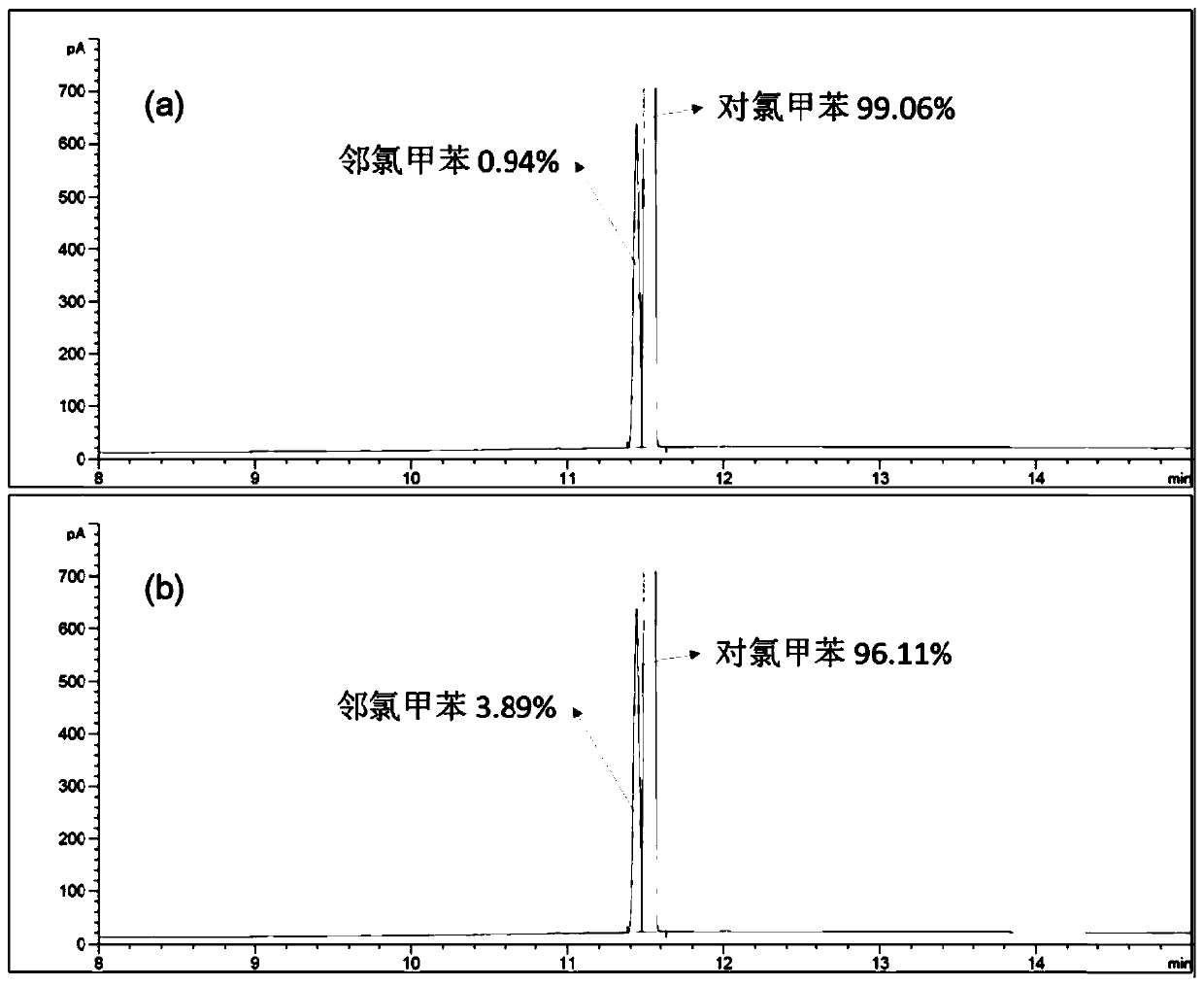
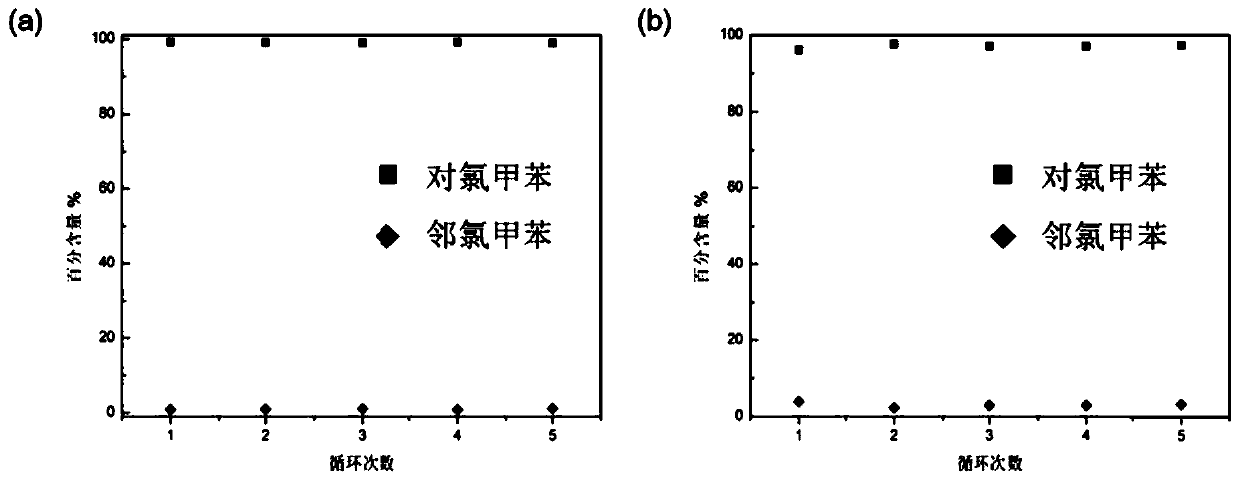
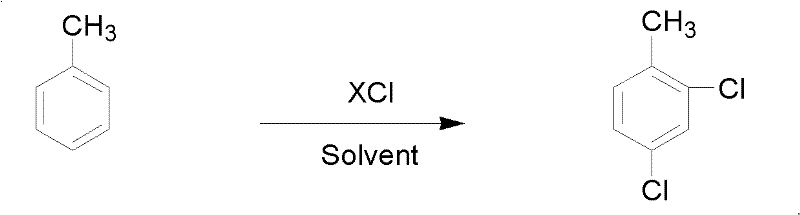
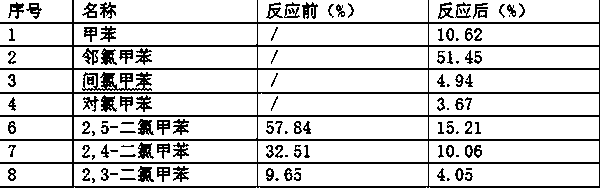
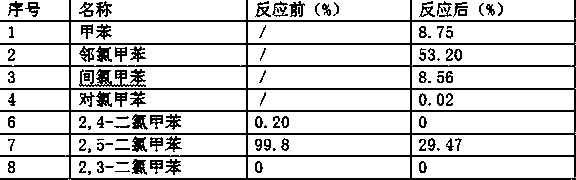
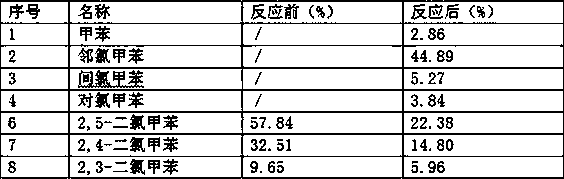
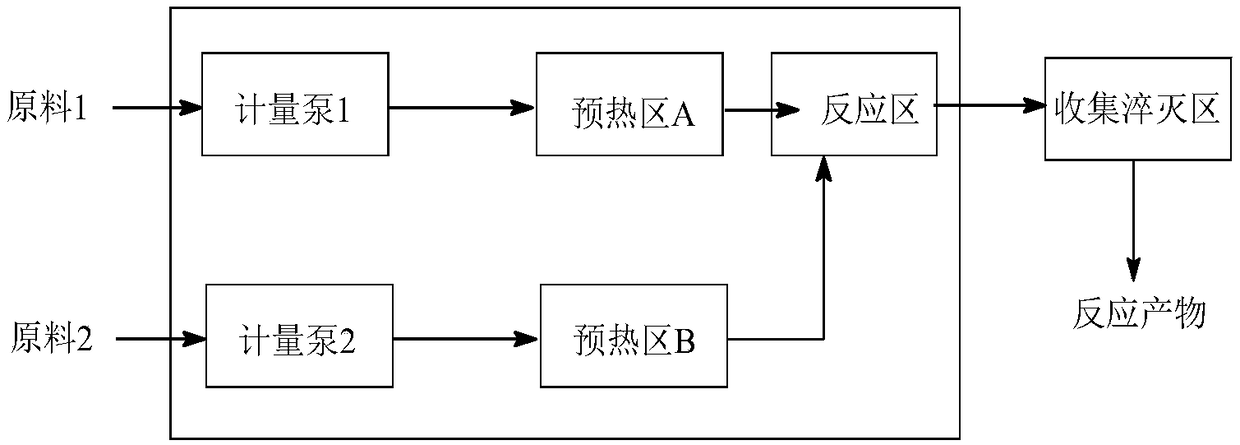
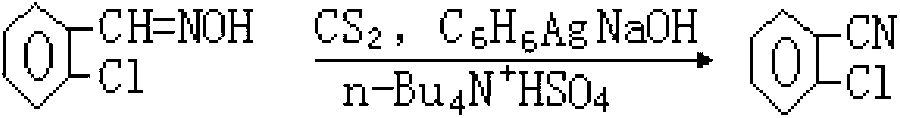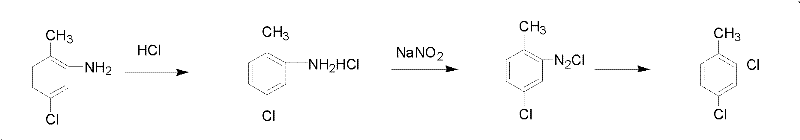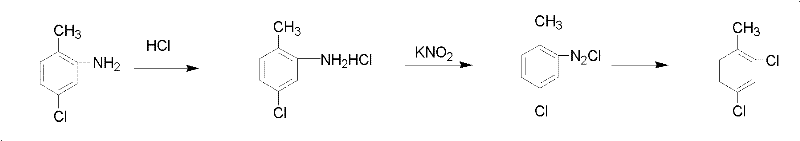Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59 results about "O-chlorotoluene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing CLT acid (6-chloro-3-aminotoluene-4-sulfoacid) by utilizing o-chlorotoluene direct nitrification method
InactiveCN101906057AReduce pollutionLow equipment requirementsSulfonic acid preparationO-chlorotolueneRaw material
The invention relates to a method for preparing 6-chloro-3-aminotoluene-4-sulfoacid (hereinafter referred to as CLT acid) in a direct nitrification way by taking o-chlorotoluene as a raw material. The method comprises the following steps of: obtaining o-chlorotoluene nitrate by taking acidic Beta zeolite as a catalyst and acetyl nitrate as a nitrifying agent; reducing the o-chlorotoluene nitrate by utilizing iron powder; recrystallizing the reduced product and separating out 6-chloro-3-aminotoluene crystal and 6-chloro-4-aminotoluene containing little 6-chloro-3-aminotoluene by adopting an acid extraction method; adding dichlorobenzene and sulfuric acid in the 6-chloro-3-aminotoluene crystal, and carrying out sulfonation at 170-190 DEG C to obtain the CHLOROT acid; and carrying out sulfonation on the 6-chloro-4-aminotoluene containing little 6-chloro-3-aminotoluene to obtain 2B acid containing little CLT acid. The invention has the characteristics of low pollution, high yield, simple process, low device requirements, and the like.
