Tobramycin liposome used for aerosol inhalation and production method thereof
A technology of tobramycin lipid and atomized inhalation, which is applied in liposome delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of low bioavailability, thermal instability, Difficulty crossing cell membranes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] A kind of tobramycin liposome for nebulization inhalation, it comprises the following components according to the total mass percentage of raw materials:
[0089]
[0090]
[0091] Preparation:
[0092] 1) Precisely weigh the protein lecithin, cholesterol and vitamin E in the prescribed amount, dissolve them in absolute ethanol (accounting for 25% of the total amount), dissolve in a water bath at 55°C, and form an organic phase solution;
[0093] 2) Accurately weigh the prescription amount of tobramycin and chitosan dissolved in pH7.0 phosphate buffer (accounting for 75% of the total amount) as the water phase;
[0094] 3) Using a syringe, slowly add the organic phase to the aqueous phase with constant temperature magnetic stirring at 55°C, and stir for 30 minutes;
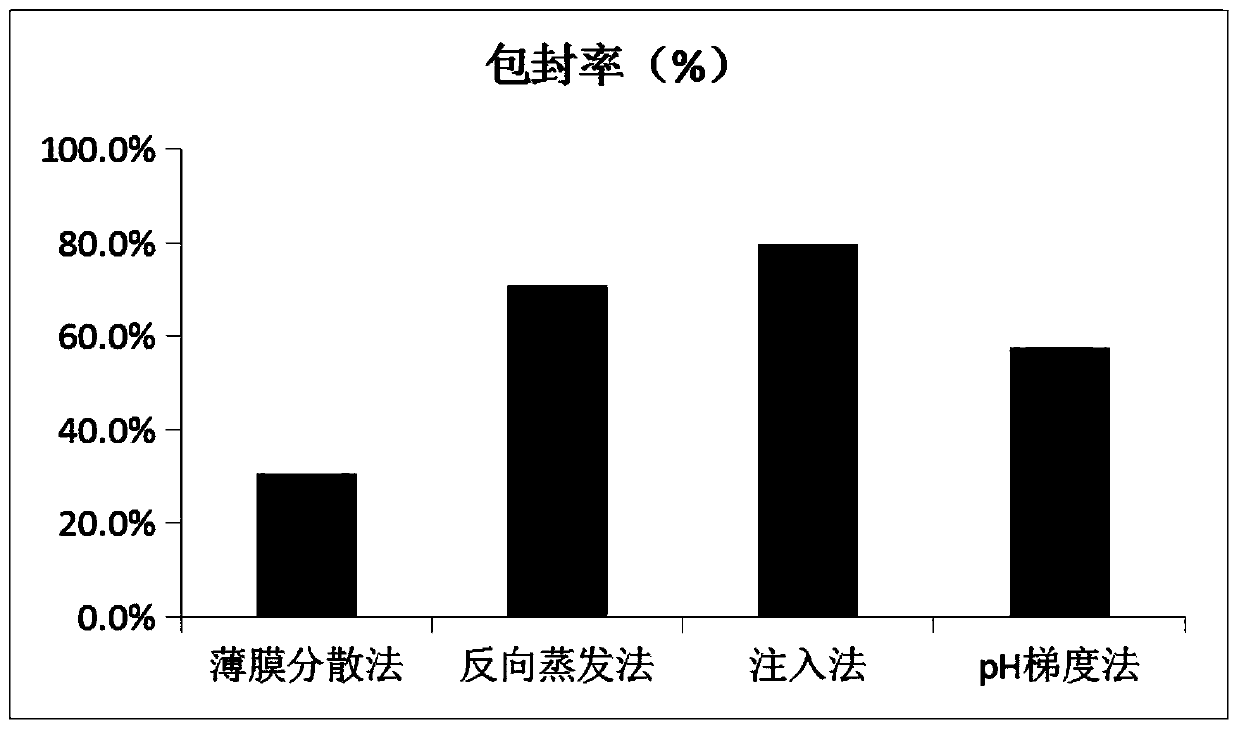
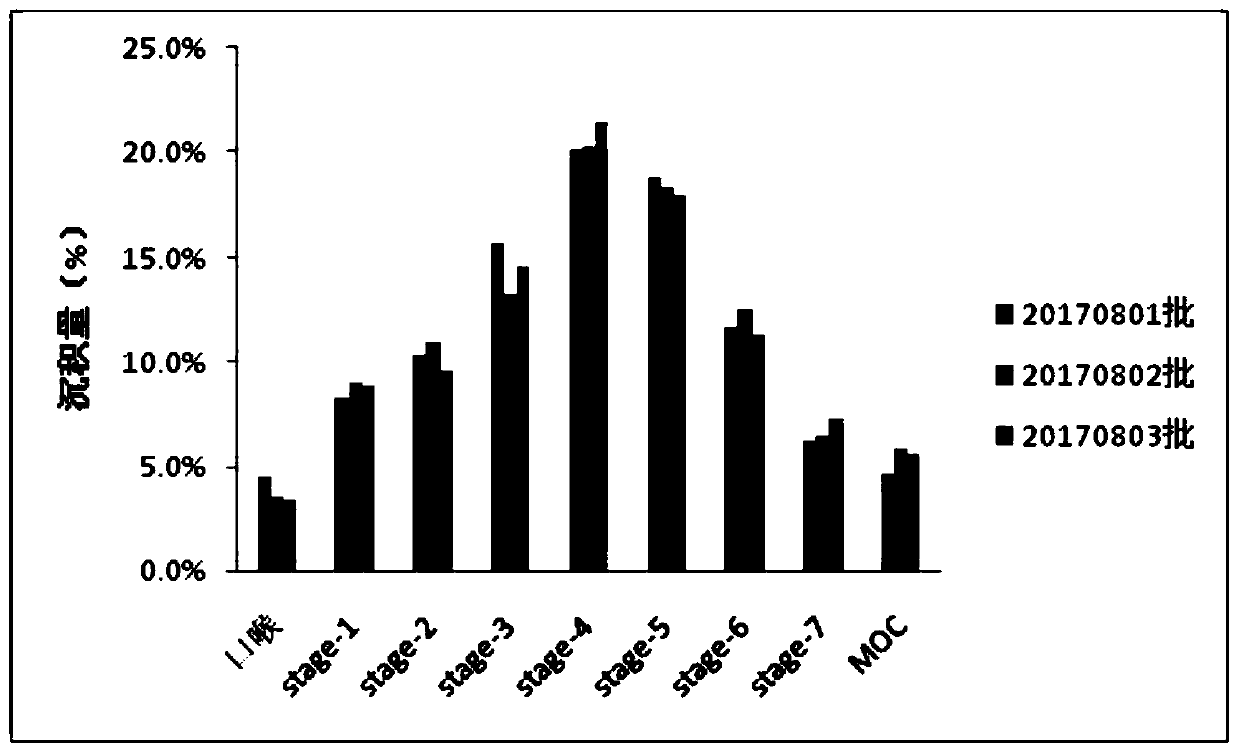
[0095] 4) Evaporate under reduced pressure to remove the organic solvent, and then filter through a 0.45 μm microporous membrane to obtain tobramycin liposomes. Its drawing is attached figure 1 sho...
Embodiment 2
[0097] A kind of tobramycin liposome for nebulization inhalation, it comprises the following components according to the total mass percentage of raw materials:
[0098]
[0099] Preparation:
