Separation method of p-chlorotoluene and o-chlorotoluene
A technology for p-chlorotoluene and o-chlorotoluene is applied in the field of separation of p-chlorotoluene and o-chlorotoluene, and can solve the problems of high energy consumption and complicated process, and achieve the effects of low energy consumption, simple operation and energy saving.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of bisdiethoxy columnar[5]arene crystal material: Weigh 2g of bisdiethoxy column[5]arene into 20mL tetrahydrofuran, heat to boiling, add tetrahydrofuran solution dropwise until completely dissolved, place the solution in Store at 0°C overnight, collect the precipitated crystals by filtration, and vacuum-dry the obtained crystals at 50°C to obtain a white powder, which is designated as EtP5.
[0029] The product characterization data prepared in this embodiment are as follows:
[0030] EtP5, 1 H NMR (400MHz, CDCl 3 , 298K, ppm) δ6.72 (s, 10H), 3.83 (q, 20H), 3.76 (s, 10H), 1.55 (t, 30H).
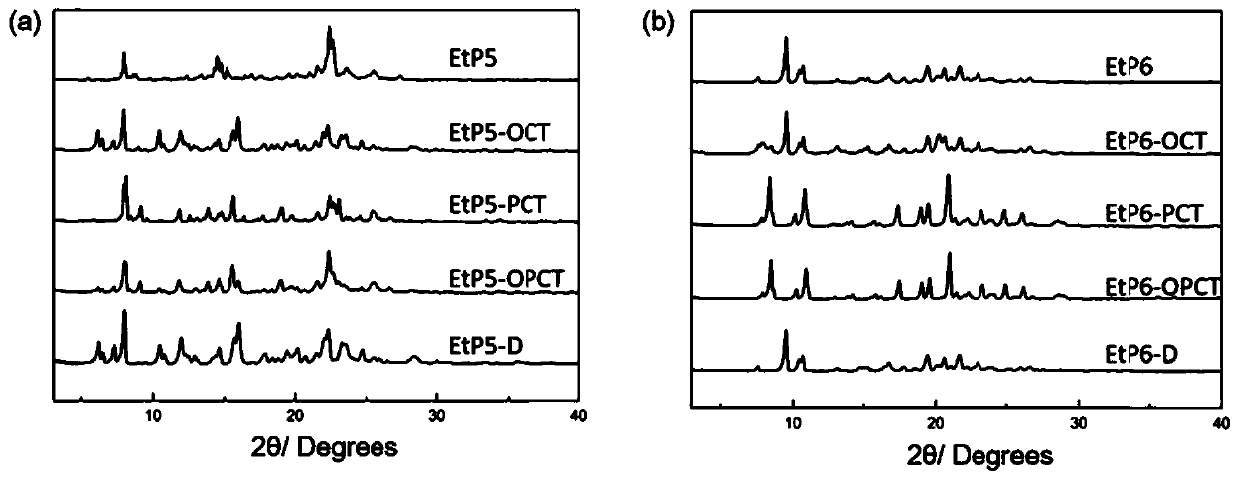
[0031] PXRD test results such as figure 1 As shown in a, the obtained bisdiethoxypillar[5]arene crystalline material has good crystallinity.
Embodiment 2
[0033]Preparation of bisdiethoxy columnar[6]arene crystal material: Weigh 2g of bisdiethoxy column[6]arene into 20mL acetone, heat to boiling, add acetone dropwise until completely dissolved, place the solution at 0 Store at ℃ overnight, collect the precipitated crystals by filtration, dry the obtained crystals in vacuum at 50 ℃, and activate at 160 ℃ for 2 hours to obtain a white powder, which is marked as EtP6.
[0034] The product characterization data prepared in this embodiment are as follows:
[0035] EtP6, 1 H NMR (400MHz, CDCl 3 , 298K, ppm) δ6.70 (s, 12H), 3.83 (q, 36H), 1.28 (t, 36H).
[0036] PXRD test results such as figure 1 As shown in b, the obtained bisdiethoxypillar[6]arene crystal material has good crystallinity.
Embodiment 3
[0038] Adsorption of p-chlorotoluene or o-chlorotoluene alone by bisdiethoxy column[5]arene crystal material: Take two 20mL strain bottles, add 1mL p-chlorotoluene and 1mL o-chlorotoluene respectively, and name them as EtP5-PCT and EtP5-OCT, take 200mg of bisdiethoxy column [5] arene crystal material and place them in two 5mL strain bottles respectively, put two open 5mL strain bottles in two 20mL strain bottles, put 20mL The strain bottle was sealed well and placed in a water bath at 25°C for 30 hours.
[0039] The product characterization data prepared in this embodiment are as follows:
[0040] EtP5-PCT, 1 H NMR (400MHz, CDCl 3 ,298K,ppm)δ7.23(d,2H)7.11(d,2H)6.72(s,10H),3.83(q,20H),3.76(s,10H),2.32(s,3H),1.55(t ,30H).
[0041] EtP5-OCT, 1 H NMR (400MHz, CDCl 3 , 298K, ppm) δ6.72 (s, 10H), 3.83 (q, 20H), 3.76 (s, 10H), 1.55 (t, 30H).
