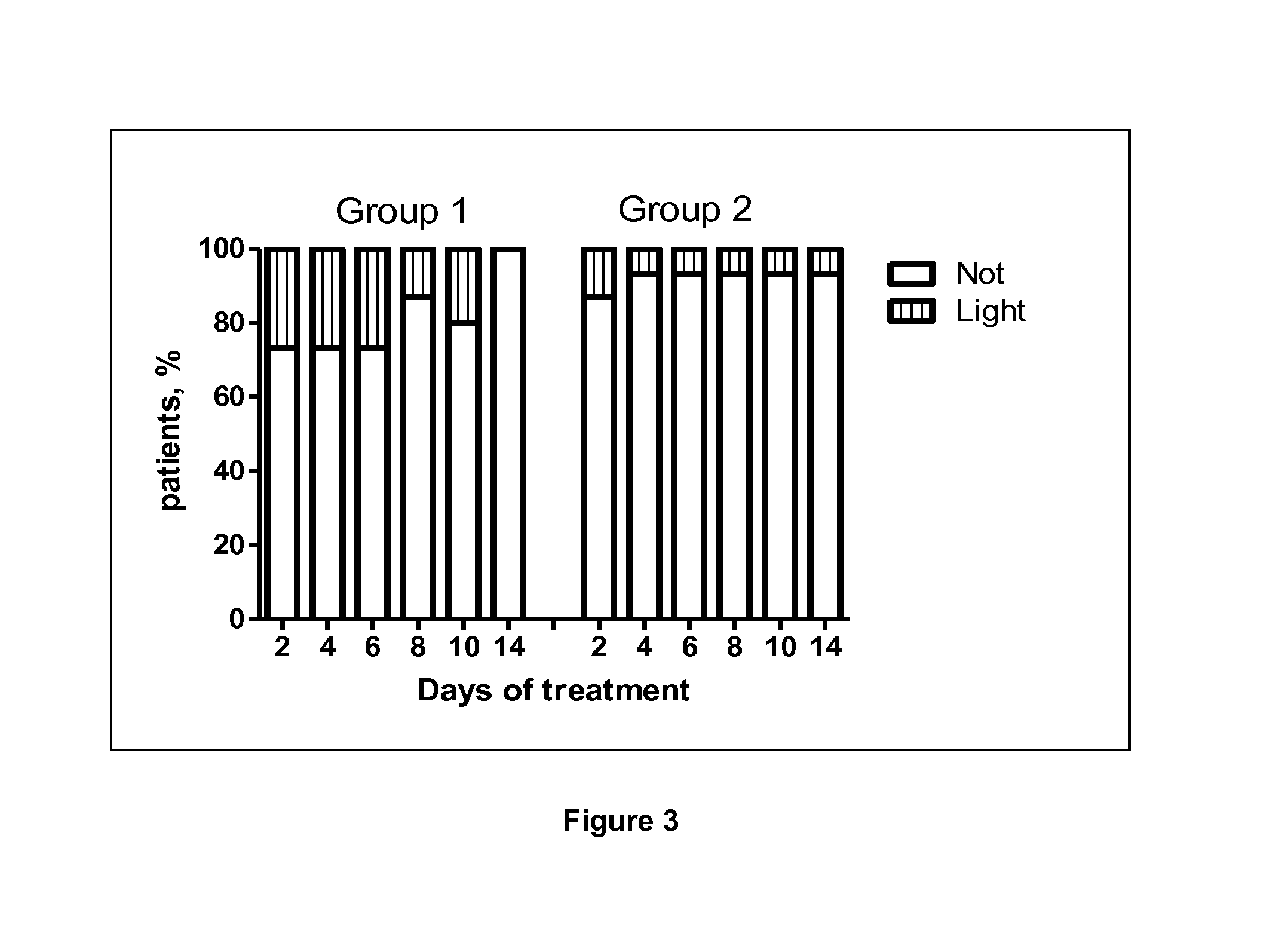
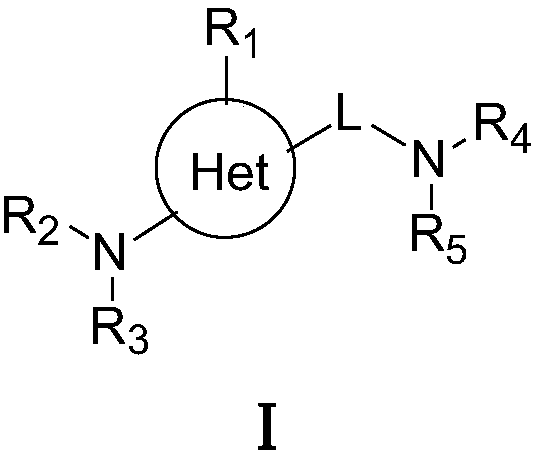
