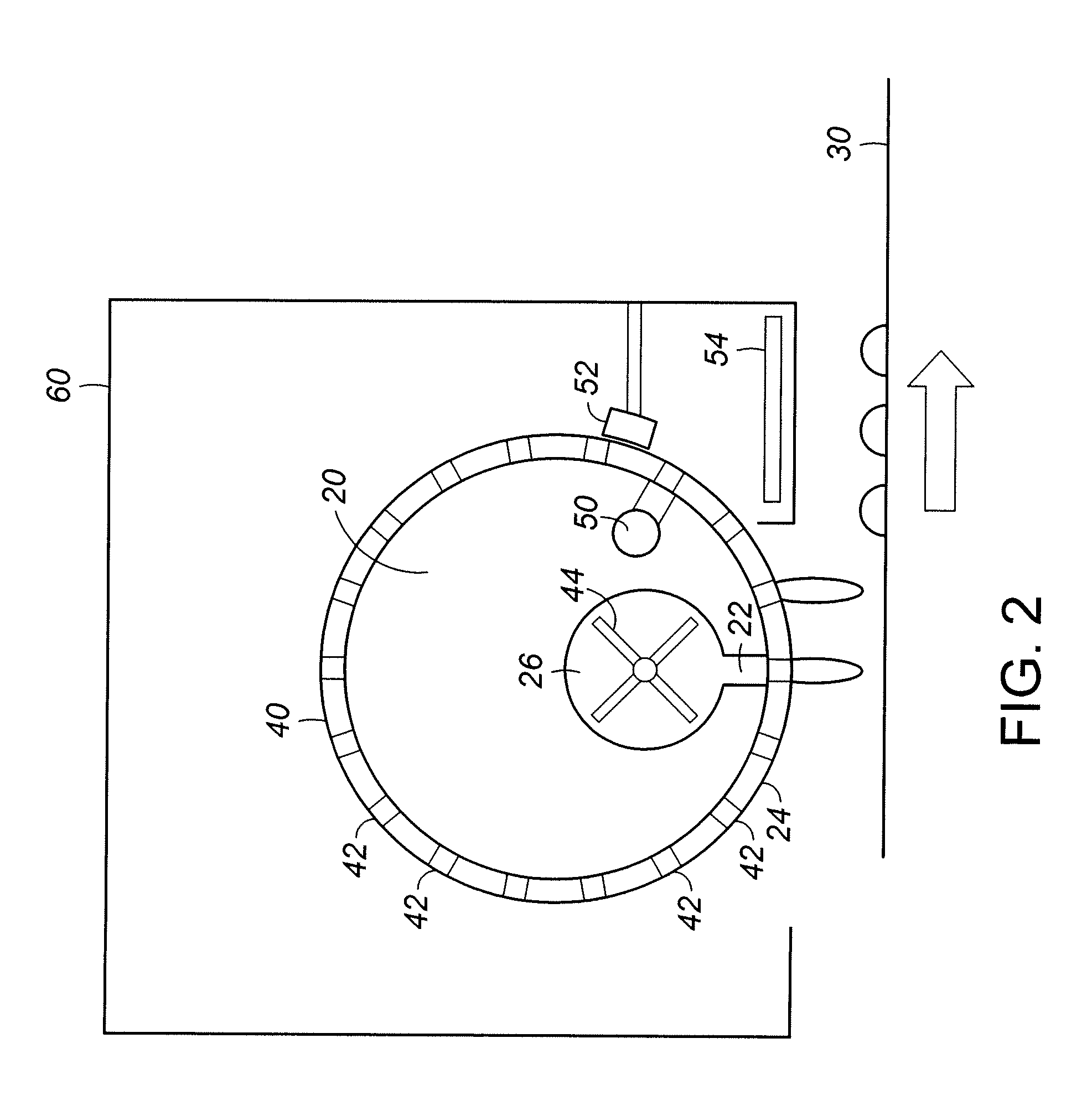
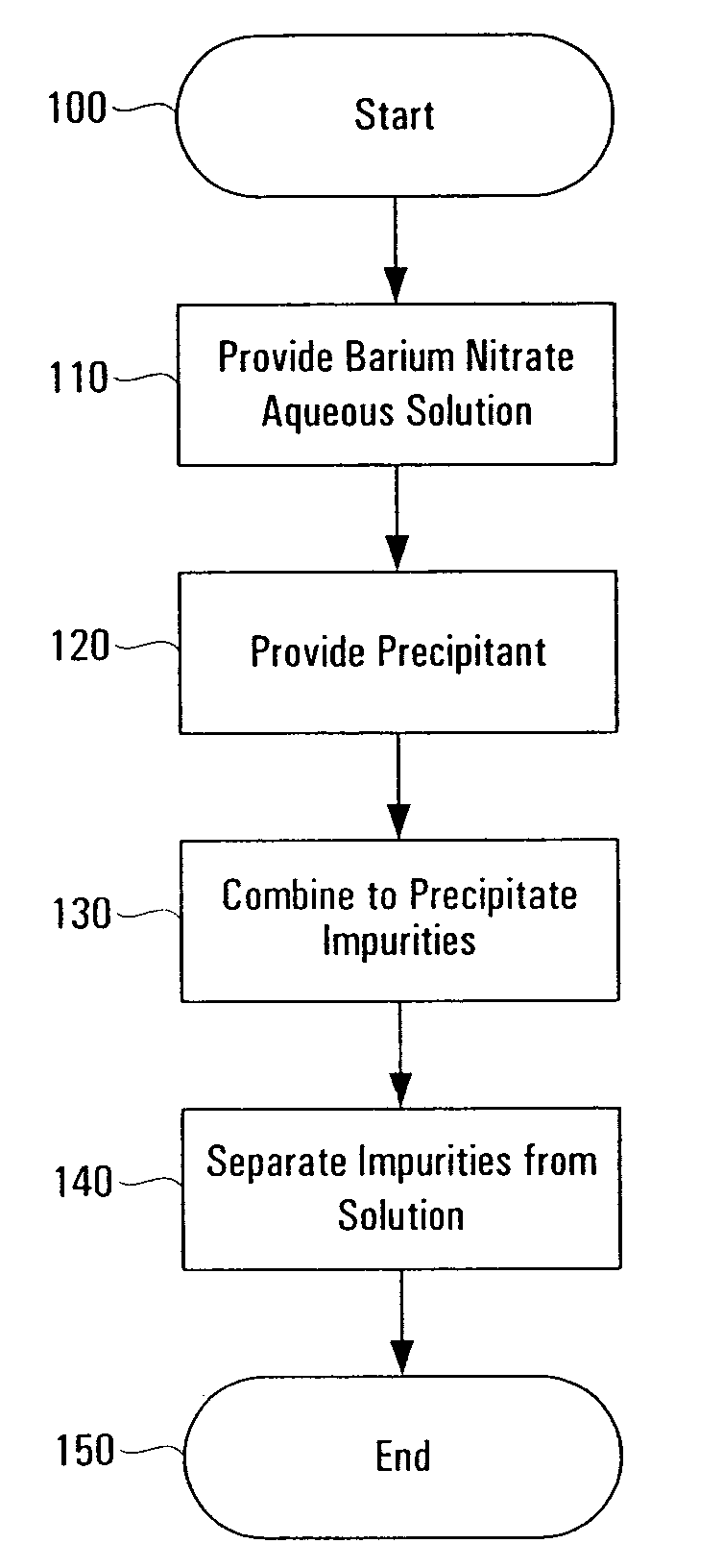
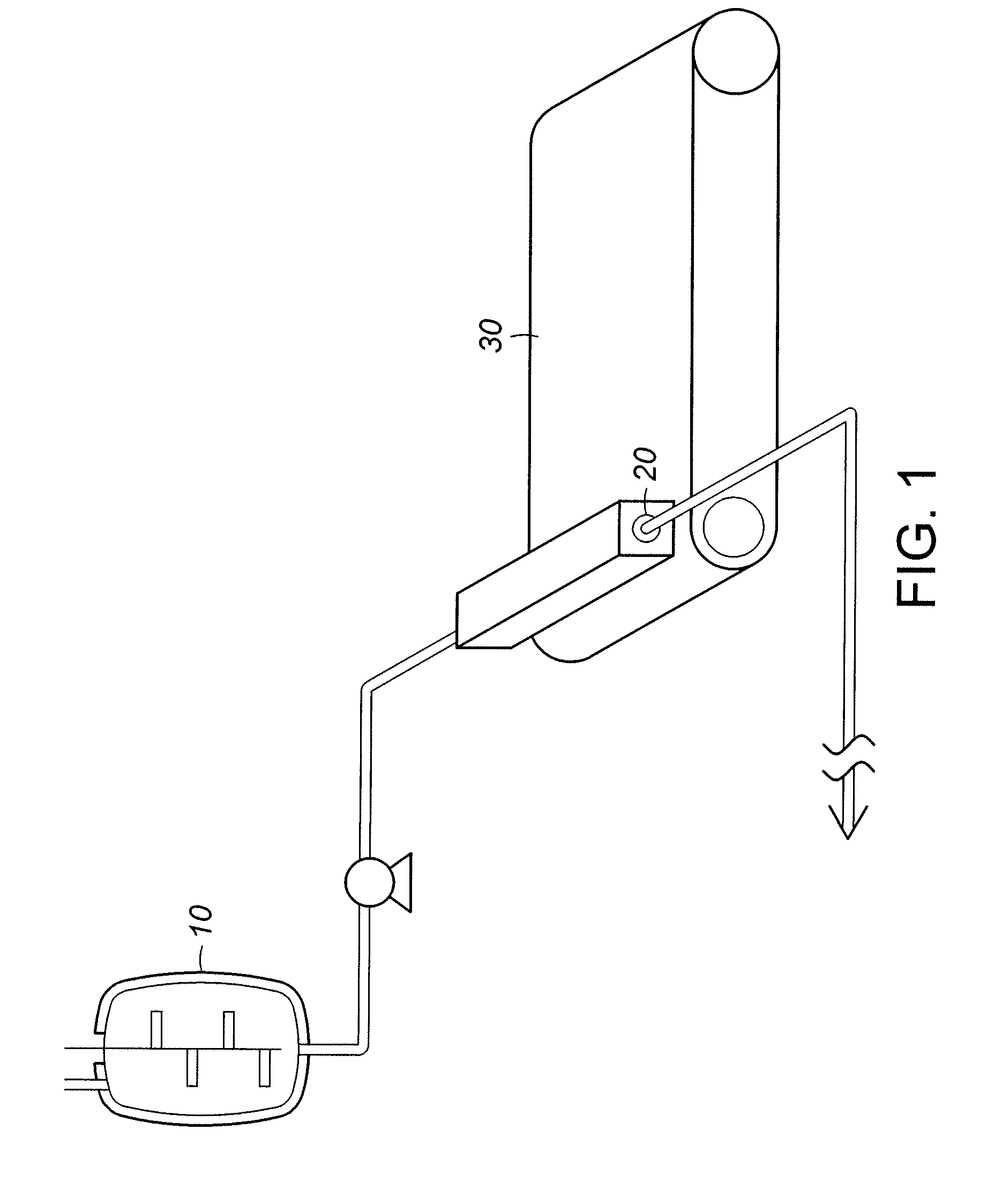
InactiveUS7648687B1Solid sorbent liquid separationLiquid solutions solvent extractionBarium nitrateOxidation state
Purification techniques have been developed for ceramic powder precursors, e.g., barium nitrate. These techniques can be performed using one or more of the following operations: (1) removal of impurities by precipitation or coprecipitation and separation using a nonmetallic-ion-containing strong base, e.g., tetraalkylammonium hydroxides; (2) reduction of higher oxidation-state-number oxymetal ions and subsequent precipitation as hydroxides that are separated from the solution; and (3) use of liquid-liquid exchange extraction procedures to separate certain impurities.
Owner:EESTOR
Strontium nitrate and method for manufacturing same
Low-cost, high-purity strontium nitrate that is low in Ba, Na, Ca, Cr, and other impurities and that is suitable for use in airbags or the like is provided. High-purity strontium nitrate having a Ba content of 0.01 wt % or lower, an Na content of 0.005 wt % or lower, a Ca content of 0.01 wt % or lower, a Cr content of less than 0.001 wt %, and a purity of 99.5 wt % or higher is produced by a manufacturing method comprising a first step for performing crystallization by adding nitric acid to an aqueous solution obtained by dissolving a strontium compound as a starting material, a second step for separating the resulting crystals, a third step for crystallizing the resulting separated solution, and a fourth step for separating the resulting crystals.
Owner:DOWA METALS & MINING CO LTD +1
Compositions and Methods Comprising High Valency Silver for Increasing Seed Germination
InactiveUS20110275518A1Promote seed germinationImproved and efficient active agent delivery systemBiocideNitrogen compoundsBiofilmGermination
The present invention is compositions and methods for improving seed germination rates or speed using a composition comprising at least one high valency silver ion. The compositions and methods of the present invention are effective in treating biofilms.
Owner:MARQUES LYRIAM L +2
Pastillation of ammonium sulfate nitrate
A process is presented for the production of ammonium sulfate nitrate. The process provides for producing a highly uniform product and having a substantially uniform size. The process includes reacting ammonium sulfate and ammonium nitrate to form an FASN slurry melt. The slurry melt is continuously stirred and heated to keep the slurry melt under a shear thinned condition and at a uniform temperature until the slurry melt is extruded, cooled and solidified.
