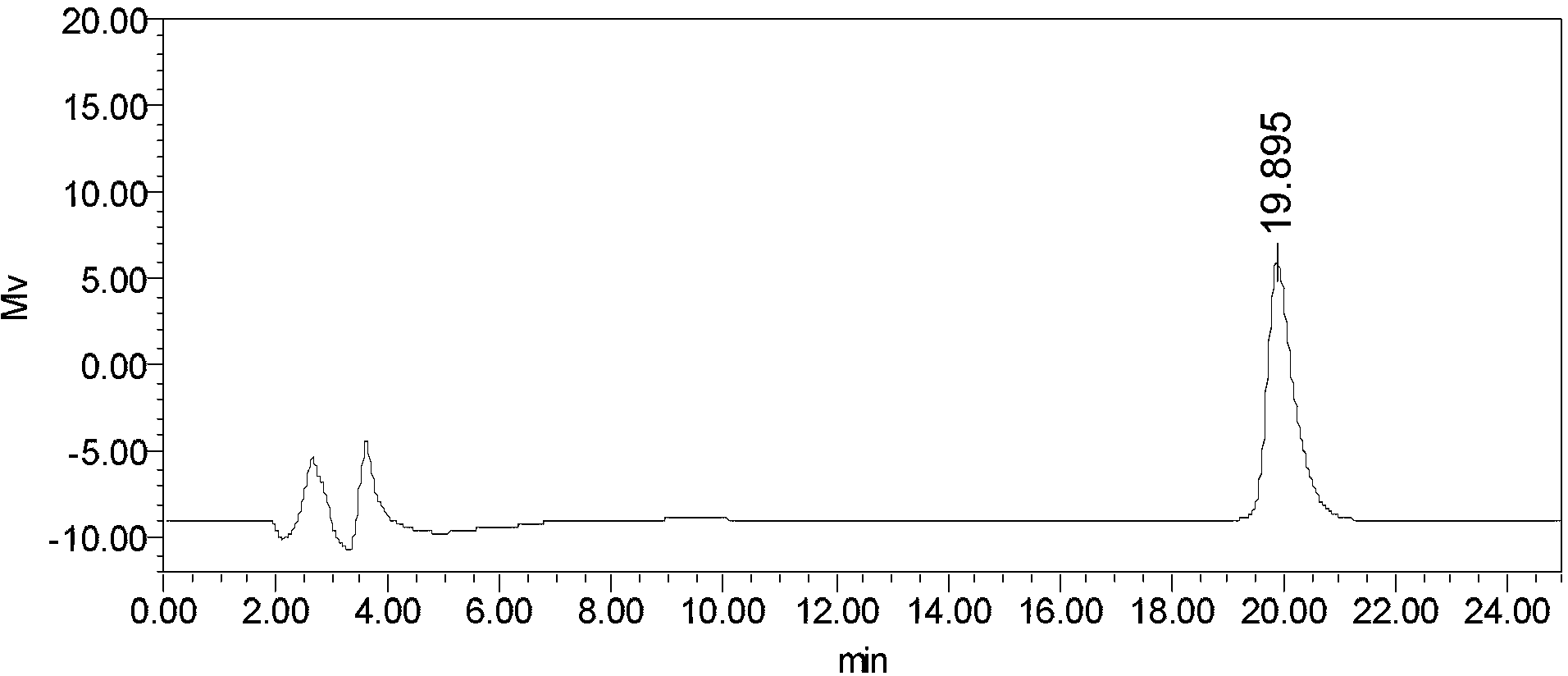
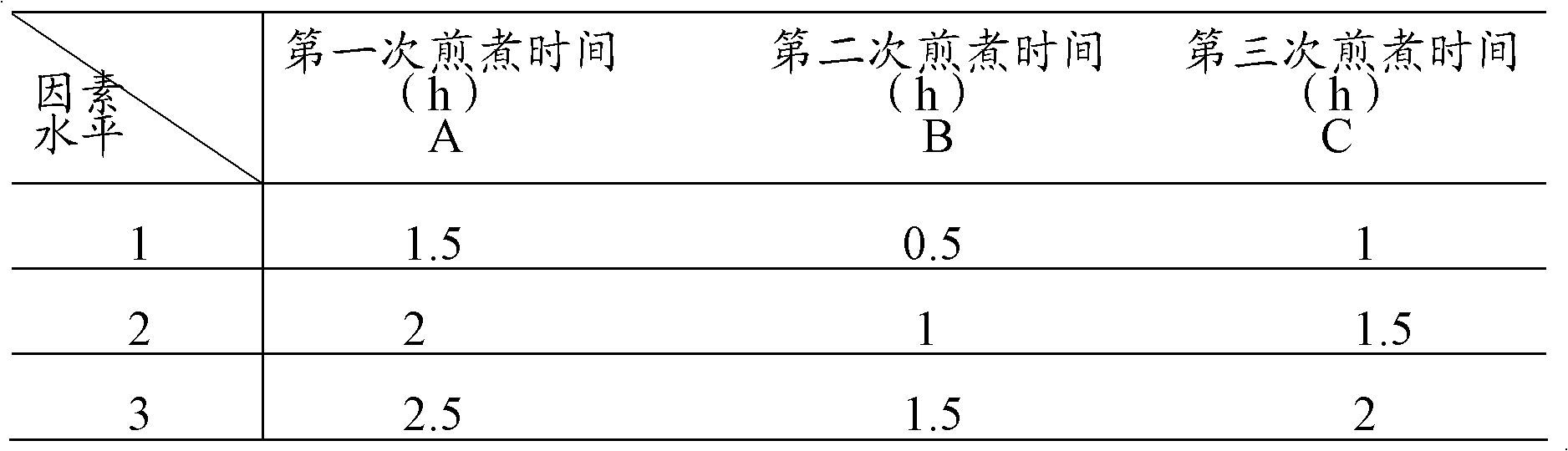
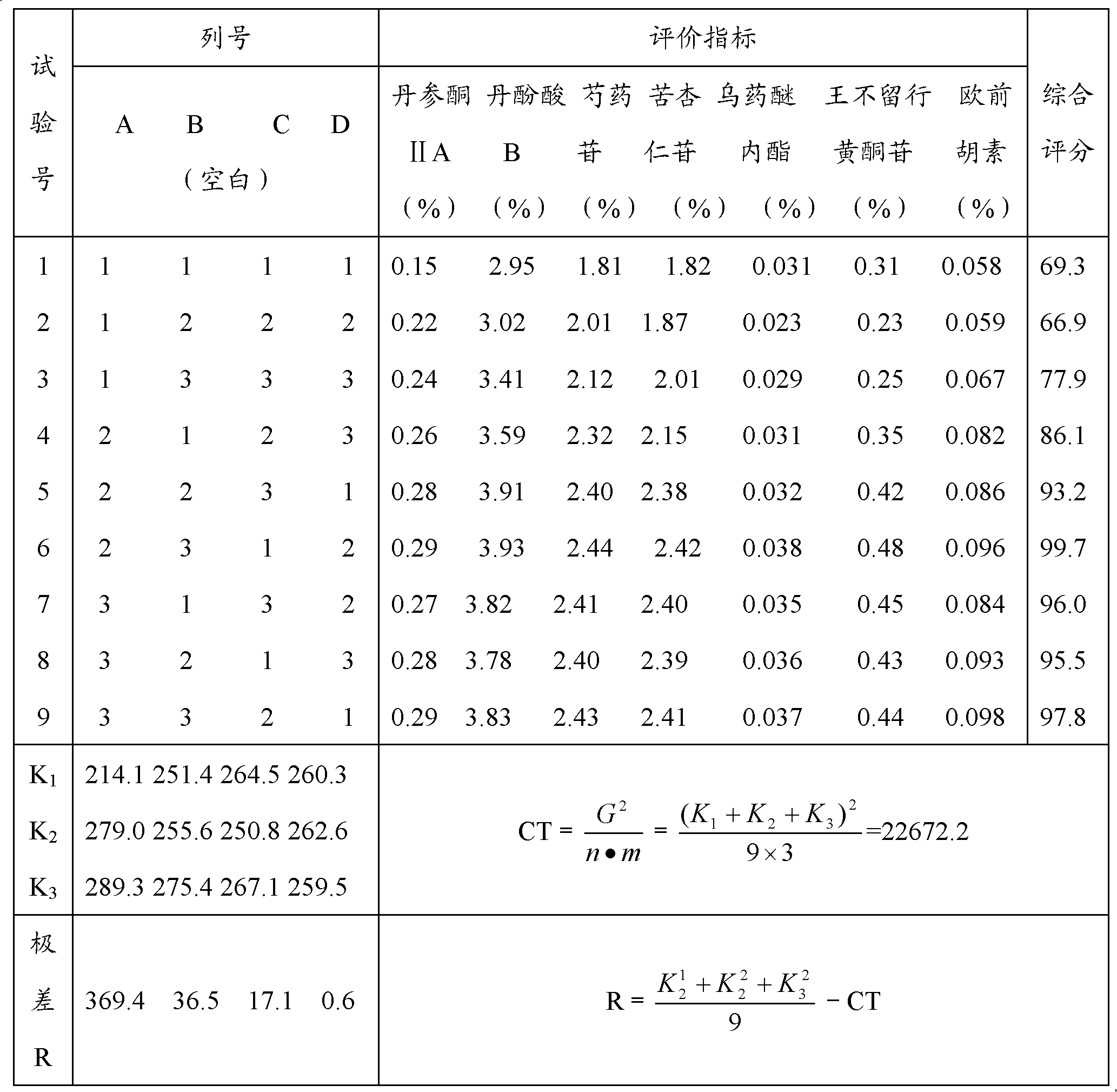
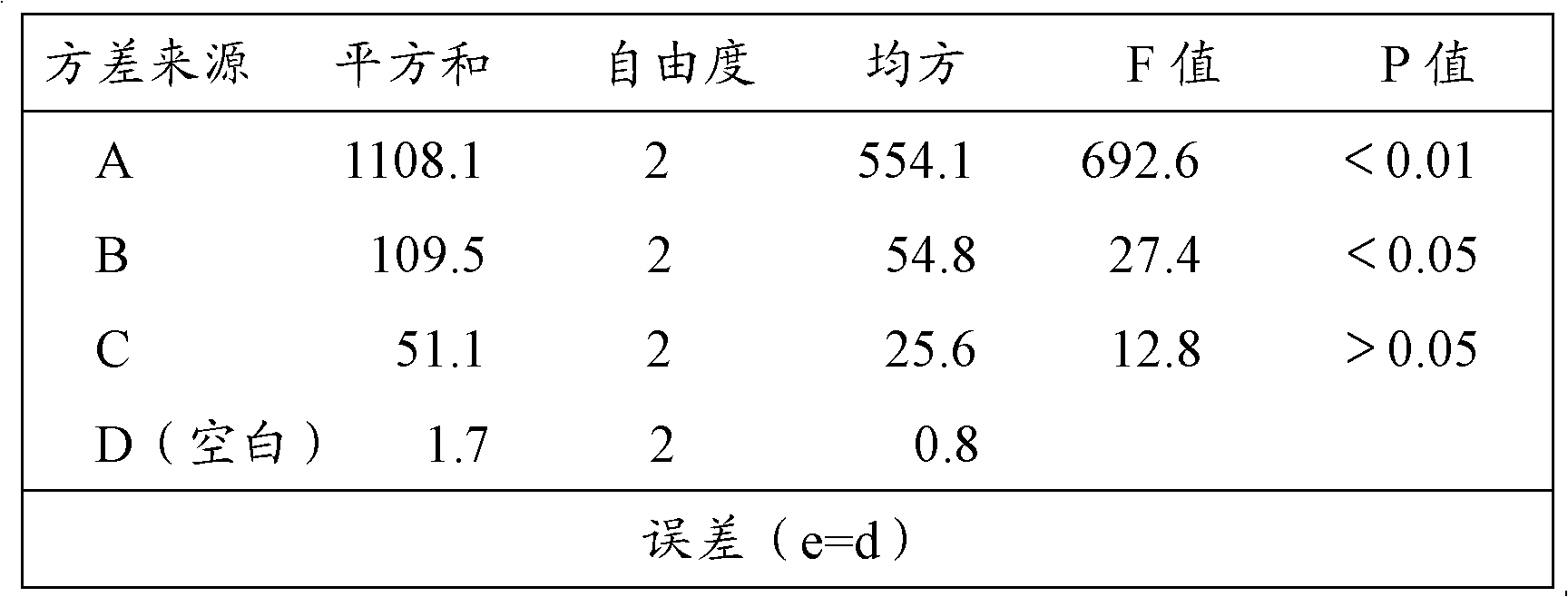
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
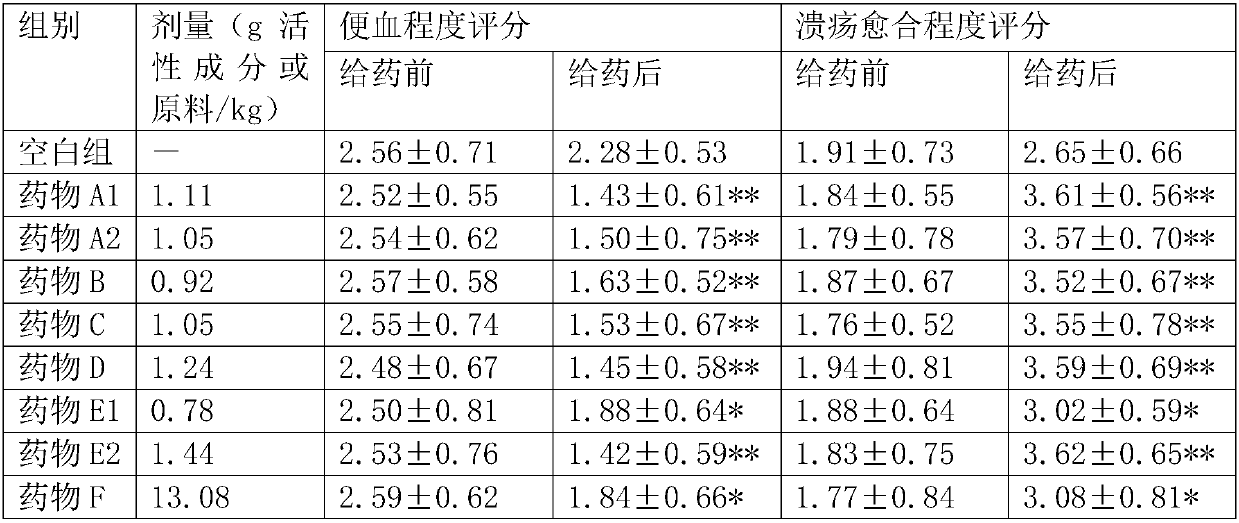
