Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
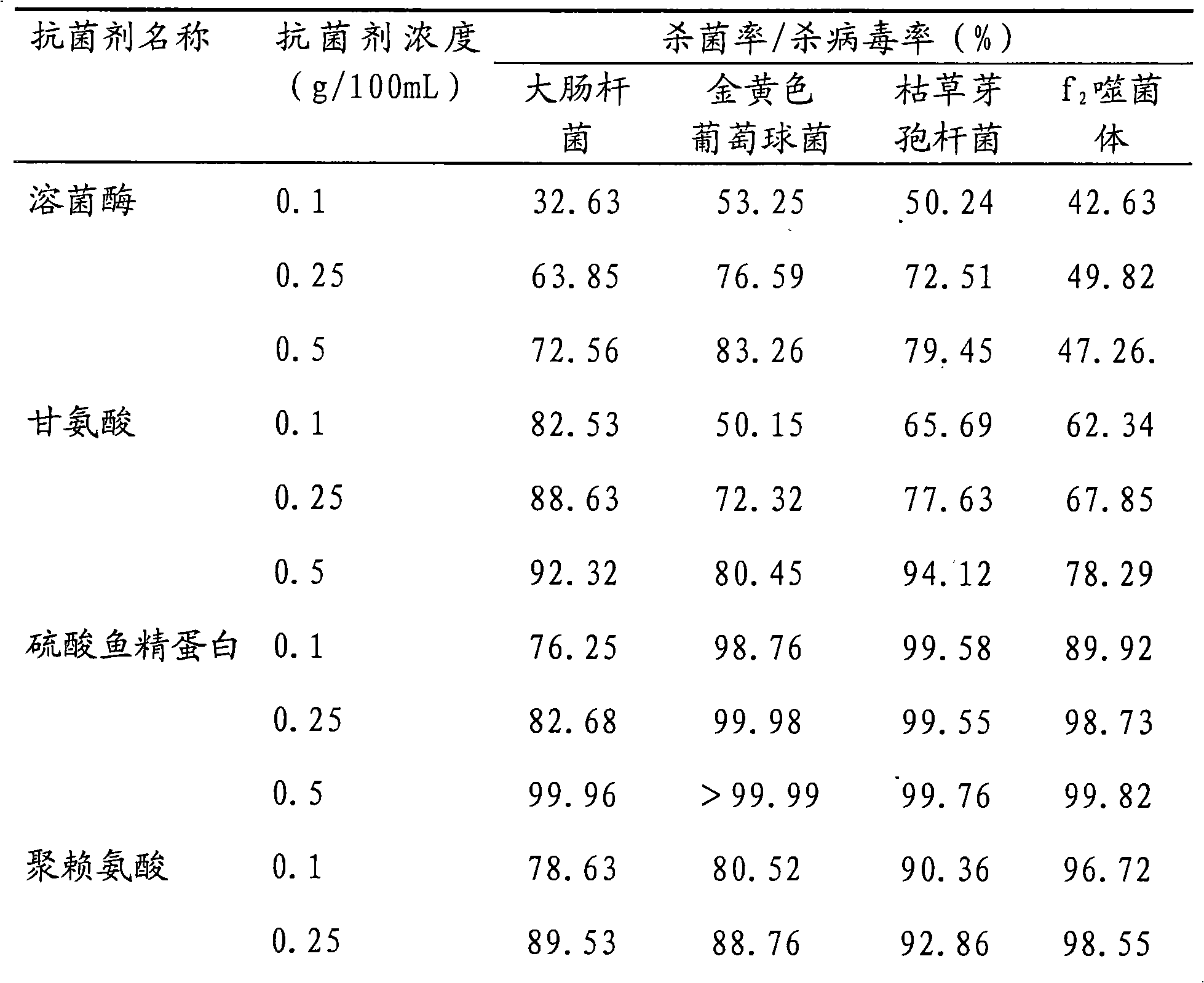
30 results about "Polymyxin E sulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polymyxin B (polymyxin b sulfate) sulfate is a drug of choice in the treatment of infections of the urinary tract, meninges, and bloodstream caused by susceptible strains of Ps. aeruginosa. It may also be used topically and subconjunctivally in the treatment of infections of the eye caused by susceptible strains of Ps. aeruginosa.
Lactating sow feed
InactiveCN103005192APromote milk productionKeep healthyAnimal feeding stuffSodium bicarbonateAnimal science
The invention discloses lactating sow feed which specifically comprises the following components in percentage by weight: 47 to 59 percent of corn, 15 to 21 percent of bean pulp, 1.5 to 2.3 percent of fish meal, 7.2 to 14 percent of bran, 8.5 to 12 percent of wheat middling, 1 to 2 percent of plant oil and 3.5 to 6 percent of a pre-mixed material, wherein the pre-mixed material consists of an additive and a pre-mixing agent; the additive comprises the following components in percentage by weight: 0.2 to 0.3 percent of a trace element, 0.03 to 0.04 percent of various vitamins, 0.05 to 0.1 percent of 10 percent of polymyxin E sulfate, 0.01 to 0.02 percent of complex enzyme, 0.01 to 0.02 percent of an acidifying agent, 0.05 to 0.1 percent of sodium bicarbonate, 0.1 to 0.25 percent of diaminocaproic acid, 0.05 to 0.1 percent of methionine, 0.2 to 0.3 percent of choline, 0.5 to 1.2 percent of codonopsis pilosula and 0.4 to 1.2 percent of astragalus mongholicus; and the pre-mixing agent comprises the following components in percentage by weight: 0.8 to 1.2 percent of mountain flour, 0.75 to 1 percent of calcium hydrophosphate and 0.25 to 0.35 percent of edible salt. The codonopsis pilosula and the astragalus mongholicus are added in the lactating sow feed to serve as traditional Chinese medicine additives, so that the lactating sow feed has the functions of supplementing qi, activating the blood, promoting the lactation and the like; the galactopoiesis of a lactating sow can be promoted; the feed utilization rate is improved; side effects are avoided; and the health of the lactating sow is guaranteed.
