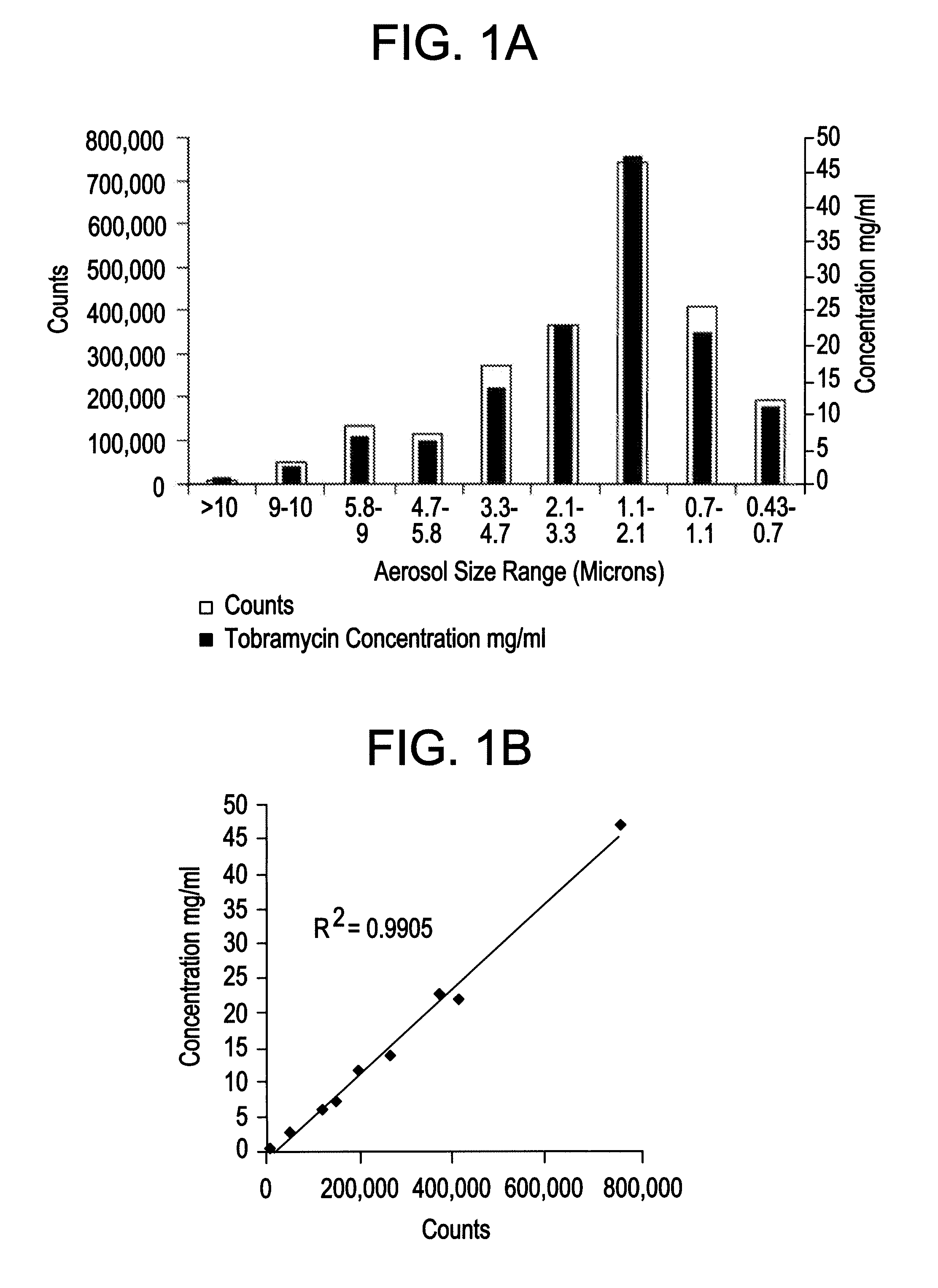
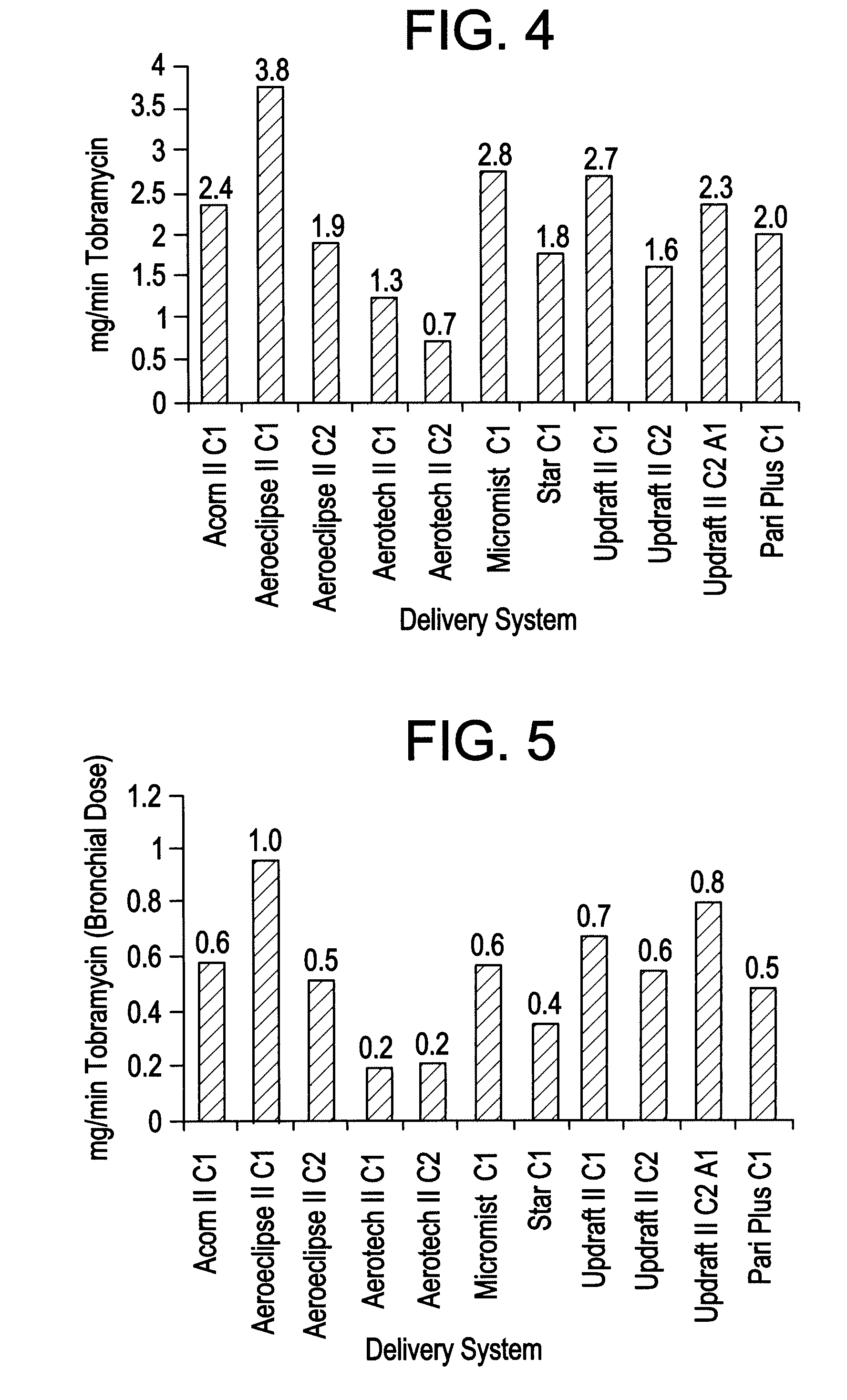
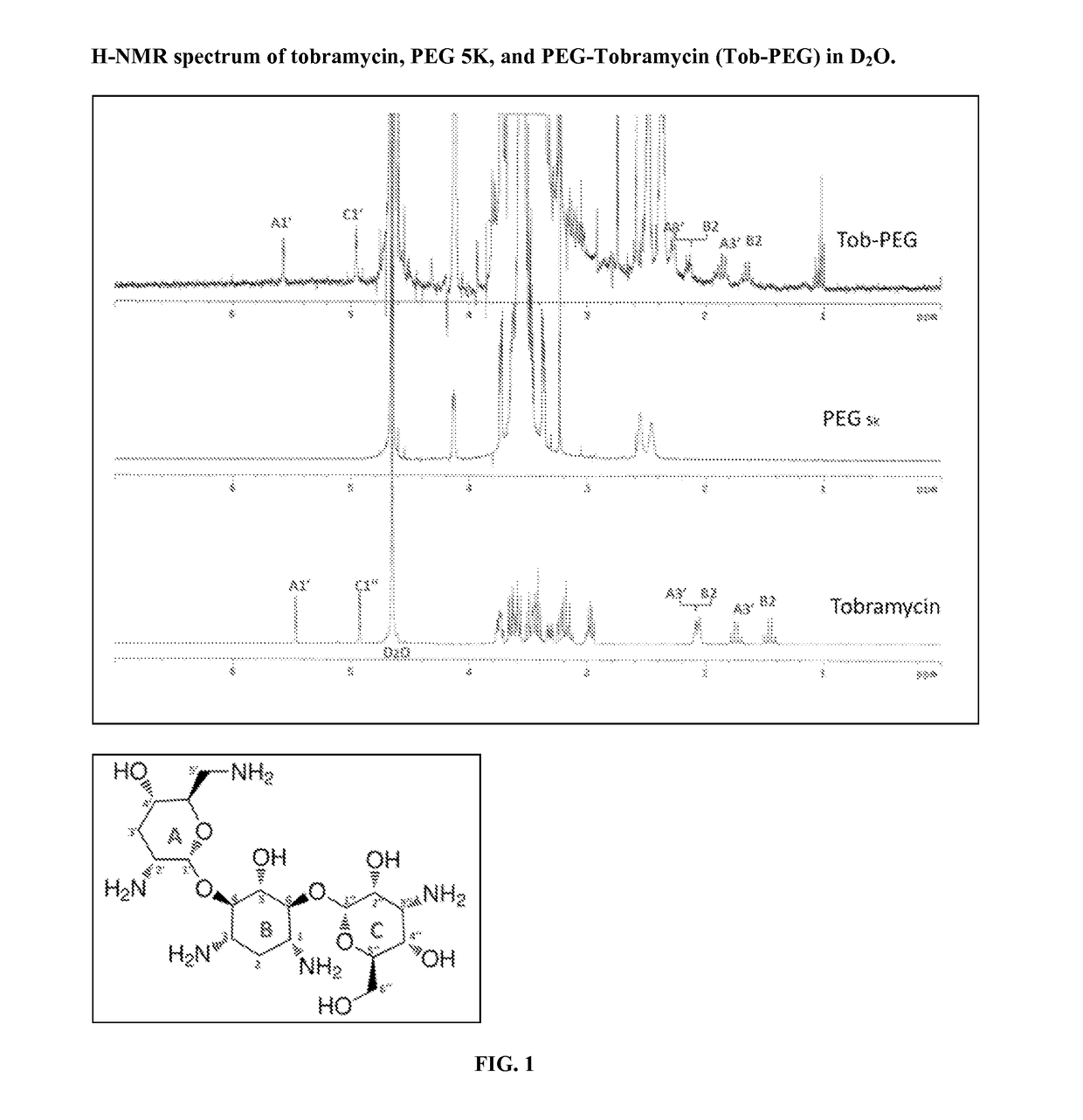
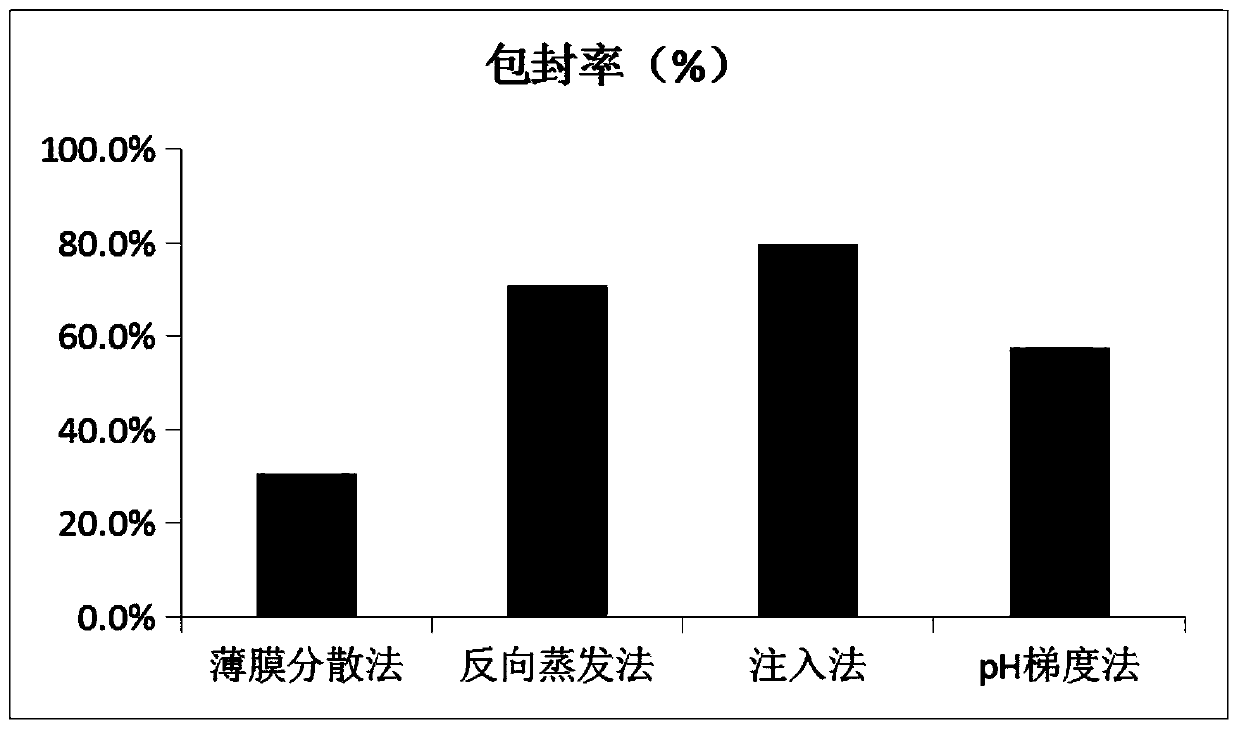
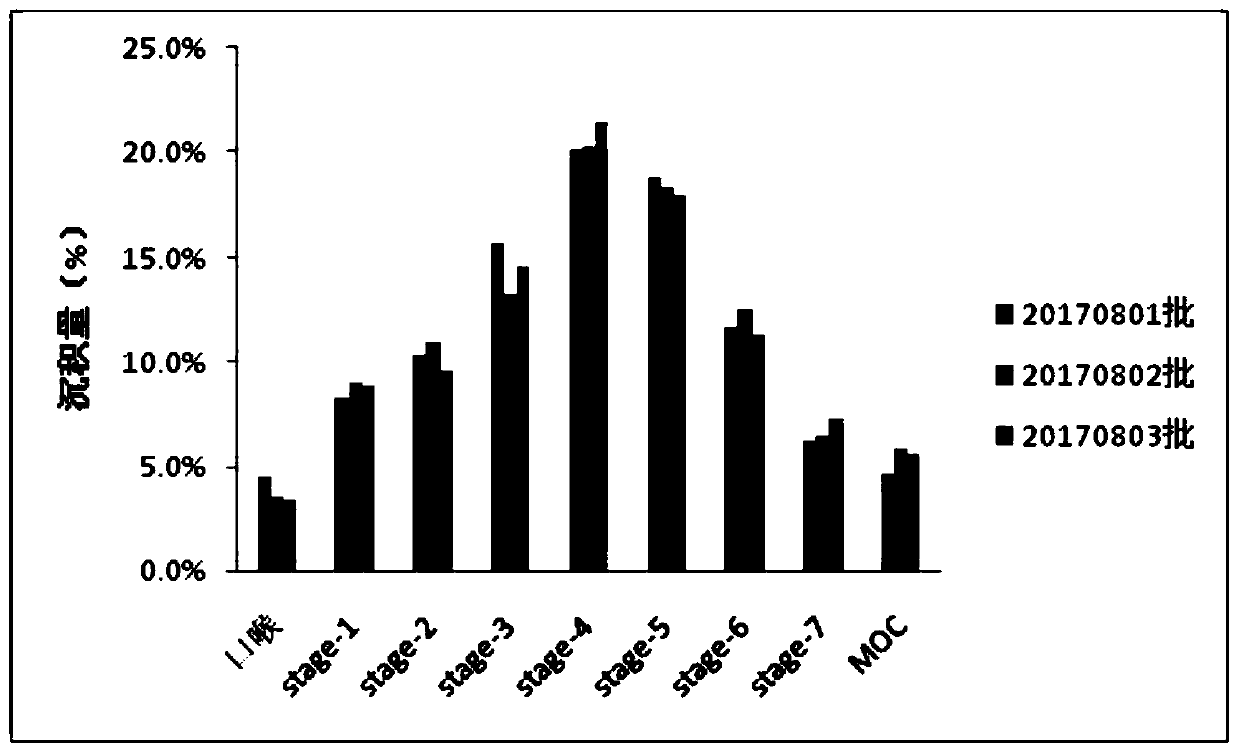
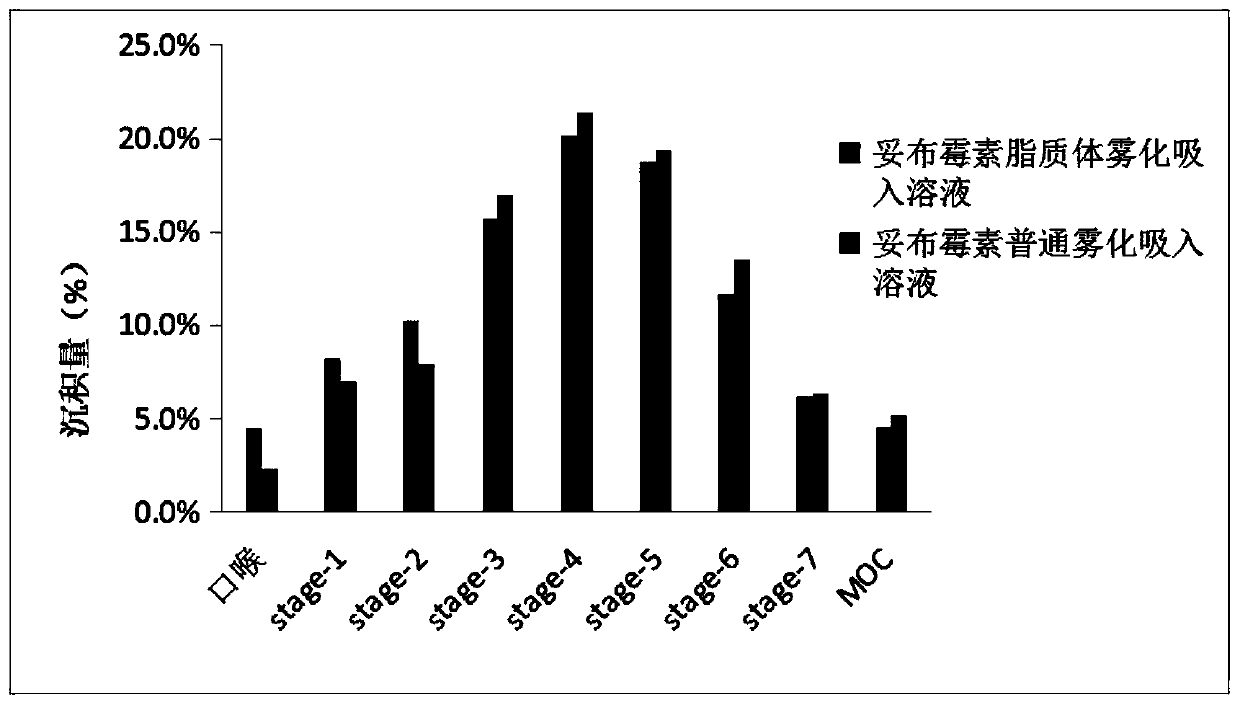
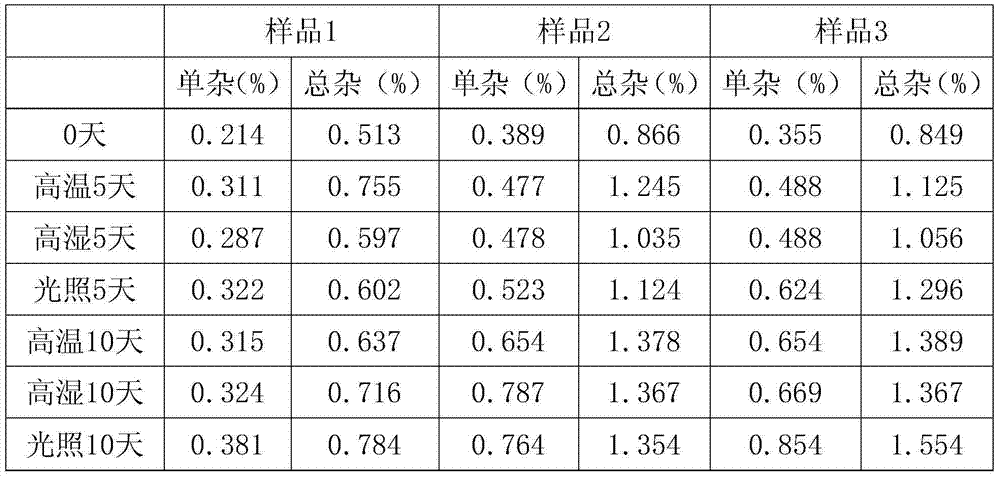
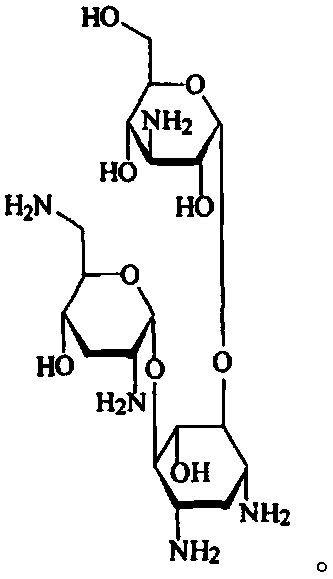
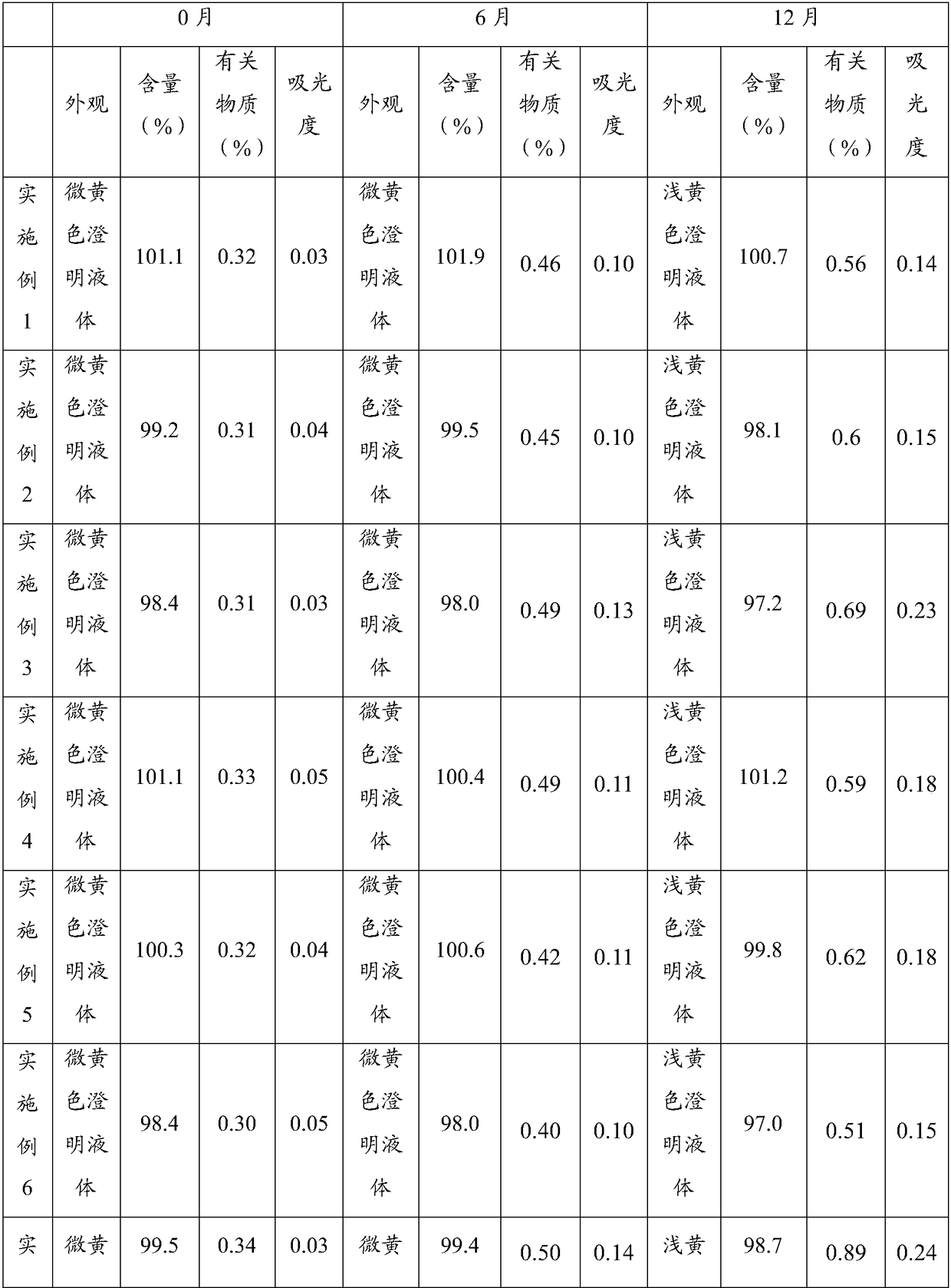
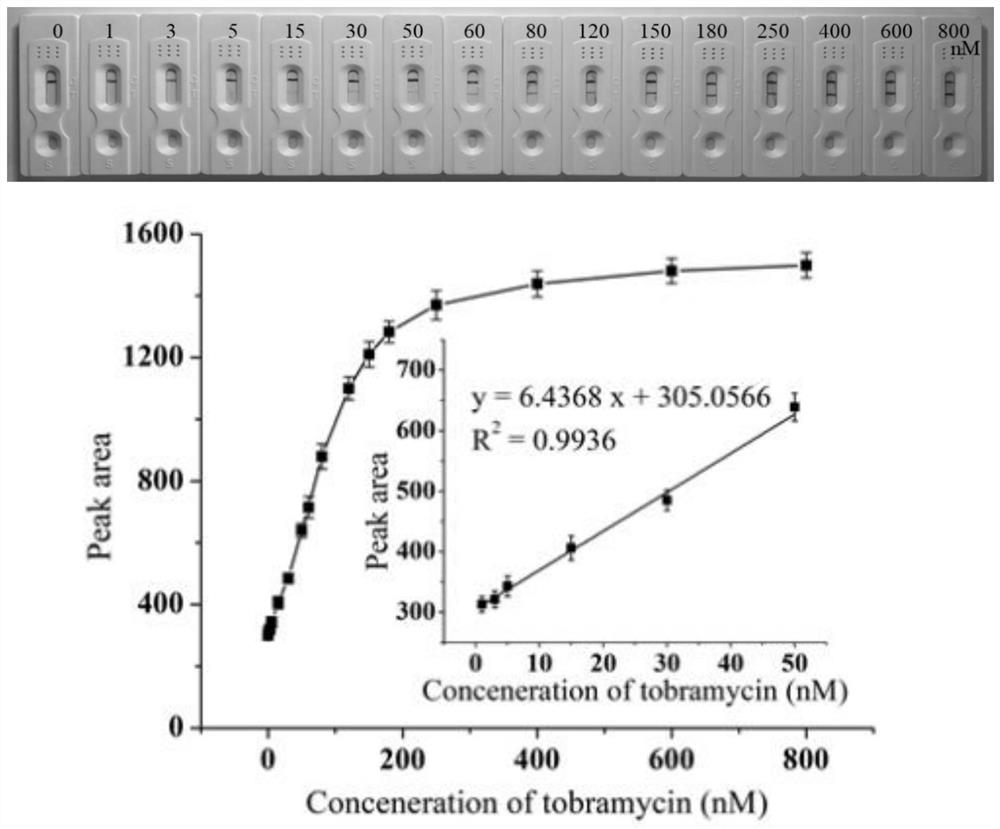
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59 results about "Tobramycinum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Surfactant-based antimicrobial solution for inhalation
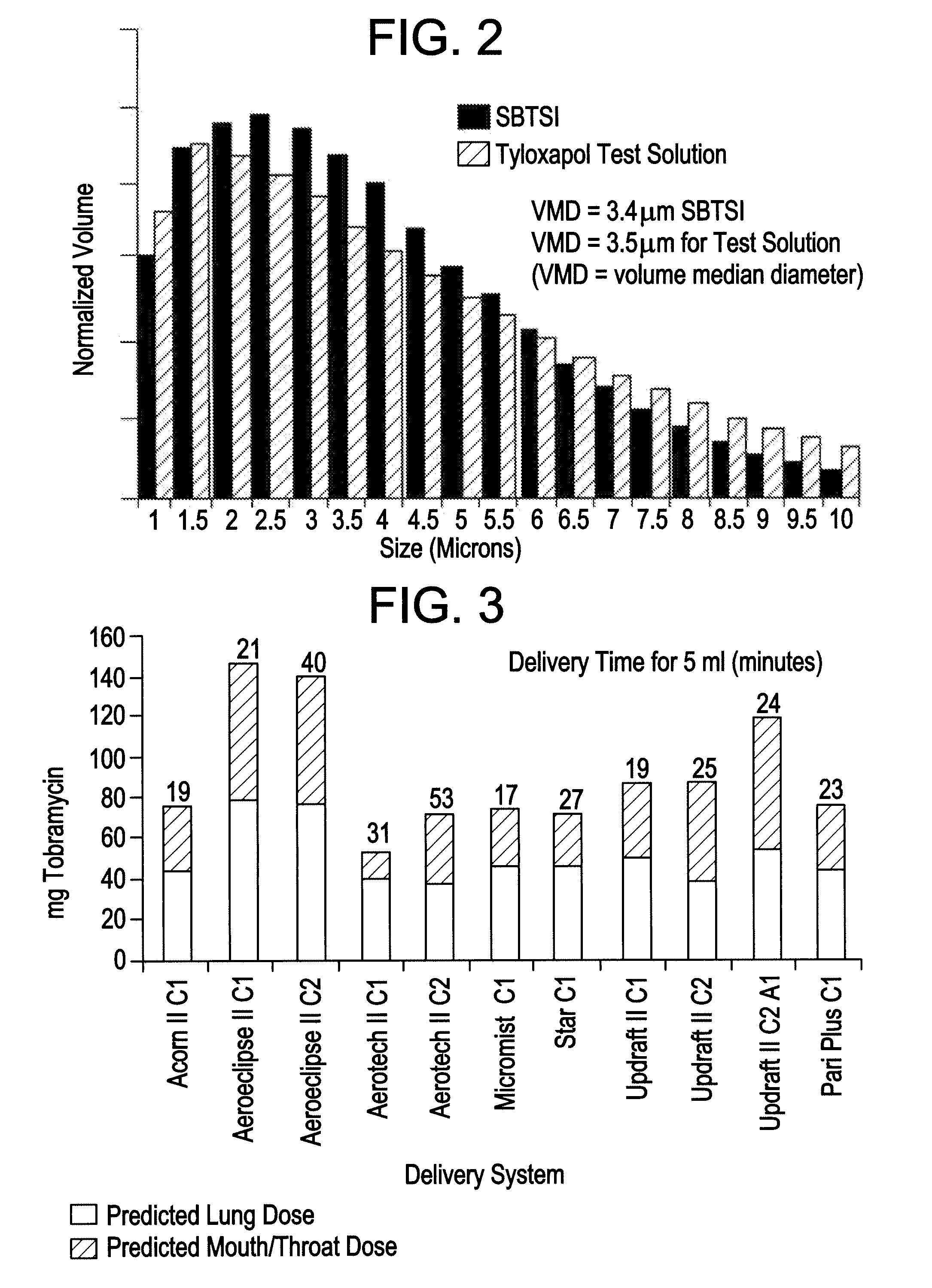
A surfactant can be added, safely and effectively, to a drug solution containing any antimicrobial agent, such as an antibiotic like tobramycin, that is suitable for administration to the lungs via inhalation. Thus, when an aerosolized drug solution includes surfactant, Marangoni flows cause the drug particles, once deposited in the lungs, to spread over a wider surface area, thereby ensuring greater antimicrobial efficacy. A solution that contains, for example, an antibiotic and tyloxapol or another surfactant providing a similar surface tension to the composition is optimally delivered by the functional combination of a breath-actuated nebulizer and a high-flow compressor.
Owner:UNIVERSITY OF PITTSBURGH
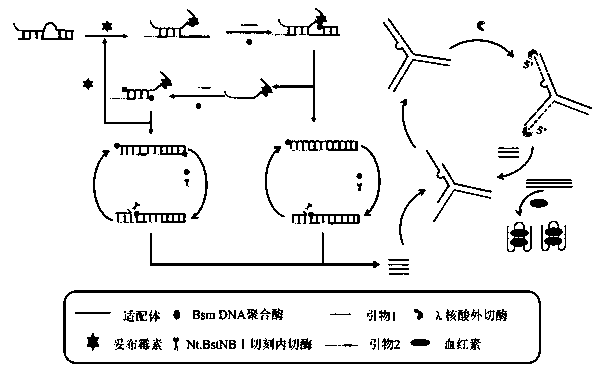
Colorimetric method for detecting tobramycin based on double strand displacement and three-dimensional DNA structure
ActiveCN110592187AAchieving multiple magnificationsExpand the scope of detectionMicrobiological testing/measurementLinear relationshipGenetics
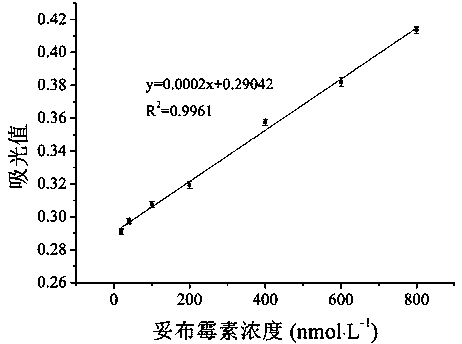
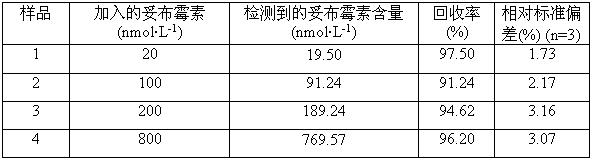
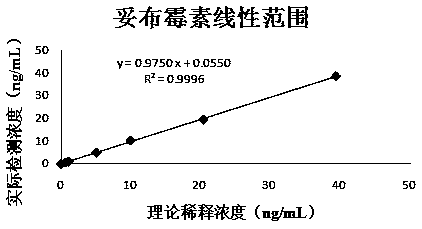
The invention discloses a colorimetric method for detecting tobramycin based on double strand displacement and a three-dimensional DNA structure, and belongs to the field of food safety, medical analysis and environmental pollution detection. The method comprises the following steps: firstly, double strands T1 / T2 are designed; when tobramycin exists, Bsm DNA polymerase synthesizes double strands which are