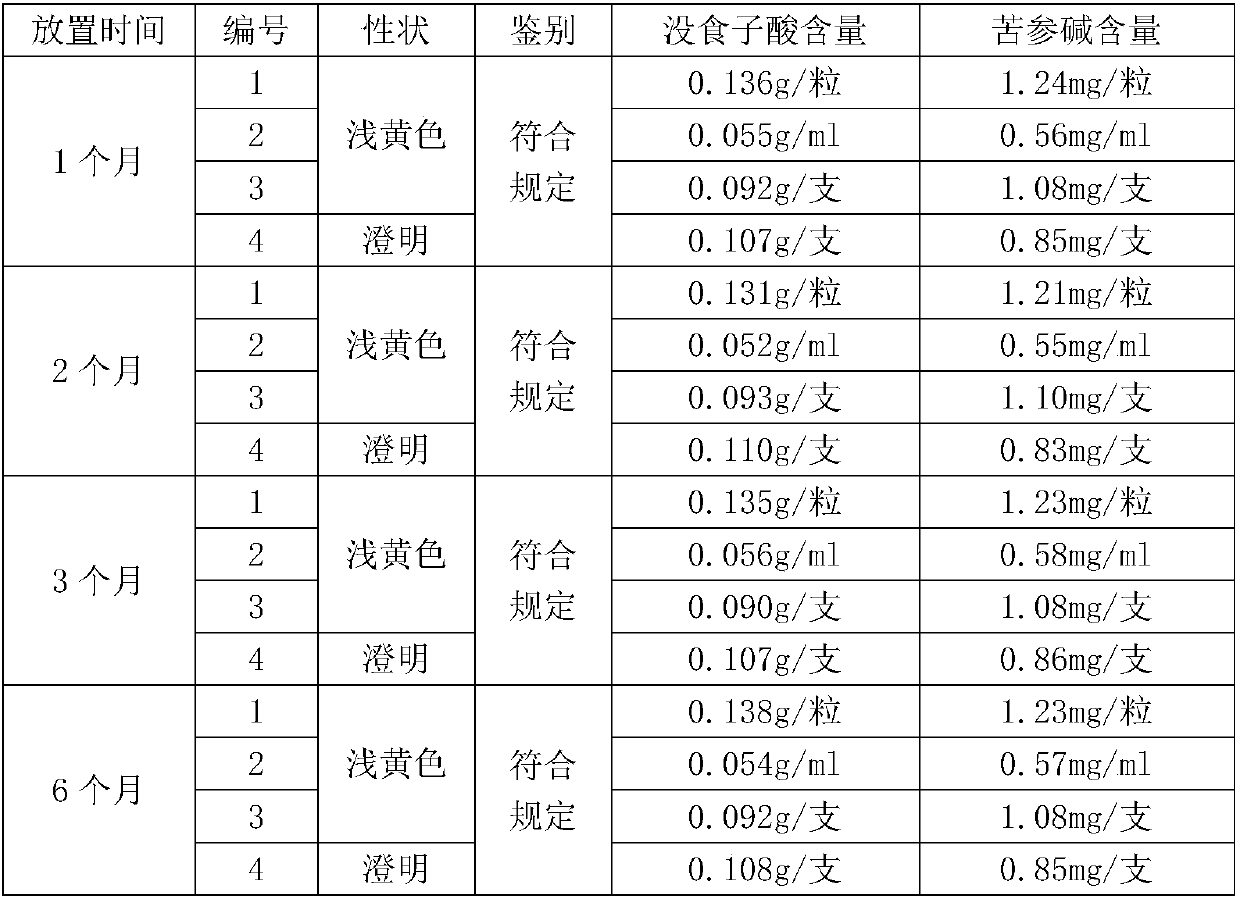
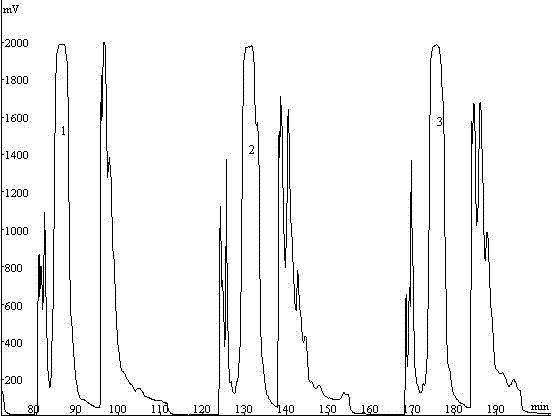
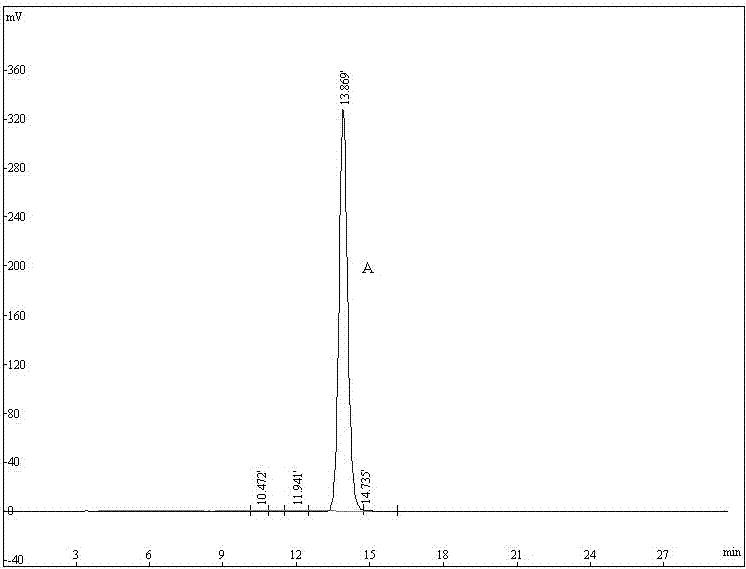
47 results about "Phellodendrine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phellodendrine is an alkaloid isolated originally from Phellodendron amurense (Rutaceae).
