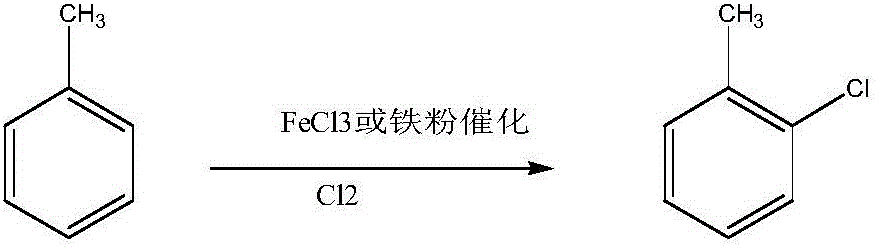
Method for preparing o-chlorotoluene through methylbenzene loop chlorination
A technology of o-chlorotoluene and ring chlorination, which is applied in chemical instruments and methods, preparation of halogenated hydrocarbons, catalytic reactions, etc., can solve the problem of reducing the total yield of p-chlorotoluene and o-chlorotoluene and insufficient selectivity of ortho-chlorination High, unfavorable industrial production and other issues, to achieve the effect of low cost, easy control, high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
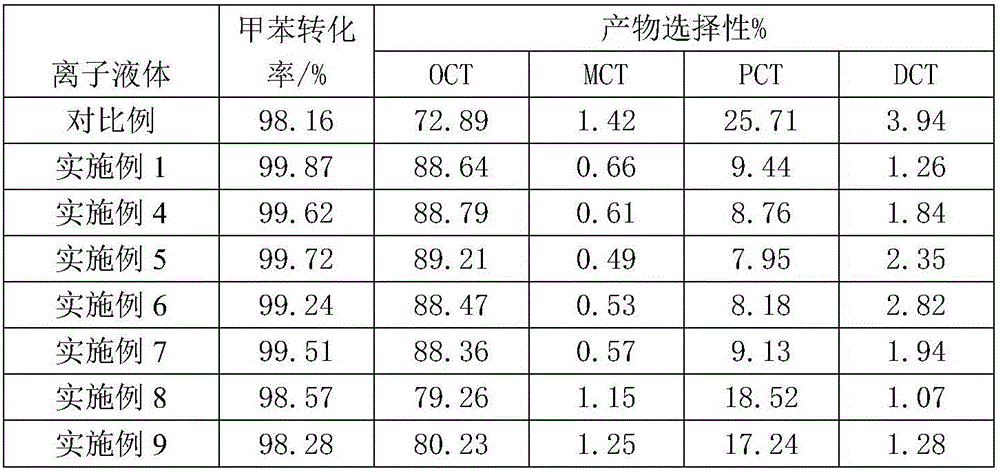
[0025] 1. Auxiliary ionic liquid [BMTM]Cl-nZnCl 2 Preparation: Under nitrogen atmosphere, mix 0.2mol 1-methyl-3-butylimidazole chloride with 0.2molZnCl 2 Add it into a three-necked flask, stir to disperse evenly, and react at 120°C for 2 hours to obtain [BMTM]Cl-ZnCl 2 ;Change ZnCl 2 The amount of 0.4mol and 0.5mol respectively prepared [BMTM]Cl-2ZnCl 2 , [BMTM]Cl-2.5ZnCl 2 .
[0026] 2. Toluene chlorination reaction steps: Add 2.0mol toluene to a 500mL four-neck flask, add a certain amount of iron powder as a catalyst, and add a certain amount of [BMTM]Cl-nZnCl 2 Ionic liquid is used as auxiliary agent (n=2), stirs to make it disperse evenly; Pass into the chlorine gas after concentrated sulfuric acid drying with the speed of 50mL / min then in the system, react at a certain temperature for a certain hour to obtain o-chlorotoluene, tail gas After condensation, the unreacted chlorine gas is absorbed by NaOH solution; the reaction is carried out under the condition of avoidi...
Embodiment 2
[0029] Adopt the method of embodiment 1, difference is only: the [BMTM]Cl-nZnCl that described employing 2 In the ionic liquid auxiliary agent (n=1); The catalyzer described in the step 2 is ferric chloride, and the volumetric amount of the ionic liquid auxiliary agent is 1% of toluene, and the mass ratio of the catalyst and the auxiliary agent is 1:5, The temperature of the chlorination reaction is 30° C., the reaction time is 2 hours, and the purity of the final product can reach 93.3%.
Embodiment 3
[0031] Adopt the method of embodiment 1, useless point only is: the [BMTM]Cl-nZnCl that described employing 2 In the ionic liquid auxiliary agent (n=2.5); The ionic liquid auxiliary agent added in the step 2 is 5% of toluene by volume, the mass ratio of the catalyst and the auxiliary agent is 5:1, and the temperature of the chlorination reaction is 90 °C, the reaction time is 6 hours, and the purity of the final product can reach 95.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com