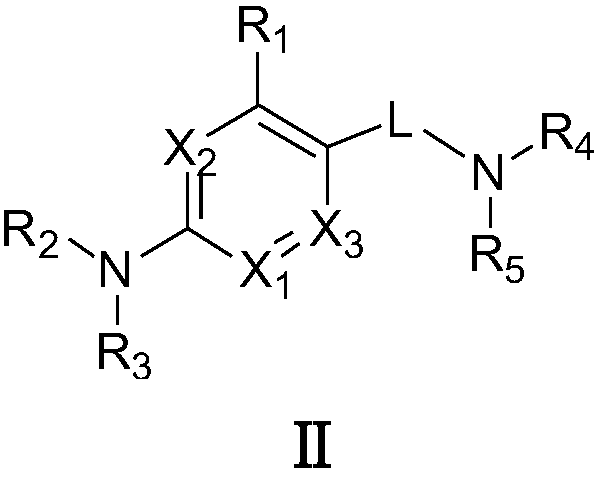
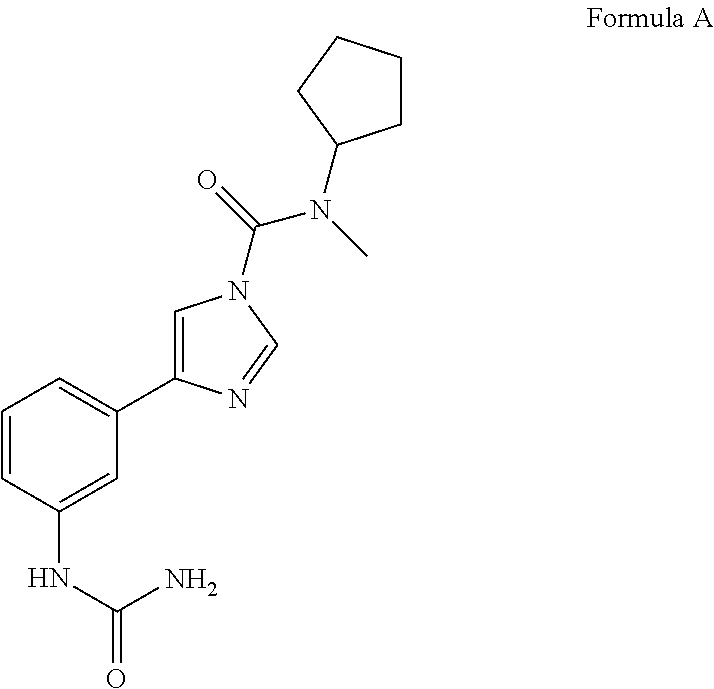