Owner:HAINING ZHENONG FEED
Method for separating and purifying method of polymyxin E methanesulfonic sodium
ActiveCN101525377ASimple processGood reproducibilityPeptidesAntiinfectivesSeparation technologyUltrafiltration
The invention relates to a method for separating and purifying polymyxin E methanesulfonic sodium from a mixture containing small-molecular weight impurities. The method adopts an ultrafiltration membrane with the cutoff molecular weight ranging from 1,000 to 10,000 to carry out separation and purification. The invention also relates to a method used for preparing high-purity E methanesulfonic sodium by controlling reaction conditions and making use of ultrafiltration membrane separation technology. The method comprises the following steps: (a) adding water to dissolve polymyxin E sulfate andthen adding formaldehyde solution in the solution to carry out reaction; (b) adding sodium bisulfite solution in the reaction products of step (a) to carry out reaction continuously; and (c) taking the reaction solution of step (b) and adopting the ultrafiltration membrane to carry out separation and purification. The method of separating and purifying the polymyxin E methanesulfonic sodium has the advantages of low pollution, low energy consumption, simple process, good repeatability and high purity and activity of obtained products.
Owner:SHANGHAI INST OF PHARMA IND +1
Method of treatment of otitis externa
This invention relates to a method of treating otitis externa using a topical combination medication, including one or more antifungal agent such as, for example fluconazole, voriconazole, itraconazole, clotrimazole, amphotericin B, caspofungin, micafungin, terbinafine, naftifine, butenafine, amorolfine, ravuconazole, posaconazole, flucytosine, econazole, enilaconazole, miconazole, oxiconazole, saperconazole, sulconazole, terconazole, tioconazole, nikkomycin Z, anidulafungin (LY303366), nystatin, pimaricin, griseofulvin, ciclopirox, haloprogin, tolnaftate, and undecylenate and one or more antibacterial agent such as neomycin sulfate, polymyxin B sulfate, colistin sulfate, gentamycin, tobramycin, chloramphenicol, Ciprofloxacin, Ofloxacin, a penicillin compound, a cephalosporin compound, a macrolide compound, a fluoroquinolone compound, streptomycin, or kanamycin.
Owner:FAIRFIELD CLINICAL TRIALS
Broad spectrum biological disinfection air filtering material and preparation method thereof
InactiveCN101293107AStable in natureAvoid pollutionDeodrantsFiltration separationAir filtrationProtamine sulfate
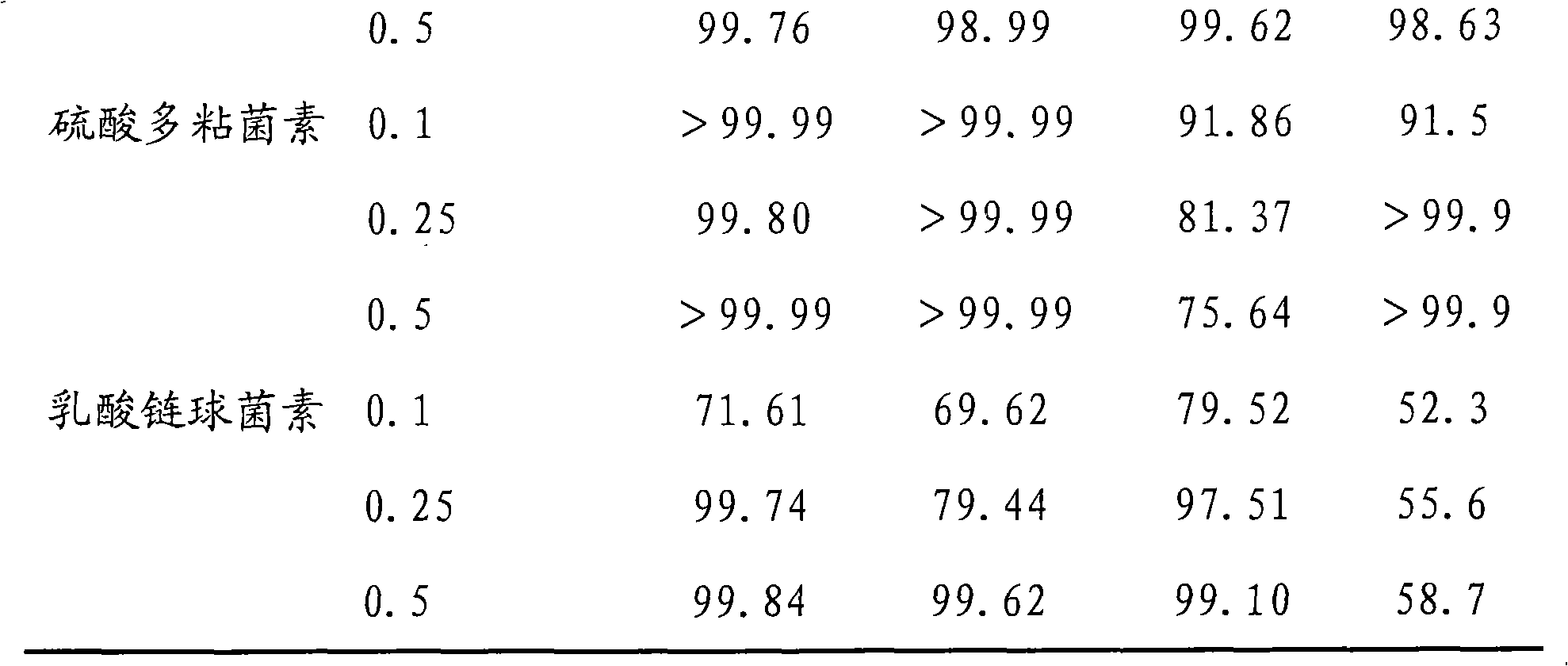
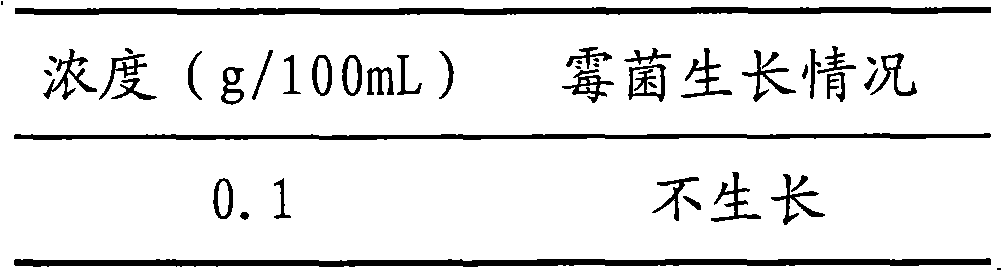
The invention discloses a broad-spectrum biological bactericidal air filtration material and a preparation method thereof. The invention is not only applicable to the general families and business departments with the need of air purification, but is also applicable to the semiconductor, pharmaceutical, hospitals and other environments with higher requirements of cleanliness. The broad-spectrum biological bactericidal air filtration material of the invention comprises at least one biological anti-bacterial agent and glass fiber filter paper, and the biological anti-bacterial agent is lysozyme, protamine sulfate, poly-lysine, nisin, polymyxin sulfate, glycine or natamycin. The preparation method of the invention is that the air filtration material is prepared by silanization, hydroformylation, condensation reaction and removal of the physically absorbed anti-bacterial agent. The biological bactericidal air filtration material prepared by the invention has low consumption of the anti-bacterial agent and no influence on the resistance of the filtration material; the sterilization rate is high, most of the killing rate of bacterial, spores and viruses is more than 99 percent, and the fungal growth is inhibited.