Medication composition of plant estrogens for treating colpitis
InactiveCN1795925AImprove vaginal flexibilityImprove the immunityOrganic active ingredientsSexual disorderCnidium monnieriPhellodendrine
A composite vegetative estrin medicine in the form of suppository, gel or film for treating vagititis and improving the toughness of vagina and its resistance to bacteria is proportionally prepared from vegetative estrin, cnidiadin, borneol, and at least one of matrine, phellodendrine, berberine, etc and their physiocologically acceptable salts.
Owner:MEDICAL COLLEGE OF CHINESE PEOPLES ARMED POLICE FORCE
Method for separating and purifying phellodendrine monomer
The invention discloses a method for separating and purifying phellodendrine monomer, which comprises the following steps of: extracting total alkaloid; removing berberine by means of acid deposition; filtering and further removing; separating phellodendrine monomer through a preparative high-efficiency liquid chromatogram; extracting dichloromethane; and drying to prepare the phellodendrine monomer. The method has simple operation, short preparation period, high separation efficiency, stable technology and low cost, and can separate the large number of phellodendrine monomer with high purity to obtain the phellodendrine monomer, the purity of which is more than 98%.
Owner:CHENGDU PUSH BIOLOGICAL TECH
Pharmaceutical composition for preventing and treating rheumatoid arthritis
InactiveCN101700249AInhibition of clinical onsetOrganic active ingredientsAntipyreticChlorogenic acidEarly rheumatoid arthritis
The invention discloses a pharmaceutical composition for preventing and treating rheumatoid arthritis. The traditional Chinese medicine raw materials for constituting active ingredients of the pharmaceutical composition by weight are as follows: 5.5-6.0mg of berberine, 0.3-0.4mg of palmatine, 1.5-2.0mg of jatrorrhizine, 1.5-1.8mg of magnoflorine, 0.1-0.2mg of phellodendrine, 0.2-0.3mg of coptisine, 55-60mg of baicalin, 1.5-2.0mg of baicalein, 5.0-7.0mg of wogonoside, 0.03-0.05mg of wogonin, 20-25mg of geniposide, 0.1-0.2mg of crocin and 0.25-0.3mg of chlorogenic acid. The results of pharmacological tests show that the pharmaceutical composition can significantly reduce the disease severity of CIA mice with the rheumatoid arthritis, significantly improve the inflammation and the immune injury situation at lesion sites in comparison with a control group and be used for preparing drugs for preventing and treating the rheumatoid arthritis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
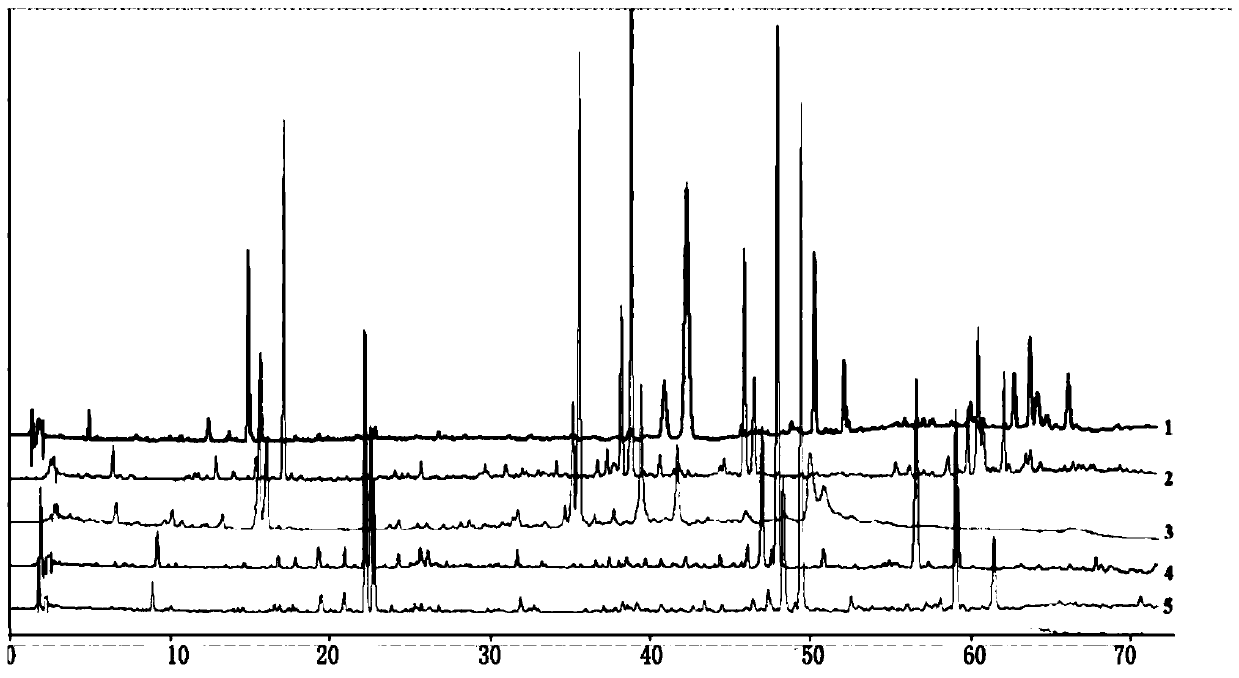
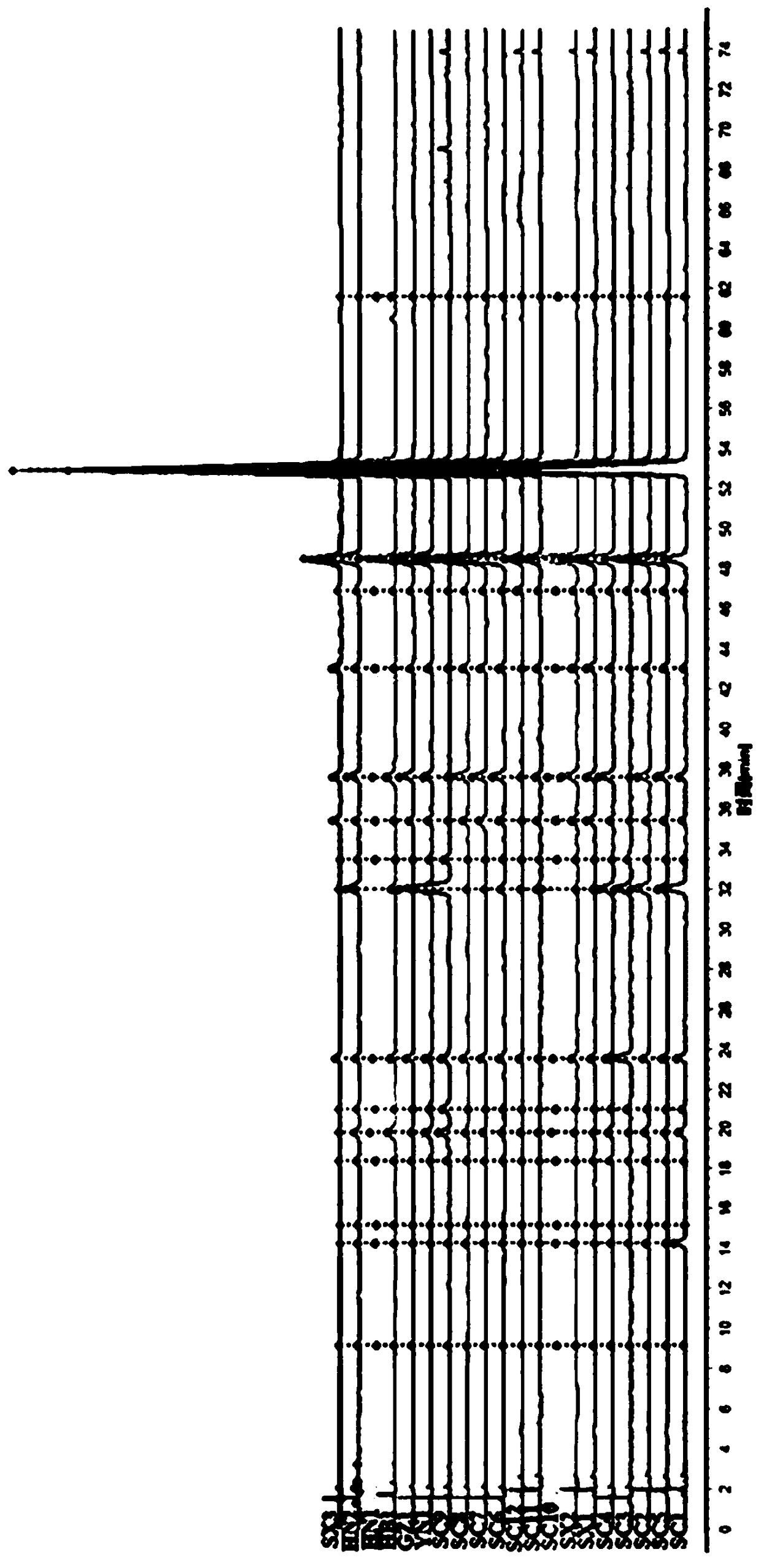
Lanqin oral solution fingerprint spectrum establishment method, fingerprint spectrum of Lanqin oral solution and application of fingerprint spectrum
ActiveCN110108825ACharacterizing qualityAvoid one-sidednessComponent separationBenzoic acidChlorogenic acid
The invention discloses a Lanqin oral solution fingerprint spectrum establishment method, a fingerprint spectrum of a Lanqin oral solution and application of the fingerprint spectrum. A test solutionis prepared from the Lanqin oral solution, a reference substance solution is a solution which dissolves adenosine, epigoitrin, phellodendrine chloride, chlorogenic acid, magnoflorine, geniposide, benzoic acid, berberine hydrochloride, baicalin, wogonoside, baicalein and wogonin, and the liquid chromatography is measured by using a high-performance liquid chromatograph; the obtained liquid chromatography is introduced into a traditional Chinese medicine chromatographic fingerprint spectrum similarity evaluation system for analysis, and the Lanqin oral solution fingerprint spectrum is obtained after multi-point correction, data matching and analysis. According to the Lanqin oral solution fingerprint spectrum establishment method and the fingerprint spectrum of the Lanqin oral solution, the quality information of the Lanqin oral solution can be comprehensively reflected, and uniform and stable product quality is ensured.