Owner:YANBIAN UNIV
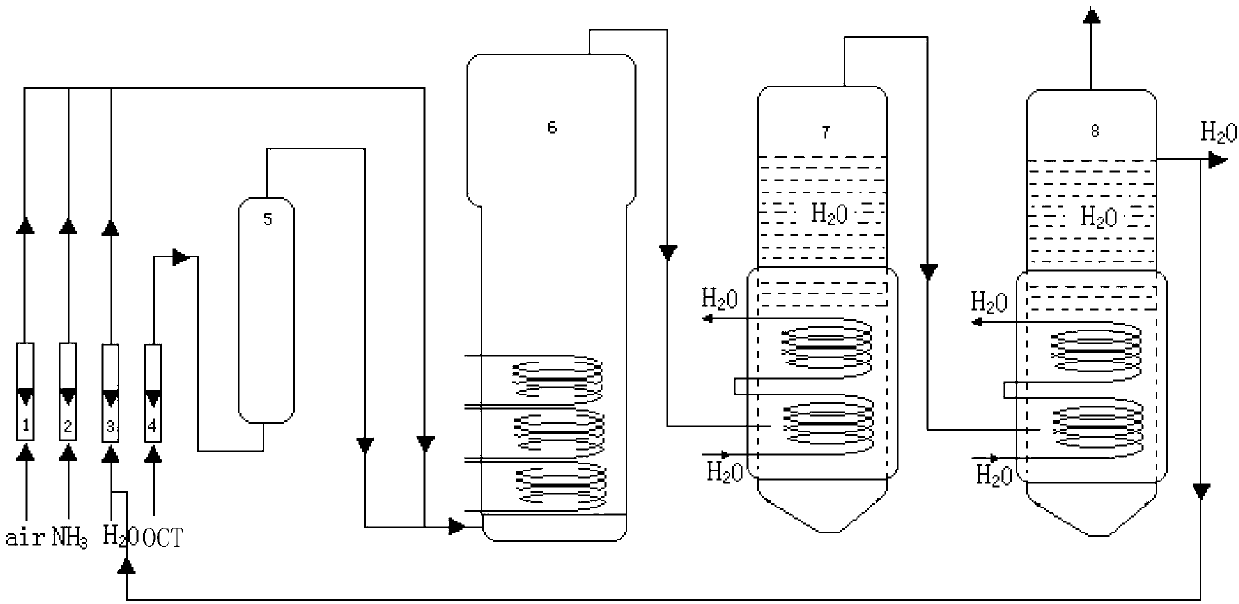
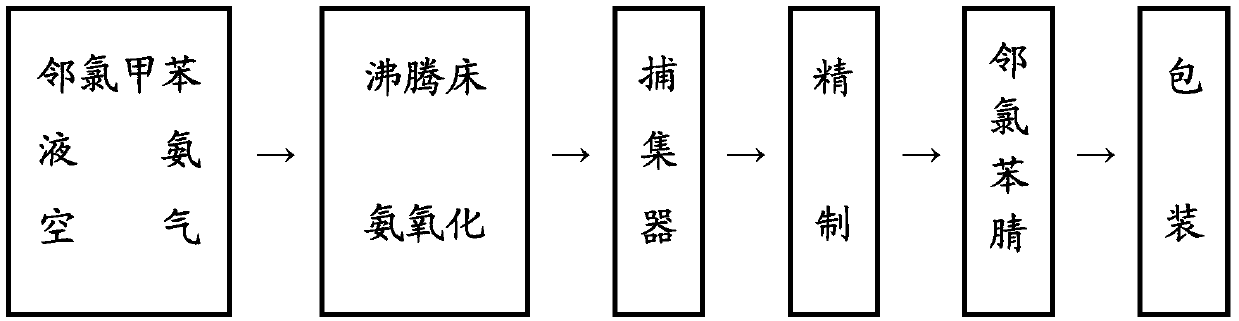
Production process for preparing chlorobenzonitrile through ammoxidation
InactiveCN103102287AMild reaction temperatureSolution to short lifePhysical/chemical process catalystsPreparation by hydrocarbon ammoxidationWater vaporFluidized bed
The invention provides a production process for preparing chlorobenzonitrile through ammoxidation. The production process comprises the following steps of: portioning air, ammonia, water vapor and o-chlorotoluene together; gasifying the o-chlorotoluene and then mixing the gasified o-chlorotoluene with air, ammonia and water vapor, and conveying the mixture to a fluidized bed reactor for reaction under the action of a catalyst; and after the reaction, cooling and crystallizing the generated product through a cold trap, thereby obtaining crude chlorobenzonitrile, wherein the catalyst is VaPbCecSbdOx multi-component catalyst, and wherein a=1, b=0.7-2, c=0.3-1, d=0.5-1, and the value of x is determined according to valence state balance when V, P, Ce and Sb are +5 valent, +5 valent, +4 valent, +5 valent and -2 valent, respectively. The production process provided by the invention employs the VaPbCecSbdOx as the active catalyst; and the catalyst is simple to fabricate, mild in reaction temperature, long in catalyst service life, high in activity, high in selectivity and the like; as a result, side reaction in the production process is suppressed, the production process of chlorobenzonitrile is improved and the yield of product is obviously improved.
