Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
267 results about "Drug granules" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
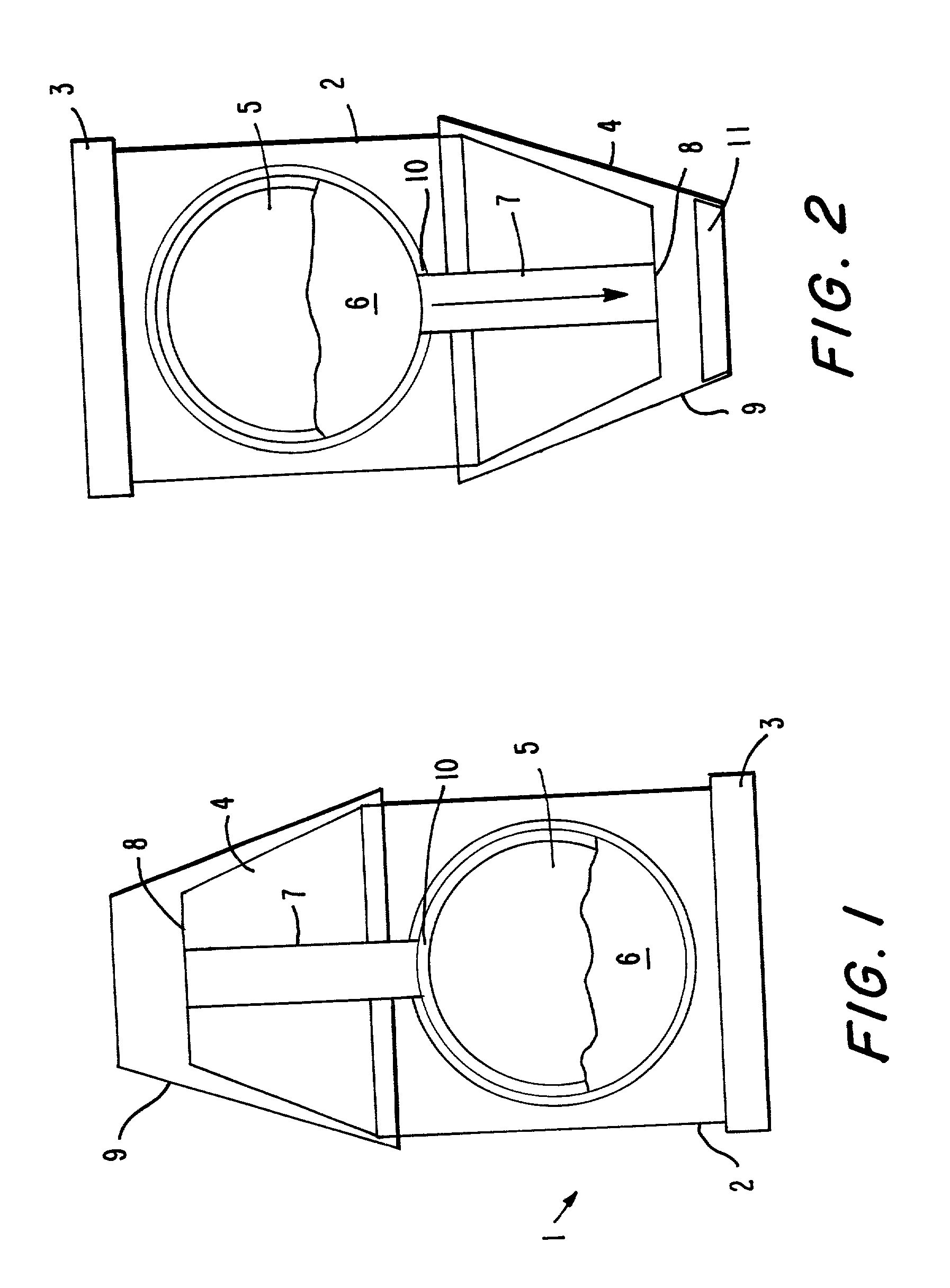
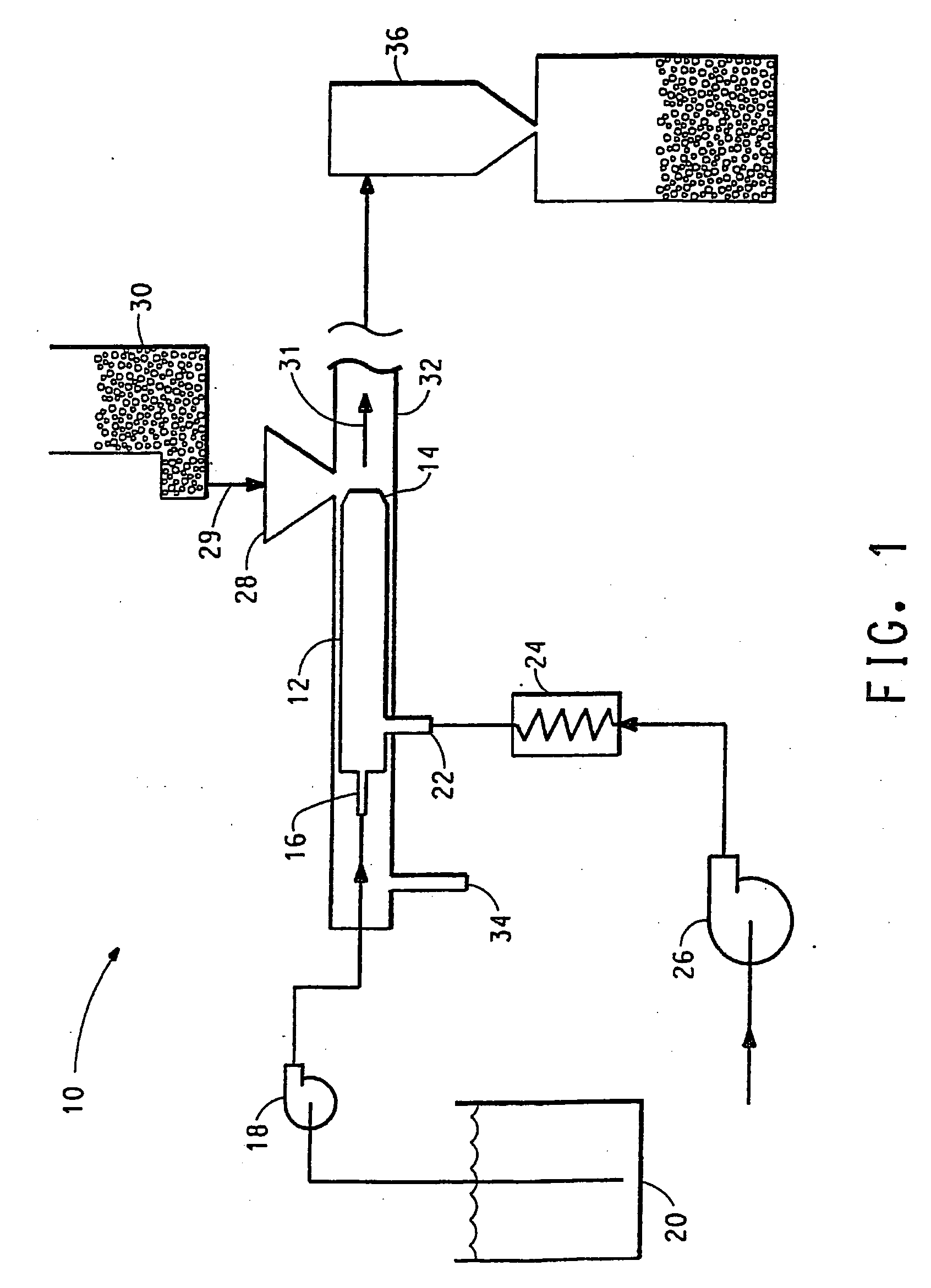
Delivery of oral drugs
InactiveUS20010020147A1Comfortable and convenient motionComfortable and convenient feelPowder deliveryLiquid surface applicatorsMean diameterHuman patient
Disclosed is a system for delivery of a drug comprising a multiple unit dosing device comprising a housing and an actuator, said device containing multiple doses of multiparticulates comprising drug particles, said device upon actuation delivering a unit dose of said multiparticulates, said drug particles having a mean diameter of greater than 10 mum to about 1 mm such that an effective dose of said drug cannot be delivered into the lower lung of a human patient. Also disclosed are novel methods, devices and dosage forms for delivering a drug.
Owner:PHARMAKODEX LTD
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS7399488B2Good treatment effectSmall dosePowder deliveryNervous disorderAdditive ingredientWater insoluble
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, a drug is modified to increase its lipophilicity. In preferred embodiments the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble, but enzymatically degradable by enzymes present in the human gastrointestinal tract. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
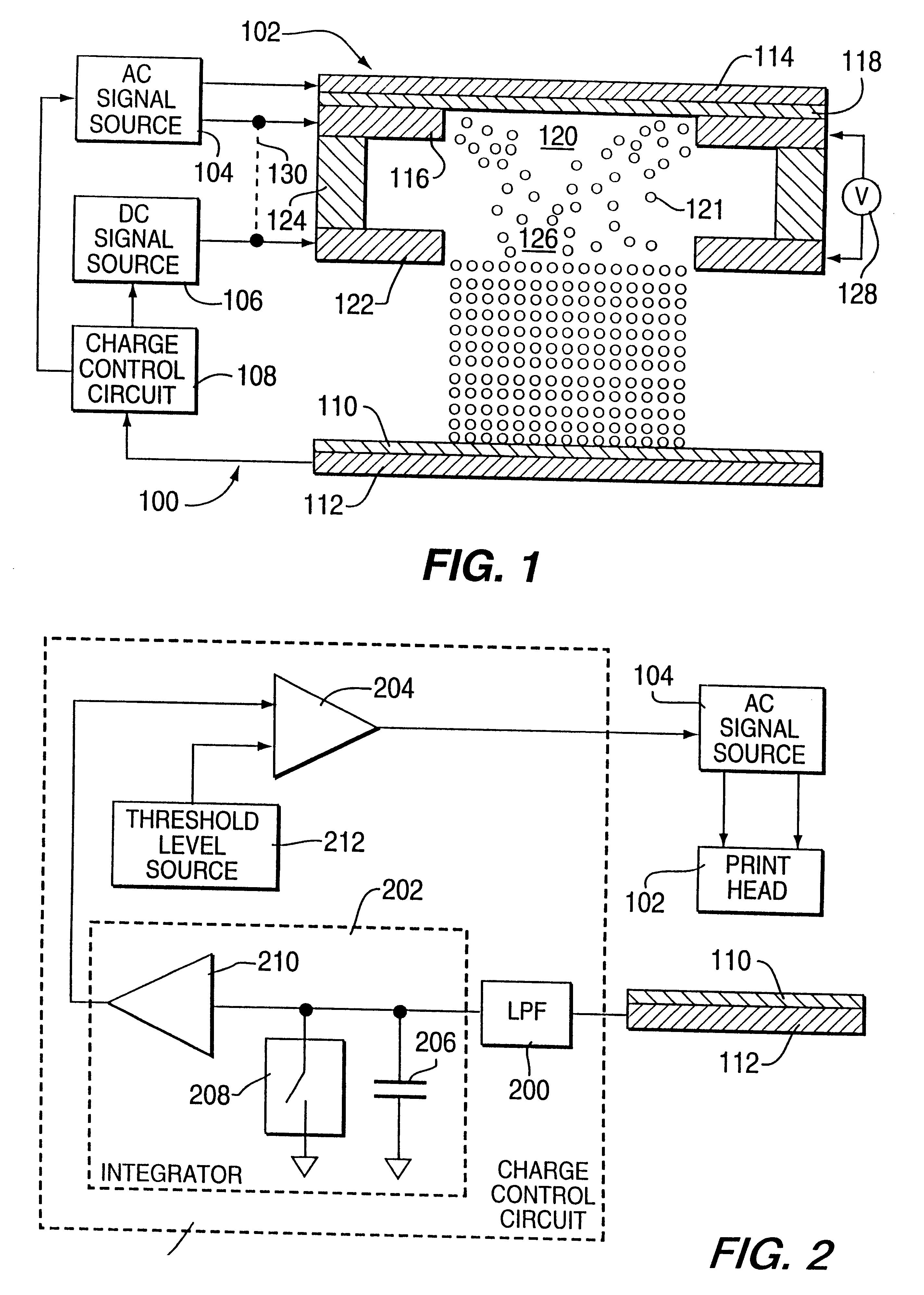
Method and apparatus for electrostatically depositing a medicament powder upon predefined regions of a substrate
A method for electrostatically depositing select doses of medicament powder at select locations on a substrate. Specifically, an apparatus contains a charged particle emitter for generating charged particles that charge a predefined region of a substrate and a charge accumulation control circuit for computing the amount of charge accumulated upon the substrate and deactivating the emitter when a selected quantity of charge has accumulated. Additionally, a triboelectric charging apparatus charges the medicament powder and forms a charged medicament cloud proximate the charged region of the substrate. The medicament particles within the medicament cloud electrostatically adhere to the charged region. The quantity of charge accumulated on the substrate at the predefined region and the charge-to-mass ratio of the medicament powder in the cloud control the amount (dose) of medicament deposited and retained by the substrate. Consequently, this apparatus accurately controls both medicament dosage and deposition location. Furthermore, since the substrate can be of any dielectric material that retains an electrostatic charge, the apparatus can be used to deposit medicament on substrates that are presently used in oral medicament consumption, e.g., substrates that are used to fabricate suppositories, inhalants, tablets, capsules and the like.
Owner:DELSYS PHARMA
Coated polyunsaturated fatty acid-containing particles and coated liquid pharmaceutical-containing particles
InactiveUS20060068019A1Powder deliveryLiquid surface applicatorsFatty acids.polyunsaturatedFatty acid
A process for coating a polyunsaturated fatty acid (PUFA)-containing carrier particle or a PUFA matrix particle, or a liquid pharmaceutical-containing carrier particle or a liquid pharmaceutical matrix particle. Also disclosed are such particles made by the process of the invention and foods, pharmaceuticals, beverages, nutritional supplements, infant formula, pet food and animal feed with incorporate such particles.