Owner:ADVANSIX RESINS & CHEM LLC
Aluminum-zirconium antiperspirant salts with low M:CI ratio
InactiveUS6991780B2Good curative effectGreat antiperspirant efficacyCosmetic preparationsToilet preparationsAntiperspirantsPerspiration
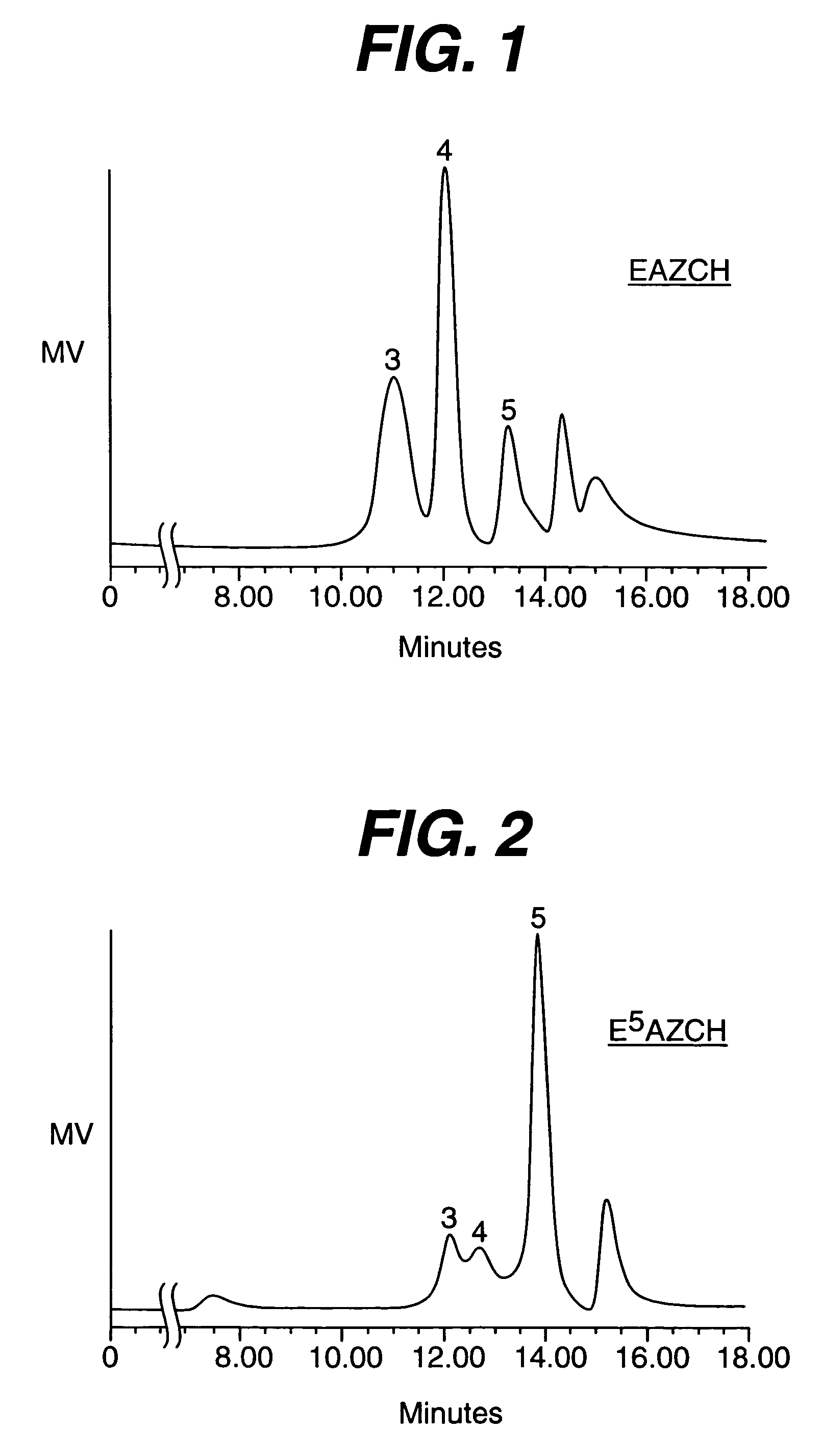
Disclosed are enhanced efficacy aluminum-zirconium antiperspirant salt compositions that have a metal (Al+Zr) to chloride (or anion) ratio of about 0.90 to about 1.00. These salts also typically exhibit an HPLC peak 5 area content of about 33% or more, preferably at least 45%, more preferably at least 50%, most preferably at least 55%. Especially preferred are aluminum-zirconium antiperspirant salt compositions which, in addition to the aforementioned high peak 5 content, also exhibit an HPLC peak 4 to peak 3 area ratio of at least 0.4, preferably at least 0.7. Also disclosed are methods of making such antiperspirant salt compositions and aqueous solutions of such antiperspirant salt compositions. Further disclosed are topical compositions comprising a dermatologically acceptable carrier vehicle and a perspiration reducing effective amount of an aluminum-zirconium antiperspirant salt composition as described above.
Owner:HENKEL IP & HOLDING GMBH
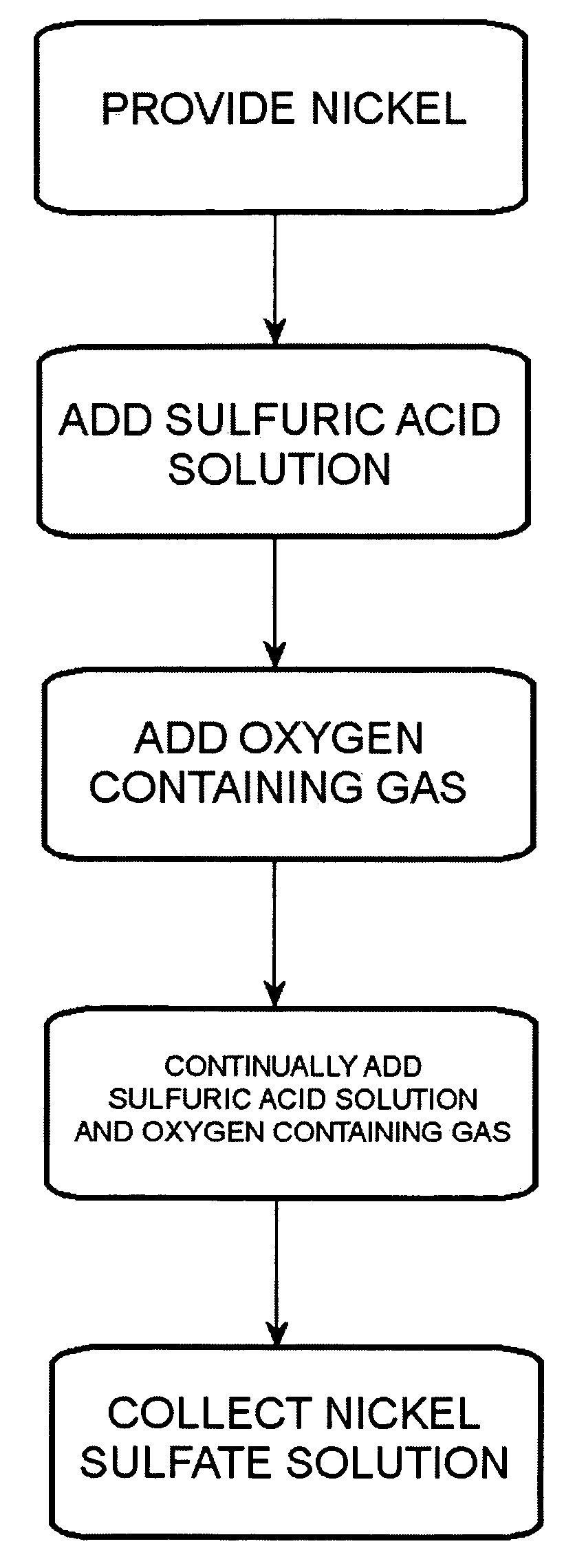
Method of producing a nickel salt solution
A method for converting nickel into a nickel salt solution. Nickel is dissolved and reacted in an oxygen-enriched acidic solution to produce a nickel salt solution as illustrated in the following chemical equation, wherein X is a conjugate base: Ni+H2X+½O2->NiX+H2O.