Owner:HUBEI JUNTAI PHARMA CHEM
Separation method of p-chlorotoluene and o-chlorotoluene
ActiveCN110092706AReduce manufacturing costImprove stabilityHalogenated hydrocarbon preparationP-chlorotolueneO-chlorotoluene
The invention discloses a separation method of p-chlorotoluene and o-chlorotoluene. A bis-diethoxy column [n] arene crystal material is utilized to adsorb and separate a p-chlorotoluene and o-chlorotoluene mixture, and the chemical structural formula of the bis-diethoxy column [n] arene crystal material is as shown in the specification, wherein n is 5 or 6. The separation method has simple separation process operation and low equipment requirement. No rectification operation is required in the separation process, the energy consumption is low, the energy is saved, and the production cost of the p-chlorotoluene is reduced. The crystal material used has high stability, and can be recycled, and the separation effect cannot be reduced.
Owner:ZHEJIANG UNIV
Preparation method of 2,4-dichlorotoluene
ActiveCN102234218AEasy to recycleImprove securityHalogenated hydrocarbon preparationNitriteDistillation
The invention discloses a preparation method of 2,4-dichlorotoluene, which comprises the following steps: mixing 5-chloro-2-methylaniline with an acid, stirring, cooling to -10-15 DEG C, adding nitrites, controlling the adding speed of the nitrites to allow the temperature to be no more than 15 DEG C, stirring, controlling the temperature to be 15-60 DEG C, and performing a thermal decomposition reaction; after the thermal decomposition reaction, separating an organic phase at the lower layer, and recovering unreacted acids by a graphite absorption tower; washing the organic phase with water to neutrality, performing distillation, removing o-chlorotoluene generated during the reaction at 180-198 DEG C, performing rectification, collecting fractions at 198-201 DEG C to obtain 2,4-dichlorotoluene. The preparation method of 2,4-dichlorotoluene of the invention has high product yield, and low production cost.
Owner:山东福尔有限公司
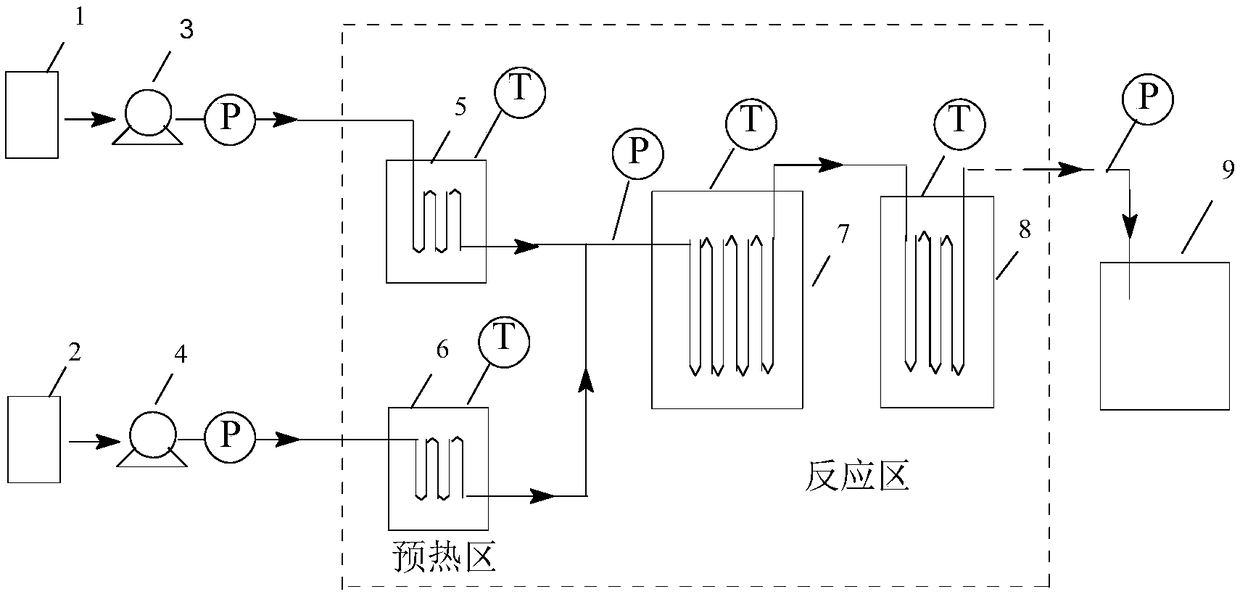
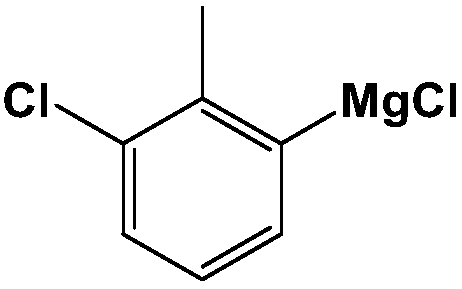
Production equipment and preparation technology for o-chlorotoluene Grignard reagent
ActiveCN104497026AHigh yieldSolve problemsChemical/physical/physico-chemical stationary reactorsMagnesium organic compoundsGrignard reagentO-chlorotoluene
The invention discloses production equipment and a preparation technology for an o-chlorotoluene Grignard reagent. The equipment comprises a first-stage reaction kettle, a second-stage reaction kettle, a tetrahydrofuran storage tank, an o-chlorotoluene storage tank, a magnesium chip storage tank and a toluene storage tank; the center parts of the first-stage reaction kettle and the second-stage reaction kettle are both provided with a separation net for obstructing magnesium chips; and pipelines are employed for connection of the reaction kettles and the storage tanks. Combination of the continuous production equipment and the corresponding preparation technology for the o-chlorotoluene Grignard reagent solves the problems that initiation is difficult and magnesium chips are difficult to separate in a conventional industrial preparation process of the o-chlorotoluene Grignard reagent, and helps to improve the yield of the o-chlorotoluene Grignard reagent.
Owner:SHANGYU HUALUN CHEM
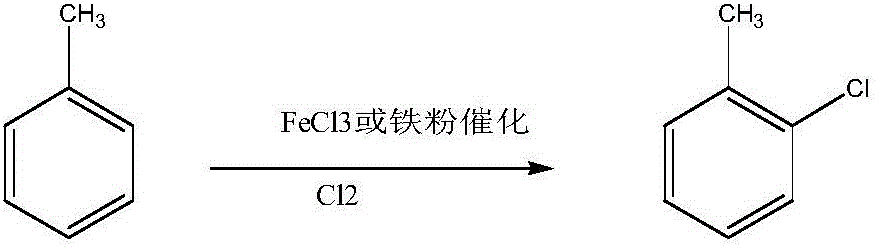
Method for preparing monochlorotoluene with toluene chloridizing method
The invention provides a method for preparing monochlorotoluene with a toluene chloridizing method. The method comprises the following specific steps of: adding iron powder or iron chloride serving as a catalyst into a toluene liquid, adding an aid, introducing chorine gas under a light-shading situation, controlling the temperature of an entire chloridizing process at 0-30 DEG C, and controlling the chlorine-introducing amount to keep the transformation rate of toluene between 96 percent and 99 percent; and cooling, and filtering an ion liquid out to obtain a toluene mono-chlorinated mixed solution. After the aid and iron or iron chloride serving as a catalyst used in the method are mixed and applied to toluene chloridizing, the selectivity of a product on toluene chloride is effectively increased, the highest selectivity on chlorotoluene / o-chlorotoluene is up to 2.1, the selectivity on m-chlorotoluene is less than 0.15 percent, and the selectivity on total dichlorotoluene is less than 1.0 percent.