Owner:YANGTZE RIVER PHARMA GRP JIA NGSU LONGFENGTANG TRADITIONAL CHINESE MEDICINE CO LTD +2
Phellodendrine monomer and preparation method of salt thereof
The invention belongs to the technical field of natural medicine extraction, and relates to a phellodendrine monomer and a preparation method of salt thereof. The method comprises the steps of: preparing the phellodendrine monomer through acid chloride solution extraction, macroporous adsorption resin enrichment, alumina column chromatographic purification and the like; and recrystallizing the phellodendrine monomer so as to obtain a phellodendrine salt monomer. The method has the advantages of low preparation cost, short period and high prepared product purity, and is convenient to operate.
Owner:SOUTHWEST JIAOTONG UNIV
Chinese medicinal capsules for treating prostatitis and prostatic hyperplasia and preparation method for Chinese medicinal capsules
InactiveCN102526305AMask bad smellImprove stabilityUrinary disorderCapsule deliverySalvianolic acid BTanshinone IIA
The invention relates to the field of Chinese medicines and discloses Chinese medicinal capsules for treating prostatitis and prostatic hyperplasia and a preparation method for the Chinese medicinal capsules. By the preparation method, the transfer rates of eight medicinal active ingredients, namely phellodendrine, tanshinone IIA, salvianolic acid B, paeoniflorin, amarogentin, linderane, vaccarin and imperatorin in phellodendron, red paeony root, root of red-rooted salvia, peach kernel, root of combined spicebush, cowherb seed and angelica dahurica are all improved to over 95 percent, and are remarkably higher than the transfer rate obtained by the conventional method in China, so that the medicinal effect is improved and the cost is reduced.
Owner:史志辉 +1
Preservative for loquat fruits
InactiveCN104642517AProtect NutrientsReduce colorFruit and vegetables preservationGastrodia Elata ExtractAdditive ingredient
The invention discloses a preservative for loquat fruits, and belongs to the field of preserving of fruits. The preservative is prepared from the following components in parts by weight: 10 to 30 parts of phytic acid, 12 to 25 parts of cinnamon, 3 to 12 parts of betel nut, 4 to 15 parts of clove, 2 to 10 parts of tea polyphenol, 4 to 12 parts of phellodendrine, and 2 to 11 parts of a gastrodia elata extract. The preservative is long in preserving cycle for loquat fruits, does not change the original taste of loquat fruits, and is safe to use; the adopted raw materials are harmless to a human body; the preparation is simple, and the cost is low.
Owner:谢光业
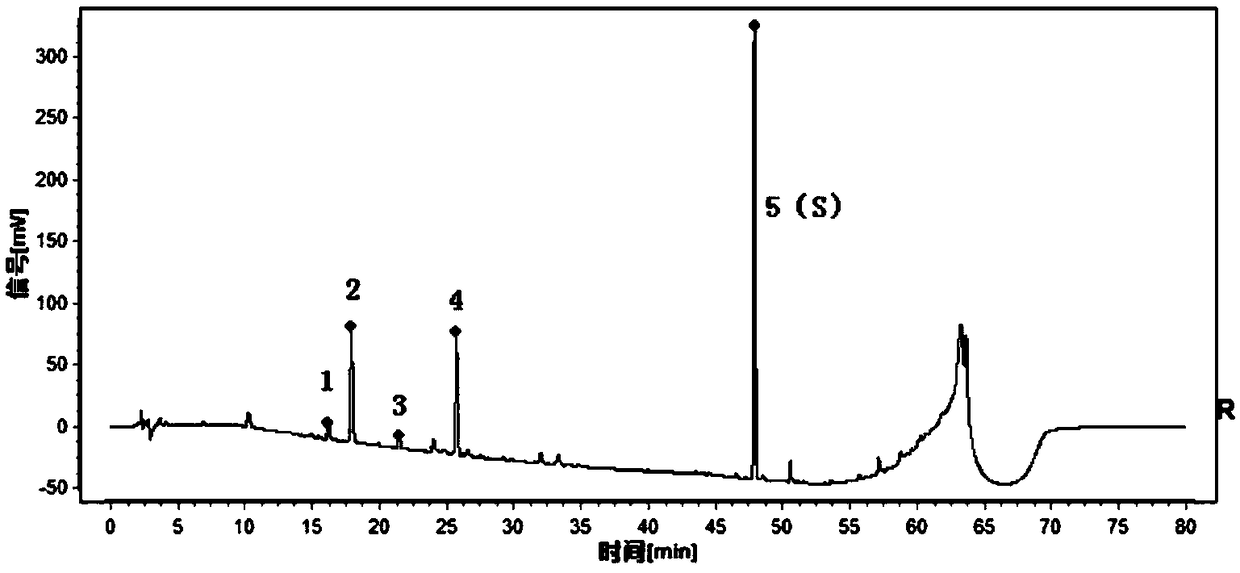
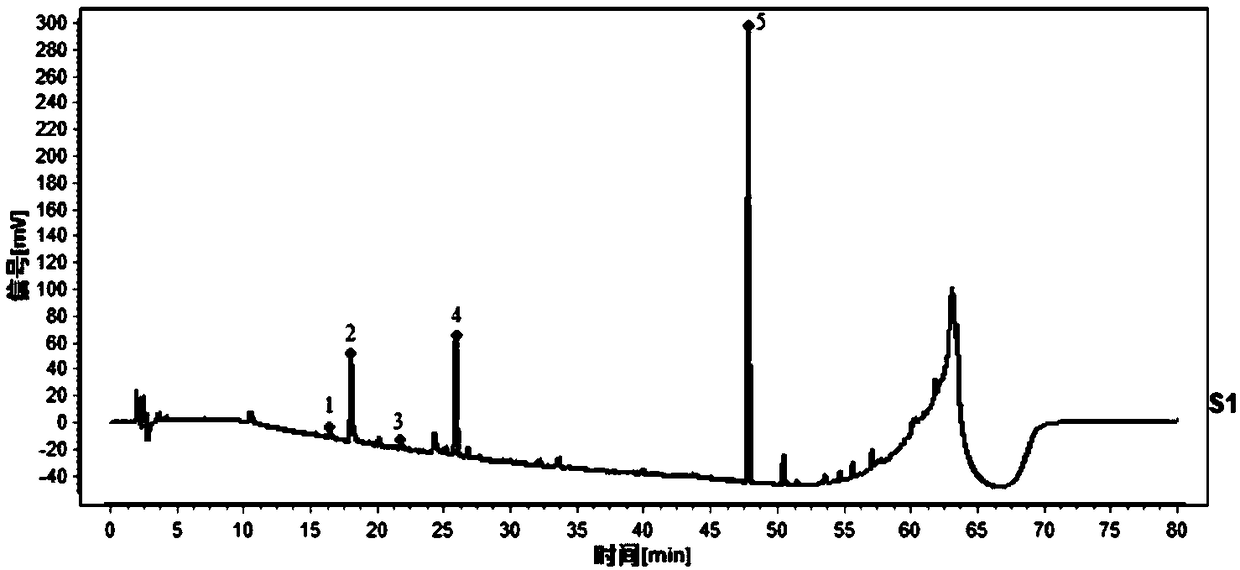
Method for analyzing all components of angelica sinensis six-yellow decoction
PendingCN112229934AAccurate analysisEasy to analyzeComponent separationAstragalosideChlorogenic acid
The invention provides a method for analyzing all components of an angelica sinensis six-yellow decoction, and belongs to the technical field of pharmaceutical analysis. An ultra-high performance liquid chromatography tandem quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS) technology is adopted to detect an angelica sinensis six-yellow decoction sample, and 15 chemical reference substances are adopted at the same time: baicalin, wogonoside, berberine hydrochloride, epiberberine hydrochloride, coptisine hydrochloride, palmatine hydrochloride, phellodendrine hydrochloride, astragaloside, calycosin-7-glucoside, ferulic acid, chlorogenic acid, ligustilide, verbascoside, baicalein and wogonin are adopted to confirm a detection result of the angelica sinensis six-yellow decoction andclearly identify 68 components; comprehensive analysis of the components of the angelica sinensis six-yellow decoction is realized, and the reliability of quality control is improved.
Owner:山东宏济堂制药集团股份有限公司
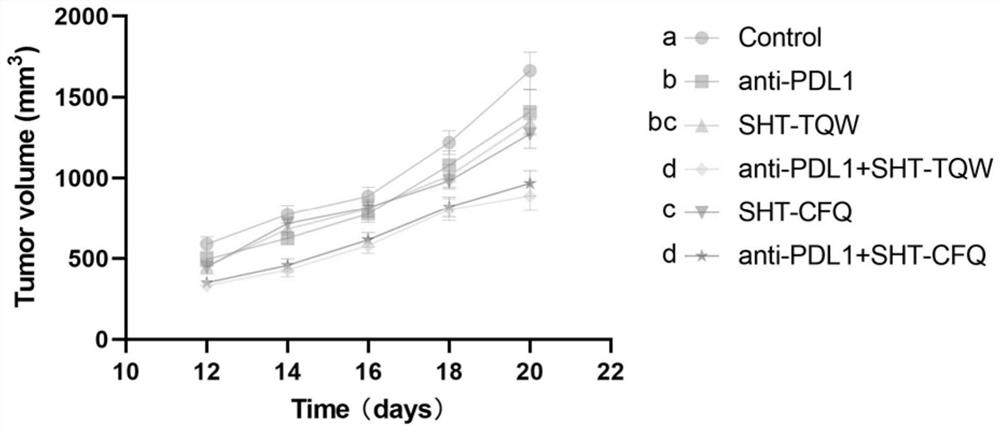
Anti-tumor combined composition, application thereof and anti-tumor drug
PendingCN114870009AEnhanced inhibitory effectGood treatment effectOrganic active ingredientsAntibody ingredientsBerberinePharmaceutical drug
The invention provides an anti-tumor combined composition, application thereof and an anti-tumor drug, and relates to the technical field of anti-tumor drugs. The anti-tumor combined composition comprises the following combined medicines: an effective component and an immune checkpoint inhibitor, the effective components comprise at least one of berberine, baicalin and phellodendrine. The invention further provides application of the anti-tumor combined composition and an anti-tumor drug prepared from the anti-tumor combined composition. The traditional Chinese medicine composition can be used for treating cancers in a drug combination manner, and can effectively enhance the inhibition effect on tumors and improve the treatment effect on the tumors.