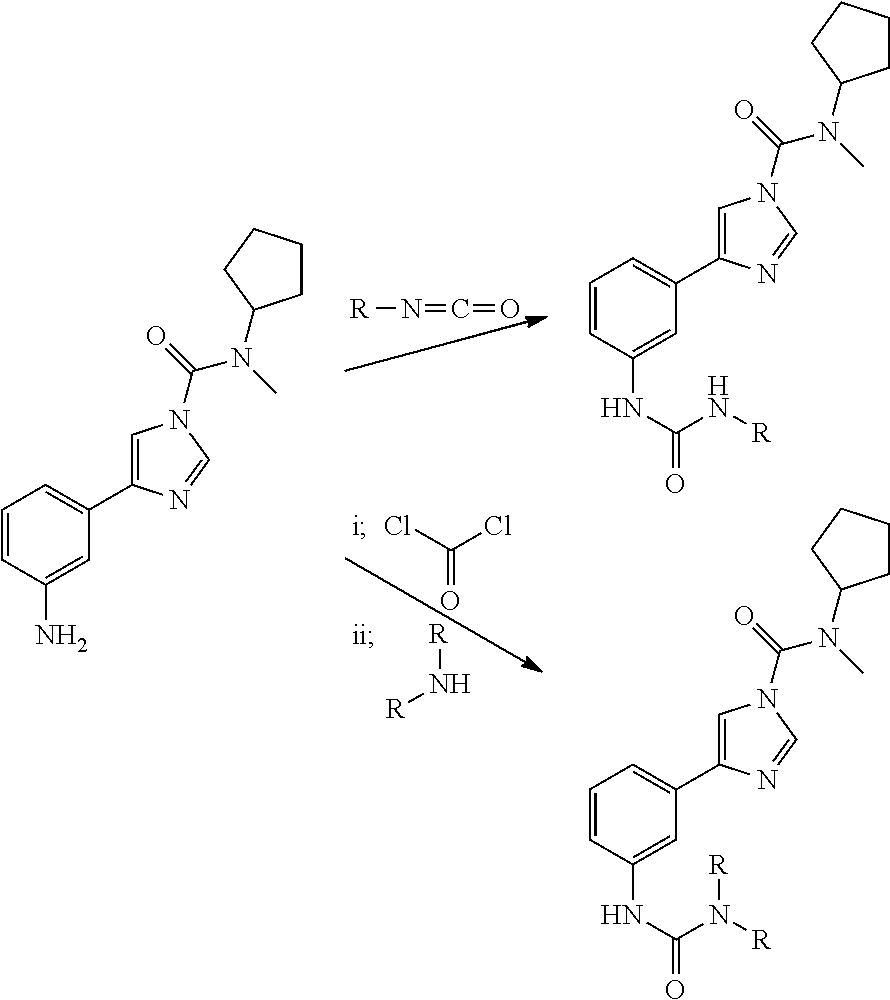