Owner:常州新东化工发展有限公司
Method for producing chloroethylene by homogeneous mercury-free catalysis
InactiveCN104926592AReduce dosageSimple processPreparation by halogen halide additionO-nitrochlorobenzeneO-chlorotoluene
The invention provides a method for producing chloroethylene by homogeneous mercury-free catalysis. According to the method, acetylene and hydrogen chloride are taken as raw materials and chloroethylene is generated under the effect of a homogeneous catalyst. One or more of PdCl2, AuCl3, FeCl3, ZnCl2 and CuCl2 are taken as catalysts, one or more of NH4Cl, NaCl, KCl, BaCl2, MgCl2 and CaCl2 are taken as cocatalysts, o-nitrochlorobenzene, o-chlorotoluene or o-dichlorobenzene is taken as a solvent, and a homogeneous catalysis system is formed. Under the reaction conditions of normal pressure and temperature of 130 DEG C, the acetylene conversion rate and the chloroethylene selectivity can reach about 95% and more than 95%, and the catalyst life is more than 3,000 hours. The method adopts the nontoxic and mercury-free homogeneous catalyst for producing the chloroethylene, so that a supported catalyst for producing chloroethylene by hydrochlorination of acetylene in existing production is avoided, the problems of easiness for generating carbon deposit sintering and the like in a conventional process carrier are avoided, and homogeneous production of the chloroethylene is realized.
Owner:YANGZHOU UNIV
Production method of p-chlorotoluene
InactiveCN102603468AHalogenated hydrocarbon preparationMetal/metal-oxides/metal-hydroxide catalystsSilicon oxideO-chlorotoluene
The invention discloses a production method of p-chlorotoluene, comprising the steps of chloridizing toluene, removing toluene and rectifying mixed chlorotoluene. Toluene is chloridized by the steps of placing toluene in a chlorination kettle and adding a catalyst in the chlorination kettle, and feeding chlorine at 40-60 DEG C and carrying out a chlorination reaction. The catalyst used in the chlorination reaction is prepared by the steps of reacting alumina, silicon oxide and sodium oxide react at 120-150 DEG C for 6-8 hours and activating in a high-temperature activation furnace at 800 DEG C for 4-6 hours. The production method provided by the invention is simple and convenient and is easy to operate. In the total content of p-chlorotoluene and o-chlorotoluene in final products, the content of p-chlorotoluene is up to above 80%. After rectification, the content of p-chlorotoluene is up to above 99.5%.
Owner:江苏优普生物化学科技股份有限公司
Synthesis process for o-chlorobenzaldehyde
InactiveCN106977381AControl generationLow costCarbonyl compound preparation by hydrolysisHalogenated hydrocarbon preparationDistillationReaction temperature
The invention discloses a synthesis process of o-chlorobenzaldehyde. The synthesis process of o-chlorobenzaldehyde comprises the following steps: feeding chlorine in o-chlorotoluene, carrying out catalytic chlorination under the condition of illumination to obtain chlorination liquid, wherein the ratio of the amount of the chlorine to the amount of the o-chlorotoluene is 2-4: 1, the reaction time is 1-2 hours, and the reaction temperature is 100-120 DEG C; distilling the chlorination liquid under reduced pressure, and separating out chlorobenzyl chloride and the o-chlorotoluene to obtain mixed liquid of o-chlorobenzyl dichloride and o-chlorobenzyl trichloride; carrying out hydrolysis reaction on the mixed liquid by using ferric chloride and zinc chloride as catalysts to obtain hydrolysate, wherein the temperature of hydrolysis reaction is 100-120 DEG C, a solvent is water, and the reaction time is 1.5-3 h; and carrying out rectification and purification on the hydrolysate in a vacuum state to obtain o-chlorobenzaldehyde. According to the technical scheme, the cost is further reduced, and the quality of the product is improved. Reasonable catalytic conditions are adopted, the synthesis process is a perfect greening technological synthesis method, generation of by-products is controlled effectively, and the quality of the product is improved further.
Owner:NANJING COLLEGE OF INFORMATION TECH
Method for selectively preparing o-chlorotoluene
InactiveCN103613482AHigh selectivityPhysical/chemical process catalystsHalogenated hydrocarbon preparationIron powderIodine
The invention provides a novel method for preparing o-chlorotoluene through chlorinating toluene. The technical scheme is as follows: the method comprises the steps of taking a mixture of elemental iodine and iron powder as a catalyst under dark conditions, adding the catalyst into a toluene solution, slowly introducing chlorine gas, controlling the temperature of a whole chlorination process to be 40-50 DEG C, and controlling an introduced chlorine gas volume so as to enable the toluene conversion ratio to be not lower than about 99%. According to the method, after the catalyst is applied to toluene chlorination, the percent content of o-chlorotoluene in a product is increased effectively, the content ratio of o-chlorotoluene to p-chlorotoluene reaches 2.0, the content of m-chlorotoluene is lower than 0.2%, and the total content of poly-chlorotoluene is lower than 5.0%.
Owner:SOUTHEAST UNIV
New synthesis technology of 2-hydroxybenzonitrile
ActiveCN106083651AHigh yieldIncrease reaction rateOrganic compound preparationPreparation by hydrocarbon ammoxidationDistillationWater vapor
A new synthesis technology of 2-hydroxybenzonitrile comprises the following steps: 1, adding o-chlorotoluene, ammonia gas, oxygen and water vapor to a free turbulent fluidized bed reactor, uniformly stirring above added materials, adding a V-Cr-O and V-P-O fine particle mixture as a catalyst, and heating and reacting the above materials; 2, washing a crude o-chlorobenzonitrile product obtained after the reaction ends with water, and rectifying the crude o-chlorobenzonitrile product to obtain pure o-chlorobenzonitrile; and 3, adding an alkali metal alkoxide solution to the pure o-chlorobenzonitrile obtained in step 2, controlling the reaction temperature, carrying out a reaction under a normal-pressure condition, carrying out reduced pressure distillation after the reaction ends to recover a solvent, and adding hydrochloric acid to the above obtained system in a dropwise manner to carry out acidification in order to obtain solid 2-hydroxybenzonitrile. Compared with the prior art, the technology has the advantages of avoiding of use of phosgene or a phosphorus-containing compound, guaranteeing of the personnel safety of operating personnel, pollution reduction, and facilitation of increase of the productivity of a device and the yield of o-chlorobenzonitrile by using the free turbulent fluidized bed reactor.
Owner:ANHUI GUANGXIN AGROCHEM
Method for preparing chlorotoluene
ActiveCN103102243AReduce dosageHigh activityHalogenated hydrocarbon preparationO-chlorotolueneEthyl Chloride
The invention relates to a method of toluene chlorination reaction and particularly relates to a method for preparing chlorotoluene through the toluene chlorination reaction. The method is characterized by adopting a made-to-order macroporous metal chelating resin as a catalyst; and the catalyst is chelated from an iron chloride water solution with the mass concentration of 10% and D418 styrene cation exchange resin with the particle size of 0.5-1.5 millimeters and the aperture of 0.6-1 nanometer at 60 DEG C under the conditions of stirring and ph of 3.5. Thus, electrophilic reaction during toluene chlorination is mainly carried out in a catalyst pore path; because chlorotoluene is smaller than o-chlorotoluene in molecular dimension and has higher diffusion velocity than o-chlorotoluene in a pore path with certain size, more para-position products are obtained; in addition, the method disclosed by the invention has low requirements on temperature and pressure; and the highest content ratio of chlorotoluene to o-chlorotoluene is 4.1:1 under the conditions of 10-80 DEG C and 0.05-0.1 MPa.