completely complementary through a strong strand displacement reaction, and Nt.BstNBI incision endonuclease cuts recognition sites on the double strands; the three-way DNA structure capture reporter probes, and regenerates and replaces a large number of S1 strands containing G-quadruplex forming sequences. Thereafter, the G-quadruplex / heme catalyzes ABTS<2-> / H2O2 chromogenic reaction, andthe tobramycin content can be determined by using the linear relationship between light absorption value and tobramycin concentration. According to the invention, an aptamer captures tobramycin to trigger double strand displacement reaction which is mediated by the Nt.BstNBI incision endonuclease and the Bsm DNA polymerase so as to generate a large number of reporter probes. Meanwhile, the reporter probes trigger lambda exonuclease-assisted loop amplification, so that multiple amplifications of colorimetric signals are realized, the detection range is widened, and the detection sensitivity isimproved.
Owner:JIANGNAN UNIV
Lotepredenol etabonate gernebcin suspension solution and method for preparing the same
The invention discloses an eye use compound drug combination containing lotepredenol etabonate and tobramycin. The combination contains less than 0.1 percent of tetra chloro metlbond. The preparation method of the drug combination is simple and is suitable for industrial production.
Owner:BEIJING D VENTUREPHARM TECH DEV
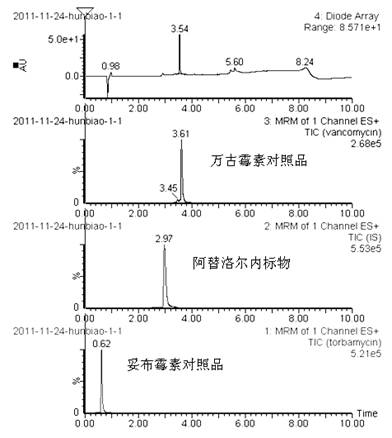
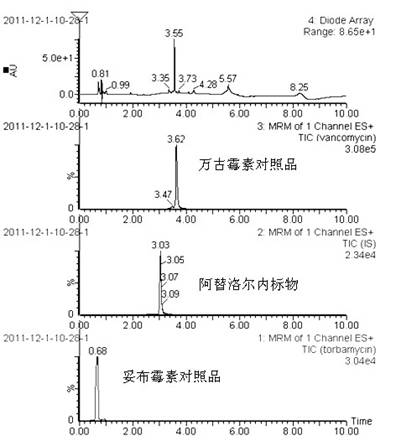
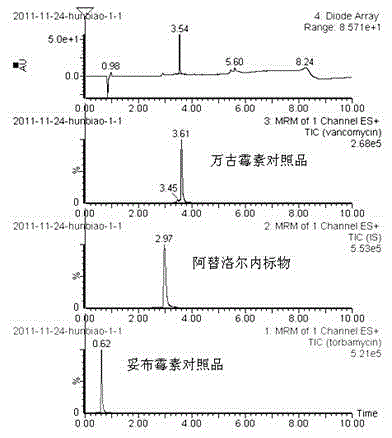
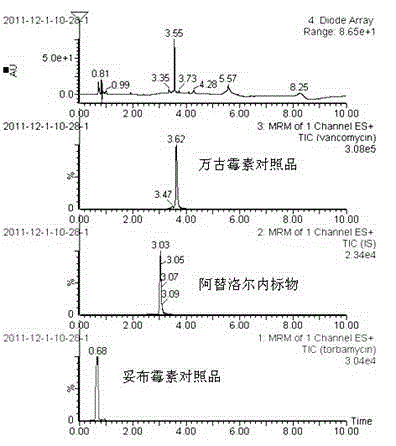
Method for simultaneously determining content of vancomycin and tobramycin in tissue drainage liquid through ultrahigh performance liquid chromatography-triple quadrupole mass spectrometry (UPLC-TQD) coupling technique
The invention relates to the field of drugs, in particular to a detection method for simultaneously determining content of vancomycin and tobramycin in tissue drainage liquid. A method for simultaneously determining content of vancomycin and tobramycin in tissue drainage liquid through an ultrahigh performance liquid chromatography-triple quadrupole mass spectrometry (UPLC-TQD) coupling techniquecomprises the following steps of: 1) preparing reference samples; 2) preparing internal standard solution; 3) preparing sample solution; 4) preparing mobile-phase solution; 5) setting chromatographicconditions; 6) optimizing mass spectrometric conditions; 7) determining the samples; 8) preparing gradient-concentration reference sample solution; 9) preparing a standard curve; and 10) analyzing and computing data. The method has the characteristics that: 1) the method is advanced; 2) the method is rapid and high-efficiency the loss is low; and 3) the clinical application prospect and the effect are good.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
Tobramycin monoclonal antibody as well as preparation method and application of tobramycin monoclonal antibody
ActiveCN104312978AEasy to prepareHigh sensitivityEgg immunoglobulinsMicroorganism based processesTobramycinCarrier protein
The invention relates to a tobramycin monoclonal antibody as well as a preparation method and application of the tobramycin monoclonal antibody and belongs to the field of food safety immunodetection. The monoclonal antibody is generated by mouse hybridoma cell line No. G (A10) with the preservation number of CGMCC No.9307. The preparation method comprises the following steps: (1) activating amino of tobramycin with a glutaraldehyde method, coupling the activated amino of the tobramycin with the amino of carrier protein, reducing C=N of Schiff base formed by glutaraldehyde and amino of the obtained conjugate into a stable C-N structure to obtain final stable conjugate serving as complete antigen; (2) immunizing, fusing and sieving the complete antigen obtained in the step (1), carrying out enlarged cultivation and mouse enterocoelia induction to generate ascites to obtain the monoclonal antibody used for specific detection of tobramycin; and (3) activating carboxyl of succinic acid with a DCC method, reacting activate fluid with ovalbumin amidogen to obtain carboxylation protein of ovalbumin-succinic acid, carrying out dialysis and condensing the tobramycin and the carboxyl of the protein by using an EDC method to prepare peridium of screened antibody.