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
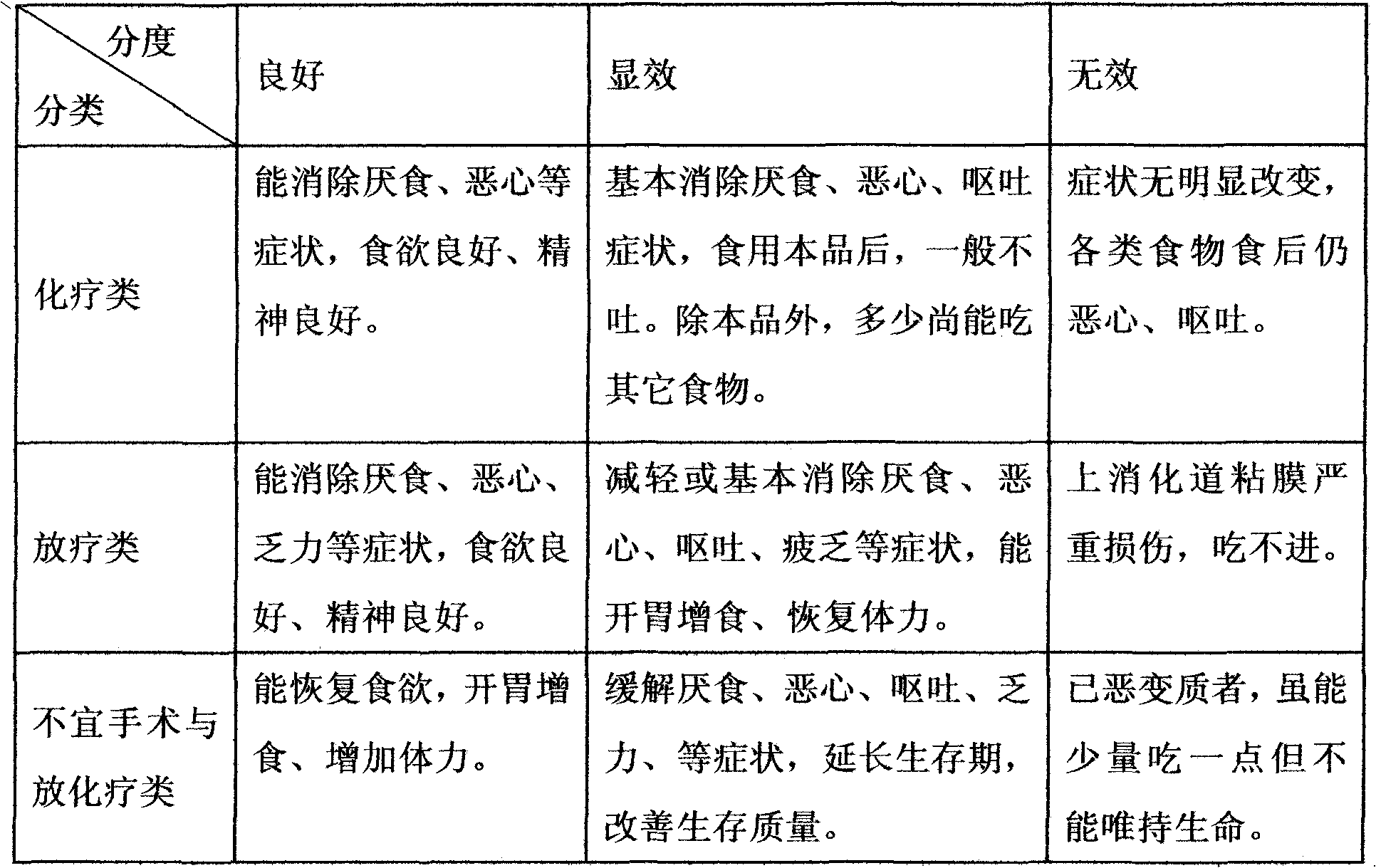