Owner:江苏超跃化学有限公司
O-chlorobenzoic acid synthesis process
InactiveCN103601639APollution without waterHigh yieldOrganic compound preparationCarboxylic compound preparationMANGANESE ACETATEAcetic acid
The invention discloses an o-chlorobenzoic acid synthesis process. The innovation point is that the process adopts o-chlorotoluene as a raw material and acetic acid as a solvent and comprises the following steps: heating to 134-136 DEG C under the effect of the composite catalysts cobalt acetate, manganese acetate and sodium bromide; filling oxygen for reaction, wherein the reaction temperature is 135-145 DEG C, the reaction pressure is 1.0MPa, and the reaction time is 5.5-6.5 hours; and after the reaction, cooling, discharging, cooling, filtering and washing to obtain the target product o-chlorobenzoic acid. The synthesis process disclosed by the invention gives overall consideration to the factors such as raw material cost, production flow, production cost and environmental pollution, and has the advantages of high yield, low cost, short flow, no water pollution and the like.
Owner:NANTONG BOTAO CHEM
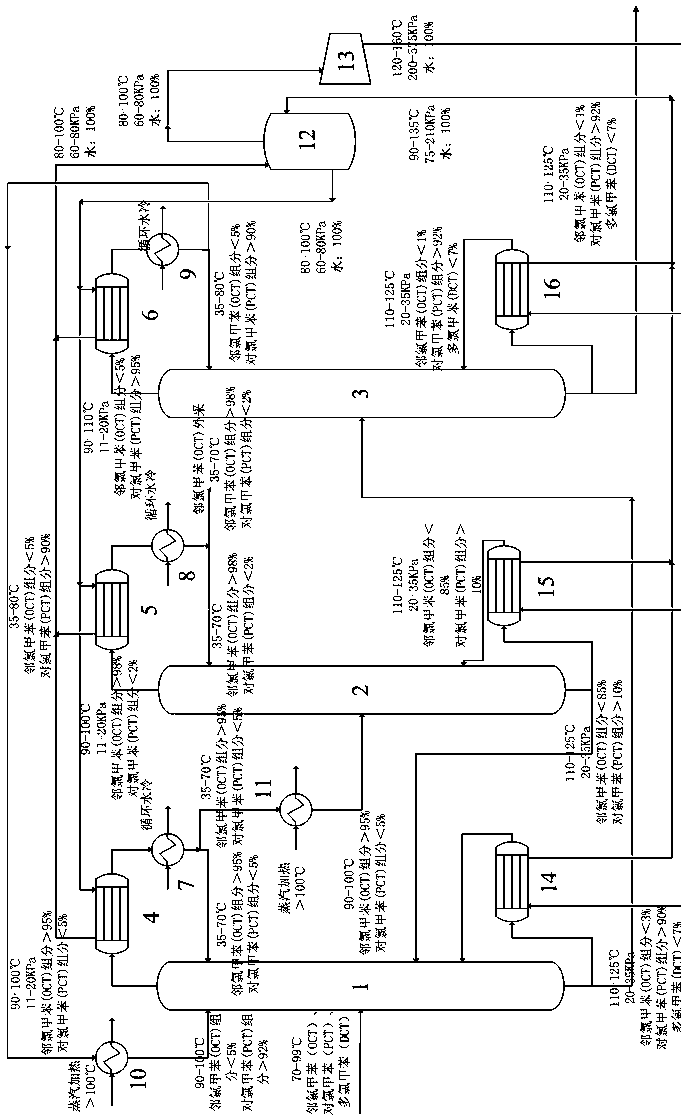
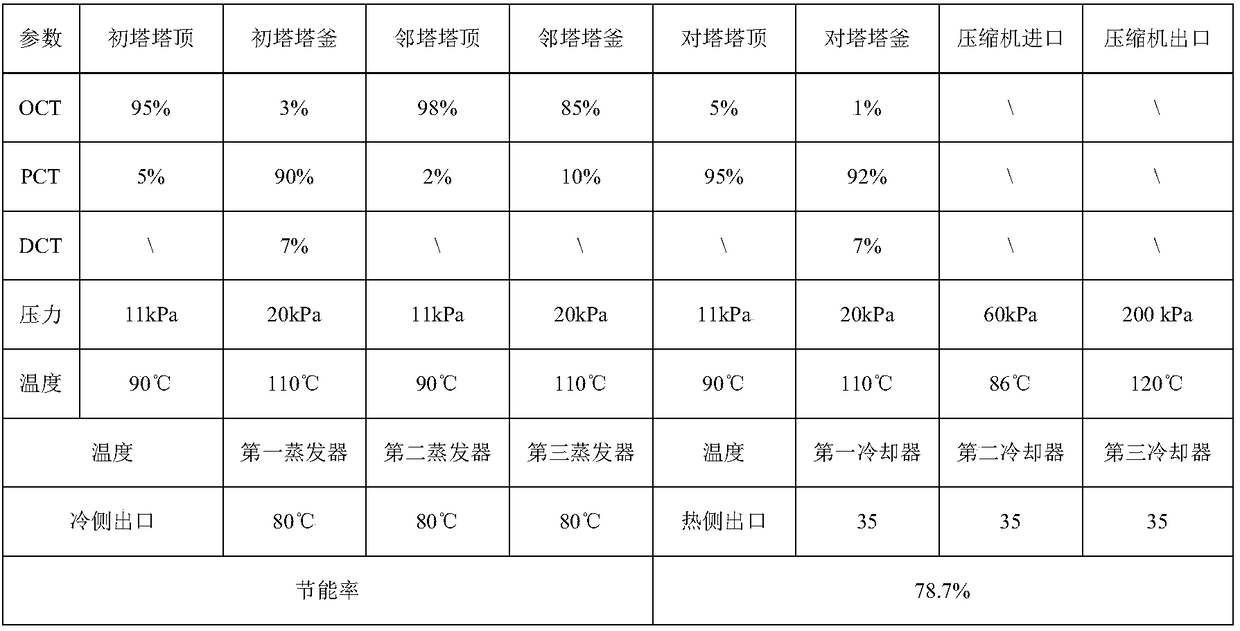
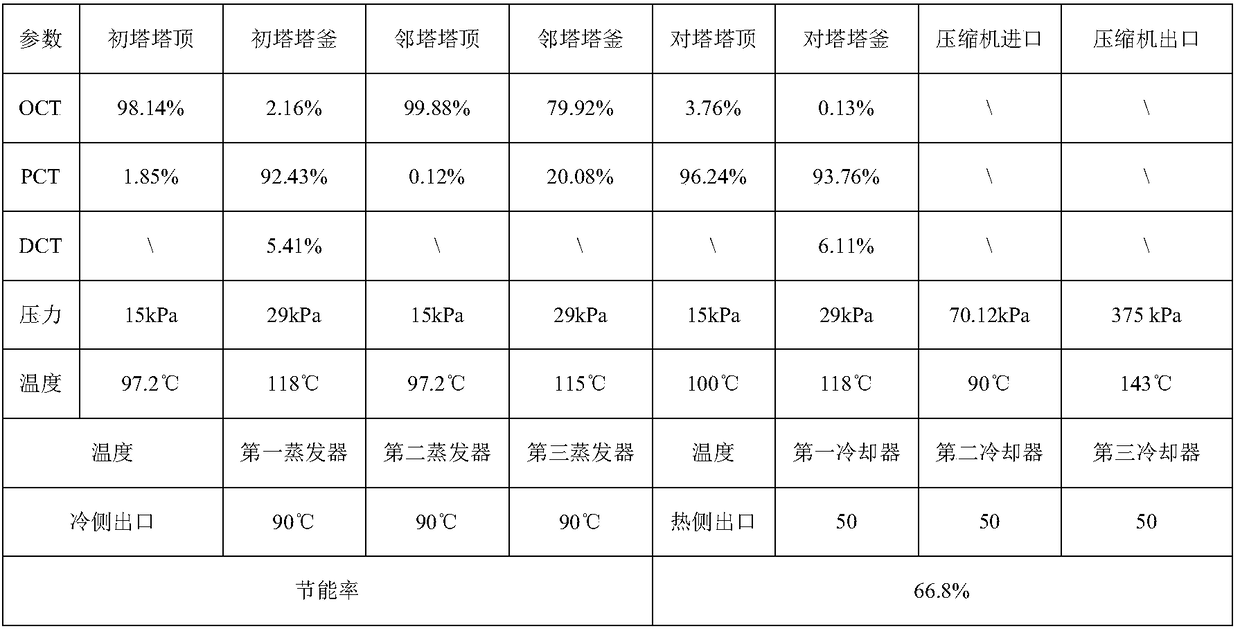
Mixed chlorotoluene MVR (mechanical vapor recompression) rectification system and method
PendingCN109134189AChemical industryHalogenated hydrocarbon separation/purificationChemical industryLiquid water
The invention belongs to the field of chemical industry and relates to a mixed chlorotoluene MVR (mechanical vapor recompression) rectification system and method. The system herein comprises a primarycolumn, an ortho-column, a para-column, evaporators, a cooler, a gas-liquid separation tank, a vapor compressor, reboilers and the like. The system is characterized in that continuous rectification via the primary column, ortho-column and para-column by means of rectification is carried out; mixed chlorotoluene mother liquor that is preheated is processed via the primary column to obtain crude o-chlorotoluene and p-chlorotoluene; the crude o-chlorotoluene and p-chlorotoluene are fed respectively into the ortho-column and the para-column for continued rectification; o-chlorotoluene with purityof higher than 98% is acquired from the top of the ortho-column; p-chlorotoluene with purity of higher than 92% is acquired from the bottom of the ortho-column; distillates from the three columns aresubjected to heat exchanging with liquid water in respective top evaporators; the liquid water absorbs heat and evaporates into water vapor; the water vapor is separated and purified by the gas-liquid separation tank before the resultant is heated and pressurized in the vapor compressor; the pressurized material enters the reboilers at the bottoms of the three columns to release latent heat to heat part of the bottom distillates. The system and method herein have the advantages of high separation efficiency, low energy consumption, low operation cost and the like.
