Purification method of polymyxin E sulfate components
A polymyxin sulfate and purification method technology, applied in the field of medicine, can solve problems such as differences, obstacles in drug administration, and defects in effectiveness and safety, and achieve the effect of mild operating conditions and weak adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (a) Weigh an appropriate amount of polymyxin E sulfate, add water to dissolve it into a solution with a concentration of 100g / L, and load it on the G-10 gel medium according to the sample loading of 30g / L;
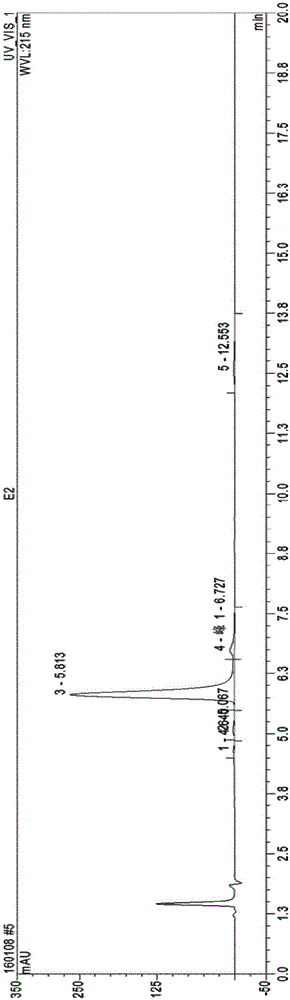
[0041] (b) Elute it with 35% methanol solution, the flow rate is 0.8BV / h, and start to collect when the white precipitate is titrated with 0.25% phosphotungstic acid. For HPLC analysis and chromatographic conditions, refer to the determination of the purity of polymyxin E in European Pharmacopoeia 8.0, and the eluents with a purity of polymyxin sulfate E2 of more than 90% and a purity of polymyxin sulfate E1 of more than 90% are combined ;
[0042] (c) Evaporate the eluent combined in the previous step to remove the organic solvent with a vacuum film at a temperature of 50° C., and then adjust the pH value to 4.0 with sulfuric acid;
[0043] (d) Spray drying, the inlet air temperature is 190°C, and the outlet air temperature is 110°C. As a result, 15g polymyxin su...
Embodiment 2
[0045] (a) Weigh an appropriate amount of polymyxin E sulfate, add water to dissolve it into a solution with a concentration of 100g / L, and load it on the G-25 gel medium according to the sample loading of 60g / L;
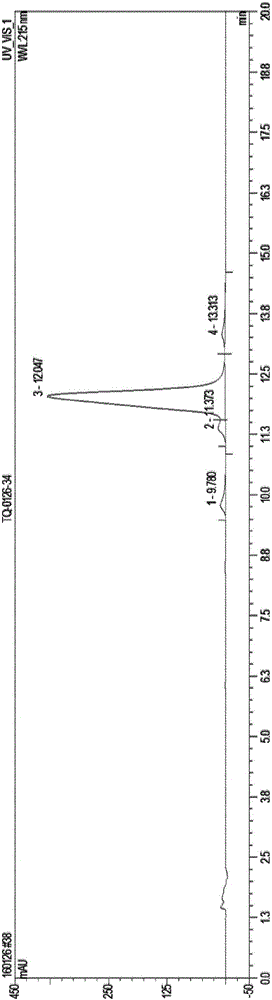
[0046] (b) Elution with 20% chloroform solution, flow rate 0.8BV / h, regularly collect a group of sample groups every 30min, use analytical HPLC analysis, chromatographic conditions refer to the purity determination of polymyxin E in European Pharmacopoeia 8.0 , combining the eluates with polymyxin sulfate E2 purity of more than 90% and polymyxin sulfate E1 purity of more than 90%;
[0047] (c) Evaporate the combined eluent in the previous step to remove the organic solvent with a vacuum film at a temperature of 40° C., and then adjust the pH value to 4.2 with sulfuric acid;
[0048](d) Spray drying, the inlet air temperature is 130°C, and the outlet air temperature is 90°C. As a result, 12 g of polymyxin sulfate E2 and 5 g of polymyxin sulfate E1 were obtained, the...
Embodiment 3
[0050] (a) Weigh an appropriate amount of polymyxin E sulfate, add water to dissolve it into a solution with a concentration of 100g / L, and load it on the G-15 gel medium according to the sample loading of 40g / L;
[0051] (b) Elution with 45% acetonitrile solution, flow rate 0.8BV / h, regularly collect a group of sample groups every 30min, use analytical HPLC analysis, chromatographic conditions refer to the purity determination of polymyxin E in European Pharmacopoeia 8.0 , combining the eluates with polymyxin sulfate E2 purity of more than 90% and polymyxin sulfate E1 purity of more than 90%;
[0052] (c) Evaporate the combined eluent in the previous step to remove the organic solvent with a vacuum film at a temperature of 60° C., and then adjust the pH value to 3.8 with sulfuric acid;
[0053] (d) Spray drying, the inlet air temperature is 180°C, and the outlet air temperature is 90°C. As a result, 18 g of polymyxin sulfate E2 and 10 g of polymyxin sulfate E1 were obtained,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com