Owner:CHEVRON TEXACO TECH VENTURES
Manufacture of water chemistries
InactiveUS20080053104A1Effective and efficient and economically feasible processEfficient and effective processNitrogen compoundsElectrostatic separationPresent methodDisinfectant
As population density increases, the transportation of hazardous chemicals, including acids and disinfectants, lead to an increased incidence of spills while the consequences of spills become more serious. While solutions of halide acids, hypohalites and halites are safer disinfectants for transportation, handling, storage and use than traditional gaseous chlorine, the manufacturing cost of these disinfectants has here-to-fore limited their use. Economical processes are presented for the manufacture of O2, halogen oxides, halide acids, hypohalites, and halates; as well as polynucleate metal compounds, metal hydroxides and calcium sulfate hydrate (gypsum). The instant invention presents methods and processes that incorporate the use of sulfur. This is while environmental regulators, such as the US EPA, require an increased removal of sulfur from hydrocarbon fuels, thereby creating an abundance of sulfur, such that the refining industry is in need of a way to dispose of said abundance of sulfur.
Owner:CLEARVALUE TECH
Process for removal of pollutants
A process for the removal of pollutants from a combustion process and, more particularly, a process for removing pollutants such as carbon dioxide, mercury, sulphur dioxide, nitrogen compounds and oxygen compounds from a combustion process. The process includes the removal of pollutants from a combustion process that produces an emission comprising: cooling the emission to a temperature of about 200° C.; removing nitrogen, water and oxygen from the emission to produce a gas containing a concentration of pollutants; contacting the gas with an aqueous magnesium chloride solution, wherein a slurry mixture is formed; and cooling the gas and the slurry mixture, wherein hydrochloric acid vapour and a sludge are formed.
Owner:CLEAN WORLD STRATEGIES CORP
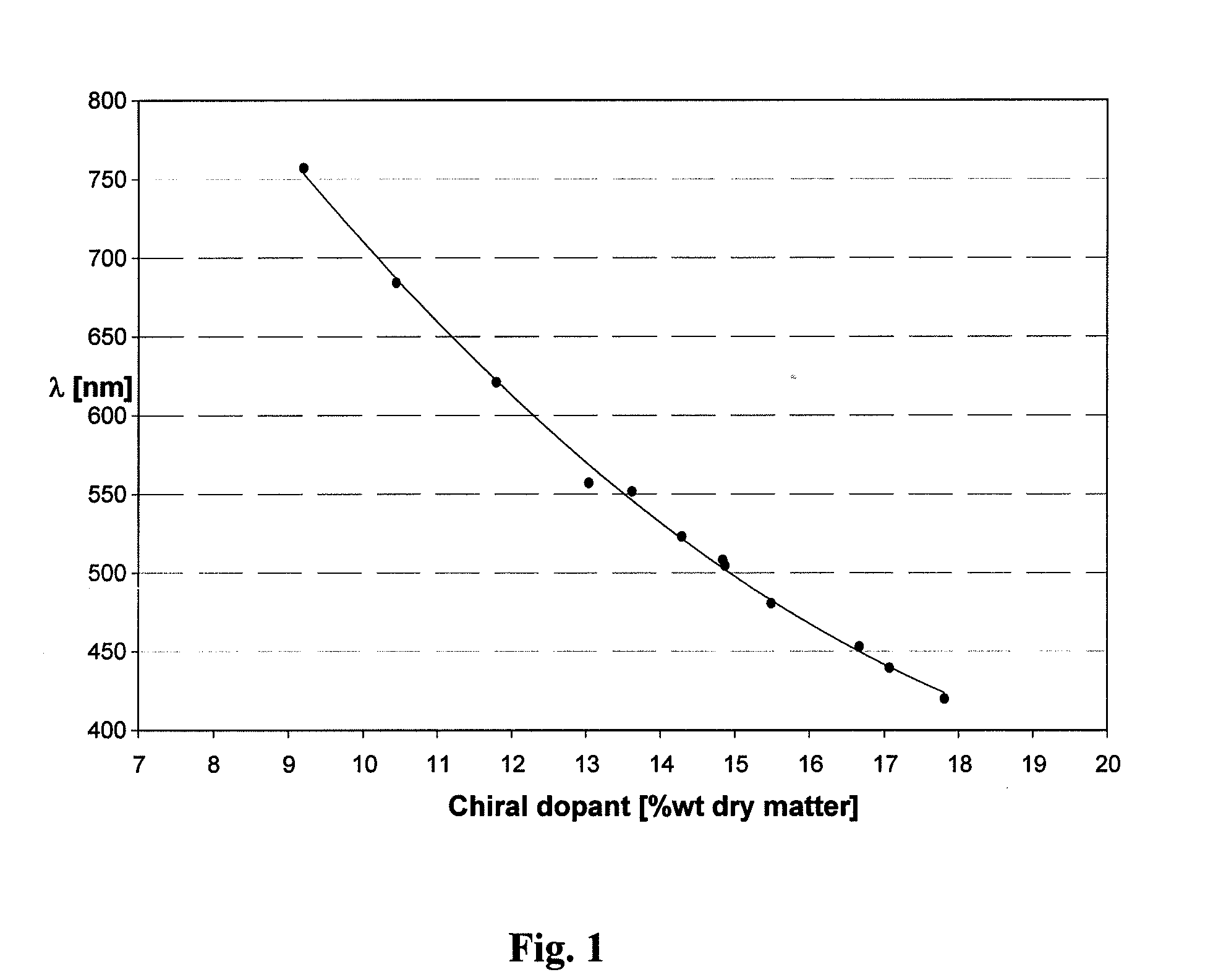
Simplified control of color shifting properties of a chiral liquid crystal polymer
ActiveUS20120141745A1Efficient and predictableNitrogen compoundsLithium compoundsSelective reflectionPolymer
A chiral liquid crystal precursor composition which comprises at least one salt that changes a position of the selective reflection band exhibited by the composition in a cured state compared to the position of a selective reflection band exhibited by a composition in the cured state that does not contain the at least one salt. This abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SICPA HLDG SA
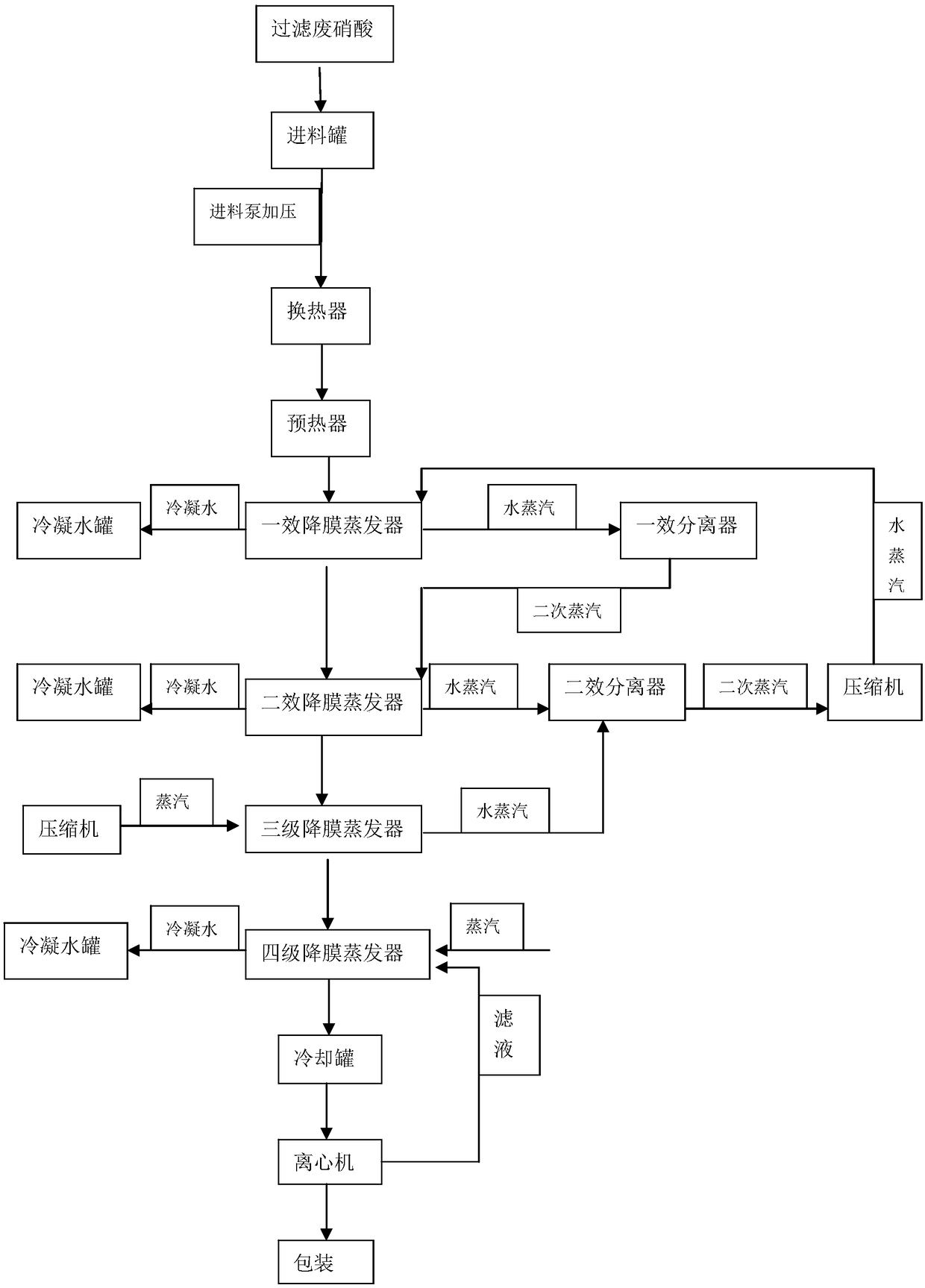
Recycling method for electrode foil-corrosion waste nitric acid
ActiveCN108715456AEasy to corrodeEfficient recyclingAluminium nitratesNitric acidThree levelSolubility