Owner:SOLAE LLC
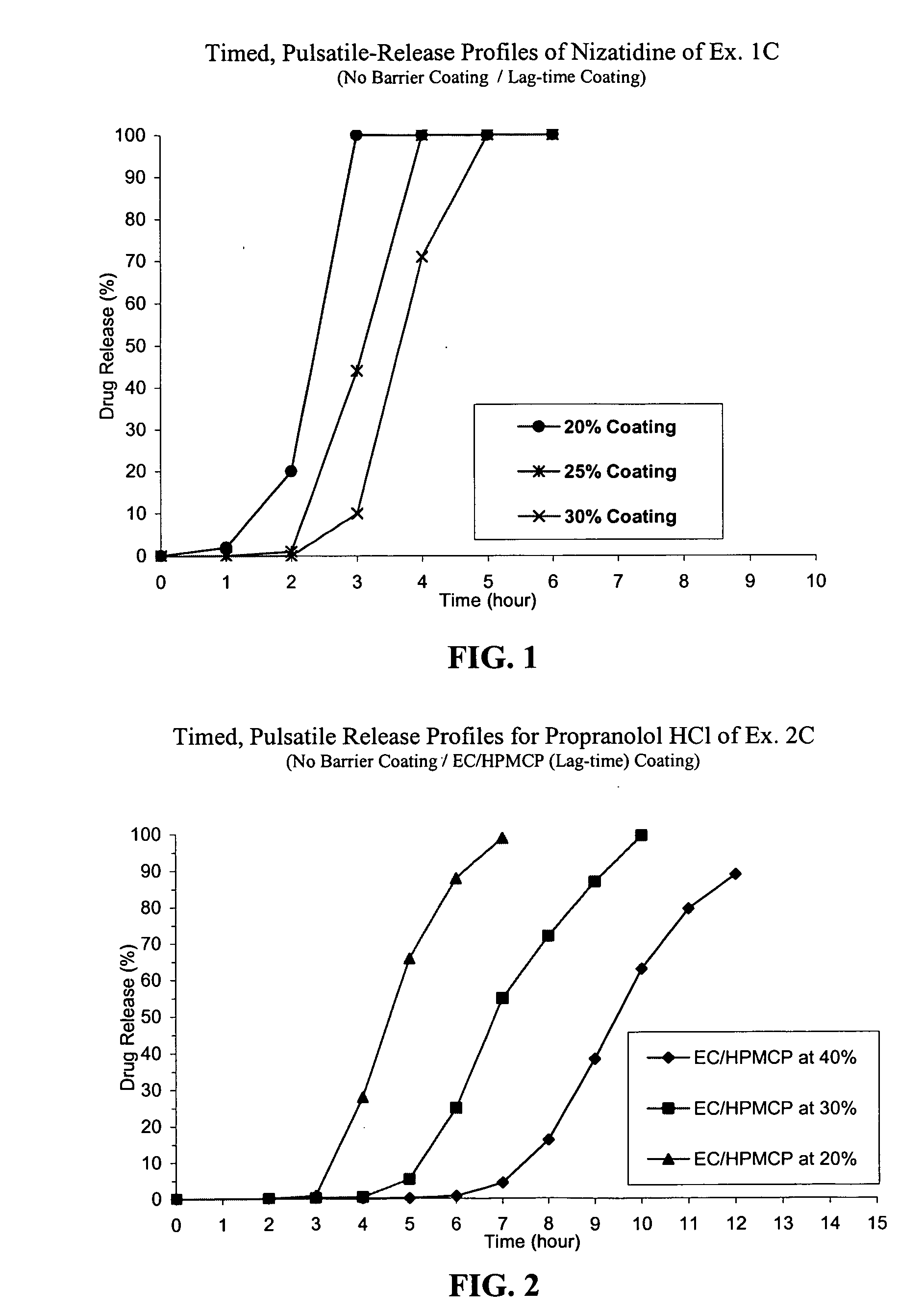
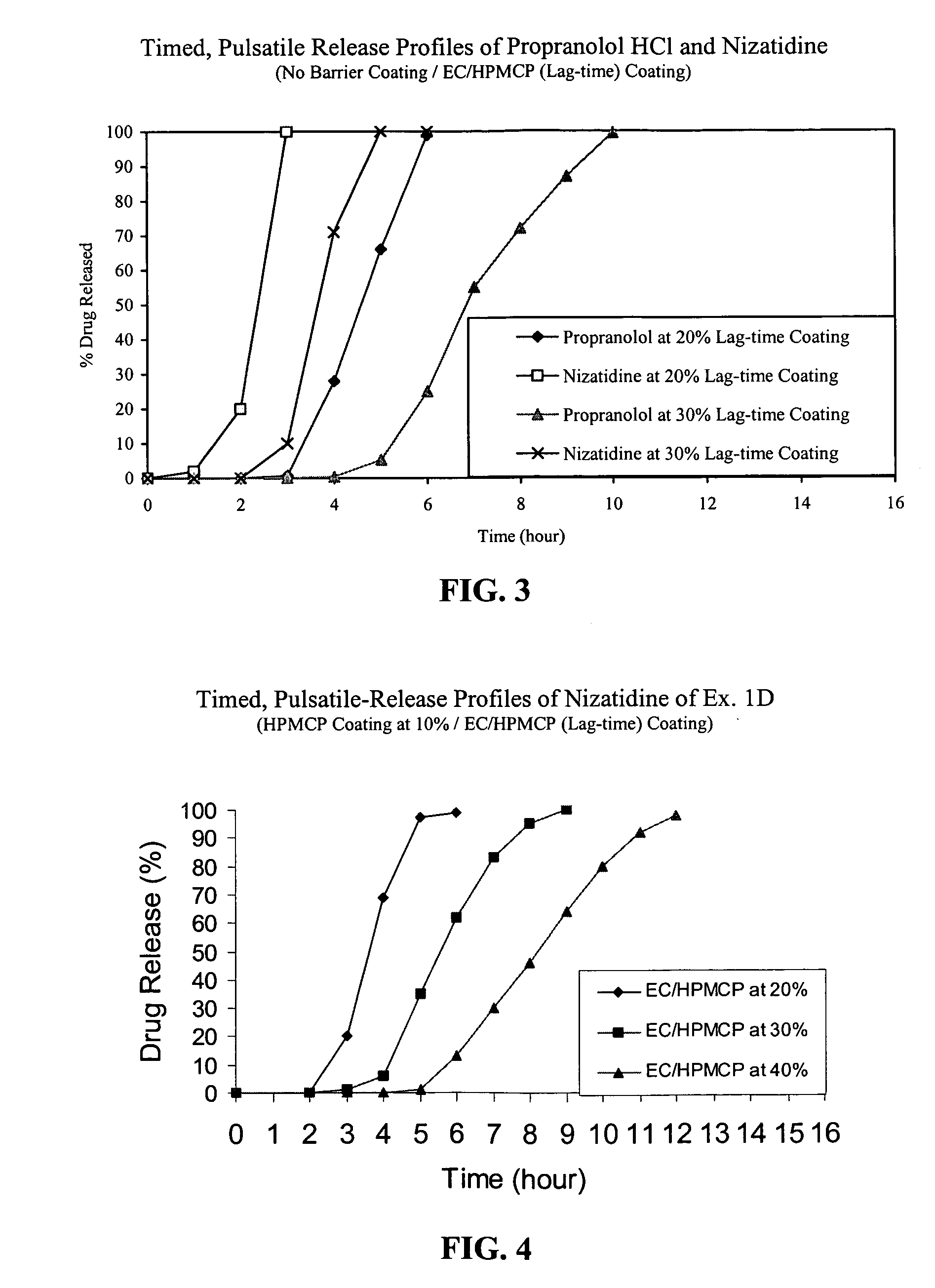
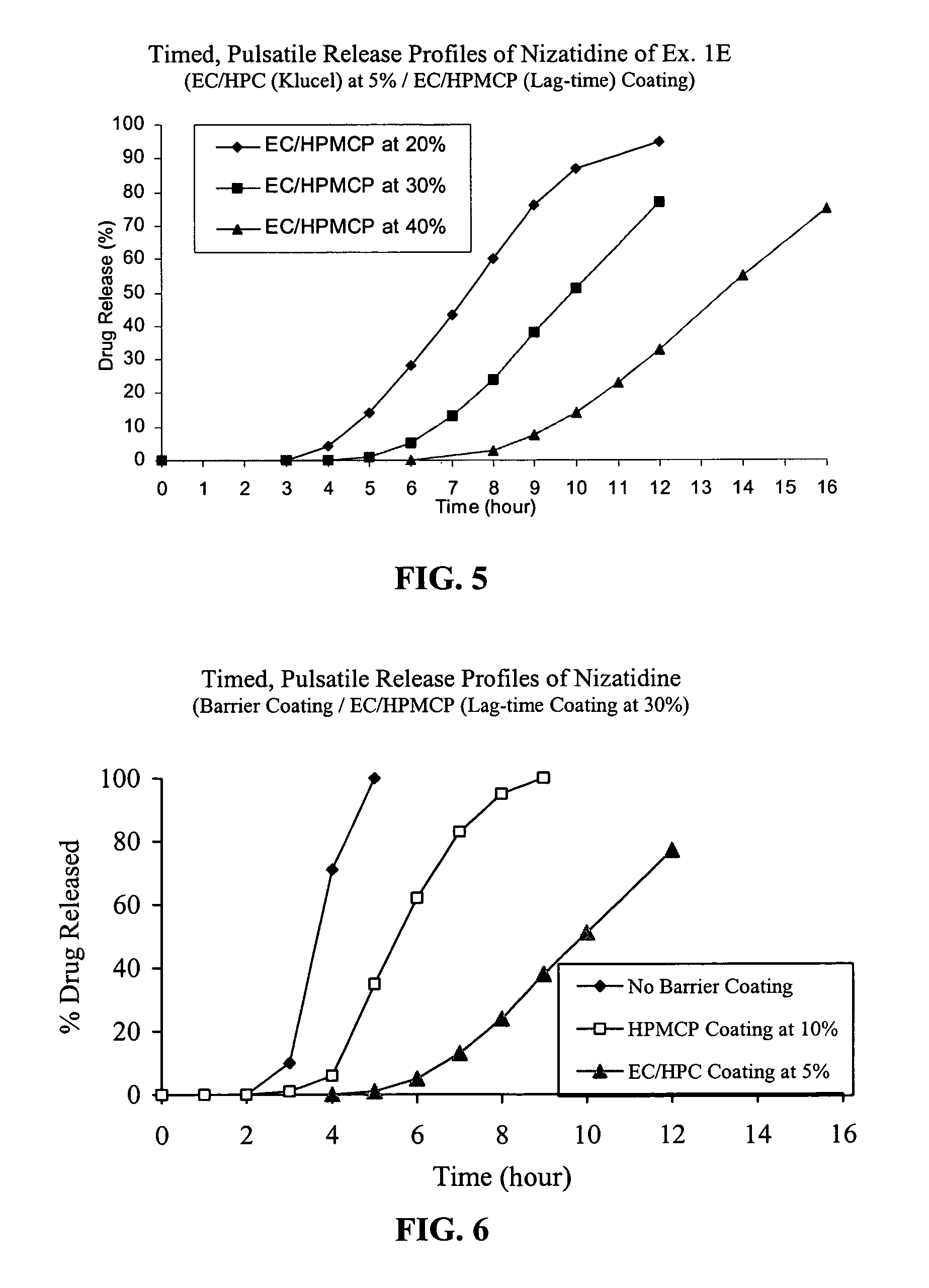
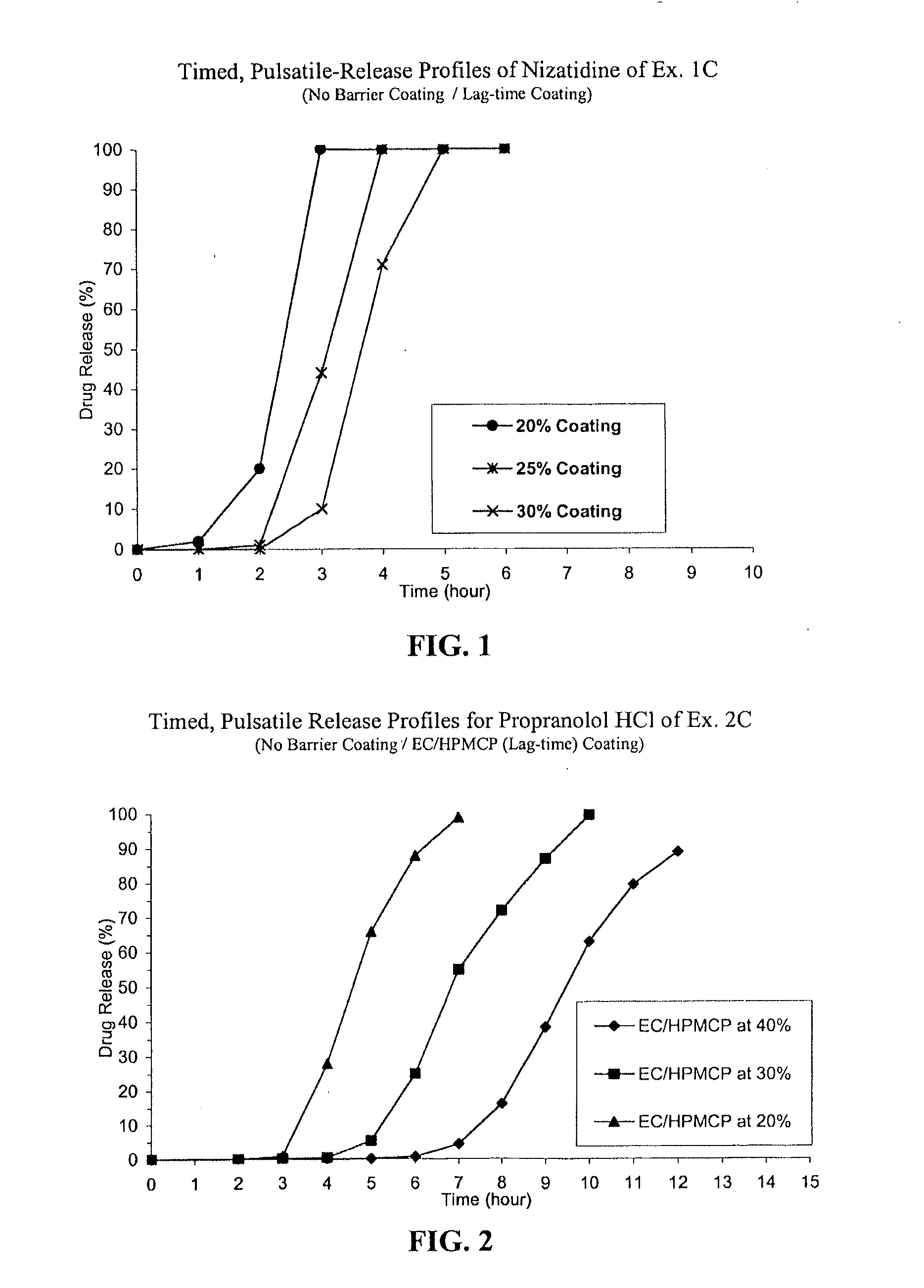
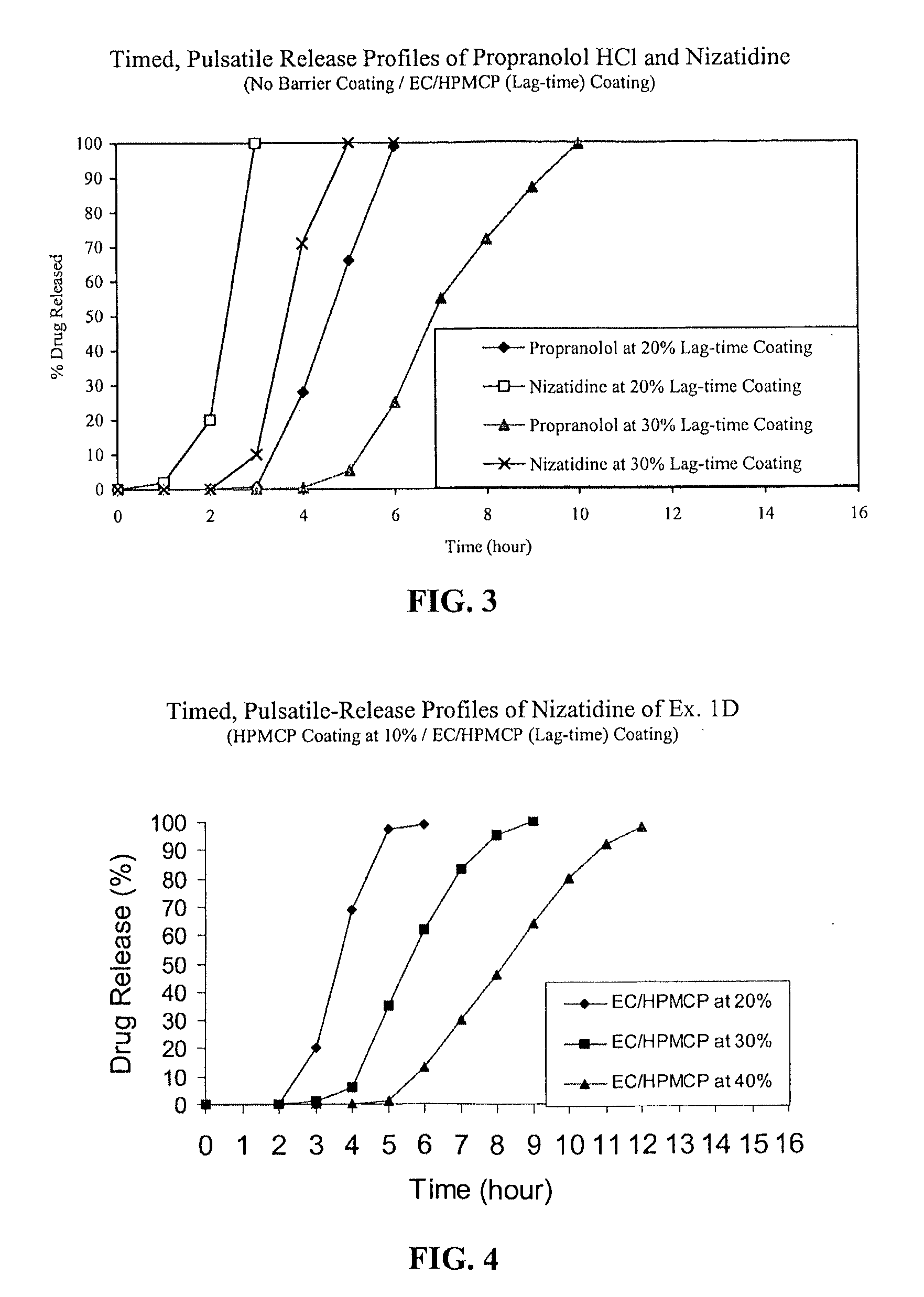
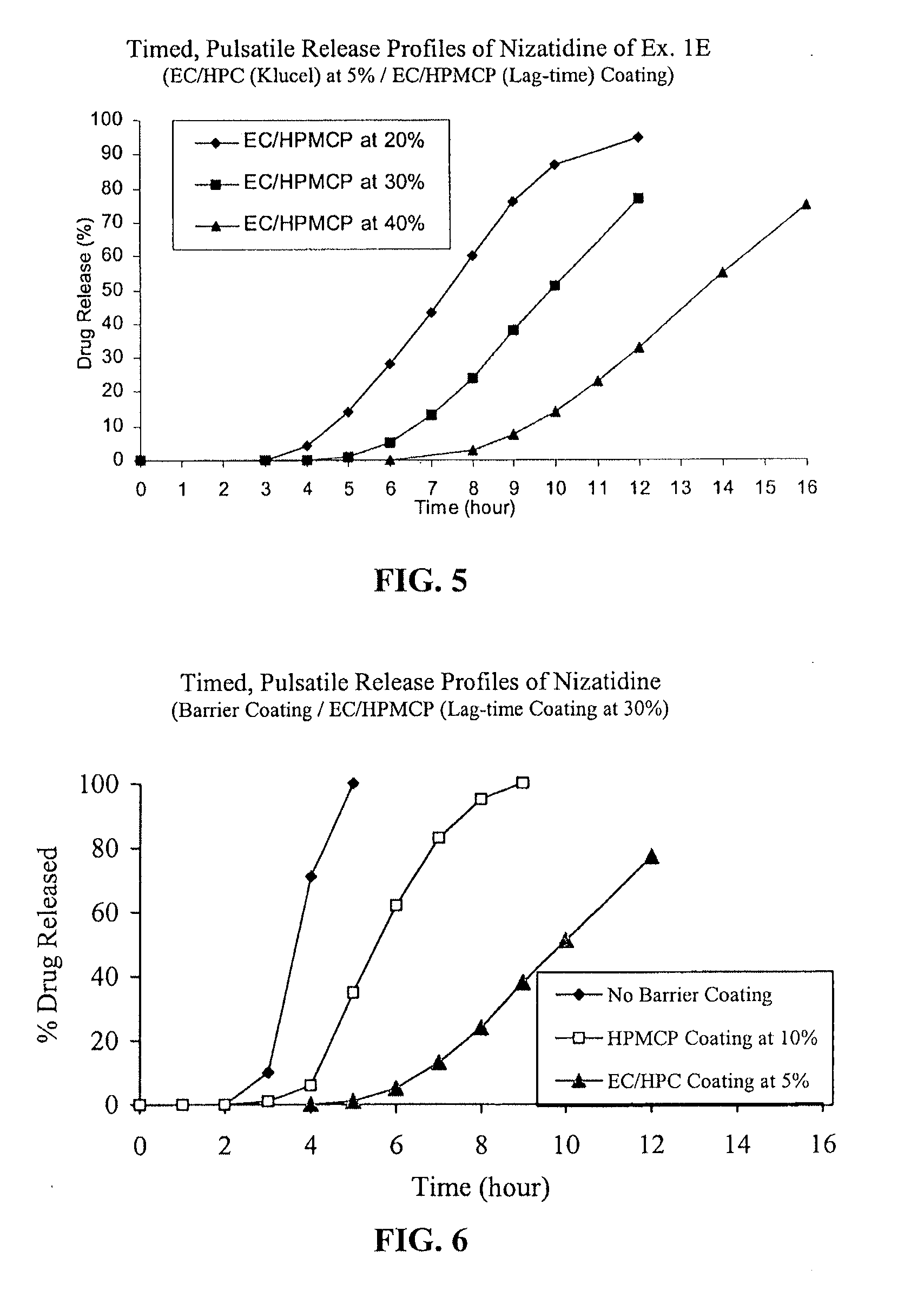
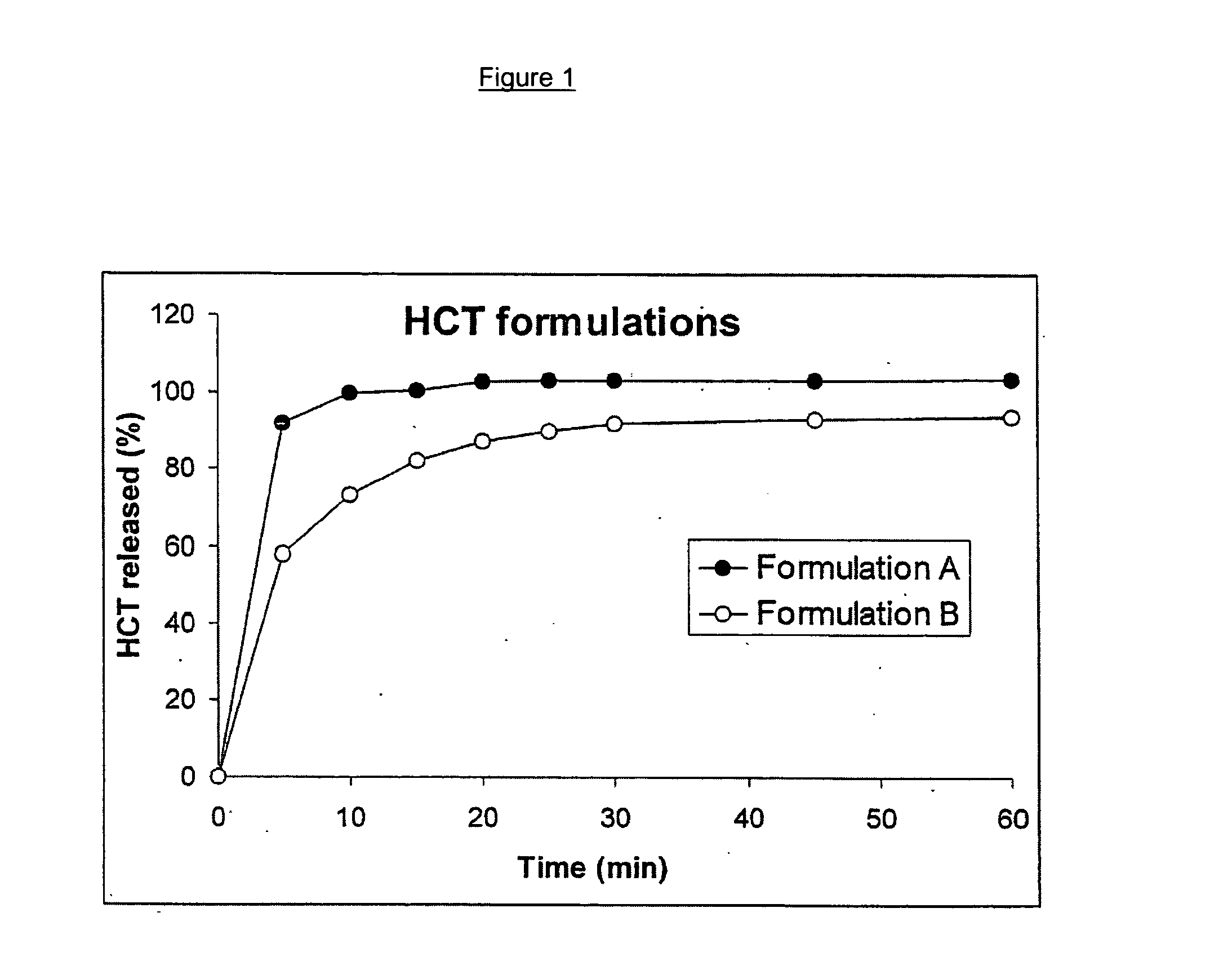
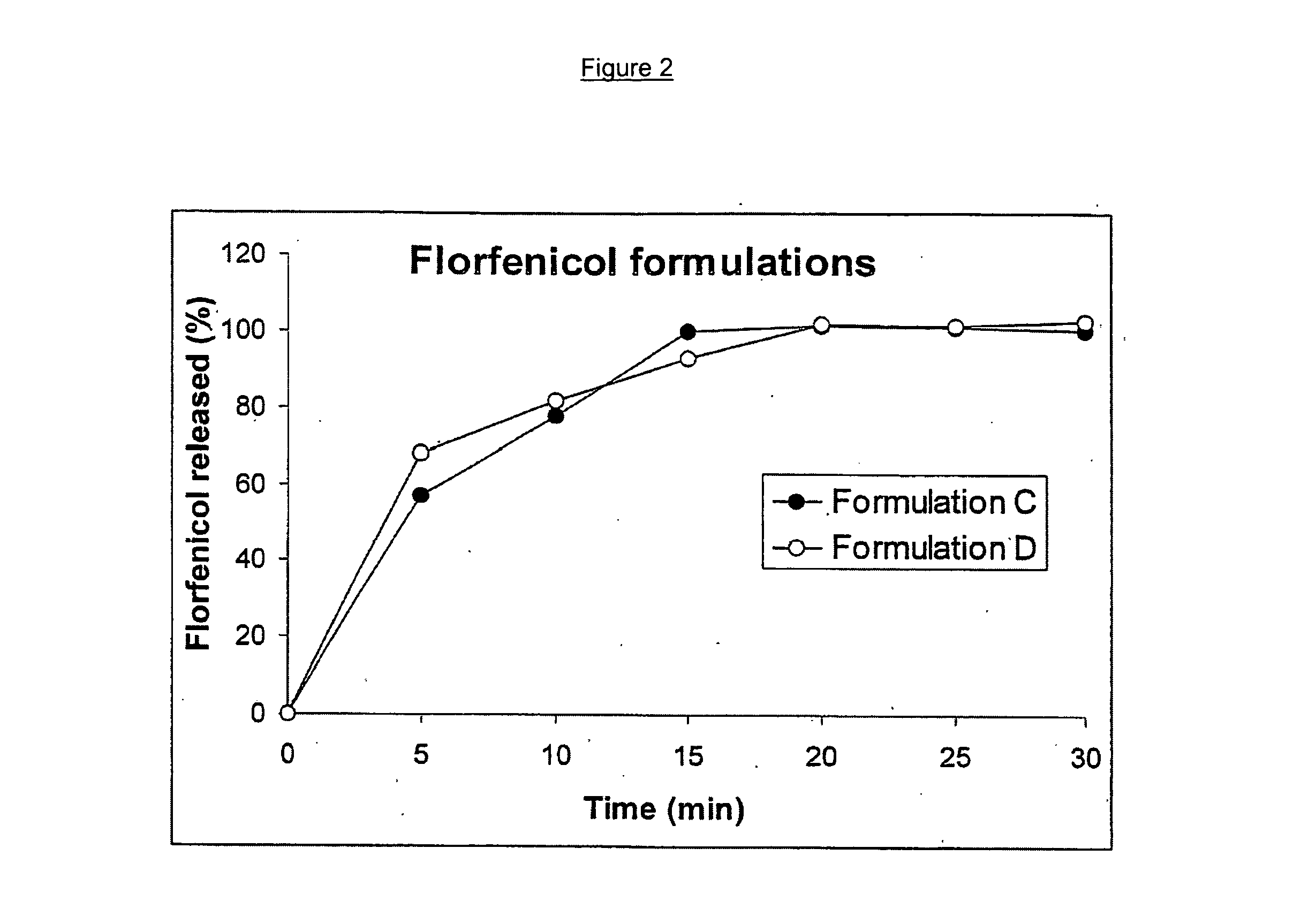
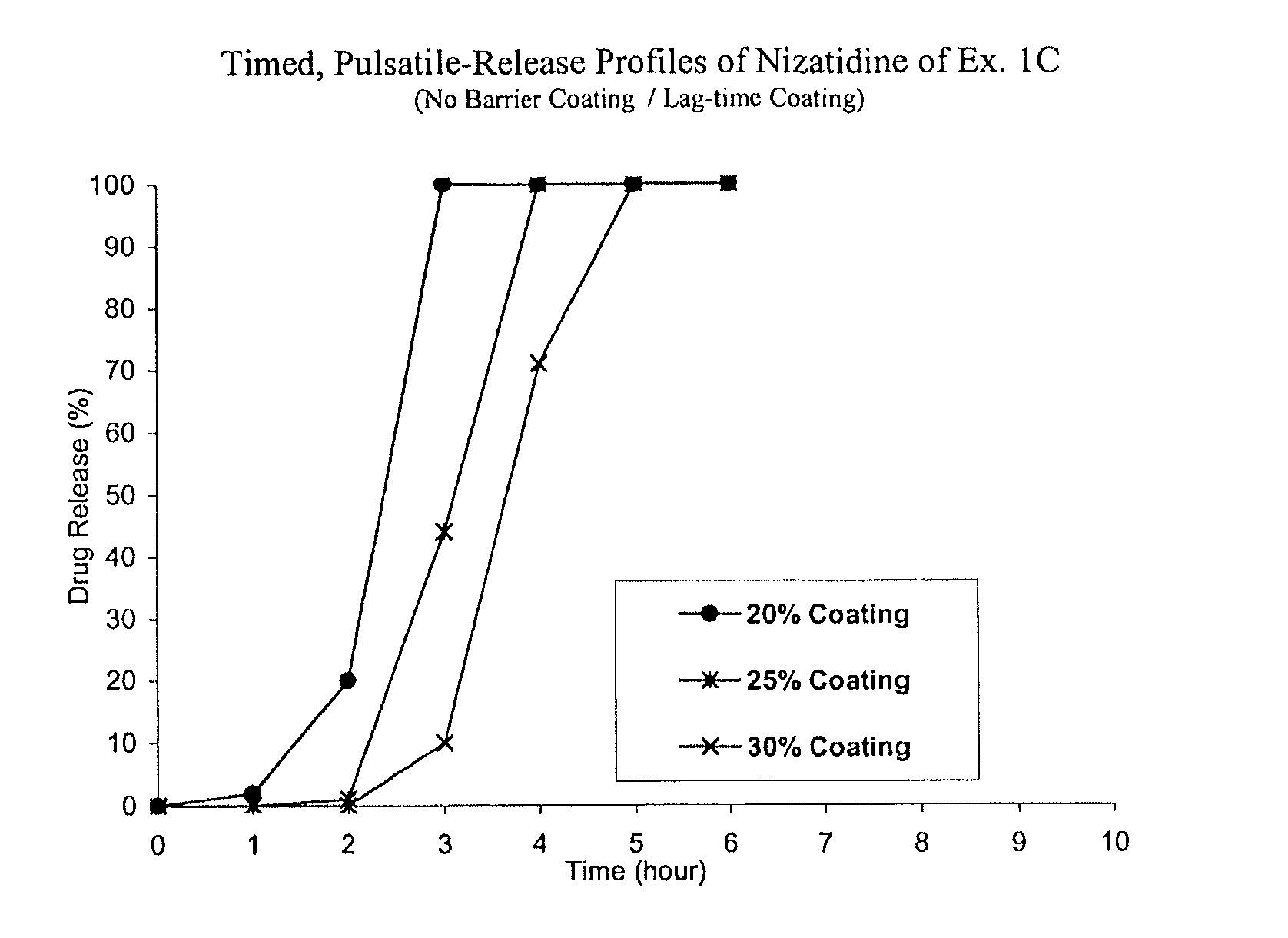
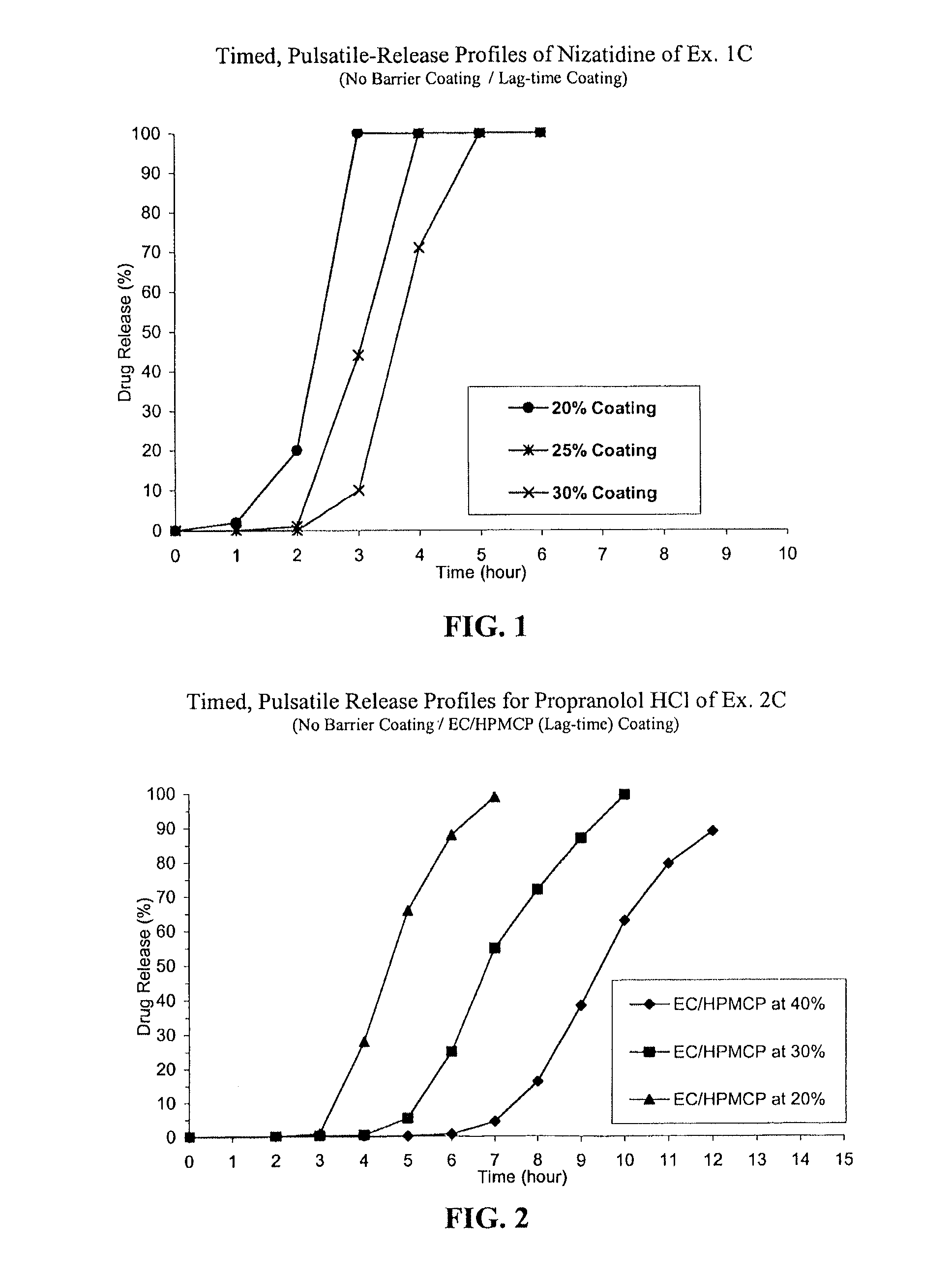
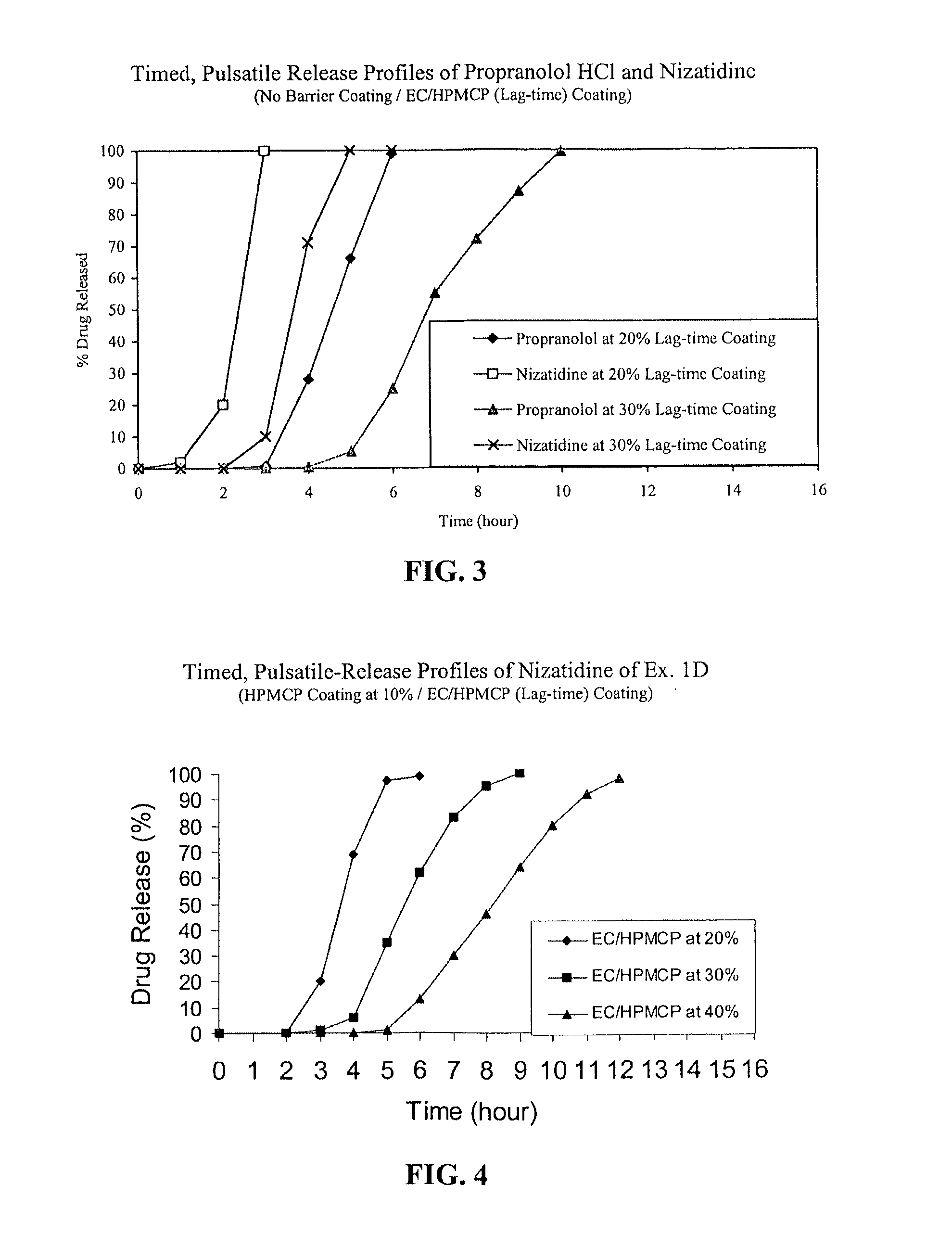
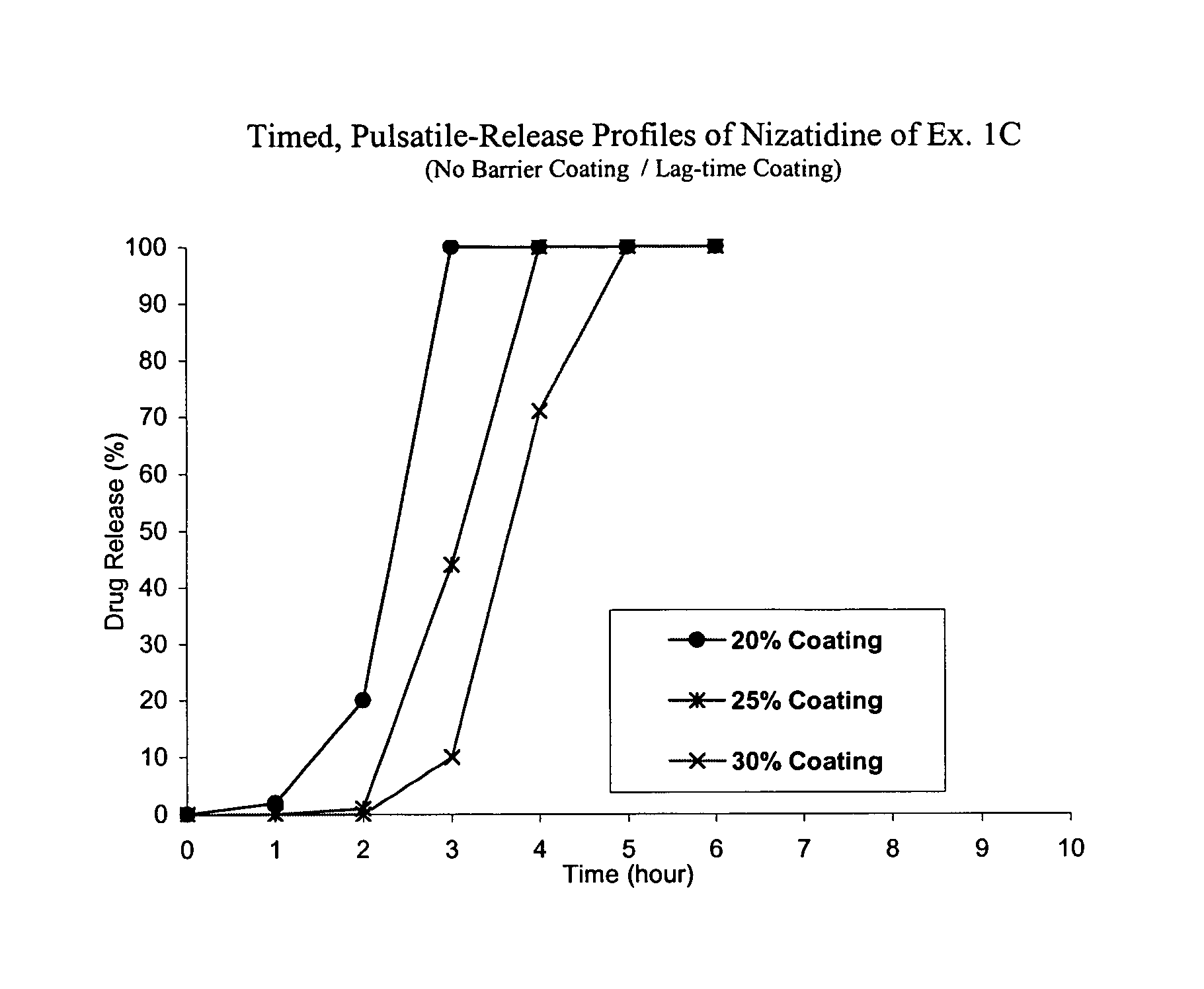
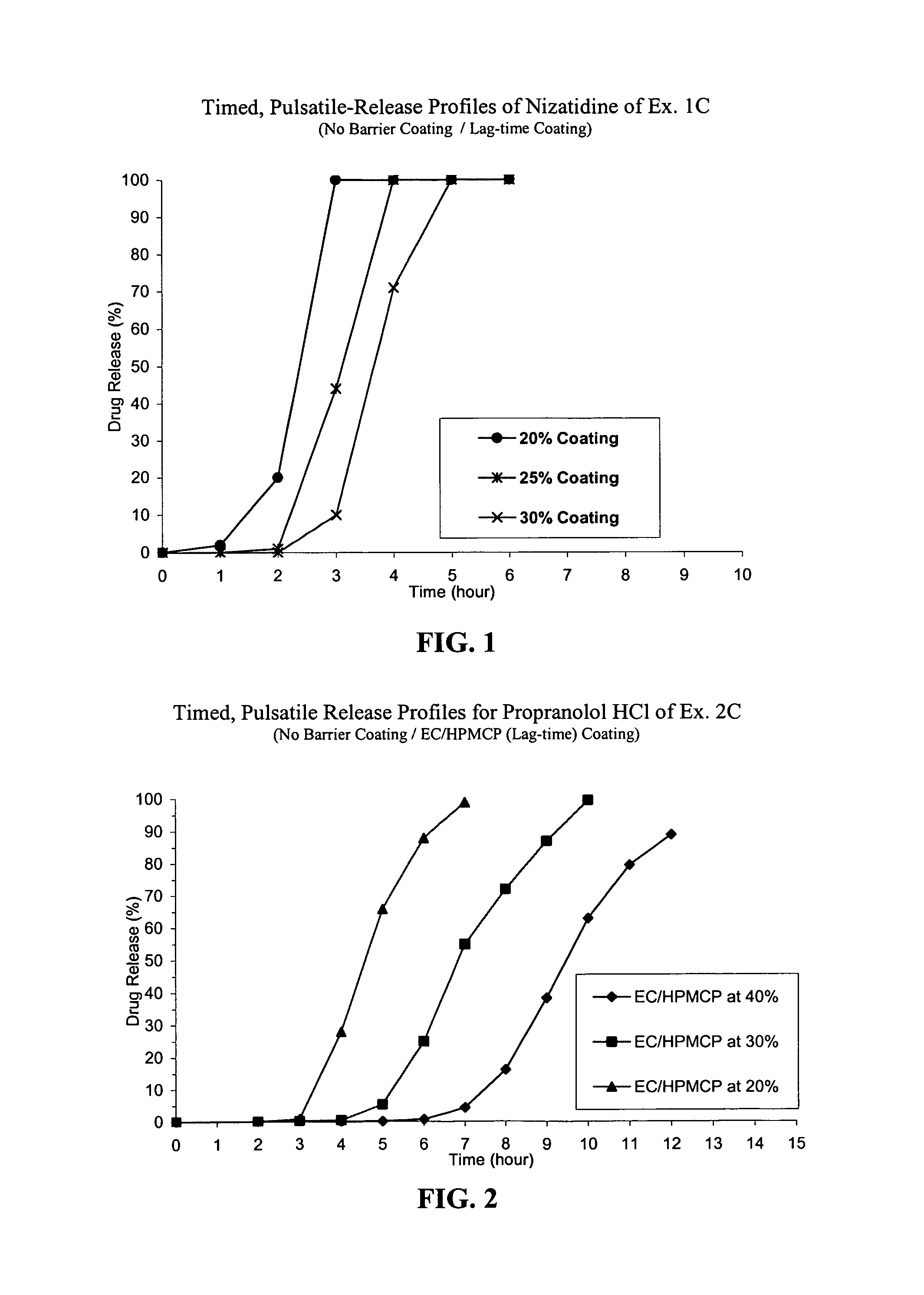
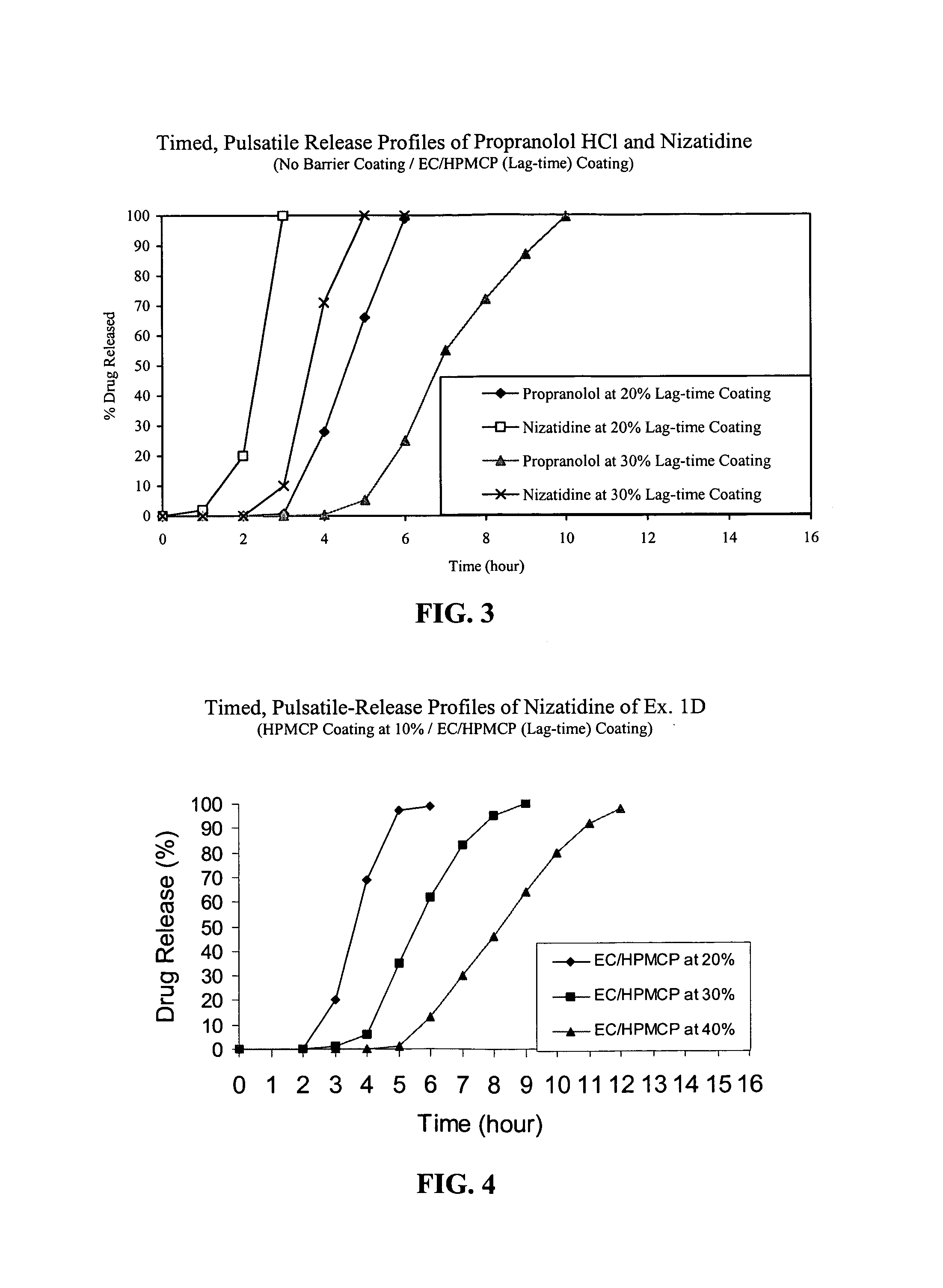
Timed, pulsatile release systems