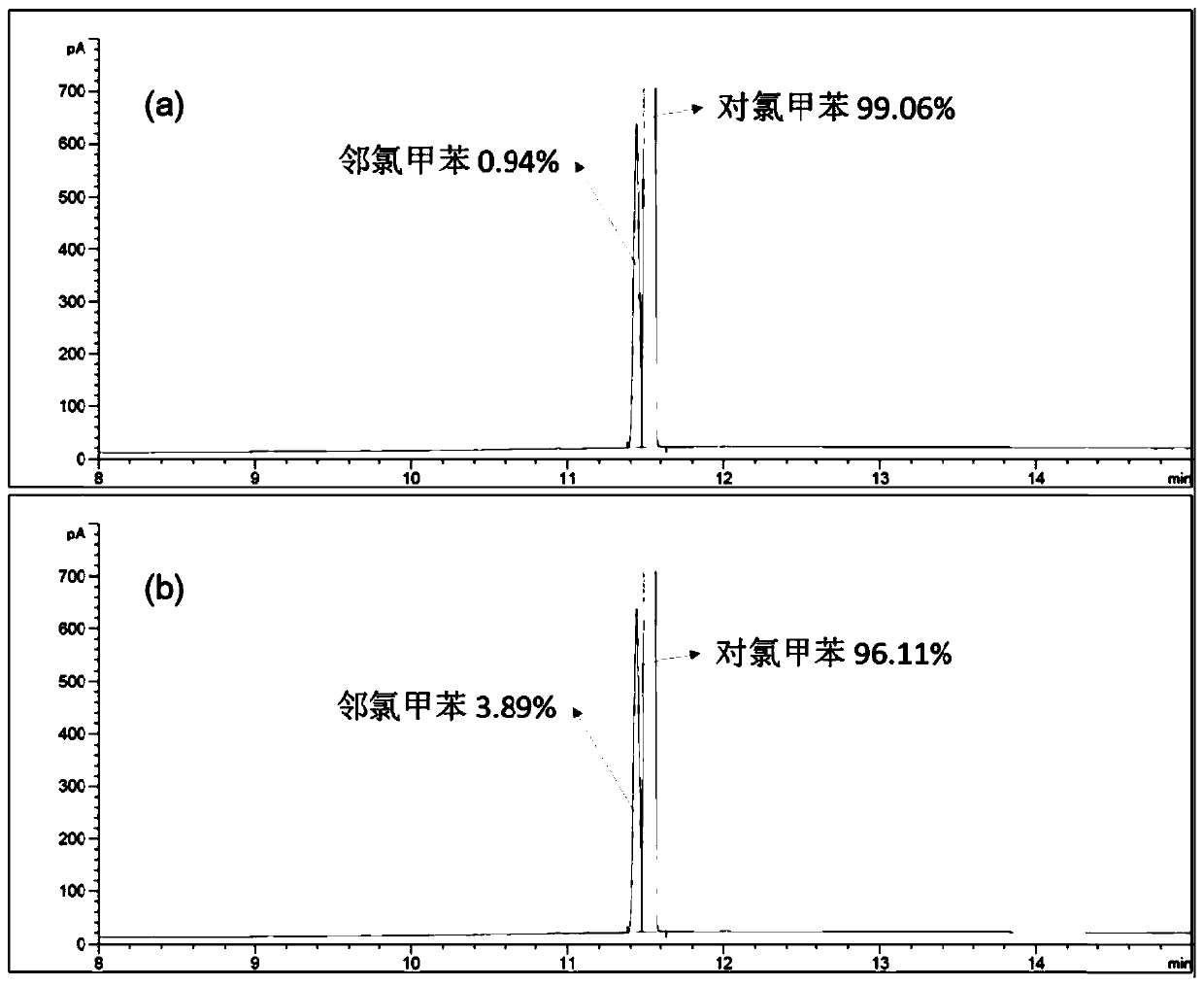
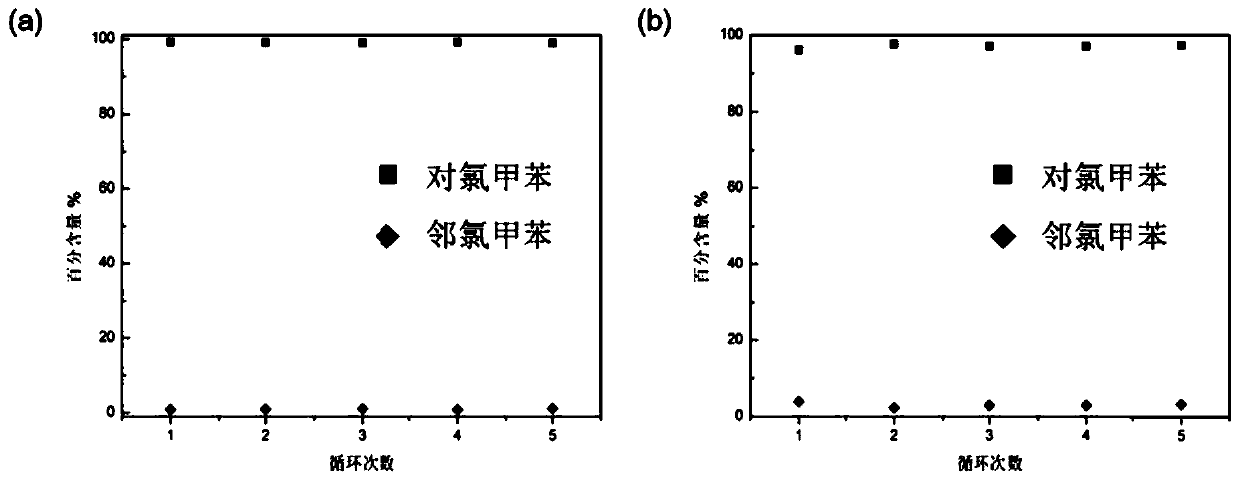
[0042] 1 H NMR results showed that bisdiethoxypillar[5]arene crystalline materials adsorbed p-chlorotoluene in a stoichiometric ratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com