The invention belongs to the technical field of resource recycling and particularly relates to a recycling method for electrode foil-corrosion waste nitric acid. By virtue of organic combination of the steps of preheating, evaporative concentration, cooling crystallization, centrifugal separation and the like, nitric acid is effectively recycled, and a byproduct, namely aluminum nitrate, is obtained. Wastewater mainly contains nitric acid and aluminum nitrate, most moisture in the wastewater is removed through two-effect three-level MVR and single-effect evaporation, and a concentrated solution with high aluminum nitrate concentration is obtained; by carrying out cooling crystallization, aluminum nitrate with relatively low solubility can be separated for crystallization; and by carrying out solid-liquid separation, filtrate containing nitric acid and aluminum nitrate crystals can be effectively separated.
Owner:常州中源工程技术有限公司
Process for manufacturing potassium nitrate fertilizer and other metal nitrates
InactiveUS20020110512A1Cost effectiveEfficient executionCalcareous fertilisersCobalt ammonia complexesWater vaporCounter current
A process for producing potassium nitrate and other metal nitrates from the chlorides, sulfates, oxides of these metals. The process uses nitrogen dioxide as a true fluidizing medium in shallow beds of the aforementioned solids at moderately elevated temperatures in a continuous counter current process to convert the metal chlorides, sulfates, and oxides, into metal nitrates and effluent gas and water vapor. The process may be carried out in a series of true fluidized beds arranged in a vertical configuration so that the solids flow downward due to the fluidized process and the nitrogen dioxide gas flows counter currently in an upward direction producing pure metal nitrates at the bottom and nitrosyl chloride gas and / or water vapor at the top.
Owner:RIGBY WILLIAM J
Process for preparing small size layered double hydroxide particles
InactiveCN107531503AWithout compromising purityCalcium aluminatesMaterial nanotechnologyPtru catalystSorbent
A process for preparing particles of a layered double hydroxide of the general formula [Mp z+ M'q y+ (OH)2]a+(Xn-)a / n.bH2O (I) wherein Mz+ and M'y+ are metal cations or mixtures of metal cations, z =1 or 2; y = 3 or 4; p + q = 1; b = 0 to 10, Xn- is an anion, n is 1 to 5 and a is determined by p, q, y and z such that a = zp+yq-2 comprises (a) mixing, in aqueous solution, Mz+ cations, M'y+ cationsand Xn- anions, with a base; and (b) allowing the layered double hydroxide of formula (I) to precipitate from the solution mixed in step (a). Preferably, M is Li, Mg, Zn, Fe, Ni, Co, Cu, Ca, or a mixture of two or more. Preferably, y is 3 and M' is Al, Ga, In, Fe or a mixture of two or more thereof. Also provided are particles obtainable by the process, especially wherein M is Ca, M' is Al and Xn- is NO3-. Particles of a layered double hydroxide wherein the particles have a particle size of not greater than 2000 nm, preferably not greater than 300 nm and especially not greater than 100 nm, are also provided. The layered double hydroxides according to the invention are useful in certain applications, for example, as adsorbents, coatings and catalyst supports.