ActiveUS20060246134A1Enhance safety , therapeutic efficacyOrganic active ingredientsNervous disorderPopulationDosing regimen
A unit multiparticulate dosage form for delivering one or more basic, active pharmaceutical ingredients into the body in need of such medications to achieve target PK (pharmacokinetics) profiles is described. The dosage form comprises one or more multicoated drug particles (beads, pellets, mini- / micro-tablets) having a barrier coating and a lag-time coating. Each Timed Pulsatile Release (TPR) bead population exhibits pre-determined lag-time followed by differing release characteristics. The composition and thickness of the barrier coating, composition and thickness of the lag-time coating, ratio of IR beads to one or more TPR bead populations and total dose may be varied depending on the alkalinity, pH-dependent solubility and elimination half-life of the active ingredients to achieve target PK profiles (suitable for a once or twice daily dosing regimen) in patients in need of such medications.
Owner:ADARE PHARM INC
Dry Powder Aerosolized Inhaler
InactiveUS20090025720A1Increase inhalation rateEffective wayRespiratorsLiquid surface applicatorsSide effectInhalation
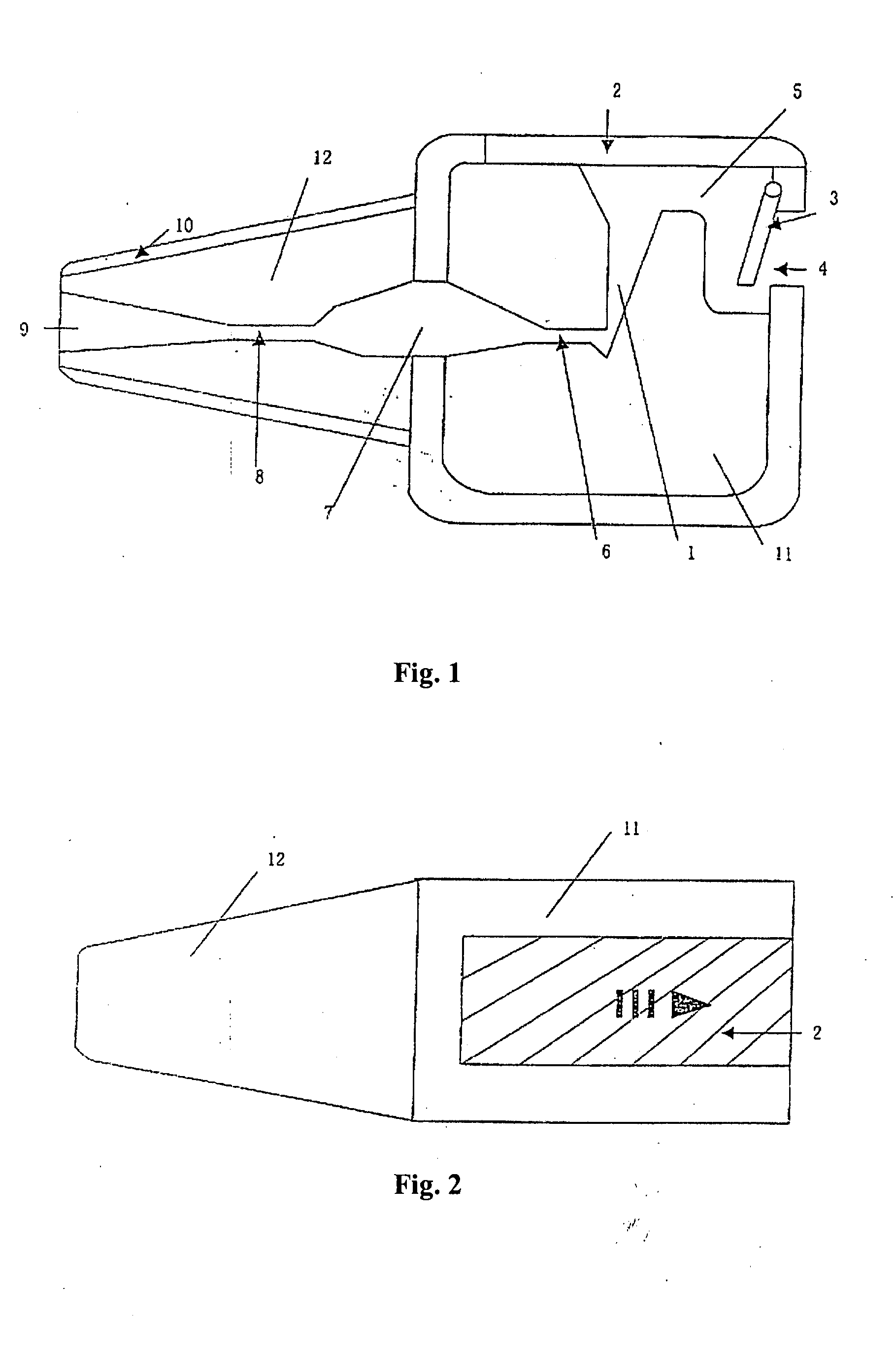
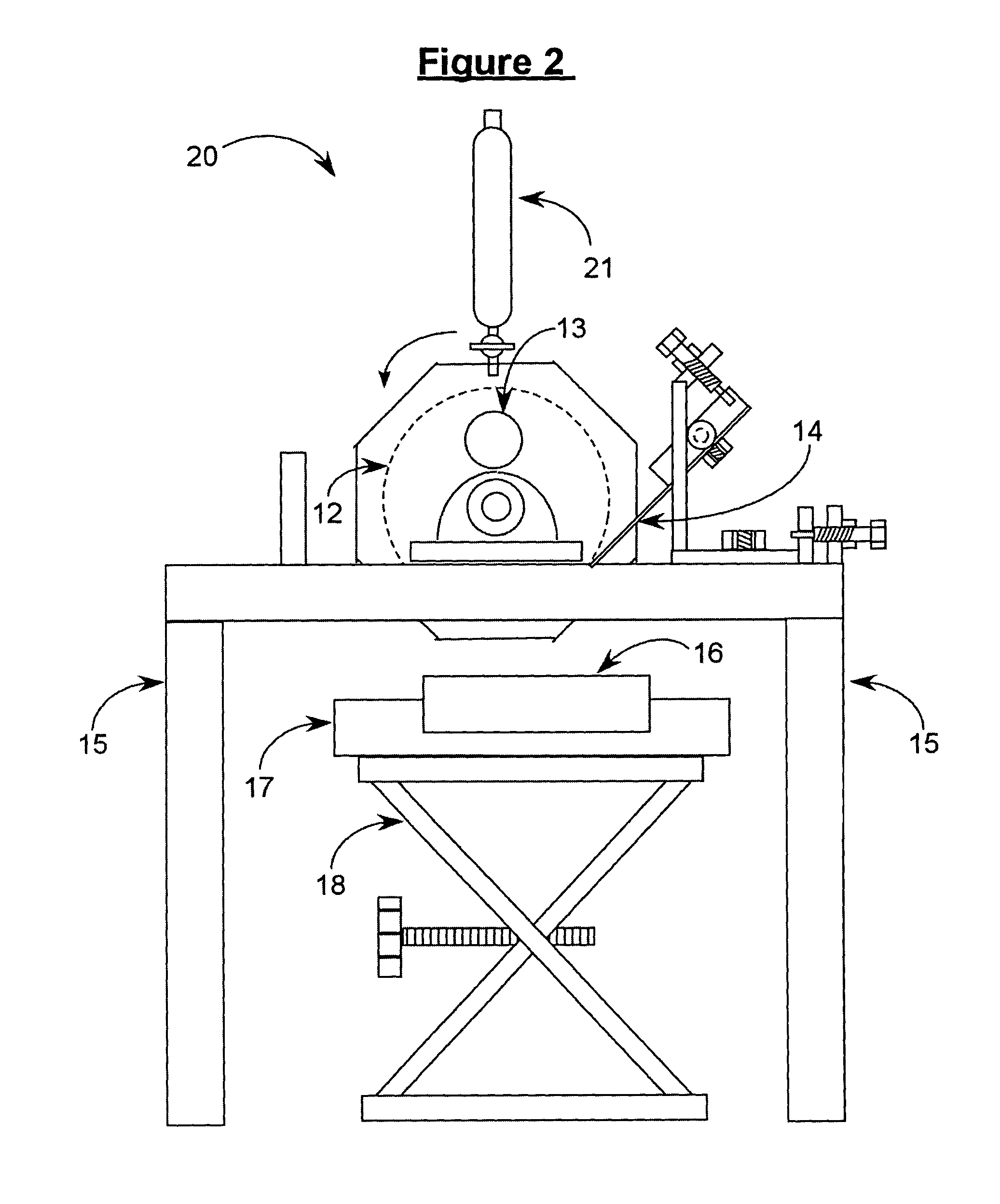
This invention provides an aerosolized inhaler which enables the drug powder to aerosolize within the inhaler by breathing in the air, comprising the main body (11) and the mouthpiece connector (12) connected thereupon, wherein the inhaler the main body (11) has drug holding opening (1), which connects with the vortex passage, and the vortex passage connects with the mouthpiece (9) on the mouthpiece connector (12). It has many obvious advantages as increasing the inhalation rate, pushing more drug granule to reach to the lower respiratory tract and even into the alveolus; and meanwhile, keeping the drug from being blown out of the inhaler when the users breathes out, preventing from cross inflection and minimizing the side effect of the drug to the whole body, and obviously lightening the burden on the users.