Owner:JIANGNAN UNIV
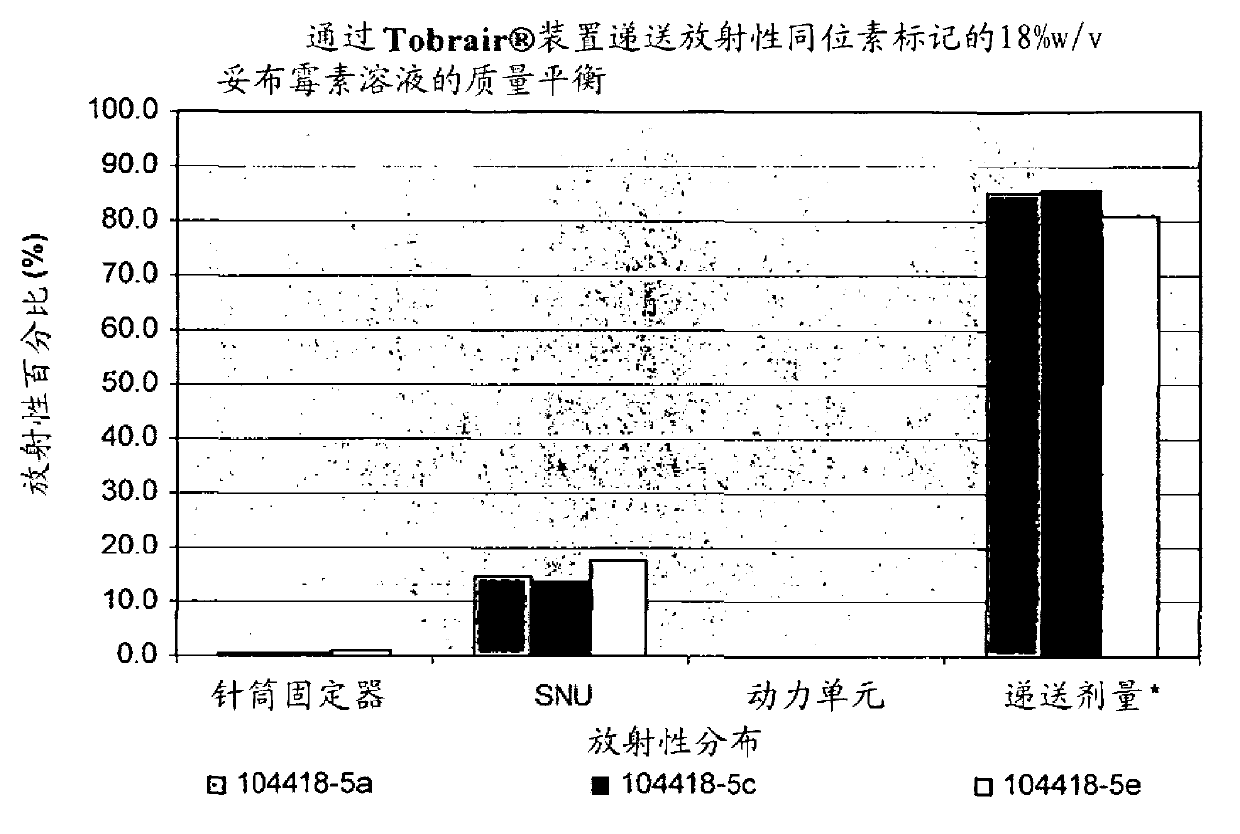
Tobramycin inhalation composition and preparation method and application thereof
ActiveCN105616345AMeets requirements for inhalation solutionsInhalation solution requirements addressedAntibacterial agentsOrganic active ingredientsInhalationTobramycin
The invention relates to a tobramycin inhalation composition and a preparation method and application thereof. The composition is prepared from tobramycin 4%-8% (w / v), sodium chloride 0.1%-0.9% (w / v), weak-reducibility organic weak acid serving as a stabilizer 0.1%-1.0% (w / v), strong acid for regulating the pH value of the composition and the balance water, wherein the pH value of the composition is 5.6-7.5. The tobramycin inhalation composition prepared by adopting the technical scheme has the pH value closer to the physiological pH of a human body and has significantly improved stability at room temperature.
Owner:GUANGZHOU JOINCARE RESPIRATORY DRUG ENG TECH CO LTD
Chemical medicine composition for treating helicobacter pylori infection
PendingCN111184867AGood synergyGood curative effectAntibacterial agentsOrganic active ingredientsHelicobacter InfectionsIsepamicin
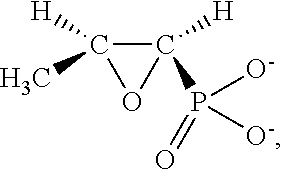
The invention discloses a chemical medicine composition for treating helicobacter pylori (Hp) infection of adults through oral administration. The composition at least comprises fosfomycin or pharmaceutical salts such as a sodium salt, a calcium salt, fosfomycin trometamol and aminoglycoside medicines of the fosfomycin of a common dosage of an adult, and the aminoglycoside medicines are selected from tobramycin, netilmicin, etimicin, isepamicin, amikacin and common salts such as sulfate, of the medicines.
Owner:龚跃明
Treatment of lung infections by administration of tobramycin by aerolisation
InactiveCN103052392ALow peak plasma concentrationHighly effective drug regimenAntibacterial agentsOrganic active ingredientsAminoglycosideAminoglycoside Agents
The present invention regards a novel administration form and a novel administration regime useful in the treatment and prevention of a bacterial lung infection in patient in need thereof, in particular by providing a composition useful for aerosolization of a highly concentrated solution of aminoglycosides such as Tobramycin.
Owner:赛利亚医药公司
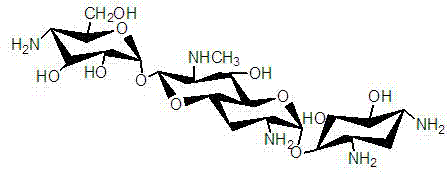
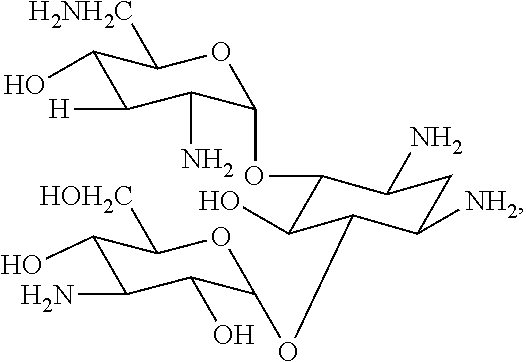
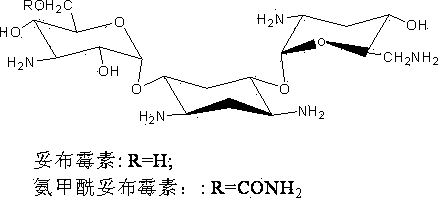
Engineering bacterium for generating carbamoyl tobramycin and application thereof
InactiveCN102373174ASimple production processReduce manufacturing costBacteriaMicroorganism based processesBiotechnologyMicroorganism
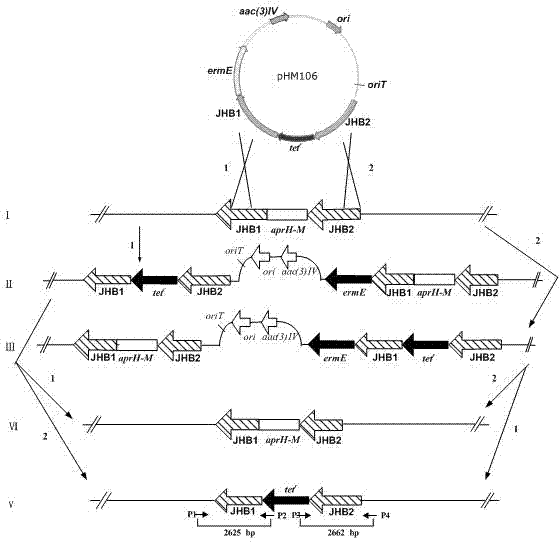
The invention relates to an aprH-aprM-inactivation-function engineering bacterium for mainly generating carbamoyl tobramycin and an application thereof, which belong to the technical field of medicine. The engineering bacterium is streptomyces tenebrarius HM106 (delta aprHM) and is registered and preserved by China General Microbiological Center Culture Collection Center on September 23, 2011, the preservation number is CGMCCNo.5246, and the engineering bacterium is used for preparing antibacterial medicine. The engineering bacterium mainly generating the carbamoyl tobramycin is obtained, theproduction process is simplified, the production cost is reduced, and simultaneously, the quality control of products is also convenient.
Owner:FUZHOU UNIV
Compounds for treating biofilm infection
ActiveUS20170143842A1Reduce and stopExtends antimicrobial efficacy of coatingCarbohydrate active ingredientsPharmaceutical non-active ingredientsBiofilmMedicine
In some aspects, pegylated aminoglycoside compounds are provided. In some embodiments, m-PEG-tobramycin compounds may be used to treat a biofilm infection or reduce or treat established biofilms. The present invention provides, in various aspects, compounds and methods for the treatment of infections, such as biofilm infections or chronic biofilm infections. The chronic biofilm infection may occur in wounds, implanted devices, immunocompromised patients, people with cystic fibrosis, eye infections, etc.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Tobramycin liposome used for aerosol inhalation and production method thereof
ActiveCN111228243AGood compatibilityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsPhospholipidLiposome
The invention discloses a tobramycin liposome used for aerosol inhalation. The tobramycin liposome used for aerosol inhalation is composed of the following components: 0.1%-15.0% of tobramycin, 0.5%-36.0% of a phospholipid, 0.05%-20.0% of a stabilizer, 0.01%-10.0% of a charge modifier, 0.01%-5.0% of an antioxidant, 5.0%-50.0% of an organic-phase medium and the balance an aqueous-phase medium. According to the tobramycin liposome used for aerosol inhalation, the aerosol inhalation technology is used, relative to oral administration, the aerosol inhalation technology has the advantages that a medicine can be directly delivered to a respiratory tract, is absorbed fast, and takes effect quickly, and a medicine concentration of the respiratory tract is increased; the bioavailability and the stability are high, and the tobramycin liposome used for aerosol inhalation has good safety; and a production process is simple, easy and reasonable, and the performance is stable, so that conditions arecreated for achieving industrial productization.
Owner:ZHUHAI ESSEX BIO PHARMA
Tobramycin sulfate injection and process for preparing same
InactiveCN103371968AGuaranteed SALAntibacterial agentsOrganic active ingredientsChemistryTobramycin Injection
The invention provides a tobramycin sulfate injection and a process for preparing the tobramycin sulfate injection. Every 10,000ml of the tobramycin sulfate injection contains the following raw materials: 400g of tobramycin, 25-38g of sodium bisulfite, 0.8-1.2g of EDTA-2Na (ethylenediaminetetraacetic acid and disodium) and the balance of water for injection. The process comprises the following steps of: adding the water for injection into a proportioning tank, wherein the amount of the added water for injection is 80% of total amount of the raw materials; introducing CO2 into the water for injection for 10-20 minutes until the water for injection is saturated; adding tobramycin into the proportioning tank; adding 25% (volume concentration) sulfuric acid into the proportioning tank; stirring to dissolve the tobramycin; adding the sodium bisulfite and solution of the EDTA-2Na dissolved with the water for injection into the proportioning tank; keeping the pH value of a solution at 4.7-5.1; adding the water for injection until the total amount of the solution reaches the preparation amount; stirring the solution uniformly; introducing CO2 into the solution; filtering the solution with a folded filtering element until the solution is clear; filling the solution into an ampoule, and sealing the ampoule, wherein N2 is introduced into the ampoule in the process of filling the solution. The steps are carried out in the aseptic preoaration condition. The process provided by the invention has the advantage that the SAL (sterility assurance level) of the sterilized tobramycin sulfate injection can be not more than 10<-3>.