Owner:SANITARY EQUIP INST ACAD OF MILITARY MEDICAL SCI PLA
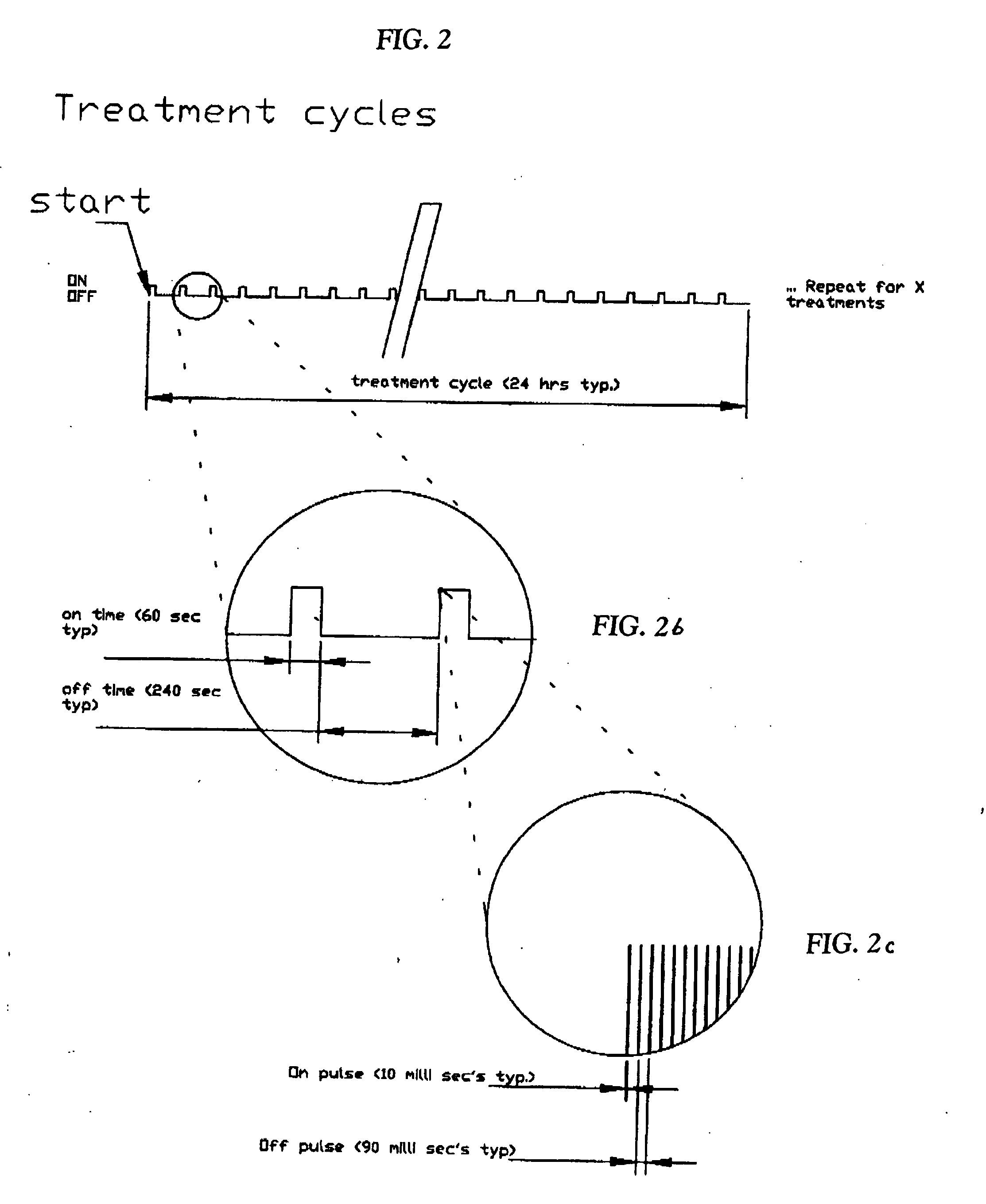
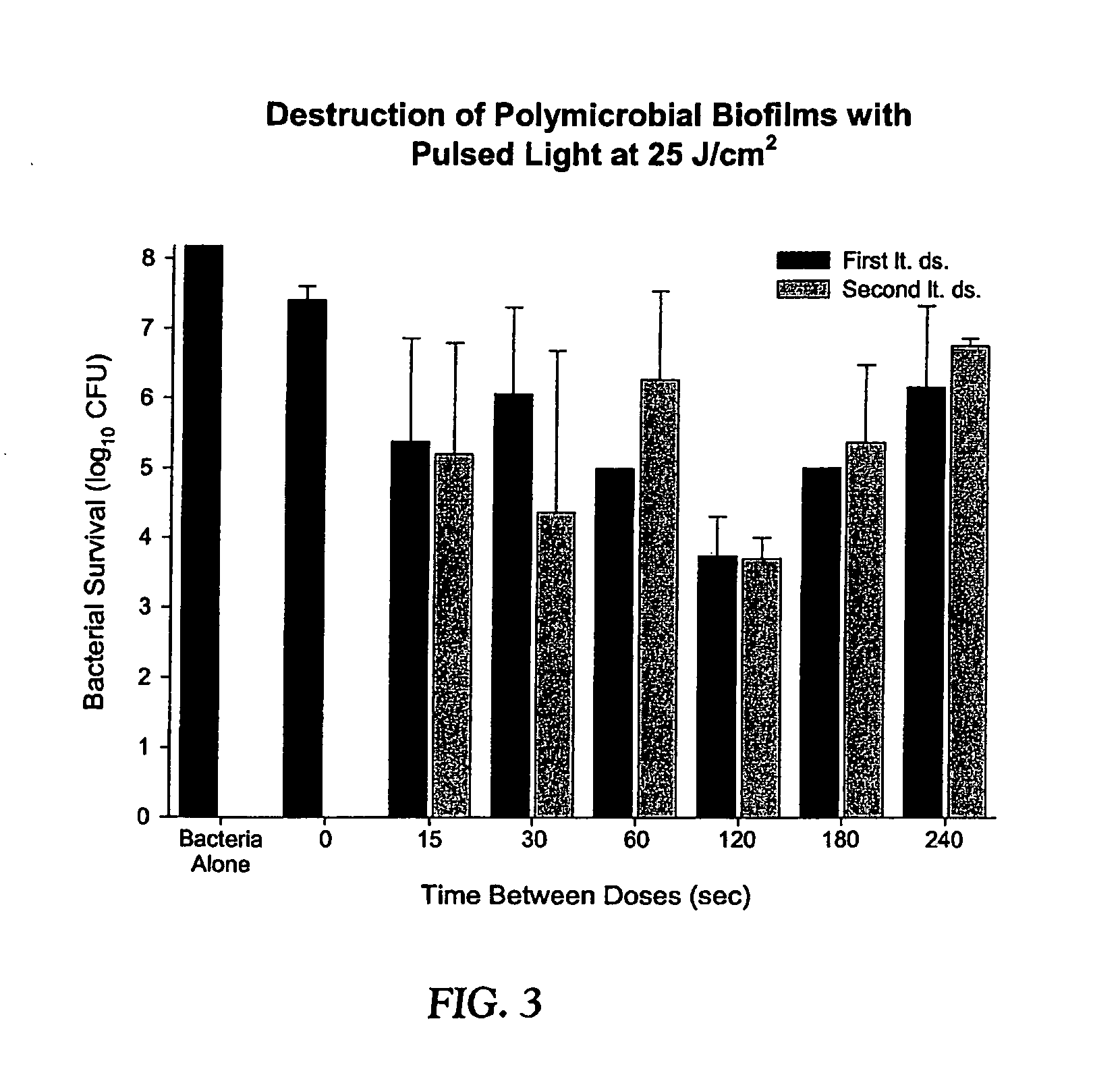
Photodynamic therapy utilizing multiple duty cycle light modulation
InactiveUS20060234959A1Improve efficiencyGood curative effectBiocideCavity massageCancer cellLight modulation
A method of photodynamic disruption of target cells within a target cell site wherein the target cells are associated with one or more of a sterilization procedure, a biofilm eradication procedure, a sterilization procedure of a medical prosthesis, a treatment of an infection at a tissue site, eradication of cancer cells and a fluid or food decontamination process. Surface acting agents such as benzalkonium chloride or polymyxin B sulfate or both may be utilized. A controllable light source capable of delivering light at a plurality of duty cycles is utilized to cause photoactivation of the photosensitive material resulting in degradation of target cells within the site.
Owner:ADVANCED PHOTODYNAMIC TECH
Weaned pig feed
InactiveCN103005196AMaintain gut healthImprove the immunityAnimal feeding stuffSodium bicarbonateAnimal science
The invention discloses a weaned pig feed which comprises the following components in percentage by weight: 50.5 to 63 percent of corn, 8 to 13.5 percent of bean pulp, 4.5 to 7.5 percent of fermented soy, 1.5 to 3 percent of plasma proteins, 4 to 6 percent of fish meal, 7.5 to 13 percent of extruded soybean, 3 to 6.5 percent of whey powder, 0.5 to 1.5 percent of wheat middling, 0.25 to 0.85 percent of plant oil and 1.25 to 4.15 percent of a pre-mixed material, wherein the pre-mixed material consists of an additive and a pre-mixing agent; the additive comprises the following components in percentage by weight: 0.35 to 0.4 percent of trace element, 0.03 to 0.04 percent of various vitamins, 0.05 to 0.06 percent of 10 percent of polymyxin E sulfate, 0.01 to 