Owner:HEZE UNIV +1
High-temperature chain lubricant and preparation method thereof
InactiveCN104342263AHas little effectImprove high temperature resistanceLubricant compositionPolyethylene terephtalateCarvacryl acetate
The invention discloses a high-temperature chain lubricant and a preparation method thereof. The high-temperature chain lubricant comprises the following raw materials in parts by weight: 10-30 parts of polydimethyl terephthalate, 20-80 parts of polyvinyl pyridine, 60-100 parts of vinyl acetate, 10-50 parts of zinc sulfide, 40-60 parts of p-phenylenediamine, 70-100 parts of zinc barium stabilizer, 10-20 parts of phellodendrine, 10-20 parts of magnoline, 5-20 parts of silane coupling agent, 6-10 parts of kaolin powder, 40-60 parts of defoamer and 40-80 parts of modifier. The preparation method comprises the following steps: uniformly mixing the polydimethyl terephthalate, polyvinyl pyridine, vinyl acetate, zinc sulfide and p-phenylenediamine, heating to react, and cooling; adding the zinc barium stabilizer, phellodendrine, magnoline, silane coupling agent and kaolin powder, and heating to react; and cooling, adding the defoamer and modifier and stirring uniformly. The melting point is 309-318 DEG C; and the high-temperature chain lubricant has the advantages of favorable high temperature resistance and stable properties, and is slightly influenced by the temperature change.
Owner:SUZHOU CHANGSHENG ELECTROMECHANICAL
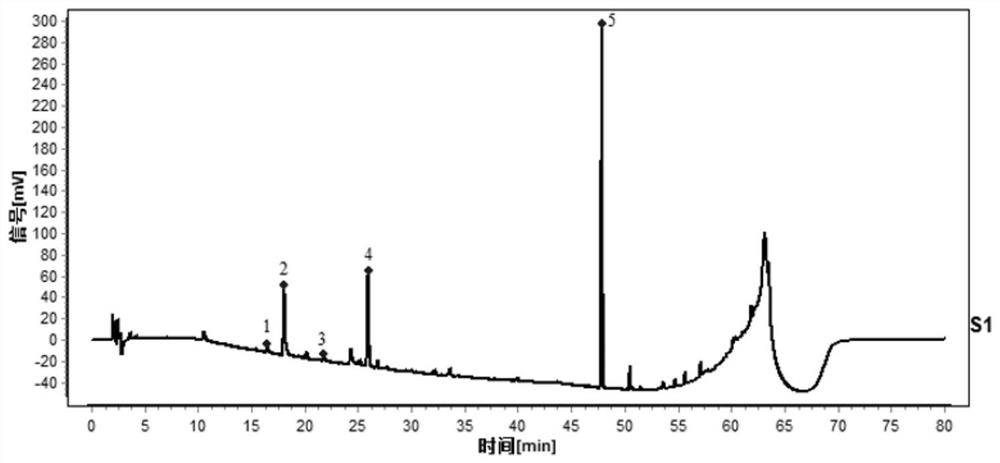
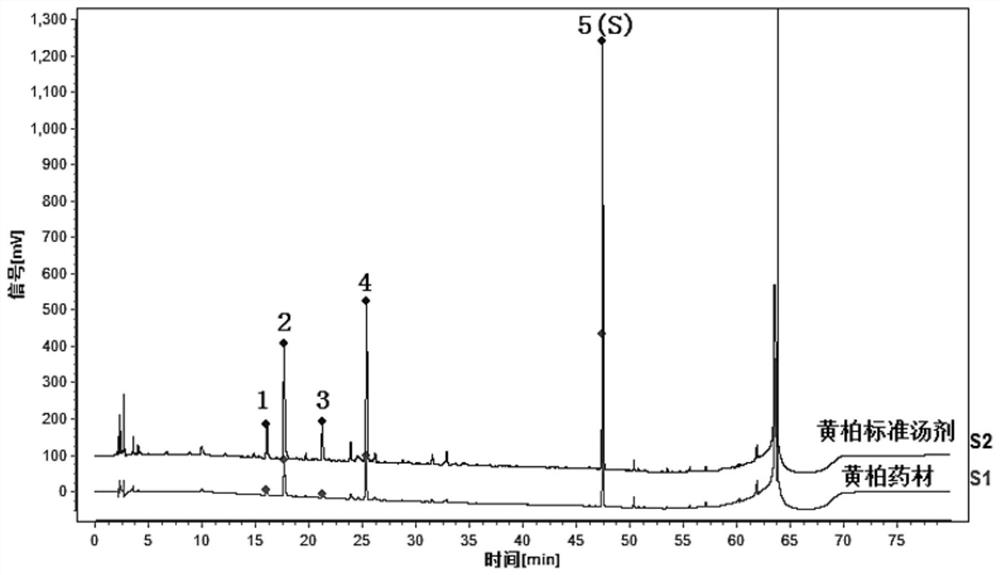
Construction method and detection method of HPLC feature map of golden cypress medicinal material
ActiveCN109374789AEasy to separateRich relevant informationComponent separationMedicineWater soluble
The invention relates to a construction method and a detection method of a HPLC feature map of a golden cypress medicinal material. The construction method comprises the following steps: reference solution preparation: preparing a golden cypress control medicinal material reference solution and a berberine hydrochloride and phellodendrine hydrochloride reference substance solution; sample solutionpreparation: preparing a golden cypress medicinal material sample solution and a decoction sample solution; high-performance liquid chromatography test: absorbing the control medicinal material reference solution, the reference substance solution, the medicinal material sample solution and the decoction sample solution; injecting into a liquid chromatograph, comparing a medicinal material samplemap with a decoction sample map, determining water soluble common peaks and obtaining the UPLC feature map of the golden cypress medicinal material. The HPLC feature map obtained by the construction method not only can qualitatively and quantitatively analyze the quality of the golden cypress medicinal material, but also can reflect the quality of golden cypress standard decoction as a reference.
Owner:GUANGDONG YIFANG PHARMA
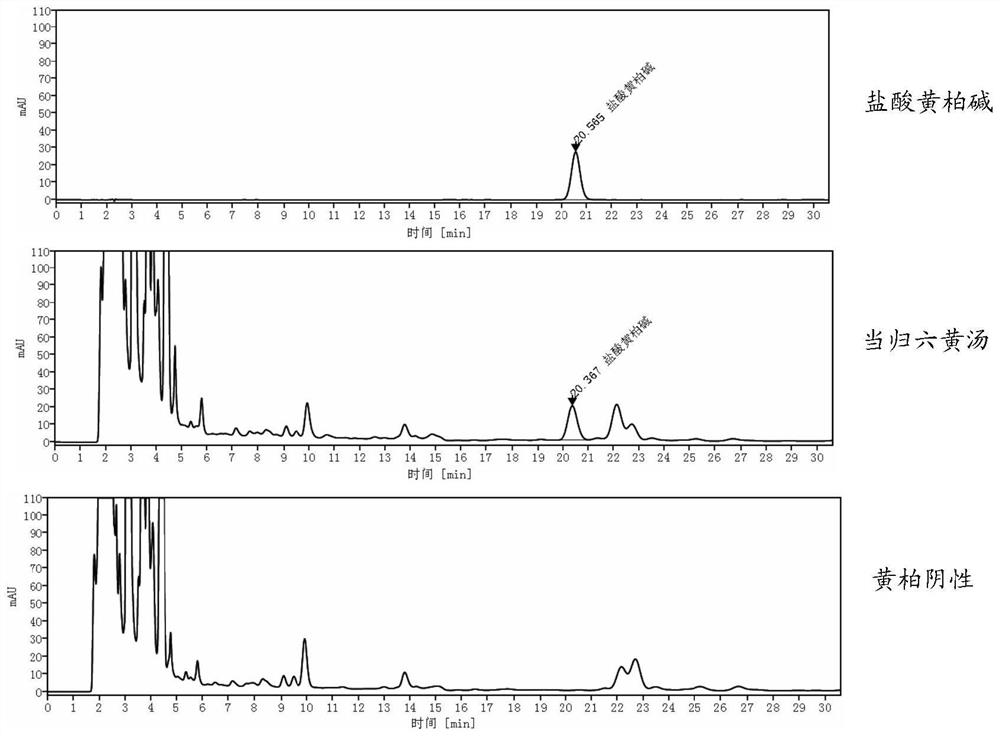
Detection method of danggui liuhuang decoction
ActiveCN112098556AImprove quality controlQuality assuranceComponent separationAgainst vector-borne diseasesBiotechnologyAstragaloside
The invention relates to a detection method of an danggui liuhuang decoction, and the detection method comprises a fingerprint spectrum and a content determination method. The fingerprint spectrum detection method comprises the following steps: a, constructing a control fingerprint spectrum of the danggui liuhuang decoction; b, employing the control fingerprint spectrum of the danggui liuhuang decoction for similarity evaluation of the danggui liuhuang decoction fingerprint spectrum detection. The content determination method comprises the step of determining the content of baicalin, coptisine, berberine, phellodendrine and astragaloside in the angelica sinensis six-yellow soup. According to the method, the fingerprint spectrum and multi-component content determination are established at the same time, the overall quality control of the angelica sinensis six-yellow soup can be realized, and the repeatability, stability, durability and the like of the method are relatively good.