Owner:上海禾丰制药有限公司
Medicine component containing tobramycin inhalation solution and application thereof
ActiveCN113952320AGood expansion effectReduce volumeOrganic active ingredientsDispersion deliveryPharmaceutical drugBronchial epithelium
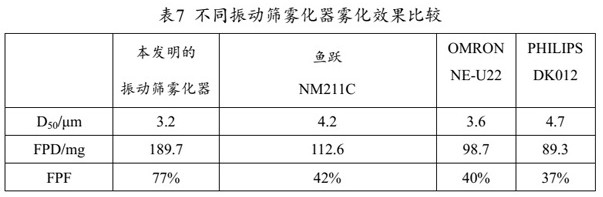
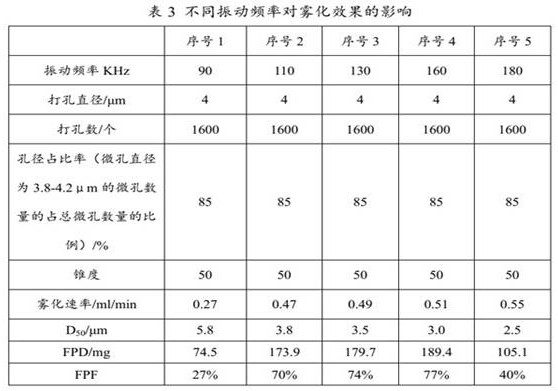
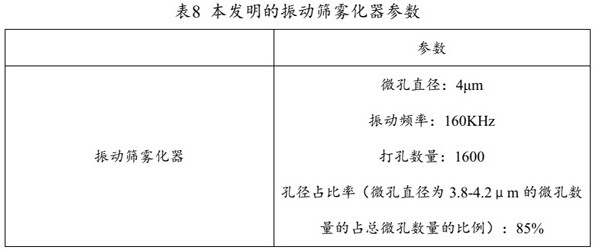
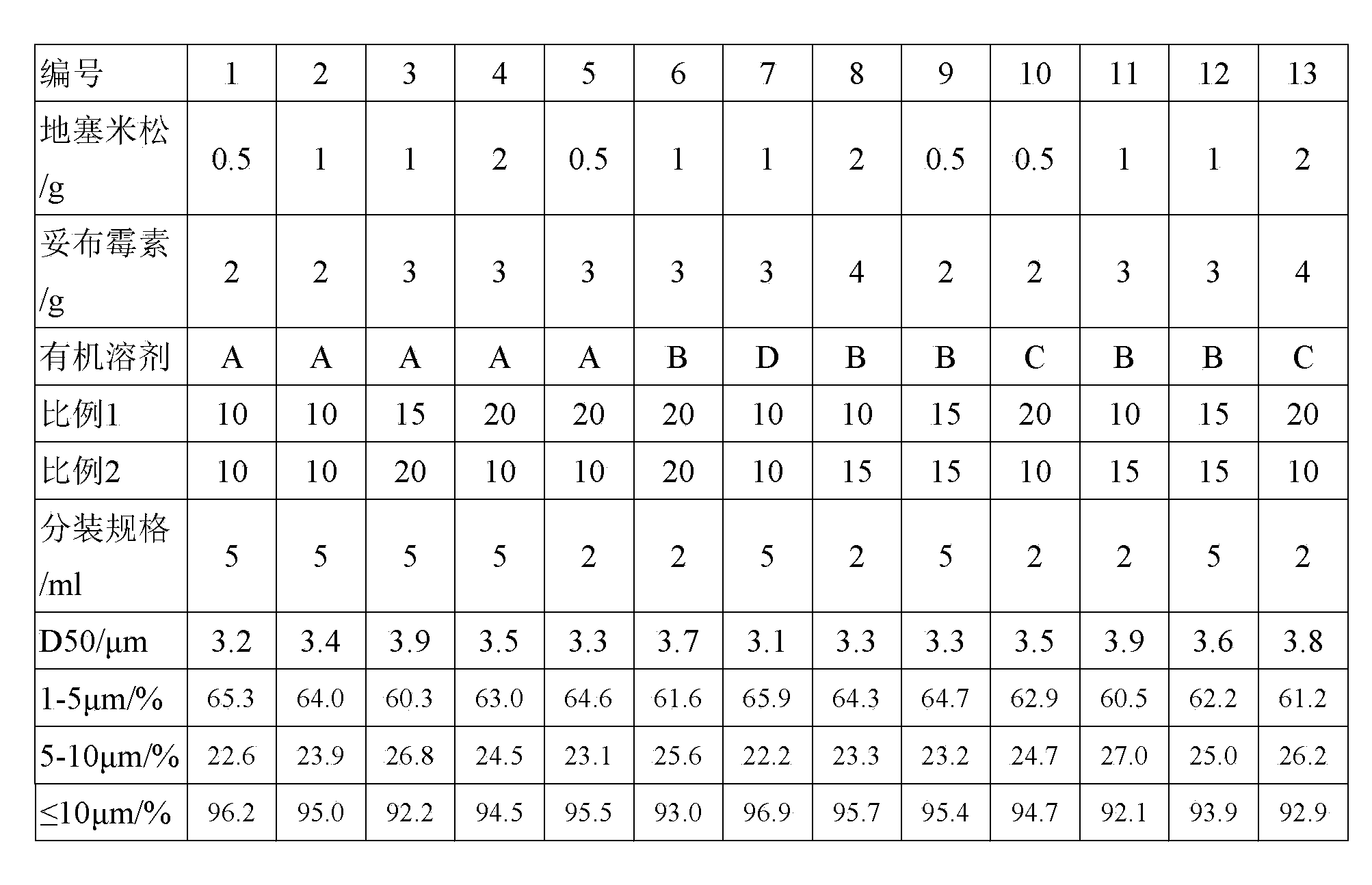
The present invention provides a medicine component containing a tobramycin inhalation solution and an application thereof. The medicine component comprises: (a) the tobramycin inhalation solution; and (b) the vibrating screen atomizer used in cooperation with the tobramycin inhalation solution. The central area of a metal mesh of the vibrating screen atomizer has 1400-1800 micropores. The fogdrop particle size of the tobramycin inhalation solution is as follows: D10 ranges from 0.5 [mu]m to 2.5 [mu]m, D50 ranges from 2.0 [mu]m to 4.2 [mu]m, and D90 ranges from 6.0 [mu]m to 9.0 [mu]m. The mass percentage of the particles with the aerodynamic particle size smaller than 5.39 [mu]m in the tobramycin inhalation solution is not smaller than 45%. The medicine component has a remarkable effect on clinically treating bronchiectasia.
Owner:JIANKANGYUAN PHARMA GROUP +1
Tobramycin-dexamethasone eye drops
InactiveCN103565816AImprove adaptabilityGood content uniformityAntibacterial agentsOrganic active ingredientsDexamethasoneOptometry
The invention discloses tobramycin-dexamethasone eye drops. The tobramycin-dexamethasone eye drops contain tobramycin micro-powder and dexamethasone as active components, and also contain one or more accessory materials for eye drops. The tobramycin-dexamethasone eye drops are characterized in that the used dexamethasone micro-powder meets the grain size conditions that D50 is in a range of 3.0-4.0 microns, the maximum grain size is less than 30 microns, a ratio of micro-powder grains having sizes of 1-5 microns is in a range of 60-66%, a ratio of the micro-powder grains having sizes of 5-10 microns is in a range of 21-26%, and a ratio of the micro-powder grains having sizes less than or equal to 10 microns is in a range of 92-98%.
Owner:TIANJIN JINYAO GRP
Method for treatment of lung infections by administration of aminoglycosides by aerolisation
InactiveCN106074461AHighly effective drug regimenImproved dosing regimenAntibacterial agentsOrganic active ingredientsPulmonary infectionGlycoside
The present invention regards a novel administration form and a novel administration regime useful in the treatment and prevention of a bacterial lung infection in patient in need thereof, in particular by providing a composition useful for aerosolization of a highly concentrated solution of aminoglycosides such as Tobramycin.
Owner:赛利亚医药公司
Preparation method and application of double-drug combined intelligent antibacterial hydrogel
ActiveCN112472705AGood biocompatibilityImprove continuityAntibacterial agentsOrganic active ingredientsSulfadiazineSulfanilamide
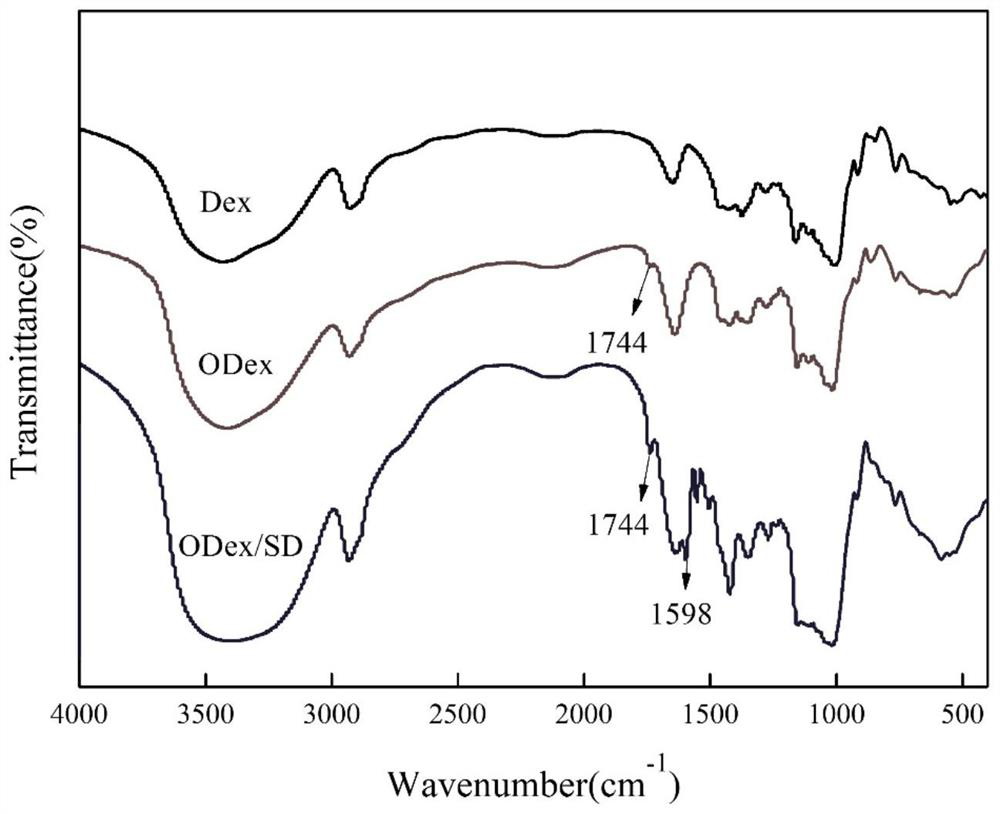
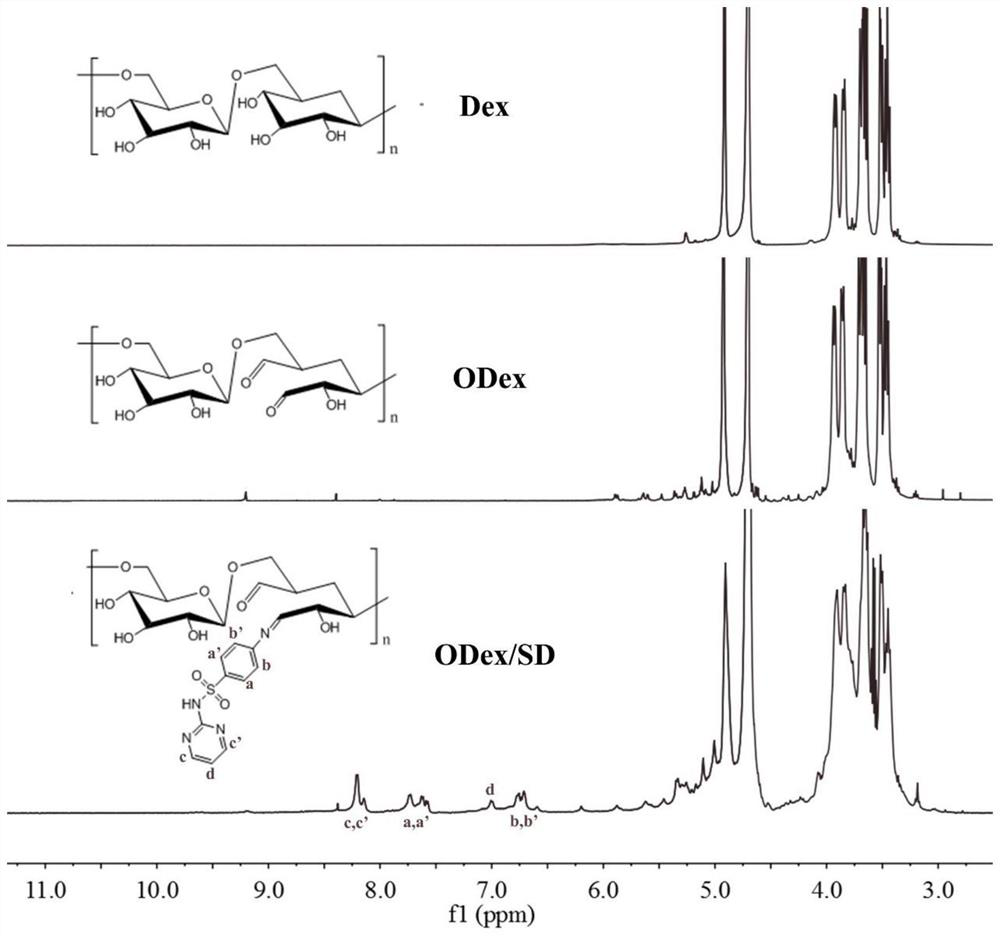
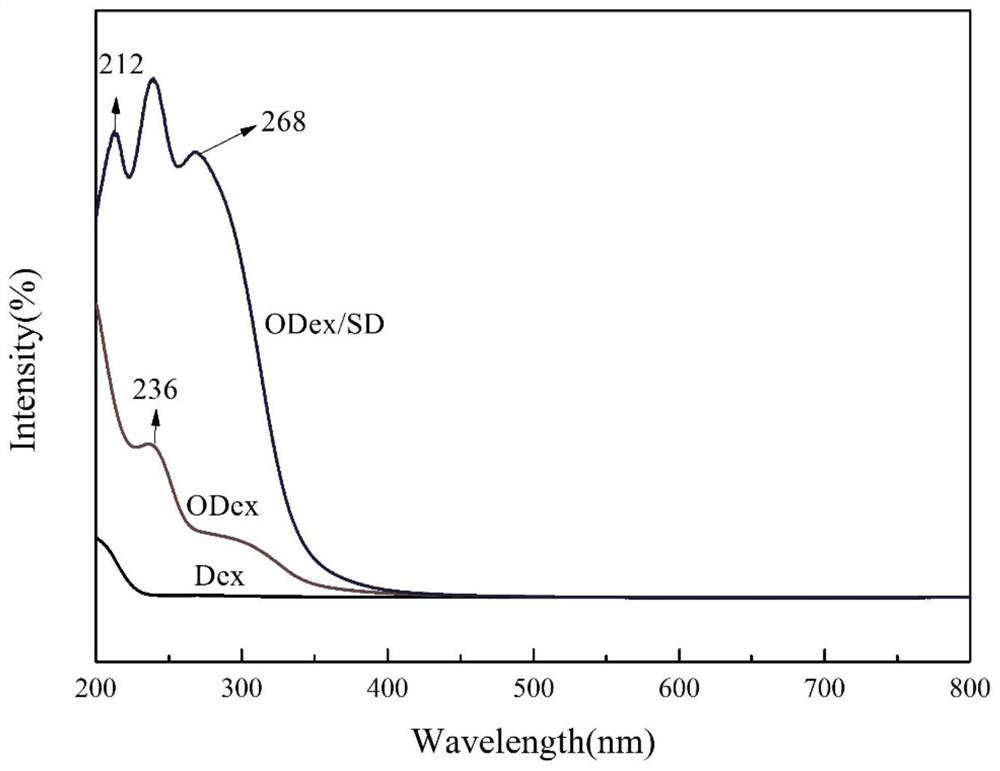
The invention discloses a preparation method and application of double-drug combined intelligent antibacterial hydrogel. The preparation process of the hydrogel comprises the following steps: oxidizing dextran (Dex) by adopting a sodium periodate oxidation method, breaking part of chains in dextran to form furfural, then reacting primary amino in sulfadiazine with part of aldehyde groups in furfural to generate pH sensitive imine bonds, and reacting and cross-linking residual aldehyde groups in furfural with tobramycin in water, and carrying out self-assembly to form the hydrogel. The hydrogelhas a pH sensitive characteristic, can effectively release drugs in an inflammatory subacid environment, realizes on-demand release of the drugs, avoids excessive use of antibiotics, reduces toxic and side effects of the drugs, simultaneously loads two bactericidal drugs sulfadiazine and tobramycin, kills bacteria through different mechanisms, improves an antibacterial effect, and has an excellent antibacterial effect.