0.02 percent of complex enzyme, 0.01 to 0.02 percent of an acidifying agent, 0.05 to 0.06 percent of sodium bicarbonate, 0.1 to 0.2 percent of diaminocaproic acid, 0.05 to 0.2 percent of methionine and 0.25 to 0.3 percent of choline; and the pre-mixing agent comprises the following components in percentage by weight: 0.7 to 1 percent of rock flour, 1.3 to 1.5 percent of calcium hydrophosphate and 0.25 to 0.35 percent of salt. According to the feed, the nutrition of weaned pigs can be enhanced, and the immunity of the weaned pigs can be improved.
Owner:HAINING ZHENONG FEED
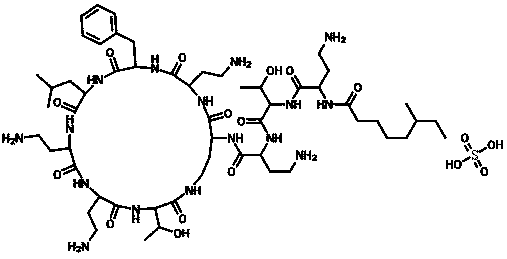
Sulfate polymyxin B crystal and preparation method thereof
The invention provides a sulfate polymyxin B crystal and a preparation method thereof. The preparation method comprises the following steps: firstly, adsorbing polymyxin B fermented liquid with resin,and adding a sulfate aqueous solution and / or an acid ethanol aqueous solution for desorption to obtain a desorption solution; secondly, adjusting a pH value of the desorption solution obtained in thefirst step to 5.0 to 7.0; and then concentrating until a solid is precipitated to obtain a sulfate polymyxin B saturated solution; and thirdly, in a stirring state, dropwise adding an organic solventinto the sulfate polymyxin B saturated solution obtained in the second step or dropwise adding the sulfate polymyxin B saturated solution obtained in the second step into the organic solvent so as toseparate out the crystal, filtering and drying a filter cake, thus obtaining the sulfate polymyxin B crystal. The solvent used in the preparation method disclosed by the invention is low in cost andeasy to obtain; the preparation method disclosed by the invention is suitable for large-scale industrial production; and the prepared sulfate polymyxin B crystal product has the advantages of higher purity and clarity and low moisture absorption.