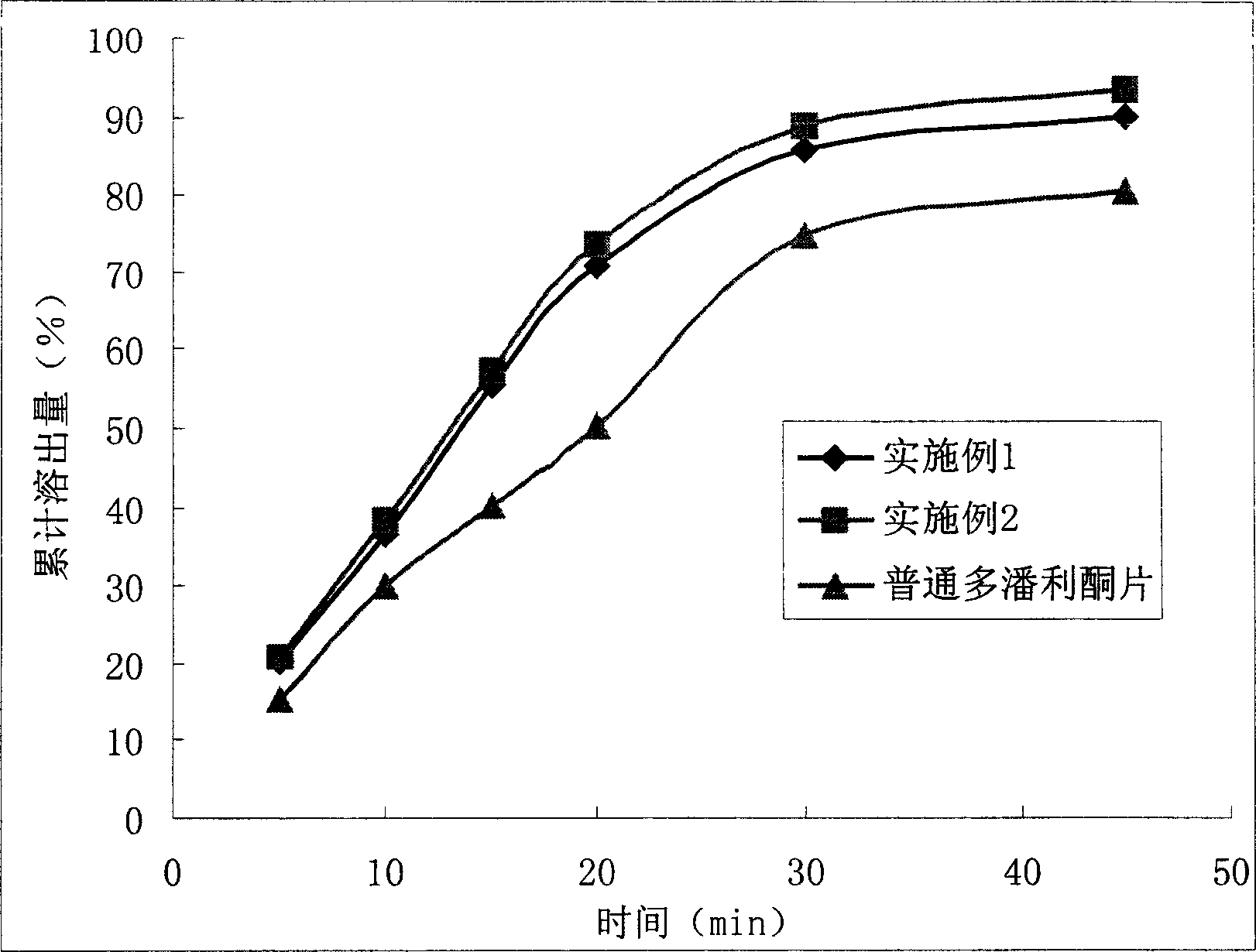
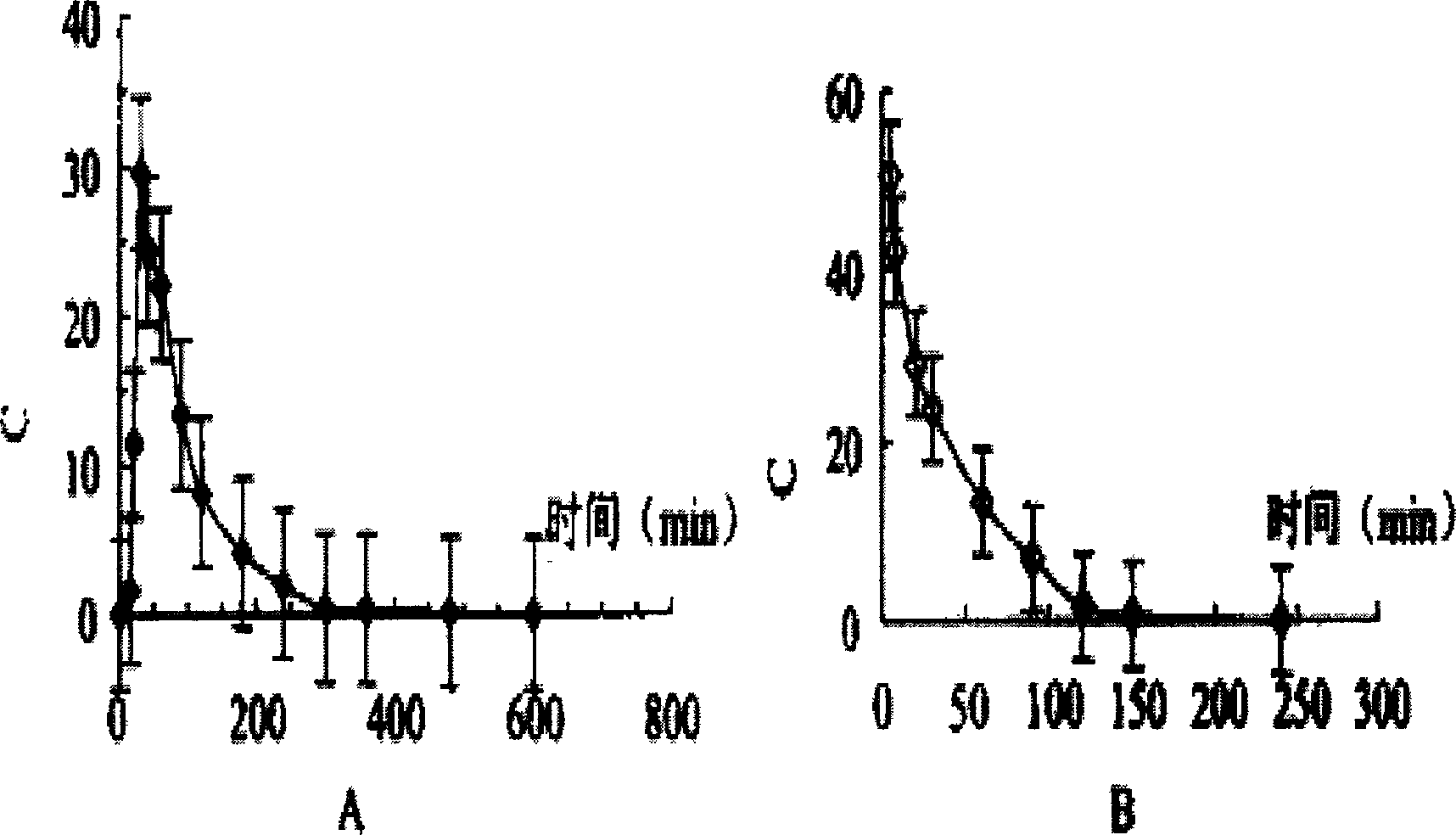