Owner:WUHAN UNIV OF TECH
Method for simultaneously determining content of vancomycin and tobramycin in tissue drainage liquid through ultrahigh performance liquid chromatography-triple quadrupole mass spectrometry (UPLC-TQD) coupling technique
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
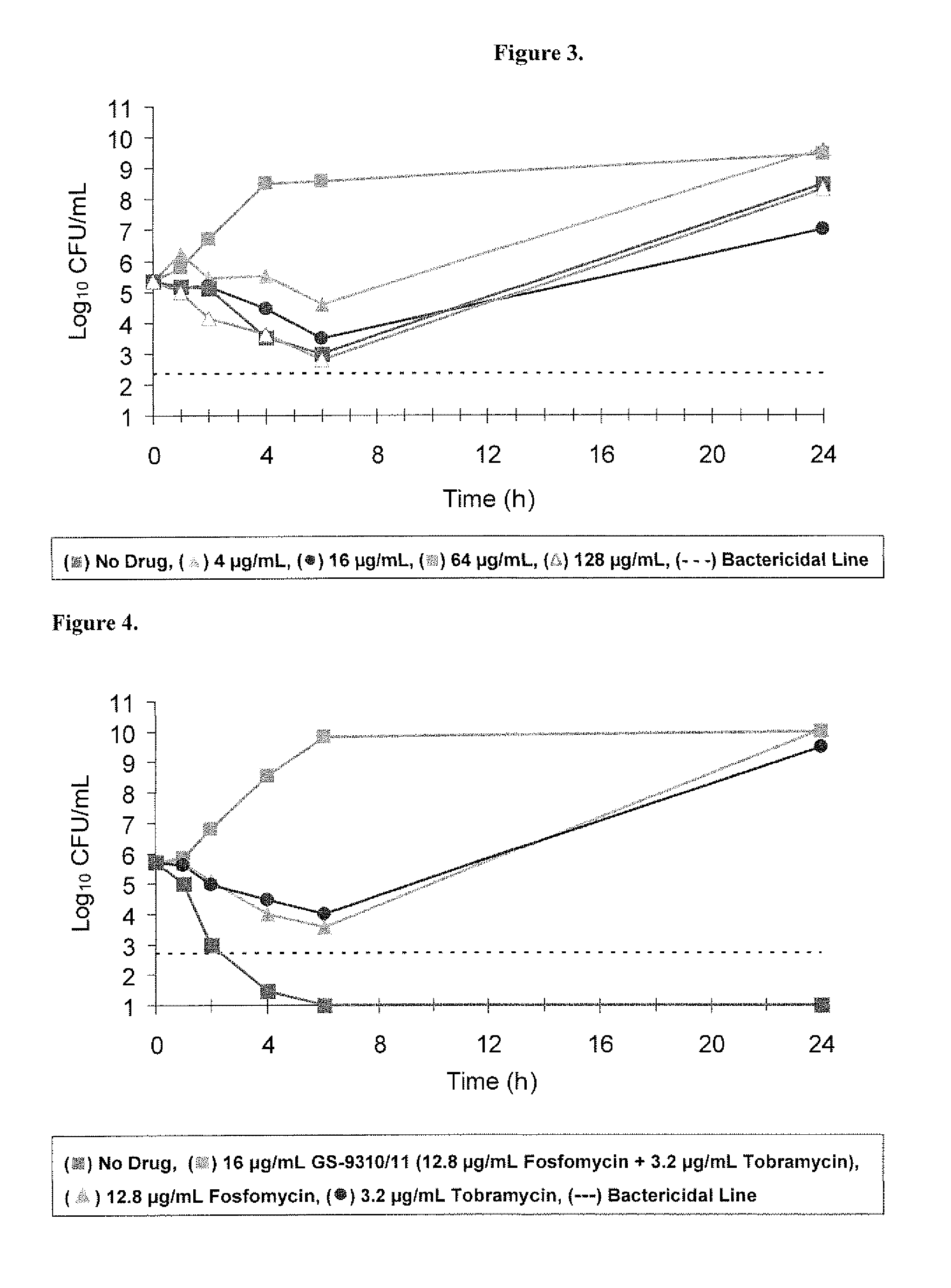
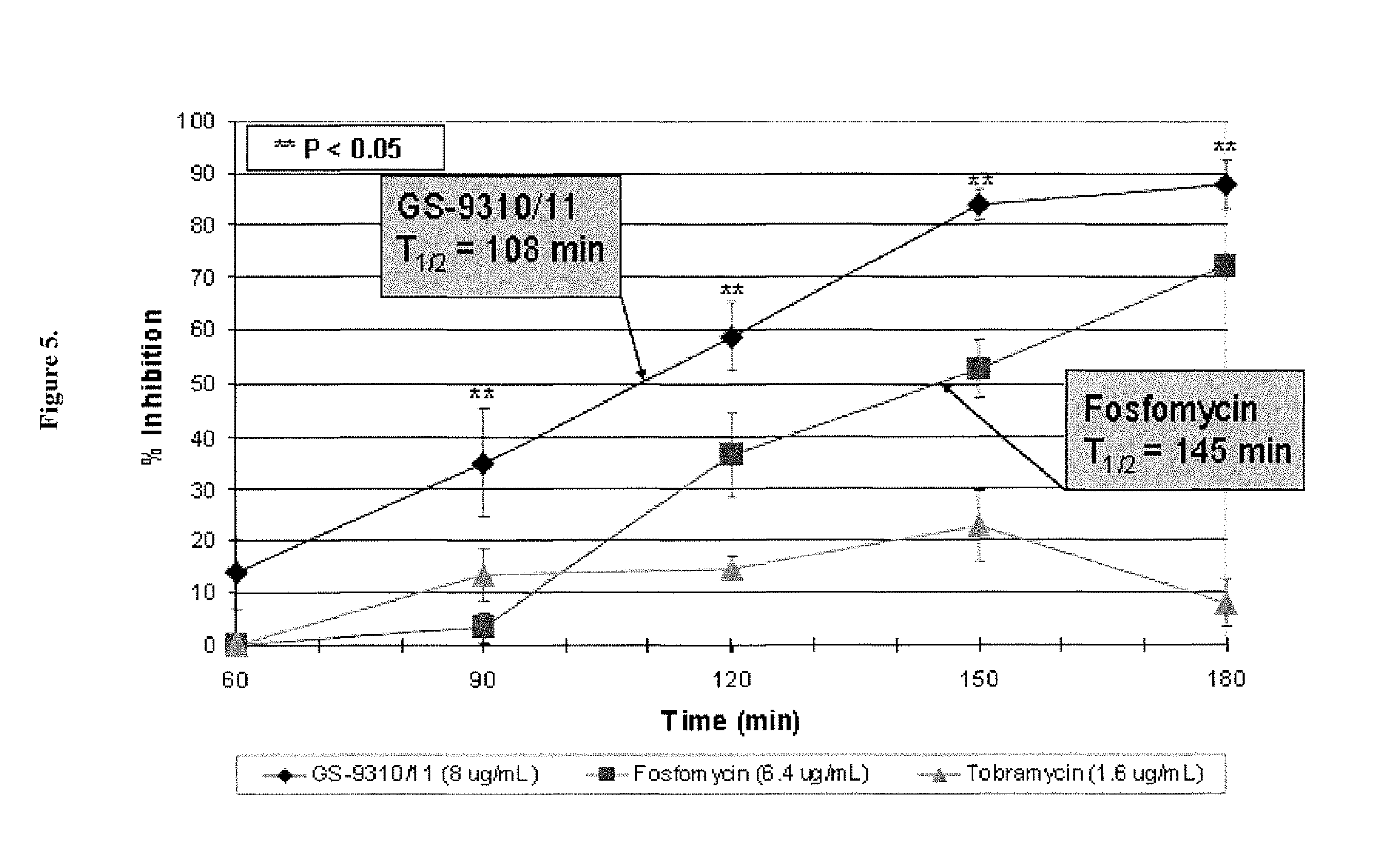
Dry powder fosfomycin/tobramycin formulation for inhalation
InactiveUS20140134253A1Reduce frequency , severity and durationBiocidePowder deliveryExacerbationCvd risk
The present invention provides an inhaled dry powder formulation containing a combination of fosfomycin salt and tobramycin-leucine compound particles. The use of such formulation for the treatment of patients who have Chronic Obstructive Pulmonary Disease (COPD) and who are experiencing or at risk of experiencing acute exacerbation, as well as patients who have other bacterial infections of the respiratory tract, particularly the lower respiratory tract, and methods for treating the same are also provided.
Owner:GILEAD SCI INC
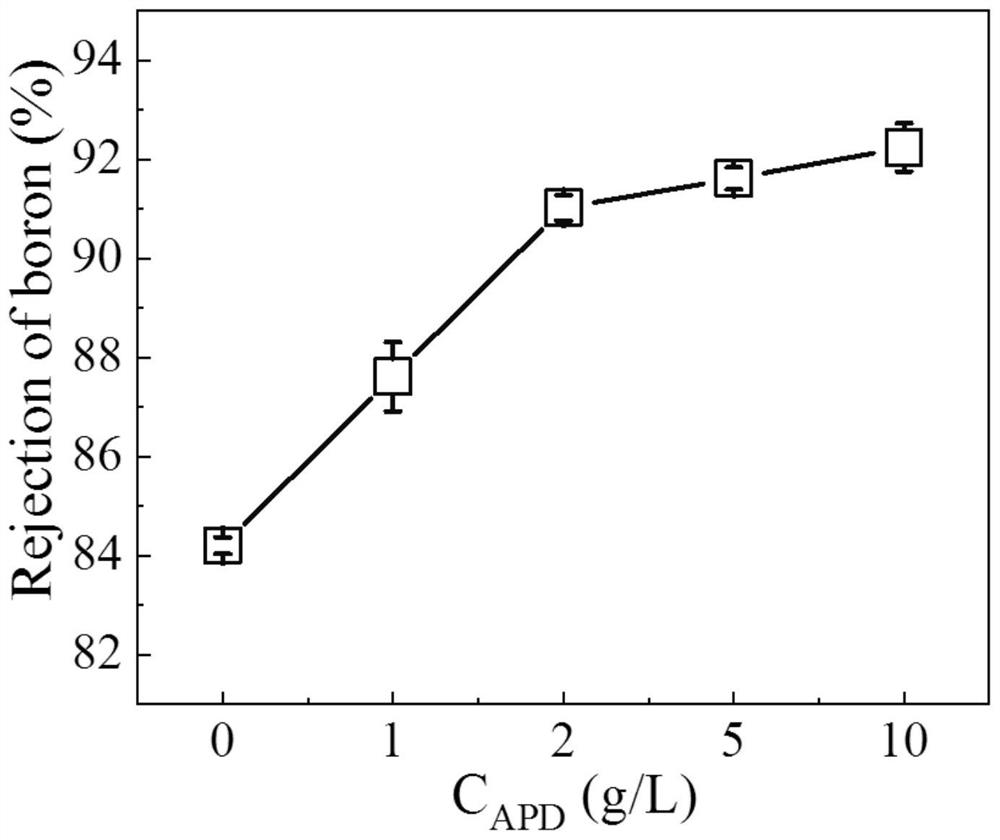
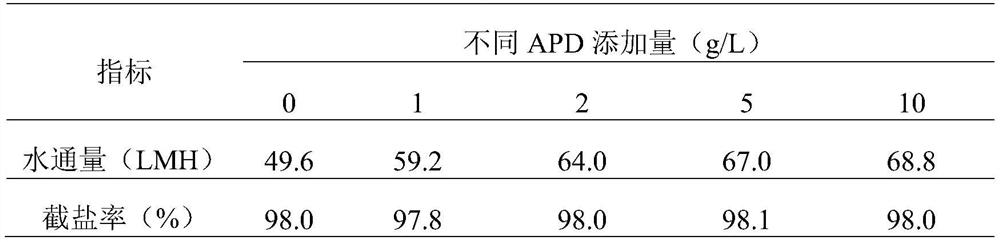
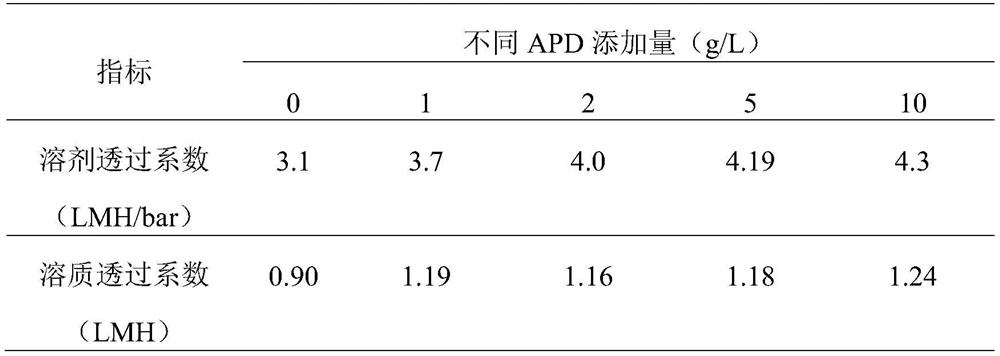
High-permeability antibacterial modified polyamide reverse osmosis membrane for efficiently removing boron and preparation method of high-permeability antibacterial modified polyamide reverse osmosis membrane
PendingCN111744374ALower boron contentLower boron content, fromMembranesWater contaminantsReverse osmosisPolyamide
The invention relates to a high-permeability antibacterial modified polyamide reverse osmosis membrane for efficiently removing boron and a preparation method of the high-permeability antibacterial modified polyamide reverse osmosis membrane. A polymerization modification layer is arranged on the surface of a reverse osmosis membrane, and the polymerization modification layer is obtained by the graft modification of a reverse osmosis polyamide layer with 3-amino-1, 2-propylene glycol (APD) and tobramycin (TOB). The surface of the composite membrane is subjected to chemical grafting modification, APD / TOB is successfully grafted to the surface of the reverse osmosis membrane, and the novel reverse osmosis membrane is obtained. Compared with that of a reverse osmosis membrane without graft modification, the hydrophilicity of the surface of the reverse osmosis membrane is greatly improved due to the fact that the APD and TOB contain a large amount of carboxyl, so that dirt is not prone tobeing deposited on the surface of the reverse osmosis membrane, therefore, the anti-fouling capacity of the obtained reverse osmosis membrane is enhanced, meanwhile, an original peak-valley structureon the surface of the reverse osmosis membrane is covered, and the surface roughness of the modified membrane is reduced; the smoother the membrane surface is, the fewer binding sites provided for pollutant deposition are, and the stronger the anti-pollution capacity of the composite membrane is.