Owner:JIANGSU LEKE ENERGY SAVING TECH CO LTD
Method for producing parachlorotoluene through continuous chlorination of methylbenzene
InactiveCN108467333AReduce manufacturing costReduce solid waste dischargeChemical recyclingHalogenated hydrocarbon preparationMolecular sieveDistillation
The invention discloses a method for producing parachlorotoluene through continuous chlorination of methylbenzene. The method comprises the following steps: with an active molecular sieve as a catalyst, performing continuous chlorination on methylbenzene in a static bed, wherein a ratio of parachlorotoluene to o-chlorotoluene in the chlorination products is 1.2-6.0; performing aeration and distillation separation on the chlorination product to obtain high-purity parachlorotoluene, and enabling the separated methylbenzene and raw material methylbenzene to enter the static bed together so as toperform continuous chlorination; periodically reproducing the catalyst in the static bed; and alternately using and activating two groups or more than two groups of static beds. According to the method disclosed by the invention, continuous chlorination production of methylbenzene is realized, the ratio of parachlorotoluene to o-chlorotoluene is 1.2-6.0, the catalyst can be repeatedly used, the production cost is reduced, and solid waste emission is reduced.
Owner:江苏优普生物化学科技股份有限公司
Method for preparing o-chlorotoluene
InactiveCN108586192AImprove catalytic selectivityImprove conversion ratePreparation by dehalogenationMetal/metal-oxides/metal-hydroxide catalystsFixed bedPalladium catalyst
The invention provides a method for preparing dichlorotoluene, and relates to the field of preparation of organic intermediates. The method provided by the invention uses more than one of 2,3-dichlorotoluene, 2,4-dichlorotoluene and 2,5-dichlorotoluene as raw materials, and uses supported palladium as a catalyst to prepare o-chlorotoluene by continuous reaction in a fixed bed. The method providedby the invention uses industrial by-products 2,3-dichlorotoluene, 2,4-dichlorotoluene and 2,5-dichlorotoluene as the raw materials; the supported palladium catalyst has good selectivity and long service time and realizes high yield of o-chlorotoluene.
Owner:江苏超跃化学有限公司
Production technology for preparing p-chlorotoluene by methylbenzene directional chlorination
PendingCN109734551AReduce generationIncrease profitHalogenated hydrocarbon preparationRefluxO-chlorotoluene
The invention relates to the technical field of chemical raw material preparation, in particular to a production technology for preparing p-chlorotoluene by methylbenzene directional chlorination. Theproduction technology includes the steps: chlorination: adding catalysts into a reaction vessel, adding methylbenzene into the reaction vessel, leading in inert gas, discharging air in the reaction vessel, placing the reaction vessel into a constant temperature device after shading treatment, leading chlorine into a constant temperature device, performing chlorination reaction and chromatographicanalysis and accordingly judging reaction endpoints to obtain chlorinated mixture for standby application; rectification: adjusting reflux ratio to be 10:1 and collecting fraction with the temperature of 158-162 DEG C to obtain o-chlorotoluene and p-chlorotoluene mixed solution for standby application; primary purification; secondary purification. The production technology solves the problems ofpoor methylbenzene chlorination directional selectivity and high cost in the prior art, and is high in p-chlorotoluene yield and conversion rate, good in para-position directional selectivity and reasonable in technological cost.
Owner:JIANGSU QIHENG AGRI & CHEM TECHCO
Method for preparing o-chlorobenzaldehyde by continuously oxidizing o-chlorotoluene
ActiveCN108794311AShort reaction timeMild reaction conditionsProcess control/regulationCarbonyl compound preparation by oxidationOrganic synthesisO-chlorotoluene
The invention discloses a method for preparing o-chlorobenzaldehyde by continuously oxidizing o-chlorotoluene and belongs to the technical field of an organic synthesis technology. The method is a process technology which takes an o-chlorotoluene compound as a raw material, one or more metal ion complexes of cobalt, molybdenum and bromine as a catalyst, hydrogen peroxide as an oxidant and acetic acid as a solvent, and is used for preparing o-chlorobenzaldehyde by continuously oxidizing o-chlorotoluene in a tubular reactor. The method disclosed by the invention has the advantages of moderate conditions, short reaction time and high raw material utilization rate; effective control in a reaction process can be realized; the method is safe and stable, is continuously operated and has high production efficiency.
Owner:CHANGZHOU UNIV
Method for producing p-chorotoluene by using element selenium as catalyst
ActiveCN102381930AIncrease percentageStable structureHalogenated hydrocarbon preparationO-chlorotolueneOrganic compound
The invention provides a method for producing p-chorotoluene by using the element selenium as a catalyst, comprising the following steps of: using a selenium-containing organic compound as a catalyst, performing a liquid phase ring chlorination reaction between toluene and chlorine, followed by aeration after the reaction, removing residual chlorine and hydrogen chloride gas in the reaction solution, rectifying to obtain o-chlorotoluene, and carrying out melt crystallization on the enriched p-chorotoluene to obtain the high-purity p-chorotoluene. By the adoption of the method, the yield of p-chorotoluene is raised; the catalyst with a stable structure can be repeatedly applied; the technology and equipment are simple; the production cost is low; and there is no pollution to the environment.
Owner:ZHEJIANG WEIHUA NEW MATERIAL CO LTD
Method for preparing o-chlorotoluene through methylbenzene loop chlorination
ActiveCN106565411AWide variety of sourcesLow costOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsIron powderO-chlorotoluene
The invention discloses a method for preparing o-chlorotoluene through methylbenzene loop chlorination. The method particularly includes the steps that iron powder or ferric trichloride is added into methylbenzene to serve as catalysts, [BMTM]Cl-nZnCl2 ionic liquid serves as an assistant (n is equal to 1, 2 or 2.5), and stirring is carried out; dry chlorine is led in, a suitable temperature is controlled under the dark condition to conduct chlorination, the o-chlorotoluene is obtained in a high-selectivity manner, and alkali liquor absorption is carried out after tail gas condensation to remove excessive unreacted chlorine; and after reacting is ended, a reacting product and the ionic liquid assistant are separated, the separated ionic liquid can be repeatedly used. Compared with the prior art, the method can well improve the methylbenzene conversion rate, and the o-chlorotoluene selectivity reaches 88%; in addition, the separated and recycled [BMTM]Cl-nZnCl2 ionic liquid is repeatedly used, the production cost is reduced, and the method is suitable for o-chlorotoluene industrial production.