Owner:HUBEI RUIHAO ANKE MEDICINE TECH DEV CO LTD
Polymyxin E composition and preparation method and application thereof
InactiveCN102190711AGuaranteed Quality ControlAntibacterial agentsPolymyxinsPolymixin EGram-negative bacterial infections
The invention belongs to the technical field of medicines, and in particular relates to a polymyxin E composition and a preparation method and application thereof. In the composition, total content of polymyxins E1, E2, E3, E1-1 and E1-7MOA is more than or equal to 80 percent, wherein the polymyxin E is preferably polymyxin E sulfate or polymyxin E sodium mesylate, and is particularly preferably polymyxin E sodium mesylate. The polymyxin E composition can be used for preparing medicines for treating Gram-negative bacterial infections.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Polymyxin sulfate B monoclonal antibody hybridoma cell strain and application thereof
InactiveCN110923208AImprove featuresHigh detection sensitivityBiological material analysisImmunoglobulins against bacteriaImmuno detectionMicrobiological culture
The invention discloses a polymyxin sulfate B monoclonal antibody hybridoma cell strain and application thereof, and belongs to the field of food safety immunodetection. The polymyxin sulfate B monoclonal antibody hybridoma cell strain OVA17 is obtained through screening and is preserved in the China General Microbiological Culture Collection Center (CGMCC), and the preservation number is CGMCC No.18516. The monoclonal antibody secreted by the cell strain has good specificity and detection sensitivity (IC50 value is 13.13 ng / mL) for polymyxin B sulfate, can realize detection of polymyxin B sulfate residue in feed and livestock and poultry food, provides a raw material for immunodetection of polymyxin B sulfate residue in food. Practical application value is achieved.
Owner:JIANGNAN UNIV
Preserving fluid suitable for preserving virus samples at normal temperature and preparation method thereof
PendingCN112314593AConducive to preservationActiveDead animal preservationDipotassium hydrogen phosphateSodium bicarbonate
The invention discloses a preserving fluid suitable for preserving virus samples at normal temperature and a preparation method thereof. The preserving fluid comprises 150-250 g of sodium chloride, 8-12 g of potassium chloride, 20-30 g of D-glucose, 3-5 g of sodium hydroxide, 0.4-0.6 g of phenol red, 2-4 g of dipotassium hydrogen phosphate, 1-1.5 g of disodium hydrogen phosphate, 8-9 g of sodium bicarbonate, 4-5 g of bovine serum albumin, 0.5-0.9 g of 2-methyl-4-isothiazoline-3-ketone hydrochloride and 0.1-0.5 g of polymyxin B sulfate. The invention relates to the technical field of clinical examination. According to the preserving fluid suitable for preserving virus samples at normal temperature and the preparation method thereof, the prepared preserving fluid has good preserving capability, can ensure that a sample virus still has activity after preservation for more than one week at normal temperature and can be preserved at 2-8 DEG C for a longer time, does not interfere a PCR detection result, has good antibacterial capability, has low requirements on production and use environments, does not need irradiation sterilization, is low in cost and easy to mass-produce, guarantees no decomposition of nutrients, and guarantees the stability of the pH value in cooperation with an HEPES buffer solution or a phosphate buffer solution.
Owner:武汉尚飞生物科技有限公司
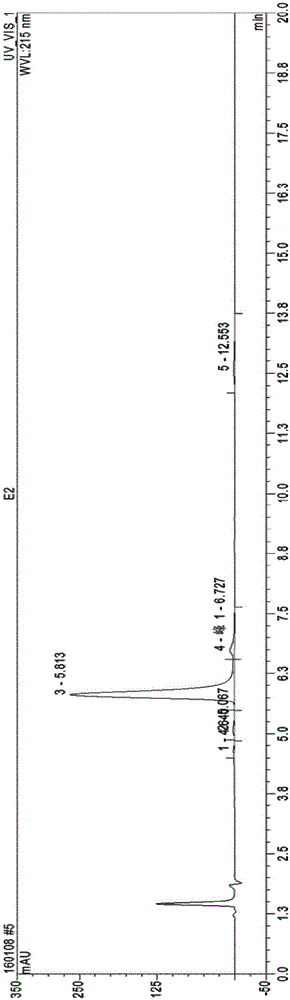
Purification method of polymyxin E sulfate components
InactiveCN105713074ANo damageReduce adsorptionPolymyxinsPeptide preparation methodsPurification methodsOrganic solvent
The invention discloses a purification method of polymyxin E sulfate components, and relates to the technical field of medicine. The purification method includes the following steps that firstly, polymyxin E sulfate is dissolved with water, and a sample is loaded to a gel medium; secondly, elution is carried out with an organic solvent after the first step is finished, and fractional collection and fusion of elution liquor containing polymyxin sulfate E1 and E2 are carried out; thirdly, the fused elution liquor obtained in the second step is taken, and the pH value is regulated with sulfuric acid after the organic solvent is removed; fourthly, spray drying is carried out after the third step is finished, and purified products of the polymyxin sulfate E2 and E1 components are obtained. According to the purification method, operation is carried out with gel which belongs to inert carriers, does not carry charges and is weak in adsorption capacity, so that the operation condition is mild, no damage is caused to the polymyxin E2 and E1 components, the polymyxin E2 and E1 components are protected to the maximum degree, and the content of the purified products of the polymyxin E2 and E1 components reaches 90%.
Owner:HEBEI SHENGXUE DACHENG PHARMA
Method for separating and purifying method of polymyxin E methanesulfonic sodium
ActiveCN101525377BSimple processGood reproducibilityPeptidesAntiinfectivesSeparation technologyUltrafiltration
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
A kind of polymyxin b sulfate production strain, preparation method and application of polymyxin b sulfate
ActiveCN111100823BStrong ability to produce polymyxin B sulfateImprove abilitiesBacteriaMutant preparationBiotechnologyMetabolite
The invention belongs to the technical field of biological fermentation, and in particular relates to a polymyxin B sulfate production strain, a preparation method and application of polymyxin B sulfate. The present invention obtains a polymyxin B sulfate production strain through mutagenesis breeding, and optimizes the carbon source and nitrogen source of the fermentation medium, precisely regulates the fermentation conditions such as the inoculum amount, fermentation temperature, fermentation time, and tank pressure, and controls the The metabolite components finally utilize the bacterial strain obtained by mutagenesis of the present invention to produce polymyxin B sulfate in a tank unit of 2.0 g / L, exceeding the industry average level by 20%. The proportions of several impurity peaks specified by the USP / EP Pharmacopoeia in the fermented liquid in tanks are far lower than the levels recorded in domestic literature. Separation and purification of the fermentation broth to obtain high-purity and high-content polymyxin B sulfate, the biometric titer of the finished product is greater than 8100u / mg, the purity and content are both greater than 90%, the single impurities and components are all qualified, and the total impurities are less than 8 %, product quality is much higher than USP / EP standard.