Owner:山东宏济堂制药集团股份有限公司
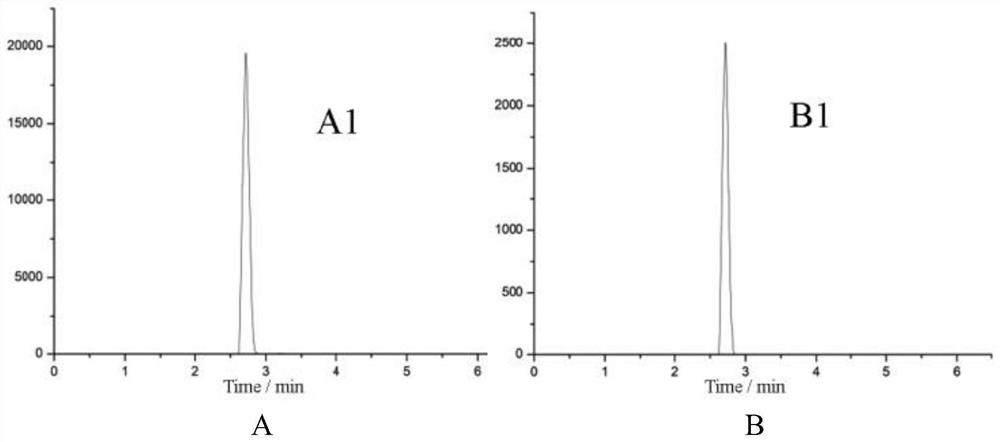
SPE-HPLC method for determining content of phellodendrine in Leshu lotion
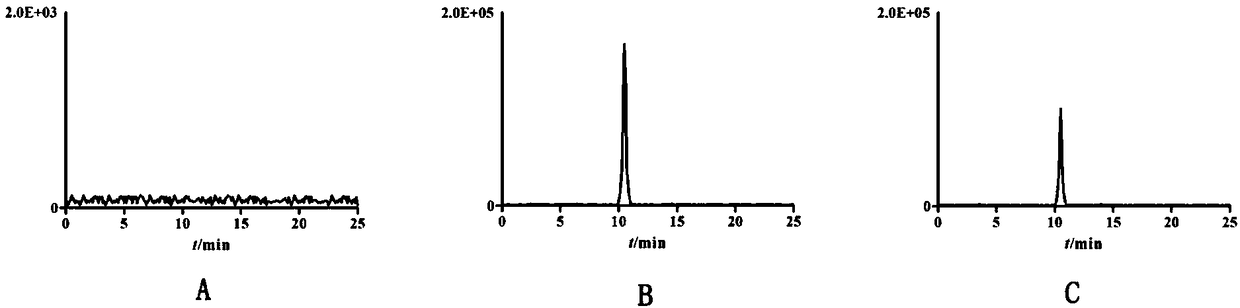
The invention relates to a SPE-HPLC method for determining a content of phellodendrine in Leshu washing lotion. The method comprises the following steps of: A, preparing aliquid of a reference substance; B, preparing aliquid of a test sample: preparing the liquidof the test sample by adopting a solid phase extraction method; C, performing the liquid chromatographic detection; D, establishing a standard curve; and E, determining a sample. The testedliquid is prepared by selecting the solid phase extraction method, the extraction recovery rate about 100 percent can be obtained, inorganic salts and organic impurities in a test sample can be effectively removed, and the service life of a chromatographic column can be prolonged. The solid phase extraction method can process the sample in batches and is easy in automation, so that the working efficiency can be improved. A majority of methods reported by the existing literatures adopt a C18 chromatographic column, and the present method selects an amino column as an analysis column, so thatcompared with the C18 chromatographic column, the amino column has the advantages of high column efficiency, sharp peak pattern and little interference. The method is simple in operation, accurate in result, good in repeatability and less in impurity interference in the analysis process.
Owner:LIUZHOU CITY HEALTHCARE HOSPITAL FOR WOMEN & CHILDREN
Pharmaceutical composition for preventing and treating cerebral infarction
ActiveCN110664809APrevent or treat cerebral hemorrhagePrevention and treatment of cerebral thrombosisOrganic active ingredientsCardiovascular disorderTherapeutic effectCerebral ischaemia
The invention relates to a pharmaceutical composition. The pharmaceutical composition is prepared from phellodendrine, paeoniflorin and optional EGCG (epigallocatechin gallate). The pharmaceutical composition can prevent or treat brain tissue damage caused by local and whole cerebral ischemia, can specifically prevent or treat cerebral hemorrhage, cerebral infarction, cerebral thrombosis and otherdiseases, especially has a very good treatment effect on cerebral infarction, and is high in safety and free from side effect.
Owner:JIAMUSI UNIVERSITY
Method for establishing fingerprint spectrum of bactericidal itching-relieving lotion
ActiveCN111175429AHigh technical contentAvoid monotonyComponent separationMedicinal herbsPharmacologic action
The invention provides a method for establishing a fingerprint spectrum of a bactericidal itching-relieving lotion. The method comprises the following steps: S1, preparing a mixed reference substancesolution, namely precisely weighing matrine, astilbin, phellodendrine, berberine hydrochloride, cnidium lactone and atractylodes lancea reference substances respectively, and adding methanol to prepare the mixed reference substance solution; S2, preparing a bactericidal itching-relieving lotion sample solution; S3, preparing a single reference medicinal material solution; and S4, determining. Thefingerprint spectrum calibrates 16 common peaks and determines the five medicinal materials belonging to a prescription, the established fingerprint spectrum has high technical content, and the singleness and one-sidedness of the existing quality control method are avoided. An effective quality control basis is provided for the production of the bactericidal itching-relieving lotion, and a foundation is laid for the research on the correlation between the pharmacodynamic material basis and the pharmacological action of the bactericidal itching-relieving lotion.
Owner:贵州长生药业有限责任公司
Method for separating phellodendrine glucuronic acid conjugate from urine
ActiveCN109942640AEasy to operateLow costSugar derivativesComponent separationChemical structureSephadex
The invention discloses a method for separating phellodendrine glucuronic acid conjugate from urine. The method comprises the steps that a urine sample is subjected to AB-8 macroreticular resin separation twice, and an ODS open column is utilized for enriching metabolites; a Sephadex LH20 gel column is utilized for purification; obtained flow components are prepared into a target product by utilizing an Agilent 1200 type preparation solution, and LC-MSn analysis verification is utilized for identifying the structure of the product. According to the method, through separation and extraction, the in-vivo phellodendrine metabolite is obtained, the chemical structure of the in-vivo phellodendrine metabolite is determined, quantitative analysis is conducted, and feasibility conditions and experiment bases are provided for analysis of the fragmentation pattern and conversion relationship of the in-vivo phellodendrine metabolite and the in-vivo pharmacokinetic study and pharmacologic action mechanism study of the in-vivo phellodendrine metabolite. The method has the advantages of simple operation, low cost, high efficiency and the like.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Quality control method of traumatology department yellow-water preparation
InactiveCN108267529AGood peak separationEasy to popularize and masterComponent separationChlorogenic acidReference product
The invention provides a quality control method of a traumatology department yellow-water preparation. The method comprises the following steps of (1) weighing phellodendrine chloride, jatrorrhizine hydrochloride, epiberberine chloride, coptisine chloride, palmatine chloride, berberine hydrochloride, geniposide and chlorogenic acid; adding absolute methanol or methanol water solution for preparinga reference product solution; (2) taking contents of the traumatology department yellow-water preparation to be tested; adding absolute methanol or methanol water solution for dissolution; performingfiltering; taking subsequent filtering liquid to be as a solution to be tested; (3) respectively taking the reference product solution and 1 to 5 muL of the solution to be tested; injecting the materials into a liquid chromatography-mass spectrometry instrument for detection; obtaining the contents of phellodendrine, jateorhizine, epiberberine, coptisine, palmatine, berberine, geniposide and chlorogenic acid through calculating a quantitative chromatogram map. The method has the advantages that the original deficient quantitative evaluation index can be completed by once quantitative determination. The quantitative chromatogram map of the method can show good wave peak separation; the identification by the method is fast and accurate.