Owner:CHEN QINGTANG +1
Delivery of oral drugs
InactiveUS7089934B2Minimization requirementsMinimize movementPowder deliveryLiquid surface applicatorsPharmaceutical drugHuman patient
Disclosed is a system for delivery of a drug comprising a multiple unit dosing device comprising a housing and an actuator, said device containing multiple doses of multiparticulates comprising drug particles, said device upon actuation delivering a unit dose of said multiparticulates, said drug particles having a mean diameter of greater than 10 μm to about 1 mm such that an effective dose of said drug cannot be delivered into the lower lung of a human patient. Also disclosed are novel methods, devices and dosage forms for delivering a drug.
Owner:PHARMAKODEX LTD
Polymer coatings containing drug powder of controlled morphology
A method for depositing a coating comprising a polymer and pharmaceutical agent on a substrate, comprising the following steps: discharging at least one pharmaceutical agent in a therapeutically desirable morphology in dry powder form through a first orifice; discharging at least one polymer in dry powder form through a second orifice; depositing the polymer and / or pharmaceutical particles onto the substrate, wherein an electrical potential is maintained between the substrate and the pharmaceutical and / or polymer particles, thereby forming the coating; and sintering the coating under conditions that do not substantially modify the morphology of the pharmaceutical agent.
PLA/PLGA shell-core microballoons prepared by oil in water-solid in oil method, and preparation method thereof
InactiveCN101461786ASmooth and rounded surfaceDisadvantages of Avoiding PollutionPharmaceutical non-active ingredientsGranular deliveryControlled releaseAcetic acid
The invention relates to a PLA / PLGA shell-core microsphere prepared by solid-in-oil-in-water in the technical field of pharmacy and a preparation method thereof. The microsphere comprises 0.01 to 50 percent of medicine, 20 to 99.99 percent of polylactic acid and / or polylactic acid-glycolic acid, or / and 0 to 30 percent of pharmaceutical excipient (weight percentage). The method comprises the steps of: adding medicine particles into a PLA and / or PLGA organic solution to be emulsified, then selecting a hydrophilic organic solvent to re-emulsify to form unhardened balls, finally hardening in another large oil phase, removing the organic solvent and collecting micropheres. The method overcomes the disadvantages of low envelope rate of the prior W / O and W / O / W, serious burst release of S / O / O, and environmental pollution, controls the grain diameter of the microsphere according to the need, does not pollute the environment, and can be applied to the preparation of slow release or controlled release microspheres of various medicines and adjuvants of vaccines.
Owner:SHANGHAI JIAO TONG UNIV
Timed, pulsatile release systems
A unit multiparticulate dosage form for delivering one or more basic, active pharmaceutical ingredients into the body in need of such medications to achieve target PK (pharmacokinetics) profiles is described. The dosage form comprises one or more multicoated drug particles (beads, pellets, mini- / micro-tablets) having a barrier coating and a lag-time coating. Each Timed Pulsatile Release (TPR) bead population exhibits pre-determined lag-time followed by differing release characteristics. The composition and thickness of the barrier coating, composition and thickness of the lag-time coating, ratio of IR beads to one or more TPR bead populations and total dose may be varied depending on the alkalinity, pH-dependent solubility and elimination half-life of the active ingredients to achieve target PK profiles (suitable for a once or twice daily dosing regimen) in patients in need of such medications.
Owner:ADARE PHARM INC
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
InactiveUS20080199530A1Reduce the possibilityImprove lipophilicityPowder deliveryNervous disorderAdditive ingredientWater insoluble
Owner:COLLEGIUM PHARMA INC
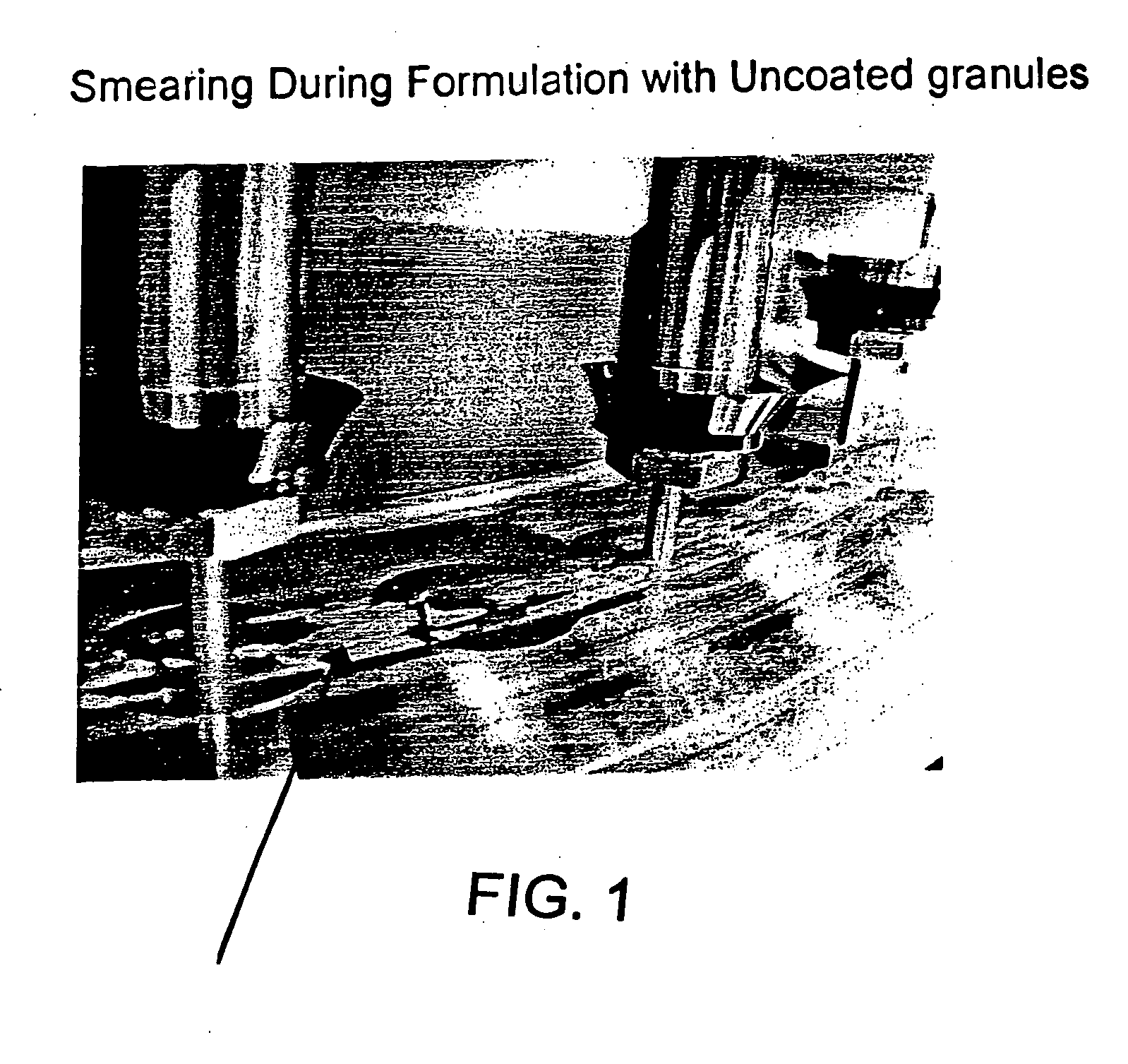
Drug granule coatings that impart smear resistance during mechanical compression
InactiveUS20050175696A1Low dissolution rateExtension of timeBiocideCarbohydrate active ingredientsSolubilityHydrophilic polymers
A drug formulation is disclosed comprising granules having a substrate and a coating, said granule substrate comprising a solubilizing surfactant or a low solubility therapeutic drug, or both, and said granule coating comprising a hydrophilic polymer. Also disclosed is a drug formulation consisting of a tablet core made by mechanical compression, wherein said tablet core comprises granules having a substrate and a coating, said granule substrate comprising a solubilizing surfactant or a low solubility therapeutic drug, or both, and said granule coating comprising a hydrophilic polymer. Also disclosed is a dosage form for oral administration of topiramate, comprising a tablet core and an osmotic delivery system. Methods for controlling topiramate release patterns by altering the composition of the topiramate dosage form are also disclosed.
Owner:ALZA CORP
Immediate release pharmaceutical granule compositions and a continuous process for making them
An immediate or fast release pharmaceutical granule composition comprising at least one drug (i) classifiable as Class II or Class IV of the Biopharmaceutical Classification System, wherein the said drug constitutes at least about 0.5% by weight and no more than 50% by weight of the composition, the said composition further comprising (ii) a first excipient being a dextrin-containing compound and a second excipient (iii) being selected from the group consisting of polyethylene glycols and polypropylene glycols having weight number molecular weights between about 300 and 10,000, glycerol, propylene glycol and glycerides.
Owner:UNIV GENT
Timed, pulsatile release systems
A unit multiparticulate dosage form for delivering one or more basic, active pharmaceutical ingredients into the body in need of such medications to achieve target PK (pharmacokinetics) profiles is described. The dosage form comprises one or more multicoated drug particles (beads, pellets, mini- / micro-tablets) having a barrier coating and a lag-time coating. Each Timed Pulsatile Release (TPR) bead population exhibits pre-determined lag-time followed by differing release characteristics. The composition and thickness of the barrier coating, composition and thickness of the lag-time coating, ratio of IR beads to one or more TPR bead populations and total dose may be varied depending on the alkalinity, pH-dependent solubility and elimination half-life of the active ingredients to achieve target PK profiles (suitable for a once or twice daily dosing regimen) in patients in need of such medications.
Owner:ADARE PHARM INC
Timed, pulsatile release systems
A unit multiparticulate dosage form for delivering one or more basic, active pharmaceutical ingredients into the body in need of such medications to achieve target PK (pharmacokinetics) profiles is described. The dosage form comprises one or more multicoated drug particles (beads, pellets, mini- / micro-tablets) having a barrier coating and a lag-time coating. Each Timed Pulsatile Release (TPR) bead population exhibits pre-determined lag-time followed by differing release characteristics. The composition and thickness of the barrier coating, composition and thickness of the lag-time coating, ratio of IR beads to one or more TPR bead populations and total dose may be varied depending on the alkalinity, pH-dependent solubility and elimination half-life of the active ingredients to achieve target PK profiles (suitable for a once or twice daily dosing regimen) in patients in need of such medications.
Owner:ADARE PHARM INC
Nanometer medicine particle, preparation method and application thereof
ActiveCN103099784AEasy to prepareEasy to operatePowder deliveryPharmaceutical non-active ingredientsLipid formationHydrophobic polymer
The invention discloses a nanometer medicine particle. The nanometer medicine particle comprises a hydrophobic polymer, a single-layer lipid molecule, an amphipathy macromolecular compound, at least one chemotherapy drug attached to the hydrophobic polymer, and at least one near-infrared photothermal conversion agent, wherein the hydrophobic polymer, the at least one chemotherapy drug attached to the hydrophobic polymer, and the at least one near-infrared photothermal conversion agent form a hydrophobic core; the single-layer lipid molecule surrounds the surface of the hydrophobic core to form an intermediate layer; and the amphipathy macromolecular compound intersperses in the intermediate layer to form a shell. The invention also provides a preparation method of the nanometer medicine particle. The nanometer medicine particle can realize the combined treatment of thermotherapy and chemotherapy and has high biocompatibility; and the preparation method is simple, convenient and easy to carry out.
Owner:珠海中科先进技术研究院有限公司
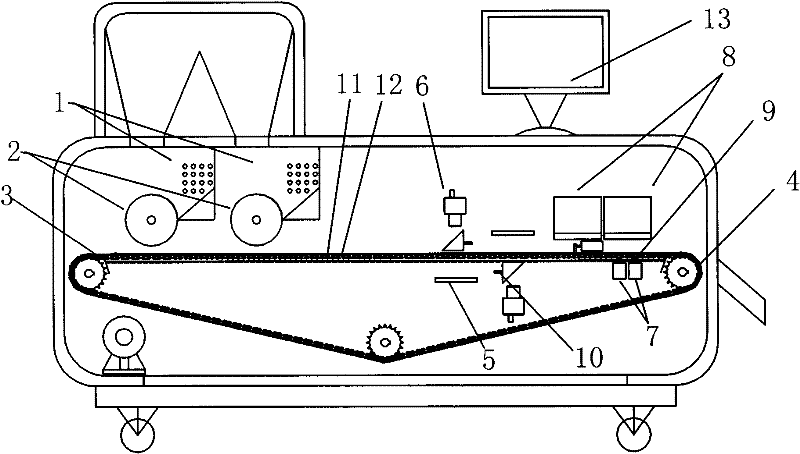
Intelligent vision capsule identification device
InactiveCN102218407ARealize monitoringImprove imaging effectOptically investigating flaws/contaminationSortingIdentification deviceVisual perception
The invention discloses an intelligent mechanical device, and specifically refers to a device which is used in fields like health-care medicines, medicines and packaging for detecting, identifying, determining and sorting hollow transparent capsule, hollow nontransparent capsule and capsule filled with drug granules. The device comprises a collating unit, a transferring unit, an identifying unit, a separating unit and an intelligent controlling unit. L-type grooves in the collating unit enable capsule to be transferred from an L-type groove at one side to another L-type groove at the other side; on the surface of a circular wheel is provided with an arc-shaped protection screen; capsule in the transferring unit can freely rotate in capsule grooves through the fraction between the transferring unit and a supporting band; the main light source of the identifying unit employs parallel light and the backlight of the identifying unit employs flat source; and the separating unit is provided with a plurality of magnet plungers respectively corresponding to capsule grooves on a linking sheet. The invention has the advantages of high efficiency in separating, improved correct rate of separating, substantial reduction of labor intensity and high automation. The intelligent mechanical device provided in the invention has a wide range of usages in the industries of pharmacy and foodstuff.