Owner:SOUTHEAST UNIV
Method for synthesizing cresol
ActiveCN103910609AEmission reductionAddress reactivityOrganic compound preparationHalogenated hydrocarbon preparationM-CresolO-chlorotoluene
The invention discloses a method for synthesizing cresol. According to the method, chlorotoluene is produced through chlorination of methylbenzene, the reaction conversion rate is controlled at 95-98%; the produced mixed chlorotoluene comprising parachlorotoluene, o-chlorotoluene and m-chlorotoluene and liquid caustic soda the volume of which is three times that of the mixed chlorotoluene are hydrolyzed, the reaction conversion rate of the mixed chlorotoluene is controlled at 95-98%; the reaction product is cooled and then separated, neutralized and layered, extracted, distilled, rectified and tert-butylated so as to prepare o-cresol, m-cresol, and 2.6 butylated hydroxytoluene respectively; the mixed chlorotoluene does not need to be separated, so that the problems of high boiling treatment caused by rectification and manpower and material resource spent on rectifying and separating mixed raw materials in the existing production technology are thoroughly solved, pollutant emission is reduced, in addition, the yield of m-cresol with high additional value in the product synthesized by the method is improved to some extent, and the economic benefits are remarkable.
Owner:ANHUI HAIHUA CHEM
O-chlorotoluene catalytic nitration method by using microwaves for assisting zeolite
InactiveCN108689855AFast and even heatingFull exposure to acidic sitesOrganic-compounds/hydrides/coordination-complexes catalystsNitro compound preparationMolecular sieveAcetic anhydride
The invention discloses an o-chlorotoluene catalytic nitration method by using microwaves for assisting zeolite. The method comprises the following steps of adding solvents into a container; sequentially adding o-chlorotoluene, acetic anhydride, nitrosonitric acid, modified zeolite molecular sieve catalysts and silicon dioxide loaded perfluorinated sulfonic acid resin; performing microwave temperature rise for 4 to 12min; performing heat insulation for 8 to 10min; terminating the reaction. The microwave technology is applied to the o-chlorotoluene nitration process; the reaction progress is greatly accelerated; meanwhile, silicon dioxide is used for loading perfluorinated sulfonic acid resin; the perfluorinated sulfonic acid resin is dispersed into SiO2 networks or pore passages; the effective surface area is greatly increased, so that the acid positions of the perfluorinated sulfonic acid resin are sufficiently exposed, so that the potential of the resin catalyst is achieved; the conversion rate and the yield are improved; in addition, the modified zeolite molecular sieve catalyst Bibeta provides acid centers and pore passage structures; the shape selection catalyst effect is provided; the reaction selectivity is improved; the yield is as high as 80.9 percent; the selectivity of the 2-chloro-4-nitrotoluene is as high as 84.1 percent. The method has the advantages that the operation is easy; the energy is saved; the efficiency is high; the control is easy; the method belongs to a green and environmental-friendly o-chlorotoluene nitration method.
Owner:LIHAI CHEM IND CO LTD OF JIANGSU JINQIAO SALT & CHEM GRP
Method for preparing o-chlorotoluene
InactiveCN105418358AHigh catalytic activityHigh activityPhysical/chemical process catalystsHalogenated hydrocarbon preparationO-chlorotolueneEthyl Chloride
The invention discloses a method for preparing o-chlorotoluene. The method comprises the following steps: adding a raw material toluene and a FeCl3-loaded macroporous metal silica gel catalyst into a reaction kettle; introducing chlorine into the reaction kettle for a liquid-phase ring chlorination reaction between the toluene and chlorine; after the reaction is complete, aerating to remove residual chlorine and hydrogen chloride gas in the reaction liquid; rectifying the reaction liquid to separate out unreacted toluene and a little dichlorotoluene to obtain mixed chlorotoluene; and then, separating high-purity p-chlorotoluene from the mixed chlorotoluene by using a melt crystallization process. According to the method disclosed by the invention, the FeCl3-loaded macroporous metal silica gel catalyst is adopted for catalyzing the selective chlorination of toluene, the reaction is fast, the catalytic activity is high, the o-chlorotoluene selectivity is good, the requirements for the technological conditions are low, the catalyst consumption is low, the catalyst has high activity and can be recycled, and the method has a good industrial prospect.
Owner:NANJING ZHONGTENG CHEM
Preparation method of high-purity chloro-2-carboxyl-benzophenone
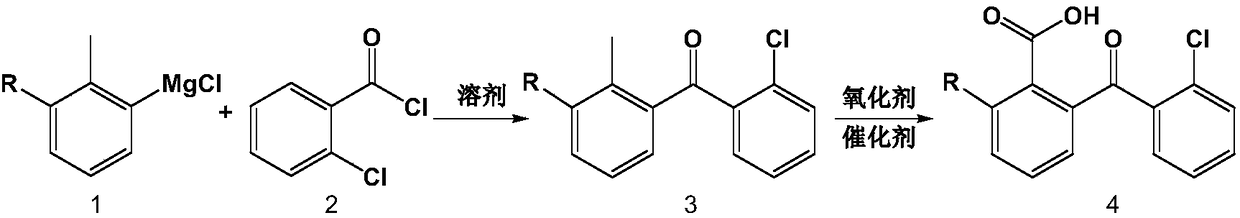
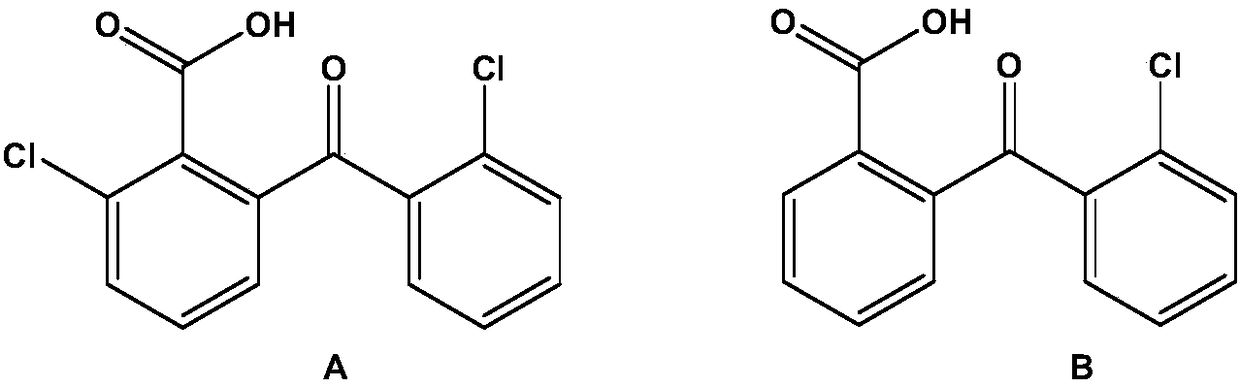
ActiveCN108530293AThe synthesis steps are simpleLow costOrganic compound preparationCarbonyl compound preparation by condensationGrignard reagentO-chlorotoluene
The invention discloses a preparation method of high-purity chloro-2-carboxybenzophenone. The preparation method comprises the following steps: preparing chlorine-substituted or non-substituted o-chlorotoluene into a Grignard reagent, and then, enabling the Grignard reagent to react with o-chlorobenzoyl chloride to obtain chloro-2-methylbenzophenone; and enabling chloro-2-methylbenzophenone to besubjected to oxidation reaction in the presence of a catalyst and an oxidizing agent to obtain the chloro-2-carboxybenzophenone. The preparation method disclosed by the invention has the advantages that the synthetic steps are simple, the cost is low, the process is environmentally friendly, and the obtained product has high purity and can be directly applied to the fields of micro-electronics andthe like.