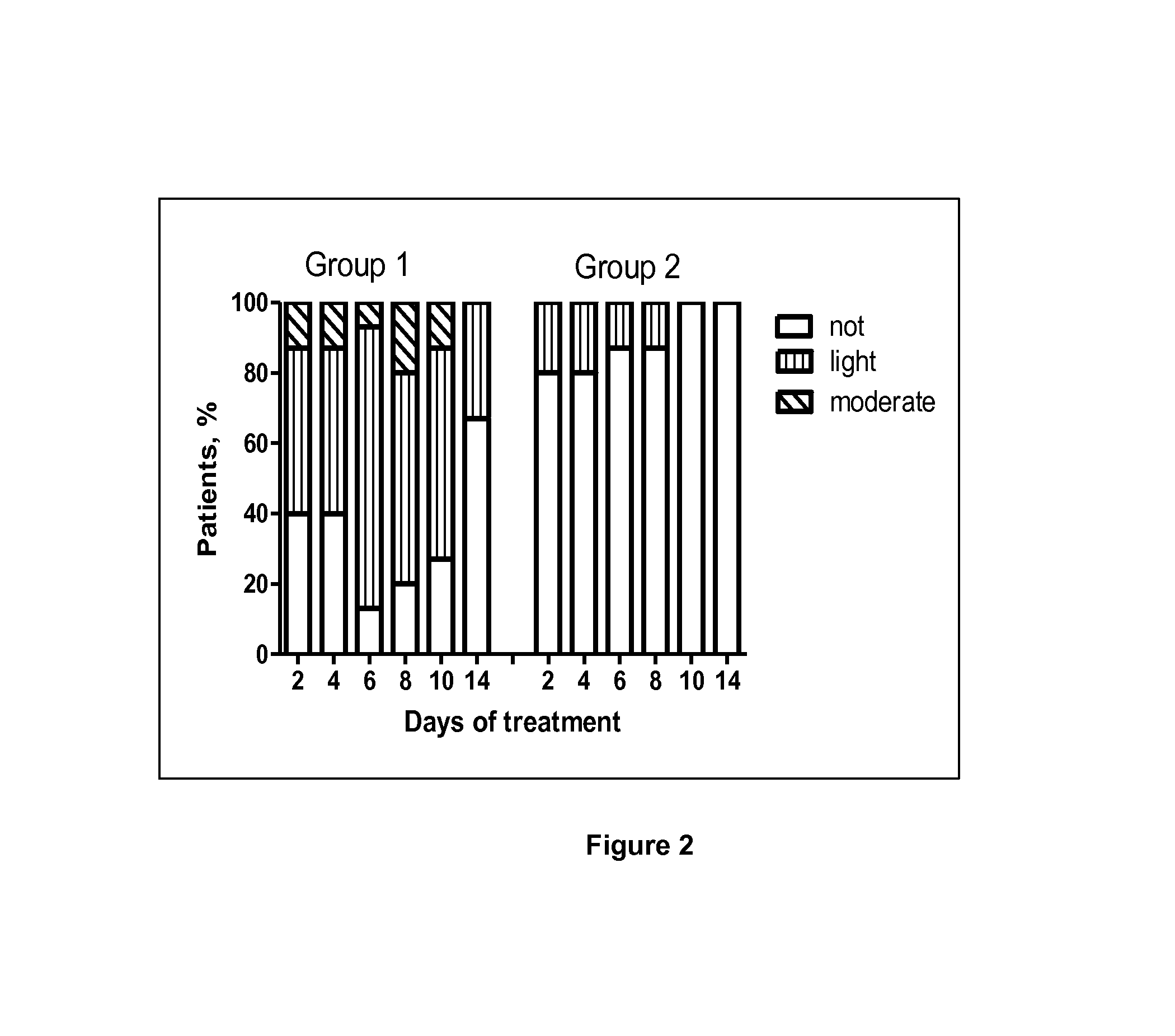
42 results about "Nausea