Owner:FOSHAN HOSPITAL OF TCM
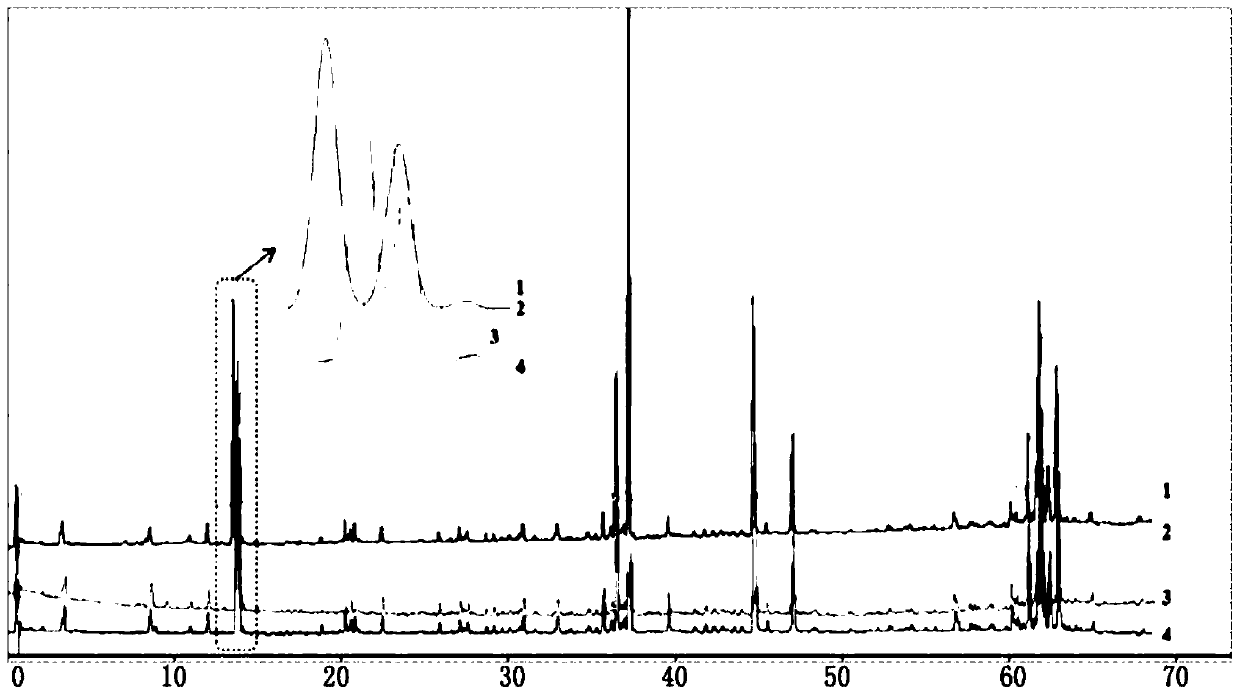
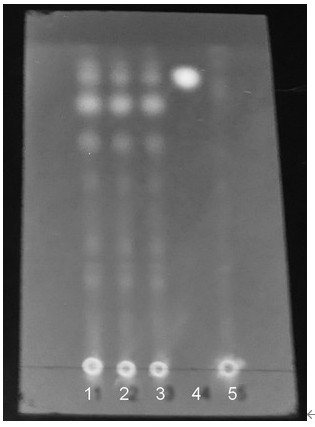
Cortex phellodendron chinese identification method and application thereof
InactiveCN109507312ACharacterizing qualityAvoid one-sidednessComponent separationPrincipal component analysisMedicine
The invention discloses a cortex phellodendron chinese identification method. According to the identification method, cortex phellodendron chinese is used for preparing a solution of a test product, solutions in which a berberine hydrochloride reference substance, a phellodendrine chloride reference substance and a magnoflorine reference substance are dissolved are used as reference substance solutions, and through high performance liquid chromatograph measurement, liquid chromatograms of the solution of the test product and the reference substance solutions are obtained respectively; the obtained liquid chromatograms are guided into a traditional Chinese medicine chromatograph fingerprint similarity evaluation system for analysis, through analysis, a cortex phellodendron chinese fingerprint is obtained, and hierarchical cluster analysis or principal component analysis or partial least square analysis is adopted for processing data of the cortex phellodendron chinese fingerprint, so that classification identification is conducted on the cortex phellodendron chinese or processed products of the cortex phellodendron chinese. According to the cortex phellodendron chinese identification method, on the basis of building the cortex phellodendron chinese fingerprint, the cortex phellodendron chinese index component content is measured, and by combining different chemical mode identification methods, the cortex phellodendron chinese, the processed products of the cortex phellodendron chinese and fake cortex phellodendri amurensis can be effectively identified and distinguished.
Owner:YANGTZE RIVER PHARM GRP CO LTD +1
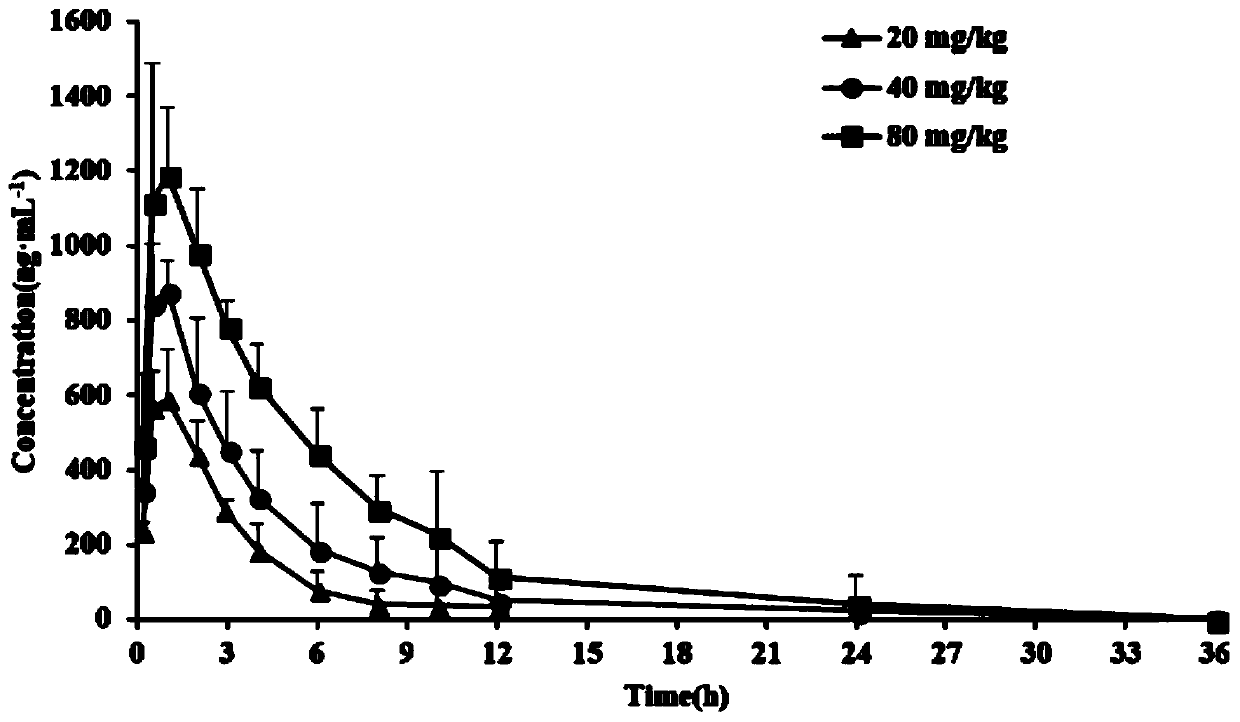
Application of phellodendrine or pharmaceutical salt of phellodendrine in tumor treatment aspect
ActiveCN108309976AInhibit proliferation and induce apoptosisInhibit apoptosisOrganic active ingredientsAntineoplastic agentsApoptosisWilms' tumor
The invention discloses application of phellodendrine or pharmaceutical salt of the phellodendrine in the tumor treatment aspect. The application finds that the phellodendrine or the pharmaceutical salt of the phellodendrine has the inhibiting effect on breast cancer, colon cancer, liver cancer, gastric cancer, KRAS mutant pancreatic cancer and leukemia, has no obvious inhibition on human normal cells and is hopeful to be developed into low-toxicity anti-tumor medicine, wherein by inhibiting the macropinocytosis effect of KRAS mutant pancreatic cancer cells and reducing the Bcl-2 / Bax expression ratio in the KRAS mutant pancreatic cancer, the phellodendrine or the pharmaceutical salt of the phellodendrine inhibits the multiplication of the KRAS mutant pancreatic cancer and induces the KRASmutant pancreatic cancer to apoptosis. Therefore the phellodendrine or the pharmaceutical salt of the phellodendrine can be developed into a medicine preparation for resisting breast cancer, colon cancer, liver cancer, gastric cancer, KRAS mutant pancreatic cancer or leukemia; the preparation contains the phellodendrine, the pharmaceutical salt of the phellodendrine or prodrug of the phellodendrine, also contains a pharmaceutically acceptable carrier or excipient and is prepared into a pharmaceutically acceptable dosage form.
Owner:CHINA PHARM UNIV
Method for determining content of multi-component chemical components in Chinese pulsatilla root decoction
ActiveCN113884587AHigh sensitivityComponent separationAgainst vector-borne diseasesBerberine hydrochlorideChemical constituents
The invention discloses a method for determining the content of multi-component chemical components in Chinese pulsatilla root decoction. The method specifically comprises the following steps: respectively establishing standard curves of anemosides B4, B5, D and A3, hederasaponin A1, alpha-hederasaponin, aesculin, aesculetin, fraxetin, phellodendrine, palmatine hydrochloride, coptisine hydrochloride and berberine hydrochloride by utilizing an HPLC-MSMS (High Performance Liquid Chromatography-Mass Spectrometry Mass Spectrometry) analysis method; measuring a Chinese pulsatilla root decoction sample extracted by a decoction method, making a standard curve, and measuring the content of the 13 chemical components. A scientific basis is provided for quality control of the Chinese pulsatilla root decoction and related preparations thereof.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Improved Longqing capsule quality detection method
PendingCN114544852AOptimized thin layer identificationOptimizing the Determination of Phellodendrine HydrochlorideComponent separationAgainst vector-borne diseasesBiotechnologyPatrinia
The invention relates to an improved Longqing capsule quality detection method. The detection method comprises the following steps: carrying out thin-layer identification inspection on coptis chinensis, cortex phellodendri, patrinia herb and rhizoma alismatis medicinal materials in a capsule, and determining the contents of phellodendrine hydrochloride, 23-acetyl alisol B and 23-acetyl alisol C in the capsule. Through methodological investigation, the detection method has the effects of being high in specificity, good in durability and good in repeatability, the quality condition of the Longqing capsules can be effectively detected, and the product quality is further improved.
Owner:贵州远程制药有限责任公司
Method for preparing cortex Phellodendri extract at room temperature
InactiveCN109223887AReduce the chance of oxidationReduce pollutionPlant ingredientsBerberineRoom temperature
The invention relates to a method for preparing the cortex Phellodendri extract at the room temperature. The method comprises the following steps: drying Phellodendron amurense, pulverizing, and putting into an infrared plasma surface modification device; The device comprises: a sealed and vacuumizable shell, an ultrasonic tank body arranged in the shell, an infrared ray emitting device and a plasma generating device arranged on the ultrasonic tank body; Water is added into the ultrasonic tank, and nanometer particles of berberine and phellodendrine are added at room temperature with vacuum degree of 10-25mmHg, star an ultrasonic device, an infrared ray emitting device and a plasma generate device, extracting and filtering to obtain phellodendron extract. The preparation method of Phellodendron amurense extract under normal temperature has the characteristics of low energy consumption, clean environment protection and high yield.
Owner:JIANGSU XINGYE POLYMERIZATION CO LTD
Medication composition of plant estrogens for treating colpitis
InactiveCN100418578CImprove vaginal flexibilityImprove the immunityOrganic active ingredientsSexual disorderCnidium monnieriPhellodendrine
A composite vegetative estrin medicine in the form of suppository, gel or film for treating vagititis and improving the toughness of vagina and its resistance to bacteria is proportionally prepared from vegetative estrin, cnidiadin, borneol, and at least one of matrine, phellodendrine, berberine, etc and their physiocologically acceptable salts.