Owner:王华平
Method for catalytic nitration of o-chlorotoluene by using modified beta zeolite
InactiveCN108658775AReduce usageReduce processingNitro compound preparationAcetic anhydrideNitration
The invention discloses a method for catalytic nitration of o-chlorotoluene by using modified beta zeolite. The method comprises the following steps: while stirring, adding a solvent into a container,then sequentially adding o-chlorotoluene, acetic anhydride, fuming nitric acid, a modified beta zeolite molecular sieve catalyst and silicon dioxide loaded perfluorinated sulfonic acid resin; performing heating reflux for 1-9h before terminating the reaction. According to the method disclosed by the invention, acetic anhydride is applied to a nitration process of o-chlorotoluene; compared with anitration process of o-chlorotoluene using nitric-sulfuric acid, the use of strong acid sulfuric acid is avoided, and the treatment amount of waste acid is reduced; meanwhile, the silicon dioxide loaded perfluorinated sulfonic acid resin is adopted, with the perfluorinated sulfonic acid resin dispersed in a SiO2 network or pore channel; since the effective surface area is greatly increased, the acid site of the perfluorinated sulfonic acid resin is fully exposed, the potential of the resin catalyst is totally played, and the conversion rate and yield are increased; moreover, the modified zeolite molecular sieve catalyst Bibeta provides an acid center and a pore channel structure as well as shape selecting catalysis, the reaction selectivity is improved, the yield reaches 85.4%, and the selectivity of 2-chloro-4-nitrotoluene is up to 84.6%; the method is easy to operate and control and is a green and environment-friendly o-chlorotoluene nitration method.
Owner:LIHAI CHEM IND CO LTD OF JIANGSU JINQIAO SALT & CHEM GRP
Treatment method and system of byproduct hydrochloric acid in chlorination process of toluene or chloro-toluene
PendingCN107879313ASave resourcesRealize the development of circular economyChlorine/hydrogen-chloride purificationChemistryOil water
The invention discloses a treatment method and system of the byproduct hydrochloric acid in the chlorination process of toluene or chloro-toluene. The byproduct hydrochloric acid produced by a tolueneor chloro-toluene chlorination system enters an extraction tower from the top of the extraction tower, the extractant enters the extraction tower from the bottom of the extraction tower, the hydrochloric acid and the extractant make countercurrent contact with each other in the extraction tower, the extractant absorbs the organic matter in the byproduct hydrochloric acid, and is extracted out from the top of the extraction tower, the raffinate is extracted out from the bottom of the extraction tower, and is fed into the lower part of a carbon fiber adsorption tower, the carbon fiber adsorbs the extractant from the raffinate, after adsorption, the hydrochloric acid purification products, the raffinate, reaches the industrial grade, and is discharged from the top of the carbon fiber adsorption tower into a storage tank; when the carbon fiber penetrates, the carbon fiber adsorption tower is desorbed by water vapor, the high temperature water vapor containing extractant enters a condenserfor condensation, the condensate enters the extraction tank for oil and water separation, the recovered oil is returned to the extraction tower. According to the method, the byproduct hydrochloric acid is treated and reaches the quality requirement of industrial grade, resources are saved, the circular economy development is realized, and environment is protected.
Owner:NANJING ZHONGTENG CHEM
Method for preparing 2,6-dichlorotoluene from o-chlorotoluene
InactiveCN105481635ALow costEliminate pollutionPhysical/chemical process catalystsHalogenated hydrocarbon preparationBoiling pointChloride
The present invention provides a method for preparing 2,6-dichlorotoluene from o-chlorotoluene, the method comprises the following steps: (a) addition of a chlorination catalyst, (b) chlorination of the o-chlorotoluene, and (c) component separation, the o-chlorotoluene is reacted with the chloride under the effect of the chlorination catalyst, adverse factors of high cost and heavy pollution of a traditional process for production of the 2,6-dichlorotoluene by a diazotization method can be eliminated, and the method for preparing the 2,6-dichlorotoluene from the o-chlorotoluene is simple in process, mild in reaction conditions and low in production cost, and has the characteristics of being free of high-temperature operation, high in efficiency, economic, energy-saving and environmentally-friendly. Difference components can be obtained by rectification and recrystallization of a reaction product in accordance with the different boiling and melting points of the components.
Owner:NANJING ZHONGTENG CHEM
A method for preparing 2,6-dichlorotoluene by catalyzing o-chlorotoluene with ionic liquid
InactiveCN106810418AImprove conversion rateHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsHalogenated hydrocarbon preparationChlorideO-chlorotoluene
The invention discloses a method for preparing 2,6-dichlorotoluene by catalyzing o-chlorotoluene with an ionic liquid. The method for preparing 2,6-dichlorotoluene uses Cl2 as a chlorination agent, and the catalyst is chloroaluminate ionic liquid Under the action of the raw material, the directional chlorination of o-chlorotoluene makes 2,6-dichlorotoluene; the intermediate of the aluminum chlorate ionic liquid is [BMIM]Cl, wherein the molar ratio of AlCl3 to [BMIM]Cl is 1 ~3, the dosage of the chloroaluminate ionic liquid is 0.1%~1% of the mass of o-chlorotoluene; the selective chlorination of o-chlorotoluene to produce 2,6-dichlorotoluene is simple in process and mild in reaction conditions. The chloroaluminate ionic liquid has good catalytic activity, good stability, is easy to separate from the product, and can significantly improve the selectivity of 2,6-dichlorotoluene. The invention has high industrial application value.
Owner:QINGDAO SENMEIKE CHEM TECH CO LTD
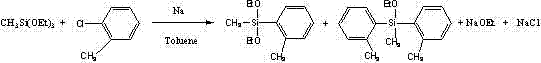
O-benzyldiethoxymethylsilane and preparation method thereof
ActiveCN102898456AMild reaction conditionsSimple processGroup 4/14 element organic compoundsO-chlorotolueneSolvent
The invention relates to the field of organic silicon chemistry and provides o-benzyldiethoxymethylsilane and a preparation method thereof. The preparation method solves the problem that in the existing sodium condensation method-based synthesis of o-substituted benzyldiethoxymethylsilane, a large amount of a solvent is used so that environmental pollution is caused and solvent recycle is difficult. The o-benzyldiethoxymethylsilane is synthesized from methyltriethoxysilane and o-chlorotoluene as raw materials by a sodium condensation method. The preparation method does not adopt a toluene solvent, allows mild reaction conditions, has simple processes and is suitable for large-scale industrial production.
Owner:HANGZHOU NORMAL UNIVERSITY
Production equipment and synthesis method of o-chlorobenzoic acid
PendingCN108558637AThe reaction process is shortNo dischargeOrganic compound preparationCarboxylic compound preparationSynthesis methodsWastewater
The invention discloses a synthesis method of o-chlorobenzoic acid. The synthesis method comprises the following steps: adding a mixed o-chlorobenzoic acid solution and o-chlorotoluene and inflating oxygen obtained by vaporizing liquid oxygen into a reactor, detecting the pressure in the reactor by using a gas pressure detecting instrument, reacting at a temperature of 125-180 DEG C after a requirement is met, condensing and stratifying o-chlorotoluene vapor by using a condenser and a water segregator, removing water, reflowing the o-chlorotoluene into the reactor, and when the content of theo-chlorobenzoic acid reaches 98.5%, discharging and passing through subsequent treatment equipment. By the synthesis method, a technological process is short, the yield is high, the cost is low, the environment pollution is low, pure oxygen replaces air, the reaction speed is high, and basically no waste gas is discharged; a solvent is not required during the reaction, a large amount of wastewaterin a diazotization method and a chlorination hydrolysis method is avoided, and an oxidation technology is greatly improved, the reaction conversion rate is high, the selectivity is good, and the crude product content is high.
Owner:ZHEJIANG ALPHARM CHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com