[0100] 1) Accurately weigh the egg yolk lecithin, cholesterol and vitamin E of the prescribed amount, dissolve them in an appropriate amount of ethanol (25%), dissolve them completely by ultrasonic, and use them as the organic phase.
[0101] 2) Precisely weigh the prescribed amount of tobramycin and dissolve it in pH 7.0 phosphate buffer as the water phase.
[0102] 3) The water phase was injected into the organic phase, and the water bath was sonicated for 4 minutes to prepare a stable emulsion.
[0103] 4) Transfer to a round-bottomed flask again, in a water bath at 50°C for 100r·min -1 , evaporated under reduced pressure to remove the organic solvent to a viscous gummy solution. Then add an appropriate amount of aqueous medium for elution. After the elution is complete, use a ...
Embodiment 3
[0105] A kind of tobramycin liposome for nebulization inhalation, it comprises the following components according to the total mass percentage of raw materials:
[0106]
[0107] Preparation:
[0108] 1) Precisely weigh the egg yolk lecithin, cholesterol and vitamin E in the prescribed amount, place them in a round bottom flask, and add an appropriate amount of ethanol (25%) for ultrasonic dissolution.
[0109] 2) Put it on a rotary evaporator again, put it in a water bath at 50°C for 100 r·min-1, and remove the organic solvent by rotary evaporation under reduced pressure, so that the lipid forms a thin film on the bottle wall.
[0110] 3) Weigh the prescribed amount of tobramycin and dissolve it in phosphate buffer solution of pH 7.0, then add it into the round bottom flask, continue to rotate and hydrate completely, and obtain a milky white suspension, ultrasonicate for 5 minutes with a 100W probe (working 3s , intermittent 3s), after using a 0.45 μm microporous membrane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com