Owner:黄石曼菲特生物科技有限公司
Polymyxin E liposome as well as preparation method and application thereof
PendingCN110974937AImprove structural stabilityGood sustained release effectAntibacterial agentsCyclic peptide ingredientsCholesterolAntioxidant
The invention relates to a polymyxin E liposome. The polymyxin E liposome comprises the following components: polymyxin E sulfate, first-class phospholipid, second-class phospholipid, cholesterol, anantioxidant, an excipient and a buffer agent. Different from other phospholipid complex products, the polymyxin E liposome provided by the invention has the following advantages: the microstructure ofthe polymyxin E liposome is that a drug is embedded into a phospholipid bilayer of the liposome and the drug and phospholipid are not simply compounded, so the polymyxin E liposome has better structural stability, slow release property and a longer half-life period.
Owner:SHANGHAI JINGFENG PHARMA
Compound ointment preparation and preparation method thereof
InactiveCN108434437AAntibacterial agentsOrganic active ingredientsButylated hydroxytolueneMillion Units
The invention provides a compound ointment preparation. According to the compound ointment preparation, per kilogram of the compound ointment preparation is prepared from the following components in parts by weight: 250-1000 million units of polymyxin B sulfate, 300-400 million units of neomycin sulfate, 25-75 million units of bacitracin, 30-50 g of lidocaine hydrochloride and a matrix added to the prescription dosage, wherein the matrix is prepared from one or more of white vaseline, yellow vaseline, ozocerite, liquid paraffin, alpha-cyclodextrin, modified chitosan, propylene glycol, methyl parahydroxybenzoats and butylated hydroxytoluene.
Owner:ZHEJIANG REACHALL PHARMA
Methods and Compositions For Topical Wound Treatment
InactiveUS20070110797A1Convenient treatmentSafe and effective amountTetracycline active ingredientsInorganic non-active ingredientsWound dressingGram
Wound Dressings for improved treatment of wounds on a patient. A wound dressing having a bandage and a healing composition. The healing composition includes from about 5 mg to about 40 mg lidocaine hydrochloride per gram of the healing composition, from about 250 units to about 2000 units polymyxin B sulfate per gram of the healing composition, and from about 10 units to about 100 units bacitracin zinc per gram of the healing composition. Article of manufacture having a packaging material and the healing composition. The packaging material includes a label indicating composition is useful for treating a patient having a skin wound and that local administration of the composition enhances healing of a skin wound.
Owner:TIPPETT ALETHA
Method for improving fermentation level of polymyxin B sulfate
ActiveCN112553277AAvoid destructionQuality improvementBacteriaMicroorganism based processesBiotechnologyInorganic salts
The invention discloses a method for improving the fermentation level of polymyxin B sulfate. The method comprises a fermentation formula and a fed-batch fermentation process, wherein the fermentationformula is divided into a basic formula consisting of a fermentation carbon source and inorganic salt and a fed-batch formula consisting of a fermentation nitrogen source; and the fed-batch fermentation process comprises the following steps: a) performing individual disinfection and sterilization on a fermentation tank; b) performing individual disinfection and sterilization on a fed-batch bottle; and c) supplementing fed-batch liquid consisting of the fermentation nitrogen source at a constant speed in the first and middle stages of fermentation. The fermentation titer of the polymyxin B sulfate obtained by fermentation culture by the method can reach 11000u / mL or above on average, the fermentation titer is improved by 30% or above compared with the original fermentation level, the component proportion of the fermentation liquor is close to the liquid phase chromatogram of a standard substance, and industrial production is easy.
Owner:NCPC NEW DRUG RES & DEV
External gel for skin wounds, and preparation method of external gel
ActiveCN112121146APromote formationHas a preventive effectAntibacterial agentsHydroxy compound active ingredientsCentella asiatica extractCollagen fibres
The invention discloses an external gel for skin wounds. The external gel is prepared from the following raw materials in parts by weight: 1-3 parts of an active component A, 4-6 parts of an active component B, 10-14 parts of a Calendula arvensis extract, 16-20 parts of a Centella asiatica extract, and 60-80 parts of an auxiliary material, wherein the active component A is at least two of polymyxin B sulfate, neomycin sulfate and bacitracin, the active component B is at least one of lidocaine hydrochloride, pramoxine hydrochloride, procaine hydrochloride and menthol, and the auxiliary materialcomprises the following raw materials in parts by weight: 0.5-3 parts of carbomer, 40-75 parts of an organic solvent, and 20-50 parts of purified water. The external gel provided by the invention hasprevention and treatment effects on the bacterial infection of skin wound parts, has analgesic and hemostatic effects on skin wounds, can promote the formation of capillaries and collagenous fibers of wound surfaces, reduces the healing days of wound surfaces, and can be used for the healing of skin wounds such as burn wounds, incised wounds and scald wounds, the healed skin is smooth and free ofscars, and thereby the healing effect is good.
Owner:SHANDONG RUIAN PHARMA CO LTD
Culture medium for fermenting polymyxin b sulfate and method for producing polymyxin b sulfate by fermentation
ActiveCN106868079BBiological metabolic pathways are smoothGeneration of effective regulationBacteriaMicroorganism based processesBiotechnologyAmylase
The invention provides a culture medium for fermenting polymyxin B sulfate. Each 100ml of the culture medium is prepared from the following components: 6g to 12g of flour, 0.03g to 0.06g of high-temperature amylase, 0.4g to 2g of cottonseed meal, 0.3g to 1g of corn pulp, 0.01g to 0.05g of dipotassium phosphate, 0.2g to 0.6g of ammonium sulfate, 0.2g to 0.6g of calcium carbonate, 0.02ml to 0.08ml of a de-foaming agent and the balance of water. A method for fermenting and producing the polymyxin B sulfate comprises the following the steps: inoculating the culture medium with strain seed liquid for producing the polymyxin B sulfate; fermenting and culturing fermentation liquid at 26 DEG C to 32 DEG C, wherein the pH (Potential of Hydrogen) value is 6.7 to 7.4 at the beginning of fermenting; controlling the pH value of the fermentation liquid to be natural during a fermentation process, wherein the dissolved oxygen volume concentration of the fermentation liquid is 10 percent to 30 percent. All the components of the culture medium are cooperatively matched and the content of impurities B2P is controlled to be lower than 1.5 percent.
