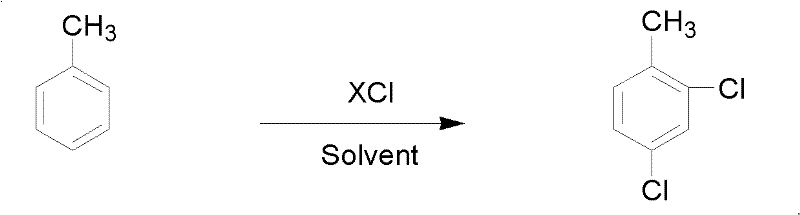
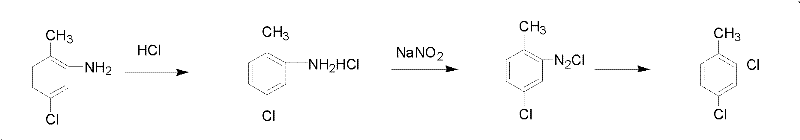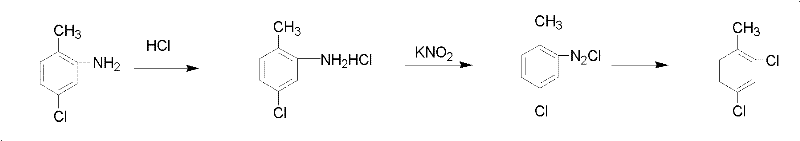Preparation method of 2,4-dichlorotoluene
A technology of dichlorotoluene and o-chlorotoluene, which is applied in 2 fields, can solve the problems of high product cost, and achieve the effects of low production cost, favorable resource recycling and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Add 3 mol of hydrogen chloride into a 1000 ml three-necked flask equipped with mechanical stirring, a reflux condenser, and a thermometer, then add 1 mol of 5-chloro-2-methylaniline dropwise into the three-necked flask, stir to form off-white particles, and cool to -10 Slowly add 1 mol of sodium nitrite after ℃, control the feeding speed so that the temperature is not higher than 10 ℃, after the addition, stir until the system is brownish red. Then thermal decomposition is carried out, the temperature is controlled at 15°C, and the following chemical reactions occur in the system:
[0015]
[0016] After the reaction is completed, first separate the lower organic phase, absorb the unreacted hydrogen chloride with a graphite absorption tower for recovery, wash the organic phase with water until neutral, remove a small amount of o-chlorotoluene generated in the reaction at 180-198 °C, and then carry out Rectification, collecting 198 ~ 201 ° C fractions to obtain 152 gr...
Embodiment 2
[0024] Add 2 mol of hydrogen chloride into a 1000 ml three-necked flask equipped with mechanical stirring, reflux condenser, and thermometer, then add 1.5 mol of 5-chloro-2-methylaniline dropwise into the three-necked flask, stir into off-white particles, and cool to - Slowly add 2 mol of potassium nitrite after 10°C, control the feeding speed so that the temperature is not higher than 10°C, and stir until the system is brown-red after adding. Then thermal decomposition is carried out, the temperature is controlled at 40°C, and the following chemical reactions occur in the system:
[0025]
[0026] After the reaction is completed, first separate the lower organic phase, absorb the unreacted hydrogen chloride with a graphite absorption tower, and recover it. Distillation, collecting 196 ~ 201 ° C fractions to obtain 227 grams of fractions.
[0027] With 2,4-dichlorotoluene as the standard, gas chromatography analysis. The analysis results are shown in Table 3 and Table 4. ...
Embodiment 3
[0034] Add 1 mol of hydrogen chloride into a 1000 ml three-necked flask equipped with mechanical stirring, reflux condenser, and thermometer, then add 2 mol of 5-chloro-2-methylaniline dropwise into the three-necked flask, stir to form off-white particles, and cool to -10 After ℃, slowly add 2 mol of ammonium nitrite, control the feeding speed so that the temperature is not higher than 10 ℃, after the addition, stir until the system is brown-red. Then thermal decomposition is carried out, the temperature is controlled at 60°C, and the following chemical reactions occur in the system:
[0035]
[0036] After the reaction is completed, first separate the lower organic phase, absorb the unreacted hydrogen chloride with a graphite absorption tower for recovery, wash the organic phase with water until neutral, remove a small amount of o-chlorotoluene generated in the reaction at 180-198 °C, and then carry out Rectify, collect fractions at 198-201°C, and obtain 303.5 grams of fra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com