Owner:MEDICAL COLLEGE OF CHINESE PEOPLES ARMED POLICE FORCE
Thin-layer chromatography identification method of Shenan capsule
InactiveCN112710772AImprove and improve the quality detection methodShort manufacturing timeComponent separationAgainst vector-borne diseasesThin layer chromatographicUrsolic acid
The invention provides a thin-layer chromatography identification method of a Shenan capsule, which comprises the following steps: carrying out thin-layer chromatography identification on the Shenan capsule, and judging whether one or more of chalepin acetate, ursolic acid, atractylodis ketone, liquiritin and phellodendrine hydrochloride are contained or not. Compared with the existing Shenan capsule medicine standard, the qualitative identification indexes of five representative components are increased, the identification method is more comprehensive in contrast, high in specificity, intuitive and clear in judgment and objective and reliable in conclusion, and a technical method is provided for perfecting and improving the quality standard of the national medicine Shenan capsule.
Owner:段复华
Fingerprint construction method and detection method of angelica sinensis six-yellow decoction composition
InactiveCN112557553AImprove the level ofEasy to separateComponent separationBiotechnologyChlorogenic acid
The invention discloses a fingerprint construction method and a detection method of an angelica sinensis six-yellow decoction composition, and the construction method comprises the following steps: (1) taking 5-hydroxymethylfurfural, chlorogenic acid, phellodendrine hydrochloride, ferulic acid, baicalin and berberine hydrochloride as reference substances, and respectively preparing reference substance solutions; (2) taking angelica sinensis, radix rehmanniae recen, prepared rehmannia root, scutellaria baicalensis, coptis chinensis, golden cypress and astragalus membranaceus to prepare an angelica sinensis six-yellow decoction composition, and preparing the angelica sinensis six-yellow decoction composition into a test solution; and (3) precisely absorbing the test solution and the reference solutions, injecting the test solution and the reference solutions into a liquid chromatograph for chromatographic analysis with a detection wavelength of 280-320 nm to obtain a test fingerprint anda reference chromatogram, and formulating a standard fingerprint of the angelica sinensis six-yellow decoction composition. The fingerprint construction method can accurately detect the standard fingerprint spectrum of the quality of the angelica sinensis six-yellow decoction composition, and can effectively control the product quality of the angelica sinensis six-yellow decoction preparation.
Owner:SINOPHARM GUANGDONG GLOBAL PHARMA CO LTD
Construction method and detection method of hplc characteristic map of Cortex Phellodendri
The invention relates to a construction method and a detection method of an HPLC characteristic map of Cortex Phellodendri. The construction method includes: preparation of reference substance solution: preparing Phellodendron phellodendron reference substance reference solution, berberine hydrochloride and phellodendron hydrochloride reference substance reference substance solution; preparation of test product solution: preparing phellodendron phellodendron medicinal material for test solution, decoction for testing Product solution; high-performance liquid chromatography test: draw described contrast medicinal material reference substance solution, contrasting substance reference substance solution, medicinal material need testing solution, decoction need testing solution, inject liquid chromatograph, compare gained medicinal material need testing collection of illustrations With the spectrum of the decoction test sample, determine the common peak of water solubility, and obtain the HPLC characteristic spectrum of Phellodendron Phellodendri. The HPLC characteristic spectrum obtained by this construction method can not only qualitatively and quantitatively analyze the quality of Cortex Phellodendri, but also use it as a reference to reflect the quality of Phellodendron Phellodendri standard decoction.
Owner:GUANGDONG YIFANG PHARMA
Medicine composition for treating hemorrhoids, medicinal preparations and application
InactiveCN107669742ASolve usabilitySolve problems such as incomplete targetingAntipyreticAnalgesicsMedicinal herbsTreatment effect
The invention relates to a medicine composition for treating hemorrhoids, medicinal preparations and application. The medicine composition and the medicinal preparations contain individual ingredients, such as honeysuckle stem, lightyellow sophora root, cortex phellodendri and Chinese gall. The medicine composition is prepared from 0.40 to 0.66 part by weight of loganin, 0.47 to 0.66 part by weight of matrine, 1.15 to 1.48 parts by weight of berberine, 0.12 to 0.16 part by weight of phellodendrine, 53.46 to 65.34 parts by weight of gallic acid, 1.86 to 2.66 parts by weight of osthole and 4.79to 5.85 parts by weight of total digua fig stem and leaf flavone. All the individual Chinese herbal medicine ingredients are chosen, excipients are added, and the medicinal preparations are prepared according to conventional preparation processes. The medicine composition and the medicinal preparations prepared therefrom disclosed by the invention have the effects of removing toxin and eliminatingdampness and stopping bleeding and astringing sores, and can achieve a good therapeutic effect when applied to internal hemorrhoids, external hemorrhoids and mixed hemorrhoids caused by the syndromeof accumulated dampness-heat and manifested uncomfortable symptoms such as bleeding, swelling, pain and itching, moreover, toxicity is low, and therefore the medicine composition and the medicinal preparations are ideal medicines.
Owner:贵州拜特制药有限公司
Method for separating and purifying phellodendrine monomer
The invention discloses a method for separating and purifying phellodendrine monomer, which comprises the following steps of: extracting total alkaloid; removing berberine by means of acid deposition; filtering and further removing; separating phellodendrine monomer through a preparative high-efficiency liquid chromatogram; extracting dichloromethane; and drying to prepare the phellodendrine monomer. The method has simple operation, short preparation period, high separation efficiency, stable technology and low cost, and can separate the large number of phellodendrine monomer with high purityto obtain the phellodendrine monomer, the purity of which is more than 98%.
Owner:CHENGDU PUSH BIOLOGICAL TECH
Pharmaceutical composition for preventing and treating rheumatoid arthritis
The invention discloses a pharmaceutical composition for preventing and treating rheumatoid arthritis. The traditional Chinese medicine raw materials for constituting active ingredients of the pharmaceutical composition by weight are as follows: 5.5-6.0mg of berberine, 0.3-0.4mg of palmatine, 1.5-2.0mg of jatrorrhizine, 1.5-1.8mg of magnoflorine, 0.1-0.2mg of phellodendrine, 0.2-0.3mg of coptisine, 55-60mg of baicalin, 1.5-2.0mg of baicalein, 5.0-7.0mg of wogonoside, 0.03-0.05mg of wogonin, 20-25mg of geniposide, 0.1-0.2mg of crocin and 0.25-0.3mg of chlorogenic acid. The results of pharmacological tests show that the pharmaceutical composition can significantly reduce the disease severity of CIA mice with the rheumatoid arthritis, significantly improve the inflammation and the immune injury situation at lesion sites in comparison with a control group and be used for preparing drugs for preventing and treating the rheumatoid arthritis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Preparation method of Chinese angelica and six-yellow preparation
InactiveCN112755092AKeep volatile componentsImprove efficacyAntibacterial agentsMetabolism disorderAngelica Sinensis RootUltrafiltration
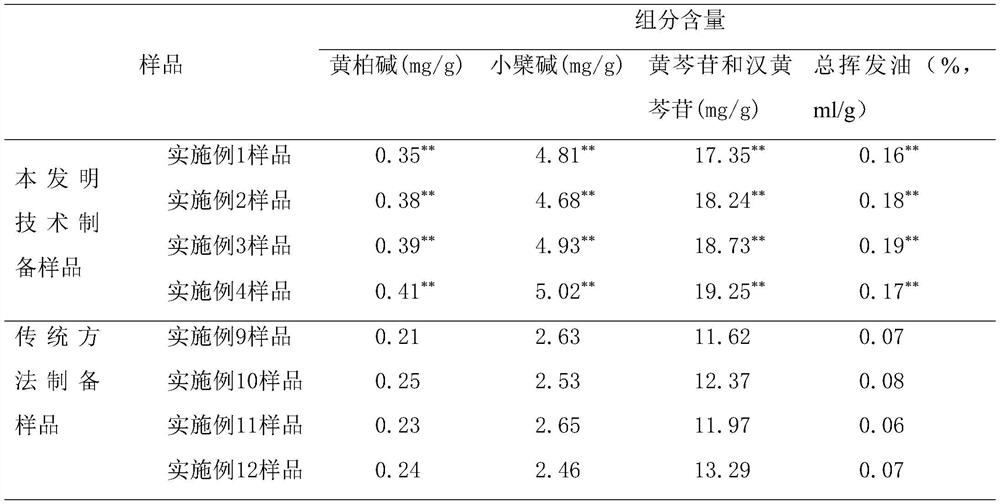
The invention relates to a preparation method of an Angelica sinensis-Liuhuang preparation. The method can improve the content of phellodendrine in the Angelica sinensis-Liuhuang preparation. According to the method, angelica sinensis, rehmannia, prepared rehmannia root, scutellaria baicalensis and astragalus membranaceus are separately treated with coptis chinensis and golden cypress, a membrane filtration technology is introduced to purify an extracting solution, so that volatile components in the angelica sinensis, the rehmannia, the prepared rehmannia root, the scutellaria baicalensis and the astragalus membranaceus are retained, the medicine effect is improved, volatile components in the angelica sinensis, the rehmannia, the prepared rehmannia root, the scutellaria baicalensis and the astragalus membranaceus are concentrated under the room temperature condition through a spiral-wound membrane ultrafiltration technology, and the volatile components and activity thereof are reserved to the maximum extent, the content of the phellodendrine in a Chinese angelica six-yellow preparation prepared by the process is remarkably improved compared with that of the Chinese angelica six-yellow preparation prepared by a traditional process, and HPLC content determination shows that the content of the phellodendrine in the Chinese angelica six-yellow preparation prepared by the process is not less than 0.3 mg / g, the content of berberine is not less than 4 mg / g, the total content of baicalin and wogonoside is not less than 15 mg / g, and the content of total volatile oil is not less than 0.1% (ml / g).
Owner:ZHONGJING WANXI PHARMA CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com