Owner:SHANDONG LUKANG PHARMA
Methods and Compositions For Topical Wound Treatment
InactiveUS20070104770A1Convenient treatmentSafe and effective amountBiocideTetracycline active ingredientsMedicineGram
Methods for improved treatment of wounds on a patient. Method includes locally administering a healing composition to the skin wound such that the healing of the skin wound is enhanced. The healing composition includes from about 5 mg to about 40 mg lidocaine hydrochloride per gram of the healing composition, from about 250 units to about 2000 units polymyxin B sulfate per gram of the healing composition, and from about 10 units to about 100 units bacitracin zinc per gram of the healing composition.
Owner:TIPPETT ALETHA
A kind of external gel for skin wound and preparation method thereof
ActiveCN112121146BPromote formationHas a preventive effectAntibacterial agentsHydroxy compound active ingredientsCentella asiatica extractAdjuvant
The invention discloses an external gel for skin wounds, which is made of the following raw materials in parts by weight: 1-3 parts of active component A, 4-6 parts of active component B, 10-10 parts of calendula extract 14 parts, 16-20 parts of Centella asiatica extract, 60-80 parts of auxiliary materials; the active component A is at least two of polymyxin B sulfate, neomycin sulfate and bacitracin; the active component Part B is at least one of lidocaine hydrochloride, pramoxine hydrochloride, procaine hydrochloride and menthol; the auxiliary materials include the following raw materials in parts by weight: 0.5-3 parts of carbomer, organic solvent 40‑75 parts, 20‑50 parts of purified water. The gel for external use of the present invention has a preventive effect on bacterial infection of skin wounds, and has analgesic and hemostatic effects on skin wounds; it can also promote the formation of wound capillaries and collagen fibers, reduce the days of wound healing, and can be used for burns, cuts, For the healing of skin wounds such as burns, the healed skin is smooth, without scars, and the healing effect is good.
Owner:SHANDONG RUIAN PHARMA CO LTD
Crystallization of polymyxin sulfate b1, b2 or mixture thereof and preparation method thereof
The invention provides an anhydride crystal of polymyxin sulfate B1, B2 or mixture thereof as well as a preparation method of the crystal. The preparation method comprises the following steps: (1) adding water into the polymyxin sulfate B1, B2 or the mixture thereof to enable the solid to be completely dissolved to obtain a saturated solution; (2) adding an organic solvent into the saturated solution slowly and dropwise, or adding the saturated solution into the organic solvent slowly and dropwise, separating out the solid and controlling the temperature of the dropwise adding process to be within the range of 0 to 60 DEG C, wherein the organic solvent is selected from one or more of C1-C4 alcohol, C3-C4 ketone or ethyl acetate, and butyl acetate; (3) filtering out the solid and vacuum-drying to obtain the anhydride crystal of the polymyxin sulfate B1, B2 or the mixture thereof, wherein the polymyxin sulfate B1 has a crystal form 1 and a crystal form A. The anhydride crystal particlesof the polymyxin sulfate B1, B2 or the mixture thereof, prepared by the method, are loose, non-sticky and particularly favorable for industrialized production of medicine products. In addition, by themethod, impurities in the medicines can be effectively removed, higher purity and clarity are achieved, and the quality of the medicine products is greatly improved.
Owner:HUBEI RUIHAO ANKE MEDICINE TECH DEV CO LTD
Crystal of polymyxin b1 sulfate, polymyxin b2 sulfate or their mixture and preparation method thereof
InactiveUS20210283213A1Effectively remove impuritiesImprove drug qualityAntibacterial agentsOrganic chemistry methodsOrganic solventPolymyxin B1
The present invention provides an anhydrous crystal of polymyxin B1 sulfate, polymyxin B2 sulfate or a mixture thereof and a preparation method thereof. The preparation method comprises using an organic solvent to precipitate a solid from a saturated solution of polymyxin B1 sulfate, polymyxin B2 sulfate or a mixture thereof, drying it under vacuum to obtain an anhydrous crystal of polymyxin B1 sulfate, polymyxin B2 sulfate or a mixture thereof.
Owner:HUBEI RUIHAO ANKE MEDICINE TECH DEV CO LTD
Lactating sow feed
InactiveCN103005192BPromote milk productionKeep healthyAnimal feeding stuffSodium bicarbonateAnimal science
The invention discloses lactating sow feed which specifically comprises the following components in percentage by weight: 47 to 59 percent of corn, 15 to 21 percent of bean pulp, 1.5 to 2.3 percent of fish meal, 7.2 to 14 percent of bran, 8.5 to 12 percent of wheat middling, 1 to 2 percent of plant oil and 3.5 to 6 percent of a pre-mixed material, wherein the pre-mixed material consists of an additive and a pre-mixing agent; the additive comprises the following components in percentage by weight: 0.2 to 0.3 percent of a trace element, 0.03 to 0.04 percent of various vitamins, 0.05 to 0.1 percent of 10 percent of polymyxin E sulfate, 0.01 to 0.02 percent of complex enzyme, 0.01 to 0.02 percent of an acidifying agent, 0.05 to 0.1 percent of sodium bicarbonate, 0.1 to 0.25 percent of diaminocaproic acid, 0.05 to 0.1 percent of methionine, 0.2 to 0.3 percent of choline, 0.5 to 1.2 percent of codonopsis pilosula and 0.4 to 1.2 percent of astragalus mongholicus; and the pre-mixing agent comprises the following components in percentage by weight: 0.8 to 1.2 percent of mountain flour, 0.75 to 1 percent of calcium hydrophosphate and 0.25 to 0.35 percent of edible salt. The codonopsis pilosula and the astragalus mongholicus are added in the lactating sow feed to serve as traditional Chinese medicine additives, so that the lactating sow feed has the functions of supplementing qi, activating the blood, promoting the lactation and the like; the galactopoiesis of a lactating sow can be promoted; the feed utilization rate is improved; side effects are avoided; and the health of the lactating sow is guaranteed.
