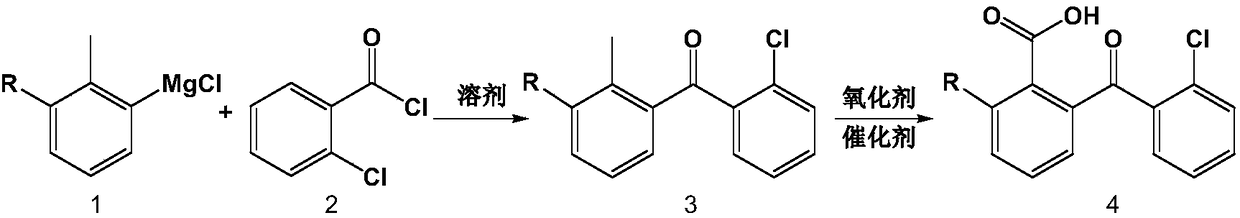
Preparation method of high-purity chloro-2-carboxyl-benzophenone
A technology of methyl benzophenone and benzophenone is applied in the preparation of carboxylate, the preparation of carbon-based compounds, the preparation of organic compounds, etc., to achieve the effects of environmental protection, high purity and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method reaction scheme of the present invention is as follows:
[0029]
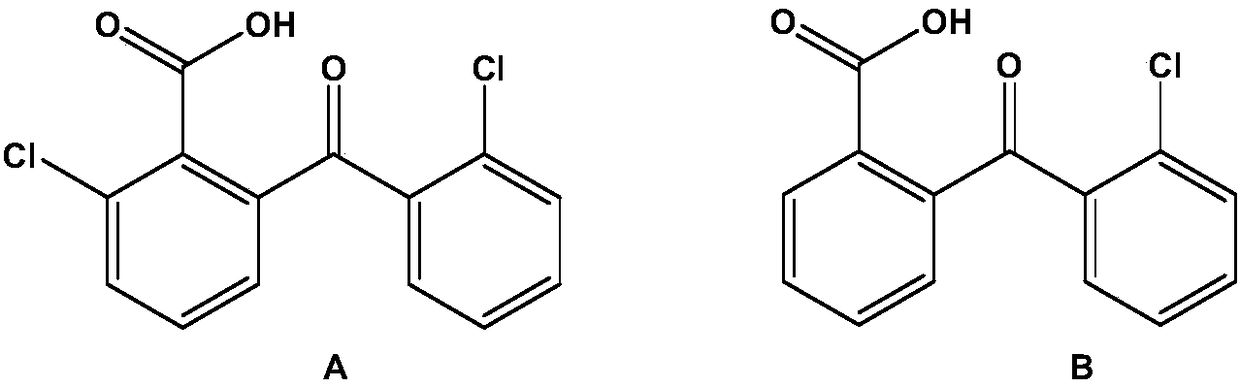
[0030] Take 2-chloro-6-(2-chlorobenzoyl)benzoic acid (compound A) and 6-(2-chlorobenzoyl)benzoic acid (compound B) as an example to illustrate the technical scheme of the present invention,
[0031]
Embodiment 1
[0032] The preparation of embodiment 1 compound A (2-chloro-6-(2-chlorobenzoyl) benzoic acid)
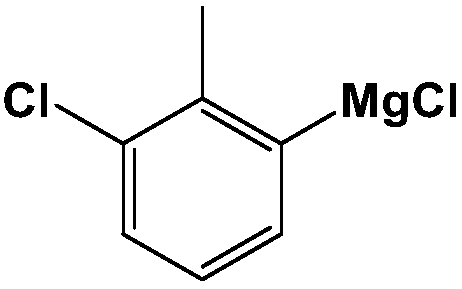
[0033] (1) Preparation of Grignard Reagents
[0034]
[0035] Get 24 grams (1.0M) of magnesium powder, put into four-neck round bottom flask, pass into dry hot nitrogen, cool after 30 minutes. Add 2-methyl m-dichlorobenzene, a small amount of tetrahydrofuran and iodine, and heat to initiate the reaction. Add tetrahydrofuran agent dropwise to the reaction bottle to make the total solvent volume 1000 ml, control the reaction temperature, react for 4 hours, cool, and the obtained Grignard reagent is ready for use.
[0036] (2) Preparation of intermediate 2-chloro-6-(2-chlorobenzoyl)toluene
[0037]
[0038] 1000 ml of tetrahydrofuran solution (concentration 1.0 M) of Grignard reagent was slowly dropped into toluene solution containing 350.0 g (2.0 M) o-chlorobenzoyl chloride under ice-bath conditions, and the reflux reaction was continued for 24 hours. After the reaction was co...
Embodiment 2
[0041] The preparation of embodiment 2 compound B (6-(2-chlorobenzoyl) benzoic acid)
[0042] (1) Preparation of Grignard Reagents
[0043]
[0044] Get 48 grams (2.0M) of magnesium powder, put into four-neck round bottom flask, pass into dry hot nitrogen, cool after 30 minutes. Add o-methyl chlorobenzene, a small amount of ether and iodine, and heat to initiate the reaction. Diethyl ether was added dropwise to the reaction bottle to make the total solvent volume 1000 ml, the reaction temperature was controlled, the reaction was carried out for 4 hours, cooled, and the obtained Grignard reagent was used for future use.
[0045] (2) Preparation of intermediate 6-(2-chlorobenzoyl) toluene
[0046]
[0047] Add 1000 ml of ether solution (concentration 2.0M) of Grignard reagent into the xylene solution containing 420.0 g (2.4M) o-chlorobenzoyl chloride slowly under ice-bath conditions, and continue the reflux reaction for 36 hours. After the reaction is completed, depressu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com