Owner:SHENZHEN CHANGLONG TECH CO LTD
Tobramycin composition freeze-dried powder needle for injection
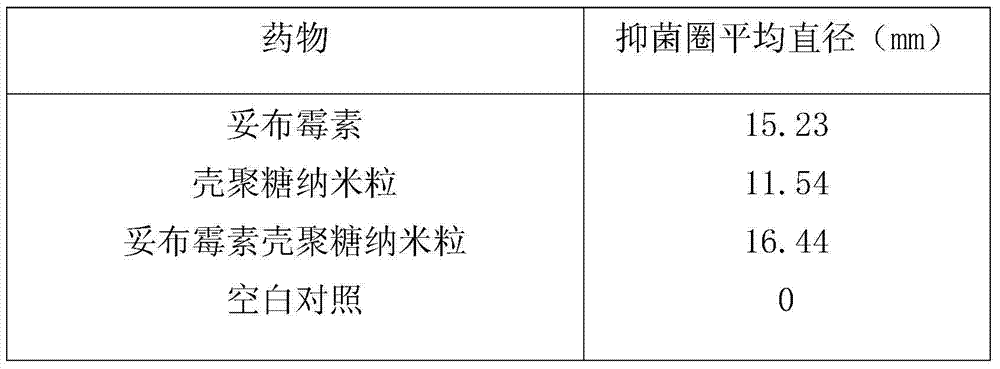
InactiveCN103585115AImprove antibacterial propertiesReduce dosageAntibacterial agentsPowder deliverySolubilityChitosan nanoparticles
The invention provides a tobramycin composition freeze-dried powder needle for injection and belongs to the technical field of medicine and medicine preparation. The tobramycin composition freeze-dried powder needle for injection comprises the following raw materials in parts by weight: 0.46-0.62 part of tobramycin, 0.23-0.31 part of chitosan nanoparticle and 98.07-99.31 parts of water for injection. The tobramycin composition freeze-dried powder needle has the advantages as below: 1) the composition of tobramycin and chitosan nanoparticle which are in a proportion of 1:0.5 can improve the solubility of tobramycin in water, shorten the dissolving time, improve the dilution stability and is beneficial to clinical application; 2) the composition can remarkably improve the antibacterial effect of tobramycin, clinically reduce the use amount of tobramycin and reduce the adverse reaction of tobramycin; 3) the chitosan nanoparticle can replace mannitol to be used as a freeze-dried framework agent of freeze-dried powder needle and can eliminate the activity of mannitol to the human body; and 4) of the situation that medicine is decomposed after being heated can be avoided, the stability of medicine is maintained, the product is loose in texture, can be dissolved rapidly in water, and is good in stability and beneficial to storage.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
A kind of tobramycin inhalation composition and its preparation method and application
ActiveCN105616345BMeets requirements for inhalation solutionsInhalation solution requirements addressedAntibacterial agentsOrganic active ingredientsInhalationRoom temperature
The invention relates to a tobramycin inhalation composition and a preparation method and application thereof. The composition is prepared from tobramycin 4%-8% (w / v), sodium chloride 0.1%-0.9% (w / v), weak-reducibility organic weak acid serving as a stabilizer 0.1%-1.0% (w / v), strong acid for regulating the pH value of the composition and the balance water, wherein the pH value of the composition is 5.6-7.5. The tobramycin inhalation composition prepared by adopting the technical scheme has the pH value closer to the physiological pH of a human body and has significantly improved stability at room temperature.
Owner:GUANGZHOU JOINCARE RESPIRATORY DRUG ENG TECH CO LTD
Tobramycin detection test paper based on aptamer and platinum modified gold nanoparticles
ActiveCN113278684ARealize detectionEasy to detectMicrobiological testing/measurementBiological testingAptamerCellulose
The invention relates to tobramycin detection test paper based on an aptamer and platinum modified gold nanoparticles. The test paper comprises a sample pad, a gold label pad, a nitrocellulose membrane, a water absorption pad, a PVC base plate, a detection line and a quality control line. Platinum-modified gold nanoparticles (Au@PtNPs) are used for loading a hybrid double strand of an aptamer (Apt) and a complementary probe (cDNA) to be used as a signal amplification label. The unique bifunctional properties (plasma optical property and nano mimic enzyme catalytic property) of Au@Pt NPs provide two different detection schemes: one is red only generated by the intrinsic color of AuNPs, and the other is more sensitive dark blue generated by a catalytic substrate, so that a detection mode adjusted as required is realized. The test paper strip provided by the invention is simple, convenient and rapid in use, is sensitive and efficient, and has huge application potential in field point detection of tobramycin residues in food and environment.
Owner:JIANGNAN UNIV
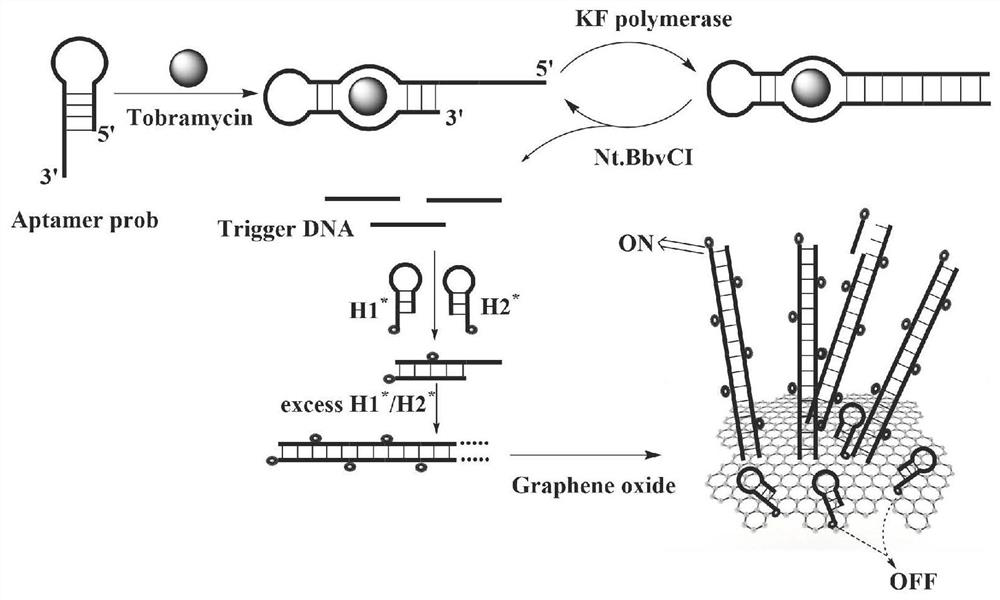
Tobramycin detection system and method based on CRISPR-Cas12a
ActiveCN113640268ARealize visualizationEasy to detectFluorescence/phosphorescenceAgainst vector-borne diseasesFluoProbesFluorophore
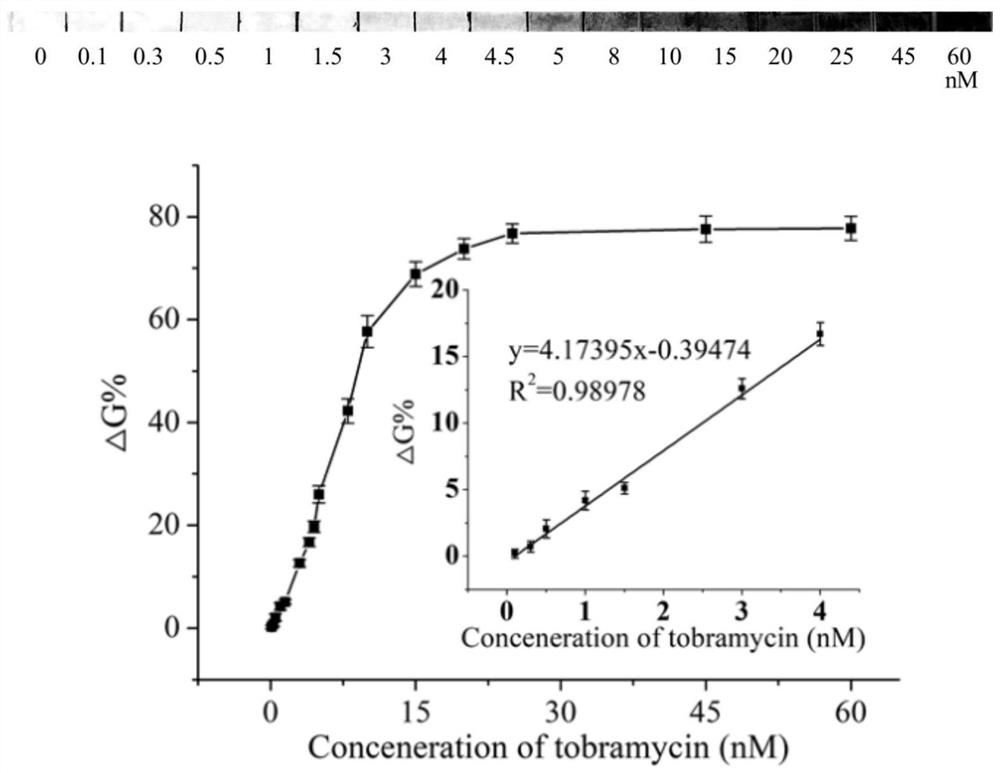
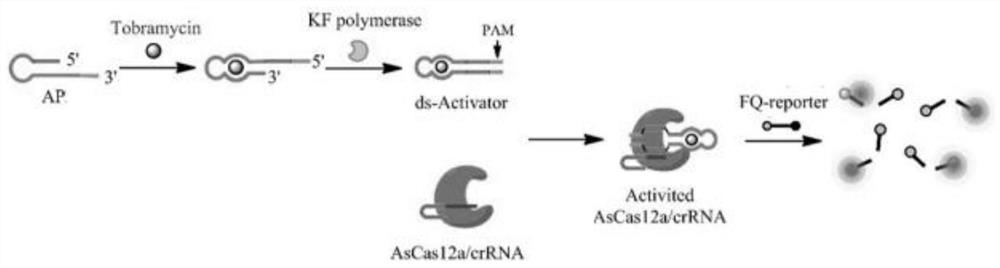
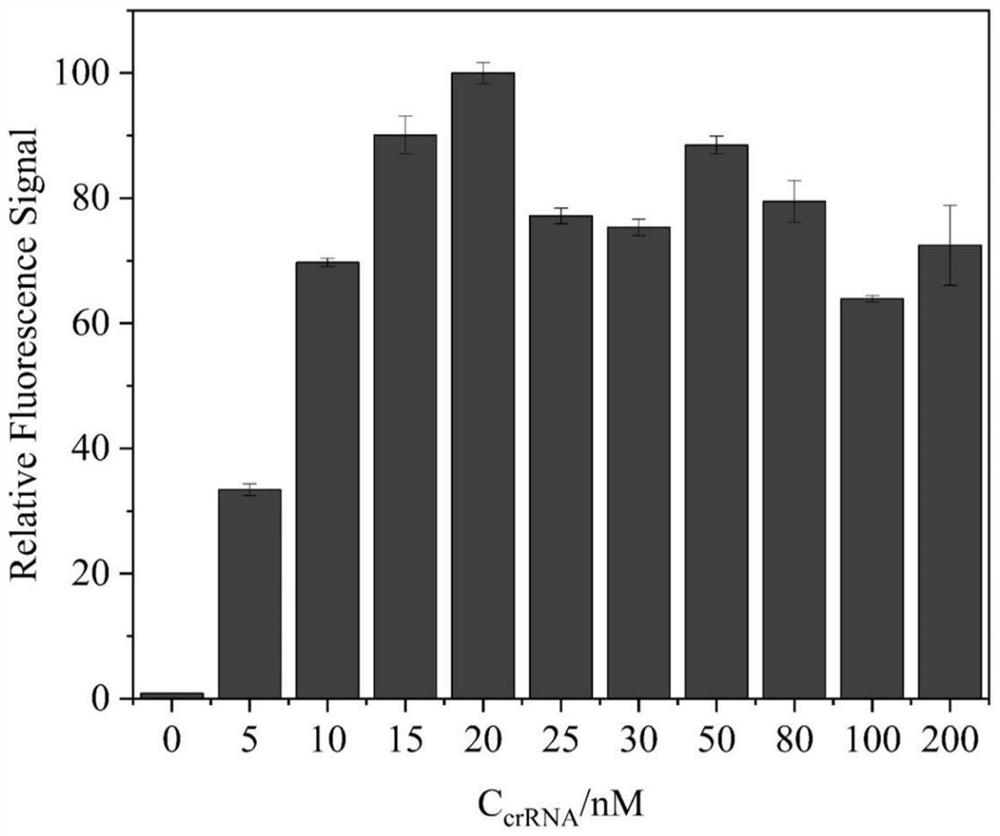
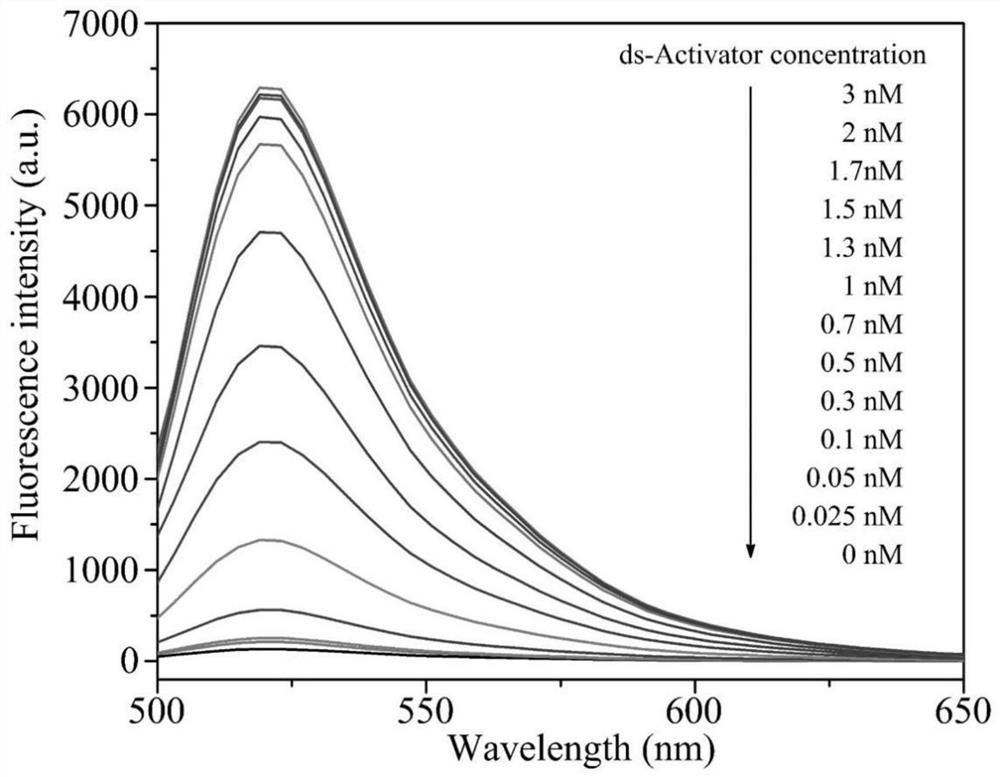
The invention discloses a tobramycin detection system based on CRISPR-Cas12a. The tobramycin detection system comprises an aptamer probe AP, CrRNA (Ribonucleic Acid), AsCas12a protein, KF polymerase and a reporter probe, wherein two ends of the reporter probe are respectively modified with a fluorophore and a quenching group; the sequence of the aptamer probe AP is as shown in SEQ ID NO:1; and the sequence of the CrRNA is as shown in SEQ ID NO:2. Compared with the prior art, the aptamer probe in the detection system can be specifically combined with tobramycin and change the configuration, trigger DNA capable of being recognized by the CRISPR-Cas12a system is generated under the action of KF polymerase, finally, the fluorescent probe is cut in the CRISPR-Cas12a system to generate a fluorescence signal, and through blue light irradiation and fluorescence intensity analysis, visual and quantitative detection of tobramycin can be realized, and the detection sensitivity is high.