Owner:HANGZHOU XUMEI INTELLIGENT TECH
Use of proton sequestering agents in drug formulations
InactiveUS20050013867A1Extended shelf lifeImprove drug stabilityPowder deliveryOrganic active ingredientsCombinatorial chemistrySpray dried
Methods are provided for preparing spray-dried, drug-containing particles comprising the steps of: (a) selecting drug, an aqueous solution, and a proton-sequestering agent; (b) adding the drug and the proton-sequestering agent to the solution to form a feed solution; and (c) spray drying the feed solution to form the spray-dried, drug-containing particles, wherein at least a portion of the proton-sequestering agent remains mixed with the drug in the spray-dried, drug containing particles, particles and pharmaceutical formulations comprising the prepared particles as well as methods of use are also provided.
Owner:NOVARTIS FARMA
Method of manufacturing drug granules, the drug granules and pharmaceutical preparation containing the drug granules
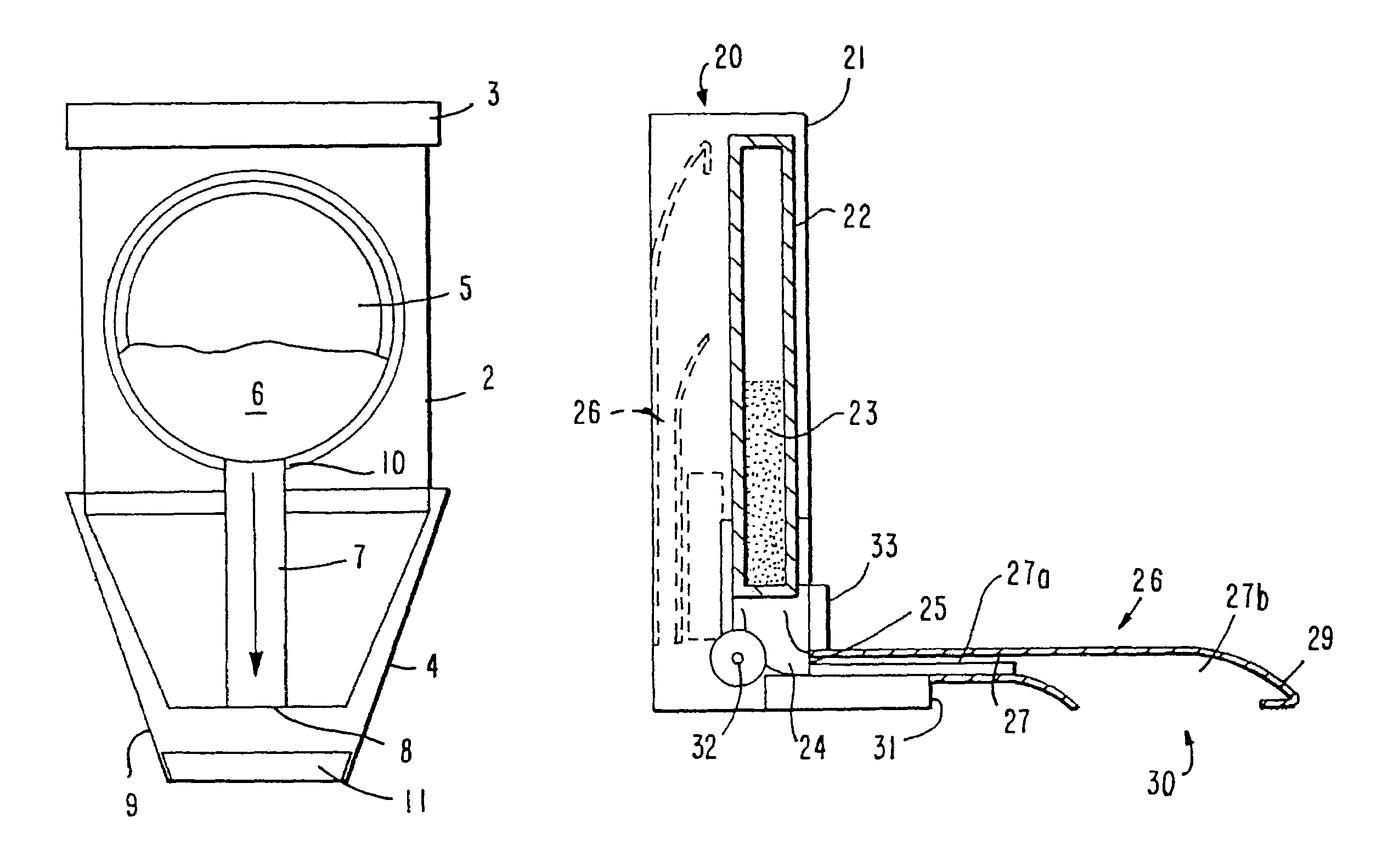
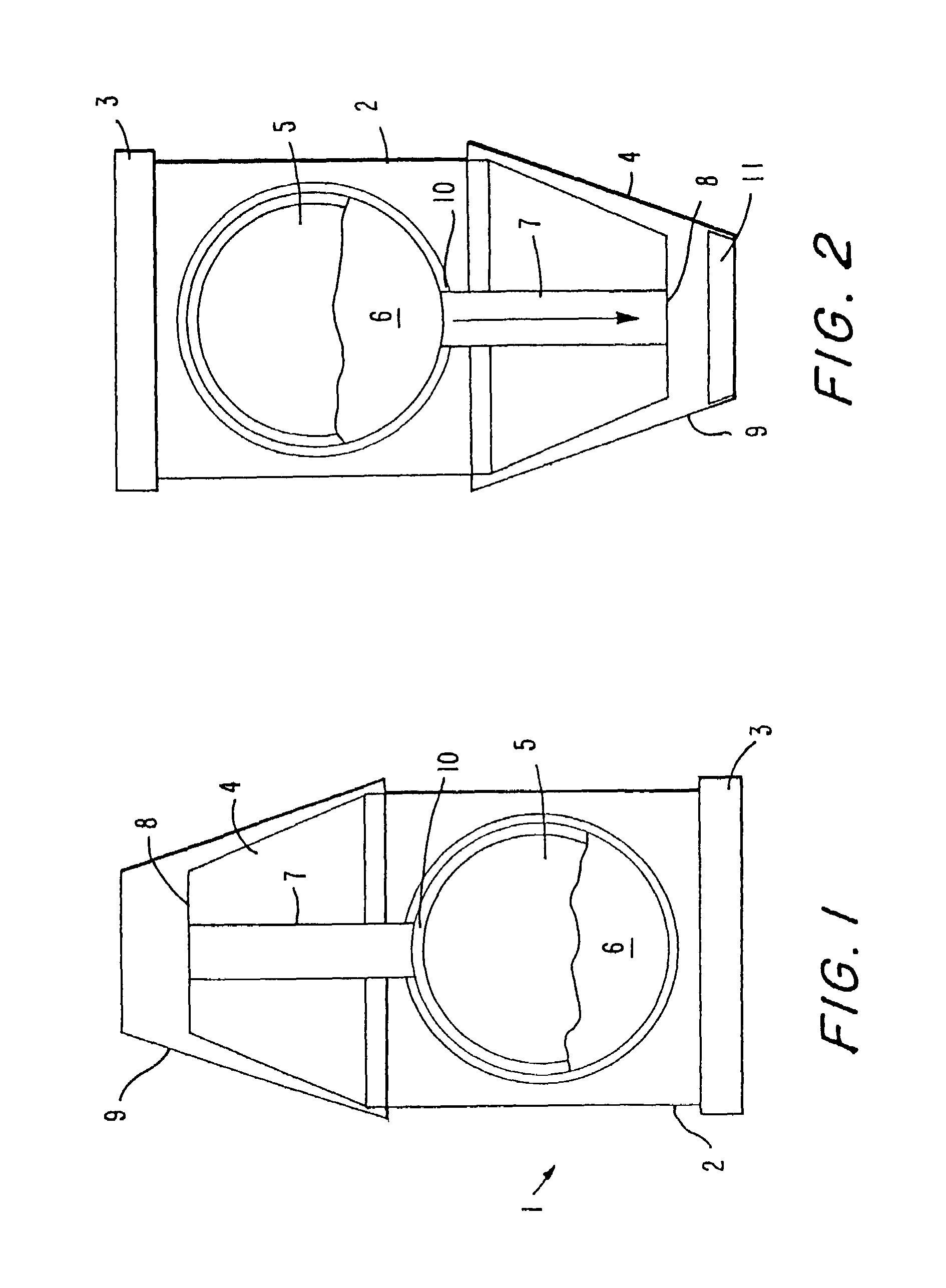
InactiveUS7192608B2High densityImprove stabilityPowder deliveryPill deliveryHigh densityWater soluble
The present invention provides coated granules using drug granules containing a water soluble drug as an active ingredient at a high density, which is superior in uniform content and stability, and which is capable of providing a pharmaceutical preparation superior in drug release control and having a smaller size than conventional preparations, and a production method of the granules, and further, a pharmaceutical preparation using the drug granules.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Micronized freeze-dried particles
InactiveUS7029700B2Prevents coalesenceMinimize timePowder deliveryPeptide/protein ingredientsSolventChemistry
A process is provided for making dry, micronized particles of an agent, such as a drug. The method includes (a) dissolving a macromolecular material, preferably a polymer, in an effective amount of a solvent, to form a solution; (b) dissolving or dispersing the agent in the solution to form a mixture; (c) freezing the mixture; and (d) drying by vacuum the mixture to form solid particles of the agent dispersed in solid macromolecular material. The micronization in this process occurs directly in a macromolecular matrix and hardening of the particles of agent by solvent removal takes place by lyophilization of the bulk matrix, which stabilizes the drug particles during hardening and prevents coalesence, thereby resulting in smaller final drug particles. The method is particularly preferred for protein agents. The process can be used in conjunction with a standard microencapsulation technique, typically following separation of the agent from the macromolecular matrix. The process yields microparticles having a homogenous size distribution, preferably less than 2 μm, and more preferably less than 1 μm, in size. The microparticles have well defined, predictable properties, which is particularly critical in drug delivery applications.
Owner:BROWN UNIV RES FOUND INC
Medicine coating balloon, preparation method and medicine coating balloon dilatation catheter
InactiveCN111298272AAvoid the risk of microembolismStrong penetrating powerBalloon catheterMedical devicesBalloon dilatation catheterPharmacy medicine
The invention discloses a medicine coating balloon. The medicine coating balloon comprises a balloon body and a medicine coating covering the surface of the balloon body, wherein the medicine coatingis formed by spraying a medicine spraying solution to the surface of the balloon body; and the medicine spraying solution is obtained by dissolving medicine, a fat-soluble excipient and a hydrophilicexcipient in a solvent. The invention further discloses a preparation method of the medicine coating balloon and a medicine coating balloon dilatation catheter. According to the medicine coating balloon, the firmness of the medicine coating is higher, the granularity of the medicine is small, the medicine loss can be greatly reduced in the medicine balloon crossing and dilatation process, the tissue absorption rate is high, and the medicine utilization rate is increased.
Owner:KOSSEL MEDTECH SUZHOU
Immediate release pharmaceutical granule compositions and a continuous process for making them
An immediate or fast release pharmaceutical granule composition comprising at least one drug (i) classifiable as Class II or Class IV of the Biopharmaceutical Classification System, wherein the said drug constitutes at least about 0.5% by weight and no more than 50% by weight of the composition, and further comprising (ii) a first excipient being a dextrin-containing compound and a second excipient (iii) selected from the group consisting of polyethylene glycols and polypropylene glycols having weight number molecular weights between about 300 and 10,000, glycerol, propylene glycol and glycerides.