Owner:SCG CHEM CO LTD
Comprehensive recycling method of coal-series solid waste
PendingCN112939046AAchieve recyclingHigh recovery ratePhotography auxillary processesAluminium oxide/hydroxide preparationChemical industryPregnant leach solution
The invention discloses a comprehensive recycling method of coal-series solid waste, and belongs to the crossing field of metallurgy and chemical industry. The method comprises the following steps: firstly, crushing and finely grinding coal-series solid wastes to obtain mineral powder, and carrying out magnetic separation and flotation on the mineral powder to obtain a carbon-removed material; and carrying out nitric acid pressurization two-stage reverse leaching reaction on the decarbonized material, and then sequentially recovering elements such as silicon, gallium, aluminum, magnesium and the like. According to the process method, the dissolution rate of extracted aluminum oxide is high, and aluminum and iron in the pickle liquor can be effectively separated; wherein aluminum nitrate and magnesium nitrate solutions obtained in the technological process are evaporated and concentrated to obtain crystals for subsequent decomposition and regeneration to obtain acid and alkali, so that acid-alkali double-medium regeneration cycle is realized; and meanwhile, elements such as aluminum, iron, calcium, magnesium and gallium in the coal-series solid waste are comprehensively utilized. The method has the advantages of low acid consumption, low impurity content in the leachate, low impurity removal cost, comprehensive recycling of each element, low equipment requirement, simple process and the like.
Owner:眉山顺应循环再生资源有限公司
Method and Compositions for Treating Plant Infections
InactiveUS20120219638A1Reducing and preventing microbial contaminationOrganic active ingredientsNitrogen compoundsMicroorganismErwinia
The present invention includes antimicrobial silver-containing compositions that are effective in treating Erwinia species bacteria. These compositions are particularly effective for treating plants susceptible to Erwinia species infections.
Owner:OLSON MERLE E +1
Thermocatalytically active coating, substrate having the thermocatalytically active coating at least in parts, and process for producing same
InactiveUS8008225B2Reduce the temperatureNitrogen compoundsSulfate/bisulfate preparationLithiumReflective layer
A substrate, such as a glass, glass ceramic, ceramic or metal substrate, is provided with a thermocatalytically active coating on at least a part of the substrate surface. The thermocatalytic coating contains an inorganic lithium salt or organic lithium-containing compound in an amount that is equivalent to not less than 2 wt. % of lithium ions, based on total coating weight. The thermocatalytic coating has a glass, glass solder or sol-gel matrix in which the lithium salt or organic lithium-containing compound is introduced. Optional barrier and IR-reflecting layers are arranged between the substrate surface and the thermocatalytically active coating.
Owner:SCHOTT AG
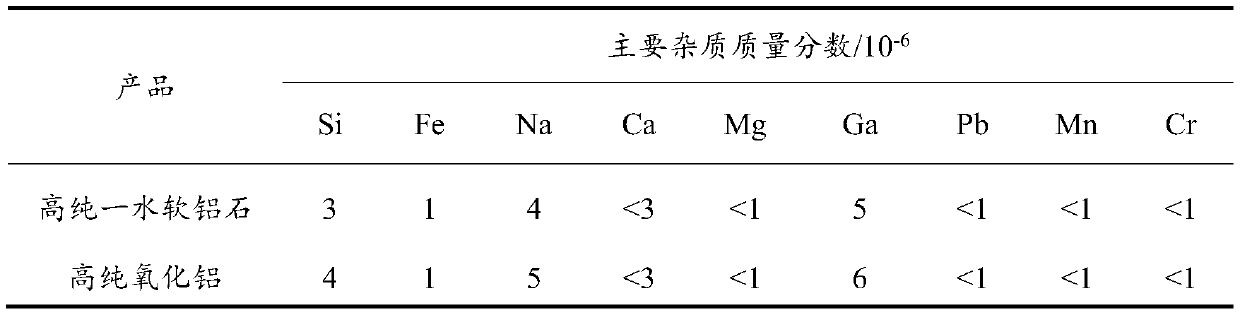
Preparation method of high-purity boehmite and high-purity aluminum oxide
InactiveCN110451538ALow priceSimple processAluminium hydroxide preparationAluminium nitratesAluminium hydroxideHigh activity
The invention provides a preparation method of high-purity boehmite and high-purity aluminum oxide. The method comprises the following steps: heating and dissolving industrial aluminum hydroxide by using analytically-pure sulfuric acid or nitric acid to obtain an aluminum sulfate or aluminum nitrate solution, respectively; carrying out cooling crystallizing twice for purifying to obtain a high-purity aluminum salt; adding a certain amount of a precipitant such as ammonia water or ammonium carbonate or ammonium bicarbonate, carrying out pre-precipitating to form a high-activity colloid, furtherremoving impurities, and carrying out solid-liquid separation to obtain a high-purity aluminum salt solution; carrying out a precipitation reaction on the high-purity aluminum salt solution and urea,carrying out filtering and carrying out washing to obtain an amorphous aluminum oxide hydrate; and carrying out hydrothermal treatment to obtain the high-purity boehmite, and roasting the boehmite toobtain the high-purity aluminum oxide. The purity of the prepared high-purity boehmite and high-purity aluminum oxide is greater than 99.99%, and the sum of the content of metal elements except an aluminum element and the content of a silicon element is not higher than 0.001%.
Owner:中铝山东有限公司
Anion-conducting material and method for manufacturing same
InactiveUS20160159659A1Reduction in ion conductivity is preventedImprove ionic conductivityNitrogen compoundsCell electrodesConductive materialsPolymer chemistry
An anion conductive material consists of a low-regularity layered double hydroxide having ion conductivity enhanced by delamination of a layer structure of a regular layered double hydroxide.
Owner:NORITAKE CO LTD
Method for treating wastewater in production of corrosion foil for aluminum electrolytic capacitor
ActiveCN105645641AAvoid mixed ingredientsAvoid large amountsCalcium/strontium/barium sulfatesTreatment involving filtrationAluminium electrolysisPhysical chemistry
The invention provides a method for treating wastewater in production of corrosion foil for an aluminum electrolytic capacitor. The method comprises the following steps: (1) injecting wastewater produced in the production of the corrosion foil for the aluminum electrolytic capacitor into a second-order reaction tank, a first-order reaction tank and a fourth-order reaction tank respectively according to the generation order; (2) adding CaCO3 to the first-order reaction tank to regulate the pH value to 2 to 4, and filtering; (3) adding the filtrate in step (2) to the second-order reaction tank, and filter pressing; (4) injecting the filtrate in step (3) to a third-order reaction tank, adding Al2O3 to regulate the pH value of a solution to 2 to 4, and filtering by an ultrafiltration membrane; (5) adding Al2O3 to the fourth-order reaction tank to regulate the pH value of a solution to 2 to 4, filtering by an ultrafiltration membrane, and concentrating by heating. The method has the advantages that the cost is low, the treated wastewater can be discharged directly, and CaSO4, Al (NO3) 3 and a concentrated AlCl3 solution produced in the treatment process have relatively high purity, and can be used as industrial raw materials to be directly sold.
Owner:淮北恒正电子材料有限公司
Method for producing basic metal nitrate
A process in which a basic metal nitrate is obtained in a high yield is provided. A process for producing a basic metal nitrate, which comprises adding an aqueous solution of a metal nitrate or an aqueous solution of a mixture of a metal nitrate and a water-soluble additive and an alkali solution to a reaction vessel in which a reaction solvent whose pH at 20° C. is adjusted to 6 or less is present, and conducting the reaction with stirring.