Owner:HAINING ZHENONG FEED
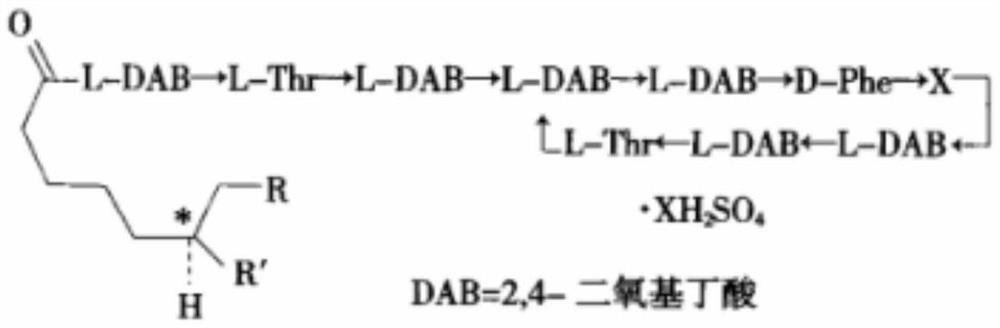
Blood purification material and preparation method based on mussel biomimetic chemistry
ActiveCN110841602BAdhesive strengthMany functionsOther chemical processesSolid sorbent liquid separationGallic acid esterPolyvinyl alcohol
The invention discloses a blood purification material based on mussel biomimetic chemistry and a preparation method thereof. The blood purification material is composed of a porous adsorption base material, an adsorption layer and a coating film in sequence from the inside to the outside, and the adsorption layer is Polymyxa sulfate. One or more of B, polylysine hydrochloride, polyethyleneimine or polydopamine, and is wound on the porous adsorption substrate in the form of molecular chains; the coating film is polyethylene Butyral-gallic acid film or polyvinyl butyral film. The blood purification material is designed based on mussel biomimetic chemistry, and has the advantages of simplicity and convenience, mild conditions, environmental protection, and basically no influence on the material body, and the blood purification material is used in the field of blood purification medical devices.
Owner:佛山市博新生物科技有限公司
Method for screening and identifying antibiotic resistant bacteria
PendingCN114350738AEasy identificationProper selectionMicrobiological testing/measurementDNA/RNA fragmentationBiotechnologyResistant bacteria
The invention belongs to the technical field of bioengineering, and particularly relates to a method for screening and identifying antibiotic-resistant bacteria. According to the method, the soil suspensions with different concentration gradients are coated, and the optimal dilution gradient for effectively selecting single colonies is determined according to the condition of the colonies growing on a flat plate, so that subsequent large-batch screening and identification of antibiotic-resistant bacteria are facilitated; according to the method, the growth speed of the resistant bacteria is controlled by controlling the concentration of erythromycin, streptomycin and polymyxin B sulfate in the resistant culture medium, so that the purpose of accurately screening the drug-resistant bacteria is achieved; the sterilization gun head is used for replacing a traditional inoculating loop to select single colonies, the bacterium selecting step can be completed by repeated blowing and beating, and the problems that a large amount of time is consumed when the inoculating loop is burnt and cooled, the cooling degree is difficult to control, and bacteria are scalded to death are avoided.
Owner:INST OF URBAN ENVIRONMENT CHINESE ACAD OF SCI
Polymyxin b sulfate crystal and preparation method thereof
InactiveUS20190256557A1Good clarity and purityReduce moisture absorption performanceAntibacterial agentsOrganic chemistry methodsOrganic solventSulfate
The present invention provides a polymyxin B sulfate crystal and a preparation method thereof. Said method comprises the following steps of: adsorbing a polymyxin B fermentation liquid with a resin, and adding sulfuric acid or acidic ethanol for desorbing; concentrating to obtain a saturated solution of polymyxin B sulfate; and using an organic solvent to precipitate a crystal from the saturated solution, filtering and drying to obtain the polymyxin B sulfate crystal.
Owner:HUBEI RUIHAO ANKE MEDICINE TECH DEV CO LTD
Weaned pig feed
InactiveCN103005196BMaintain gut healthImprove the immunityAnimal feeding stuffSodium bicarbonateAnimal science
The invention discloses a weaned pig feed which comprises the following components in percentage by weight: 50.5 to 63 percent of corn, 8 to 13.5 percent of bean pulp, 4.5 to 7.5 percent of fermented soy, 1.5 to 3 percent of plasma proteins, 4 to 6 percent of fish meal, 7.5 to 13 percent of extruded soybean, 3 to 6.5 percent of whey powder, 0.5 to 1.5 percent of wheat middling, 0.25 to 0.85 percent of plant oil and 1.25 to 4.15 percent of a pre-mixed material, wherein the pre-mixed material consists of an additive and a pre-mixing agent; the additive comprises the following components in percentage by weight: 0.35 to 0.4 percent of trace element, 0.03 to 0.04 percent of various vitamins, 0.05 to 0.06 percent of 10 percent of polymyxin E sulfate, 0.01 to 0.02 percent of complex enzyme, 0.01 to 0.02 percent of an acidifying agent, 0.05 to 0.06 percent of sodium bicarbonate, 0.1 to 0.2 percent of diaminocaproic acid, 0.05 to 0.2 percent of methionine and 0.25 to 0.3 percent of choline; and the pre-mixing agent comprises the following components in percentage by weight: 0.7 to 1 percent of rock flour, 1.3 to 1.5 percent of calcium hydrophosphate and 0.25 to 0.35 percent of salt. According to the feed, the nutrition of weaned pigs can be enhanced, and the immunity of the weaned pigs can be improved.
Owner:HAINING ZHENONG FEED
High-purity polymyxin B1 sulfate crystal form and preparation method thereof
PendingCN112940083AUniform particle size distributionImprove liquidityPolymyxinsPeptide preparation methodsPhysical chemistryPolymyxin B1
The invention provides a preparation method of a high-purity polymyxin B1 crystal form, specifically, a DAC preparative column is adopted for separation, then macroporous resin desalination treatment is conducted, and then the polymyxin B1 sulfate crystal form is crystallized after nanofiltration concentration, the crystal form provided by the invention is uniform in particle size distribution and good in fluidity, preparation of a preparation is facilitated, the preparation method of the crystal form is simple, and the obtained crystal has high purity.
Owner:CHONGQING QIANTAI BIOLOGICAL MEDICINE
Blood purification material based on mussel biomimetic chemistry and preparation method thereof
ActiveCN110841602AAdhesive strengthIncrease stickinessOther chemical processesSolid sorbent liquid separationGallic acid esterPolyvinyl alcohol
The invention discloses a blood purification material based on mussel biomimetic chemistry and a preparation method thereof. The blood purification material sequentially comprises a porous adsorptionbase material, an adsorption layer and a coating film from inside to outside, the adsorption layer is one or more of polymyxin B sulfate, polylysine hydrochloride, polyethyleneimine or polydopamine, and the adsorption layer is wound on the porous adsorption base material in a molecular chain form; and the coating film is a polyvinyl butyral-gallic acid film or a polyvinyl butyral film. The blood purification material is designed on the basis of mussel biomimetic chemistry, has the advantages of simplicity, convenience, mild conditions, greenness, environmental protection and basically no influence on material bodies, and is applied to the field of blood purification medical instruments.
Owner:佛山市博新生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com