Owner:南京百利特生物科技有限公司
Antibiotic bone cement for treating orthopedic infection
InactiveCN110975003AIncrease vacuolesFacilitated releaseSurgical adhesivesOrthopedic departmentPolymethyl methacrylate
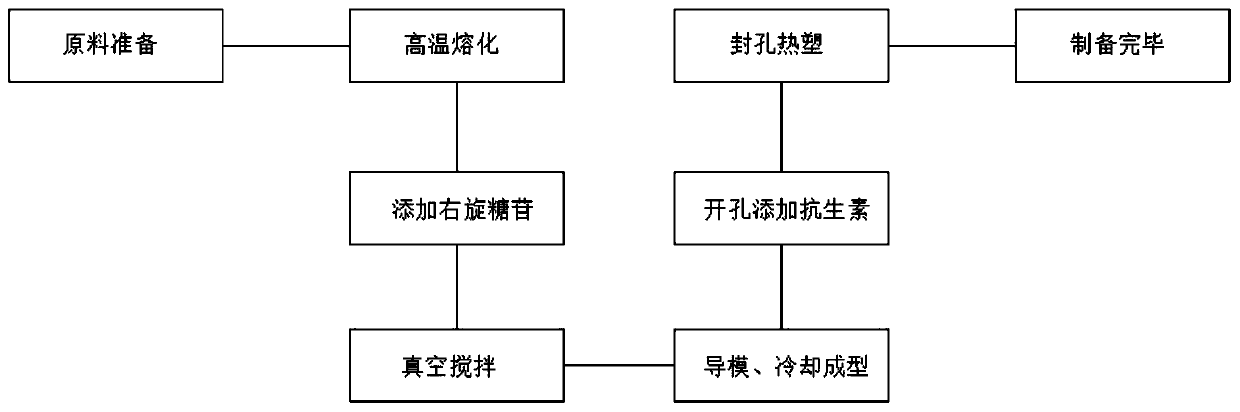
The invention discloses antibiotic bone cement for treating orthopedic infection. The antibiotic bone cement for treating orthopedic infection comprises the following main components: polymethyl methacrylate, vancomycin, tobramycin and dextran. According to the invention, the dextran is added into original polymethyl methacrylate, so cavitation bubbles in a cured product are increased, and the release of antibiotics can also be promoted, while mechanical properties are reduced; by adoption of vacuum stirring, bubbles are reduced, while mechanical properties of the bone cement can be reinforced; by combination of vacuum stirring with the dextran, the effect that the mechanical properties and the antibiotic release rate are relatively moderate can be achieved; moreover, by addition of the vancomycin and the tobramycin at two sides respectively, releasing of antibiotics at a using position through mutual cooperation of two antibiotics can be realized, and the anti-infection capacity is improved; meanwhile, by combined application of two antibiotics, the antibacterial spectrum is expanded, and drug resistance is reduced; and certain using prospects are achieved.
Owner:苏州众泽医疗科技有限公司
Tobramycin immunoassay reagent and preparing and detecting method thereof
The invention discloses a tobramycin immunoassay reagent and a preparing and detecting method thereof. The tobramycin immunoassay reagent comprises enzyme-labelled tobramycin and an indication reagentfor detecting a tobramycin antibody-enzyme-labelled tobramycin compound. The enzyme-labelled tobramycin is formed by coupling tobramycin and glucose dehydrogenase. By means of the tobramycin immunoassay reagent, the content of tobramycin in human blood and other samples can be precisely and rapidly determined. Compared with existing detecting reagents on the market, the immunoassay reagent has the advantages of being convenient and rapid to use, high in sensitivity, high in specificity, accurate in quantifying and the like, and is beneficial for clinical use and popularization.
Owner:太原瑞盛生物科技有限公司
Metabolic controlled fermentation process for carbamoyl tobramycin production
InactiveUS20020197683A1High purityGuaranteed economic efficiencyAntibacterial agentsSugar derivativesNitrogen sourceStreptomyces tenebrarius
A metabolic controlled fermentation process has been developed for the production of carbamoyl tobramycin by the application of different Streptomyces tenebrarius strains in submerged cultures at a temperature within about 37-41° C. on a medium containing assimilable carbon and nitrogen sources, mineral salts and controlling the assimilable carbon and nitrogen sources by feeding in an optimal range. As a result of this invention a high yield production of carbamoyl tobramycin with high purity could be achieved.
Owner:TEVA PHARMA IND LTD +1
Polyether ether ketone bone defect repair material and preparation method
ActiveCN108310457BEnhance osteogenic activityBiodegradableTissue regenerationCoatingsOsseointegrationMicrosphere
The invention belongs to a polyetheretherketone bone defect repair material and a preparation method thereof in the field of medical materials. The surface of the polyetheretherketone bone defect repair material has a micropore-nanopore multilevel hole structure, and is also loaded with simvastatin, Polylactic acid porous micromembranes and tobramycin microspheres. The present invention overcomes the defects of low bioactivity, no antibacterial effect and poor osseointegration ability with bone tissue in the existing polyether ether ketone bone defect repair materials, and the provided polyether ether ketone bone defect repair materials have good antibacterial properties performance and good osteogenic activity, and the preparation method of the polyetheretherketone bone defect repair material provided has simple process and low cost.
Owner:SICHUAN UNIV
Engineered bacterium for producing tobramycin by direct fermentation and construction and application of engineered bacterium
ActiveCN103740628APrevent secondary occurrenceSimple production processBacteriaMicroorganism based processesBiotechnologyMetabolite
The invention discloses an engineered bacterium for producing tobramycin by direct fermentation and construction and application of the engineered bacterium. By utilizing genetic engineering technology, octose synthatase gene aprK in apramycin biosynthesis gene cluster of streptomyces tenebrarius apramycinis subjected to knocking-out in frame, and further carbamoyl transferase gene tobZ in apramycin biosynthesis gene cluster is knocked out, so that the engineered bacterium (S.tenebrarius318 (delta aprK + delta tobZ)), of which the fermentation metabolite does no generate apramycin any longer and mainly generates tobramycin, is obtained. Tobramycin is directly produced by the engineered bacterium through fermentation, so that a conventional production process of preparing tobramycin from carbamoyltobramycin is avoided, the production flow is substantially simplified, the production cost is reduced, pollution is reduced, the product quality is improved, and the engineered bacterium has vita practical significance and social significance on industrialized production.
Owner:福州市鼓楼区荣德生物科技有限公司
Fosfomycin/tobramycin combinations for the treatment and prevention of ophthalmic, otological and dermatological infections
InactiveUS20110230431A1High activityAvoiding potential for ototoxicityAntibacterial agentsBiocideTobramycinDermatology
Provided are topical fosfomycin-tobramycin compositions for the treatment and / or prevention of ophthalmic, otological, and dermatologic inflammation and / or bacterial infections and methods of treating ophthalmic, otological and dermatological inflammation and / or bacterial infections.
Owner:GILEAD SCI INC
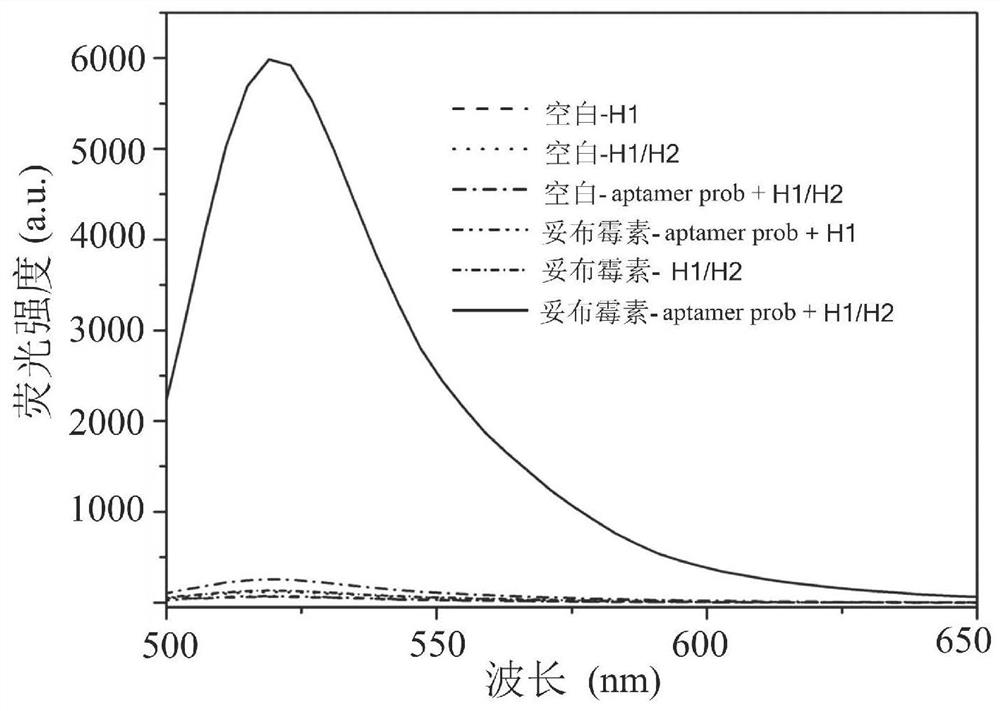
Biosensor for detecting tobramycin and detection method
ActiveCN113341128ADetection adaptationMeet the sensitivity requirementsMicrobiological testing/measurementBiological testingAptamerKlenow fragment
The invention discloses a biosensor for detecting tobramycin. The biosensor comprises an aptamer probe, a hairpin probe H1, a hairpin probe H2, Klenow Fragment polymerase, Nt.BbvCI endonuclease, a buffer solution and graphene oxide. The sequence of the aptamer probe is as shown in SEQ ID No: 1, the aptamer probe is formed by hybridizing an aptamer Aptamer with the sequence as shown in SEQ ID No: 2 and an amplification template T with the sequence as shown in SEQ ID No: 3. The sequence of the hairpin probe H1 is as shown in SEQ ID No: 4, and the sequence of the hairpin probe H2 is as shown in SEQ ID No: 5. When the biosensor is used for detecting tobramycin, the method is simple, the practicability is good, the stability is high, the detection lower limit reaches 0.06 nM and is lower than that of the existing similar sensors, and the biosensor has wide application prospects in the fields of environmental monitoring and food safety.
Owner:JIANGSU INSTITUTE OF EDUCATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com