Owner:DAICEL CHEM IND LTD
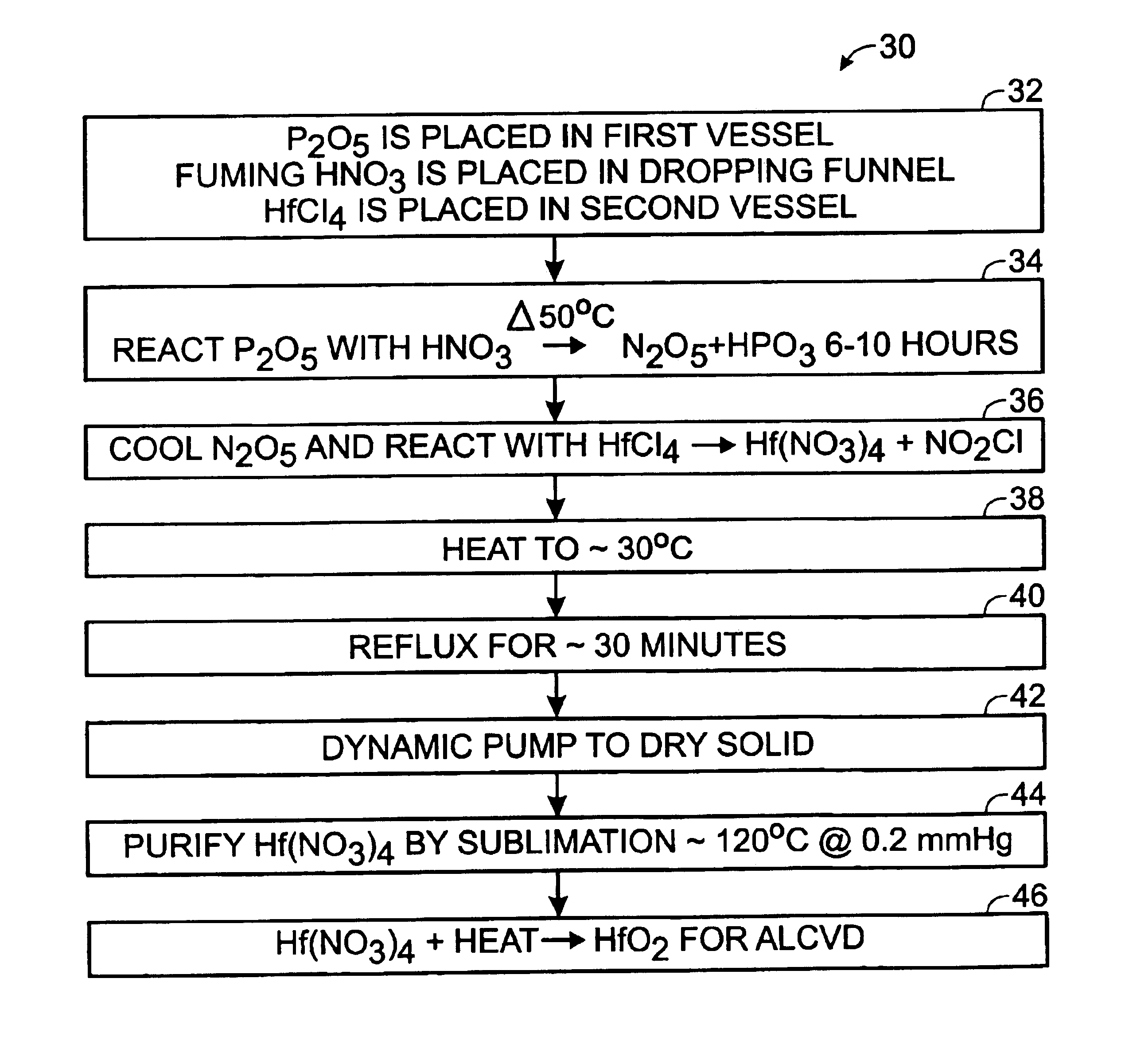
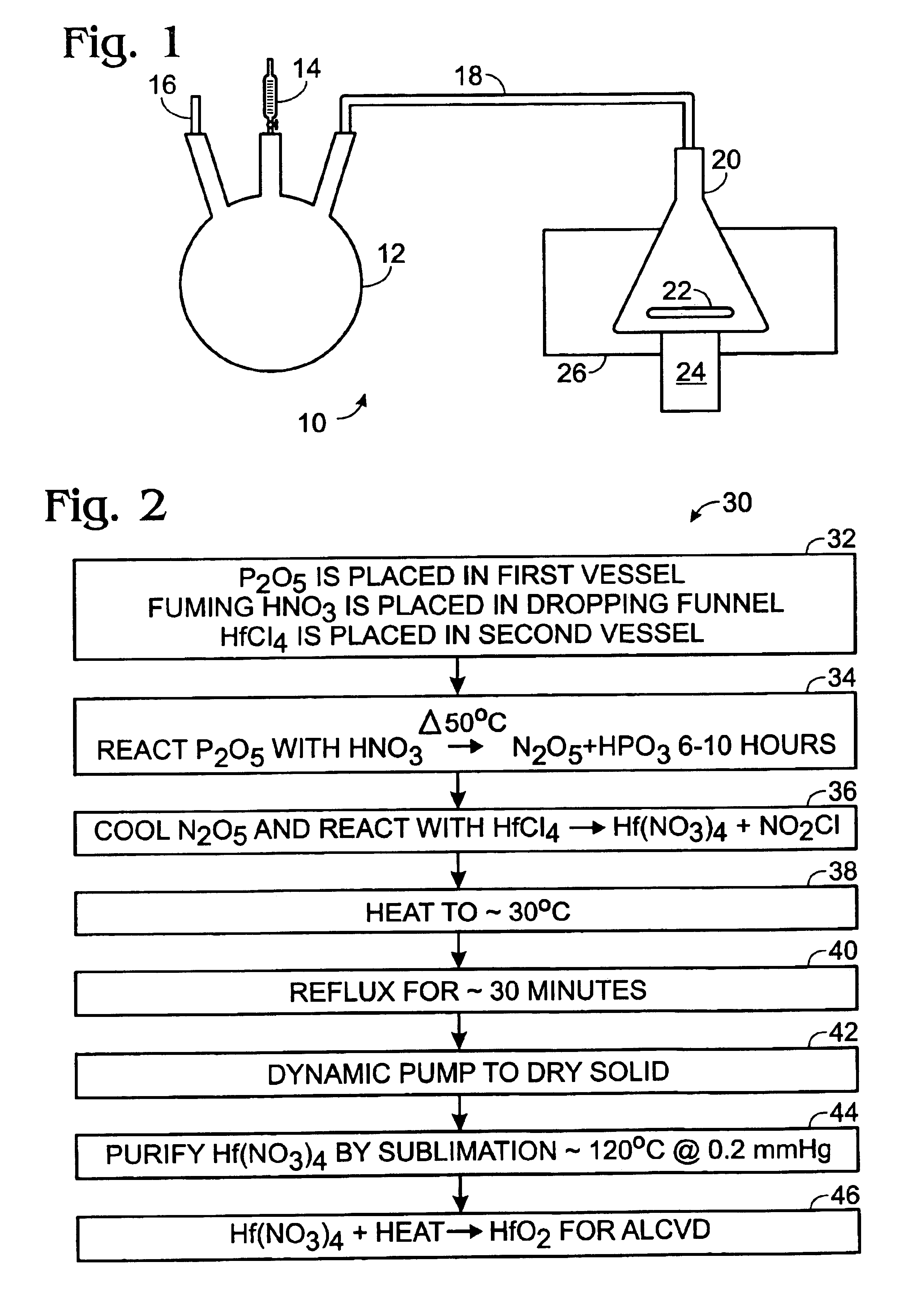
Method of synthesis of hafnium nitrate for HfO2 thin film deposition via ALCVD process
A method of preparing a hafnium nitrate thin film includes placing phosphorus pentoxide in a first vessel; connecting the first vessel to a second vessel containing hafnium tetrachloride; cooling the second vessel with liquid nitrogen; dropping fuming nitric acid into the first vessel producing N2O5 gas; allowing the N2O5 gas to enter the second vessel; heating the first vessel until the reaction is substantially complete; disconnecting the two vessels; removing the second vessel from the liquid nitrogen bath; heating the second vessel; refluxing the contents of the second vessel; drying the compound in the second vessel by dynamic pumping; purifying the compound in the second vessel by sublimation to form Hf(NO3)4, and heating the Hf(NO3)4 to produce HfO2 for use in an ALCVD process.