Owner:UNIV GENT
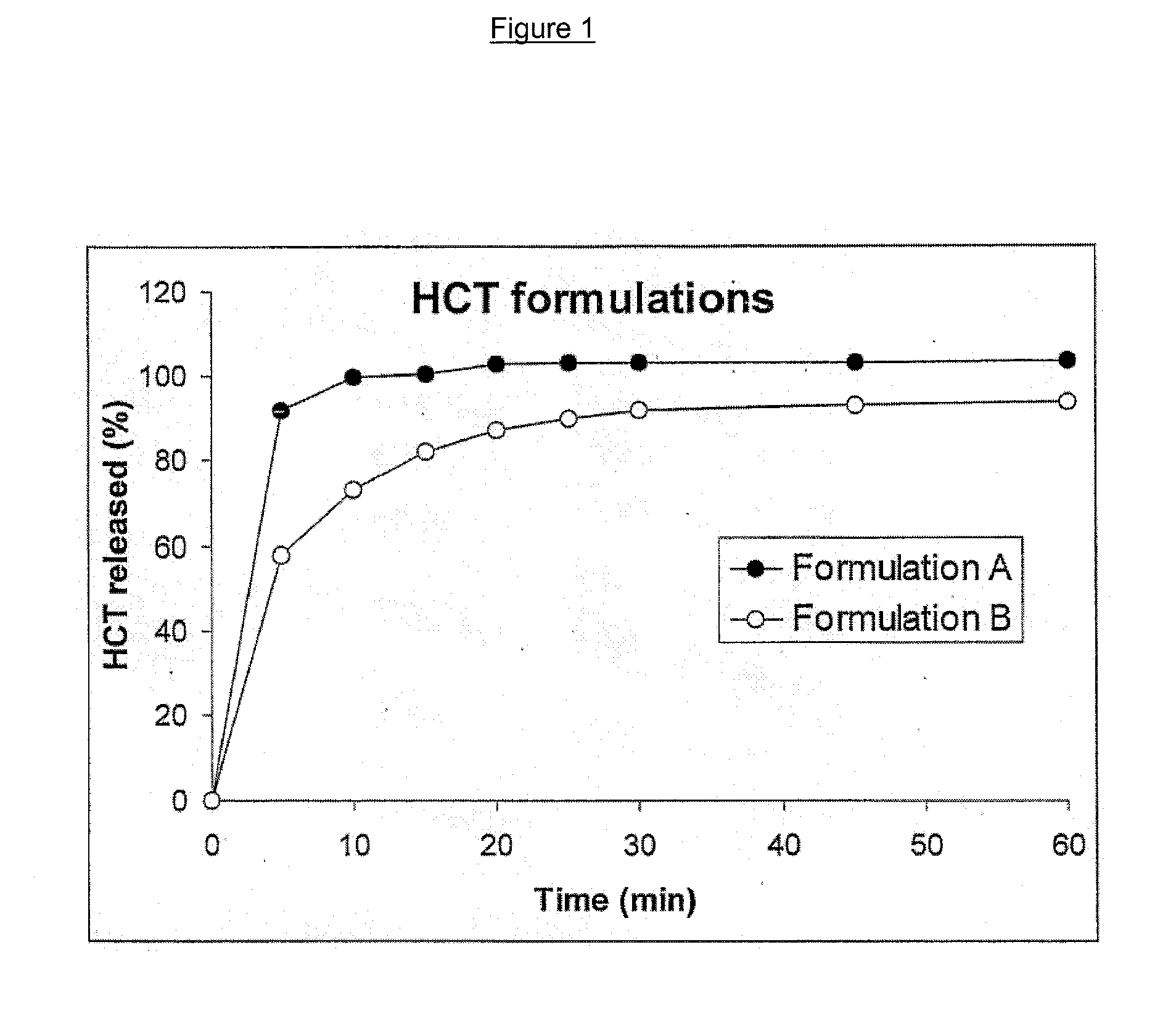
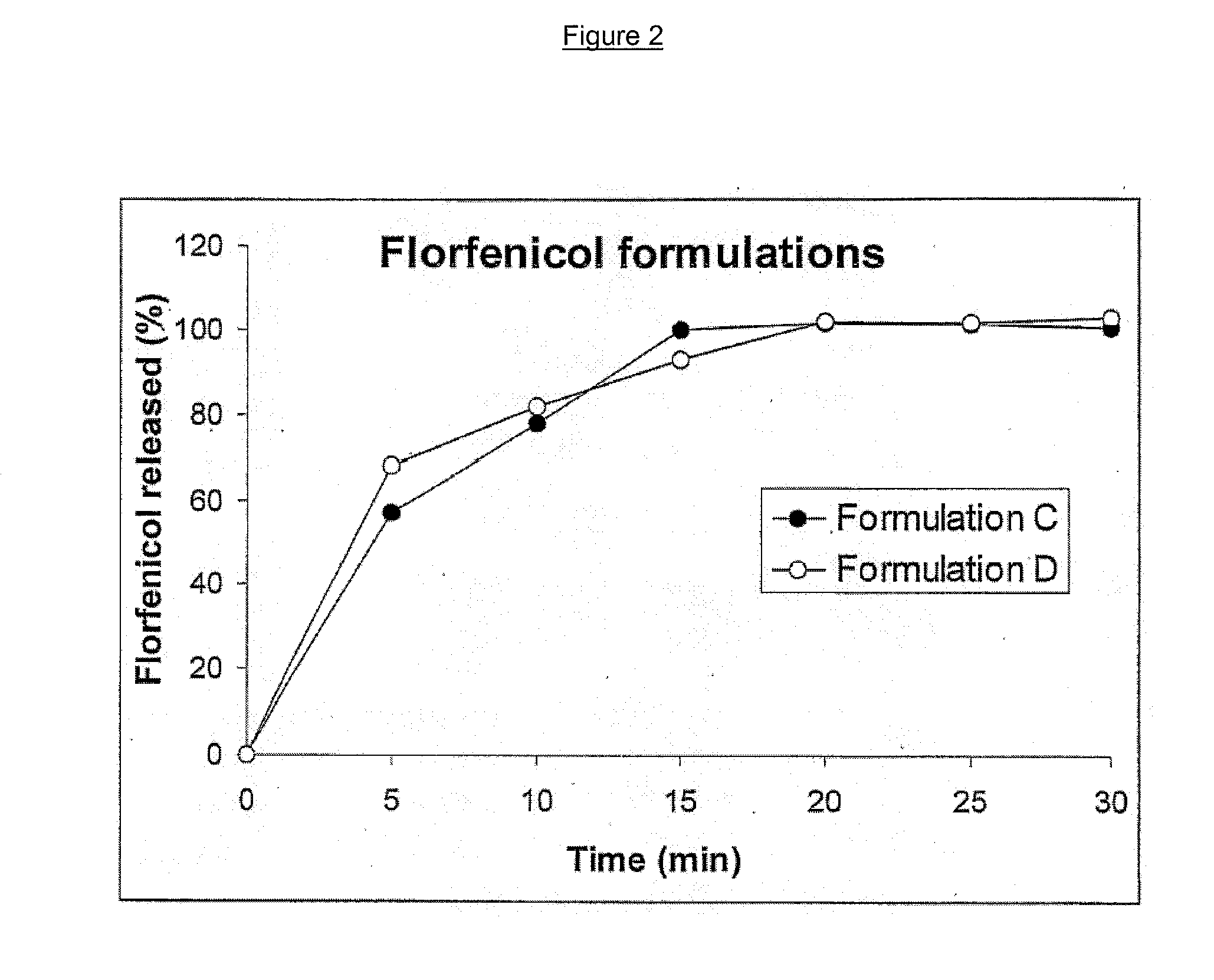
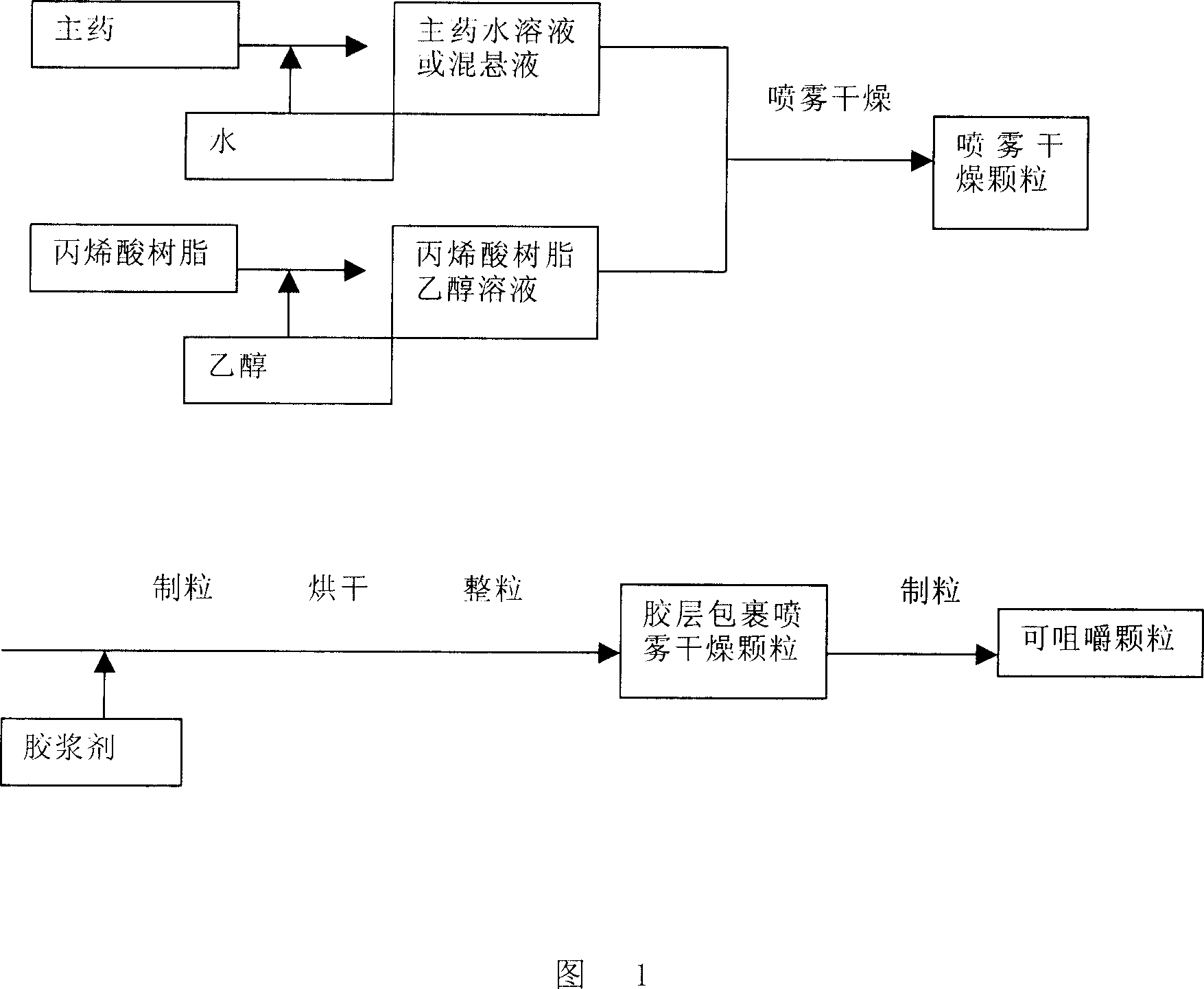
Unfavorable taste-masking drug granule, chewable formulation and preparation process thereof
InactiveCN1994468AStrong stickinessAvoid destructionPharmaceutical non-active ingredientsPill deliveryRotary evaporatorAcrylic resin
The invention relates to a particles used to shade bad taste of drug, wherein it is formed by corn element with active component and continuous polymer dress; the corn element is formed by active component and acrylic resin at 1:0.5-1:20 mass ratio; the dress is soluble gel slurry; and the drug is soluble in acid solution with bad taste. And the production comprises that: dissolving drug and soluble base material into solvent; using atomizing drier or rotation evaporator to obtain dry particles; then graining them at neutral condition via gel material; forming dress that separates drug and taste bud on the surface of particles; preparing oral agent via general method. The invention can avoid releasing bad taste in mouth but release drug in stomach.
Owner:牛祝琴 +2
Pharmaceutical suspensions containing drug particles, devices for their administration, and methods of their use
ActiveUS20170172961A1Convenient treatmentDisperse fastPowder deliveryOrganic active ingredientsDrug suspensionDrug delivery
Owner:SYNAGILE CORP
Surfactant-based antimicrobial solution for inhalation
A surfactant can be added, safely and effectively, to a drug solution containing any antimicrobial agent, such as an antibiotic like tobramycin, that is suitable for administration to the lungs via inhalation. Thus, when an aerosolized drug solution includes surfactant, Marangoni flows cause the drug particles, once deposited in the lungs, to spread over a wider surface area, thereby ensuring greater antimicrobial efficacy. A solution that contains, for example, an antibiotic and tyloxapol or another surfactant providing a similar surface tension to the composition is optimally delivered by the functional combination of a breath-actuated nebulizer and a high-flow compressor.
Owner:UNIVERSITY OF PITTSBURGH
Nano-drug carrier, reduction response nano-drug granules, nano-drug granular preparation and preparation method thereof
The invention relates to a nano-drug carrier, reduction response nano-drug granules, a nano-drug granular preparation and a preparation method thereof. The nano-drug carrier comprises a main chain which is formed by polysaccharide and a side chain which is formed by polyethylenimine on the main chain. Because of the characteristics of the nano-drug carrier, when the nano-drug carrier carries drug, the drug is connected with the nano-drug carrier through a disulfide bond (-SS-), and the disulfide bond is disconnected in a cell in the reduction environment so as to well release the drug to a target location.
Owner:SHENZHEN INST OF ADVANCED TECH
Method for preparing ultrafine particles of water-insoluble or insoluble medicine
ActiveCN102836128AImprove solubilityImprove bioavailabilityPowder deliveryOrganic active ingredientsDrugs solutionWater insoluble
The invention discloses a method for preparing ultrafine particles of a water-insoluble or insoluble medicine. The method mainly comprises the following steps: dissolving the water-insoluble or insoluble medicine into other solvents to prepare a medicine solution; allowing the medicine solution to enter an aqueous solution, into which a stabilizing agent is dissolved, through a dispersing film to form a solution on which ultrafine medicine particles are suspended; and drying the solution on which ultrafine medicine particles are suspended to obtain the product. The invention also discloses the ultrafine medicine particles prepared by the method. By the method, the particle size range of the medicine particles can be controlled precisely. The method has the characteristics of simple process, low cost and environmental friendliness and meets the requirement of industrialized production.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
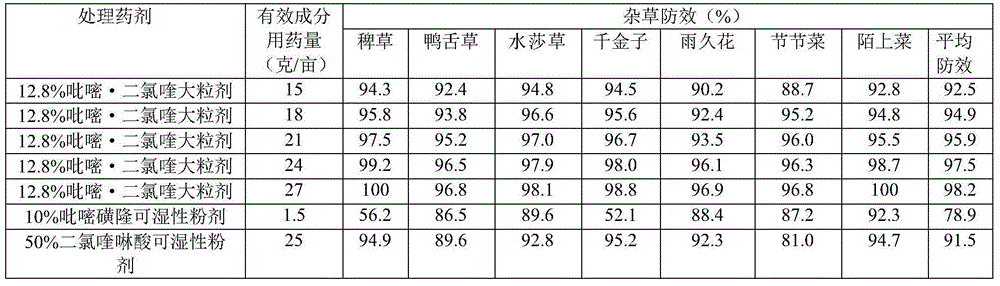
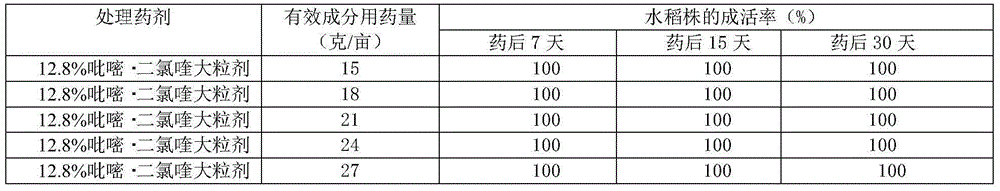
Paddy field weed control macro-granule containing pyrazosulfuron ethyl and quinclorac
InactiveCN104430446AReduce the amount of application per muResidue reductionBiocideAnimal repellantsPhytotoxicityPyrazosulfuron-ethyl
The invention discloses a paddy field weed control macro-granule containing pyrazosulfuron ethyl and quinclorac. The paddy field weed control macro-granule containing pyrazosulfuron ethyl and quinclorac comprises the following components in percentage by weight: 12-14% of quinclorac, 0.5-2.0% of pyrazosulfuron ethyl, 2-4% of silicon dioxide, 9-11% of oil substances, 1.5-5% of surface active agents, 20-30% of solid core materials, 20-30% of additives and the balance of a filler. The paddy field weed control macro-granule containing pyrazosulfuron ethyl and quinclorac uses floating material glass beads, so that the drug granules can float on the water and can freely disperse under the actions of wind power and the auxiliaries; the dispersing agent is added so that the active ingredients of the medicament can be dispersed to the whole paddy field within short time; the pesticide effect is permanent, and the labor and the time are saved. Compared with a quinclorac wettable powder single dosage, the paddy field weed control macro-granule has the advantages that the application amount of quinclorac is reduced so that the residue of the quinclorac in the soil is reduced, and phytotoxicity probably generated to subsequent crops is avoided.
Owner:NANJING GAOZHENG AGROCHEM
Drug particles from freezing onto a surface
ActiveUS9175906B2Improve drug bioavailabilityIncrease ratingsPowder deliveryImpression capsOrganic solventWater soluble drug
The present invention is a method for preparing micron-sized or submicron-sized drug particles comprising contacting a solution comprising a poorly water soluble drug substance and at least one freezable organic solvent with a cold surface so as to freeze the solution; and removing the organic solvent. The resulting particles are also disclosed, as are several embodiments of an apparatus that can be used in performing the method of the present invention.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Process for producing granules containing branched amino acids
The problem of the present invention is to provide pharmaceutical granules containing, as active amino acid ingredients, three kinds of branched chain amino acids of isoleucine, leucine and valine, which has a smaller specific volume than conventional products, and a production method thereof. As a result of intensive studies, granules containing, as active amino acid ingredients, three kinds of branched chain amino acids of isoleucine, leucine and valine and a production method thereof have been provided, which are characterized by adding an acid to a particle mixture of three kinds of branched chain amino acids of isoleucine, leucine and valine and granulating the mixture, which has solved the above-mentioned problem.
Owner:AJINOMOTO CO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com