Owner:SHARP LAB OF AMERICA INC
Nitrates
InactiveUS20090189117A1Solution rate in fasterAmmonium nitratesNitrogen compoundsNitrate saltsNuclear chemistry
A method of producing anhydrous calcium nitrate, anhydrous magnesium nitrate or mixture thereof involves removing water from a solution of calcium nitrate, magnesium nitrate or mixture thereof in a pulse combustion drier. The invention also provides a mixture of anhydrous calcium nitrate, anhydrous magnesium nitrate and the individual anhydrous nitrate salts in a sealed container.
Owner:FORSYTH ALASTAIR JAMES
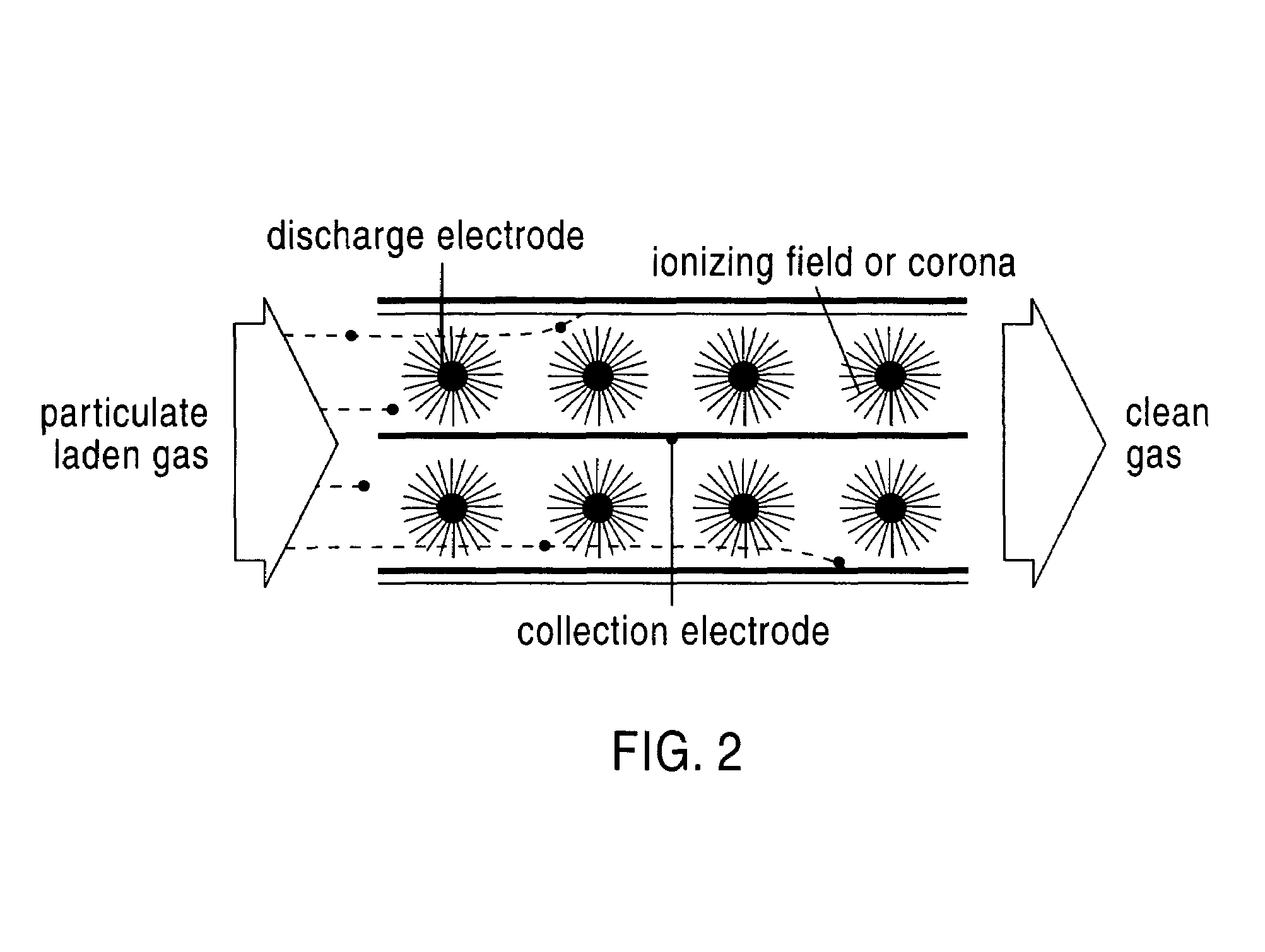
Flue gas desulfurization process and apparatus for removing nitrogen oxides
InactiveUS6958133B2Reduce the temperatureCombination devicesAmmonium nitratesNitrogen oxidesFlue gas
An apparatus and process for removing acidic gases and NOx from flue gases produced by utility and industrial plants. The process and apparatus convert NOx, and particularly nitric oxide, to nitrogen dioxide, which is then reacted to form a valuable byproduct. The process generally entails contacting a flue gas with a scrubbing medium to absorb acidic gases from flue gas and produce an intermediate flue gas. The intermediate flue gas is then cooled to cause nitric oxide present therein to be oxidized to form nitrogen dioxide, which is then absorbed from the flue gases to produce a nitrogen dioxide-containing solution and a scrubbed flue gas. The nitrogen dioxide in the nitrogen dioxide-containing solution is then reacted with ammonium hydroxide to form ammonium nitrate as a valuable byproduct.
Owner:BANK OF MONTREAL AS ADMINISTATIVE AGENT +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com