vomiting" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
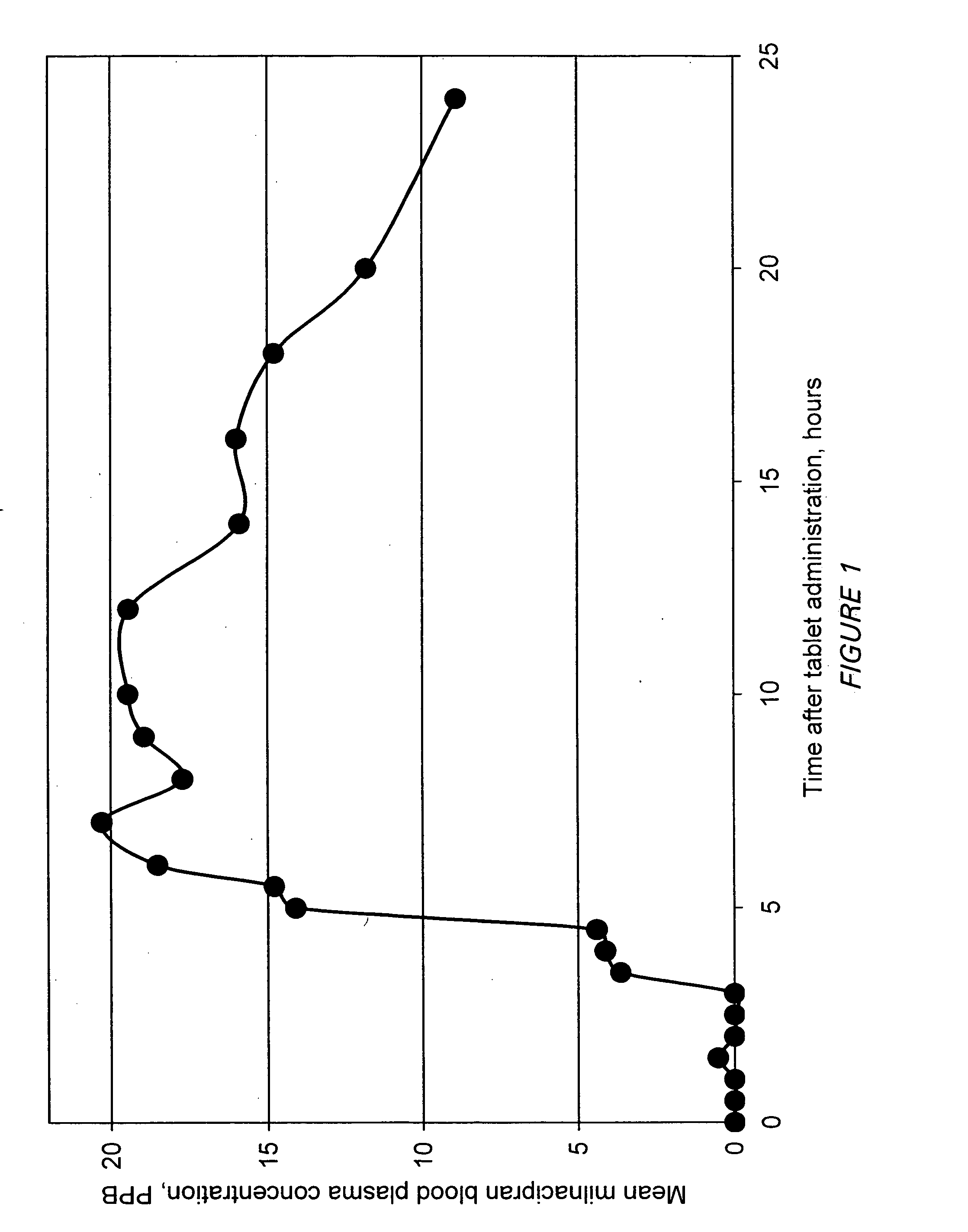
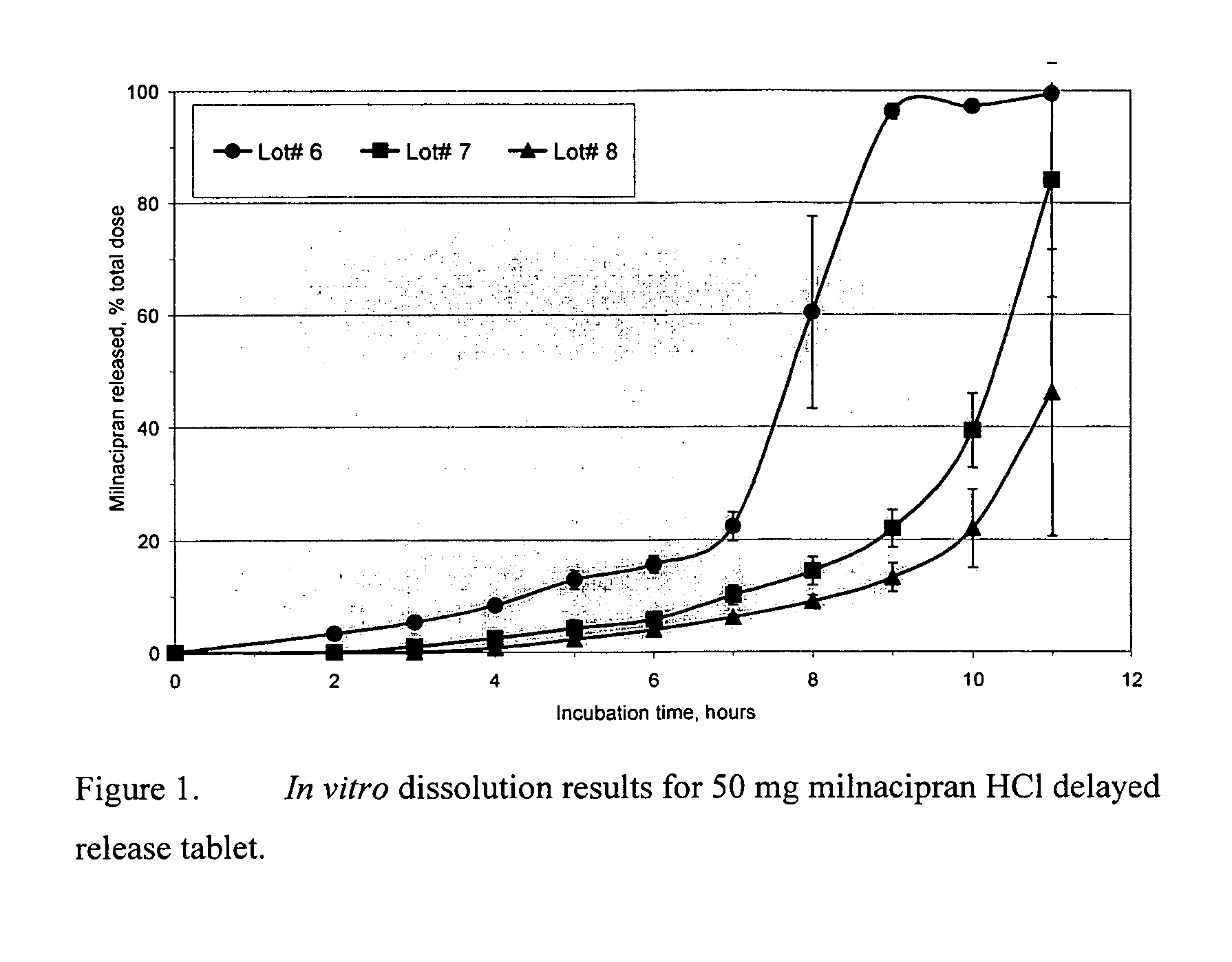
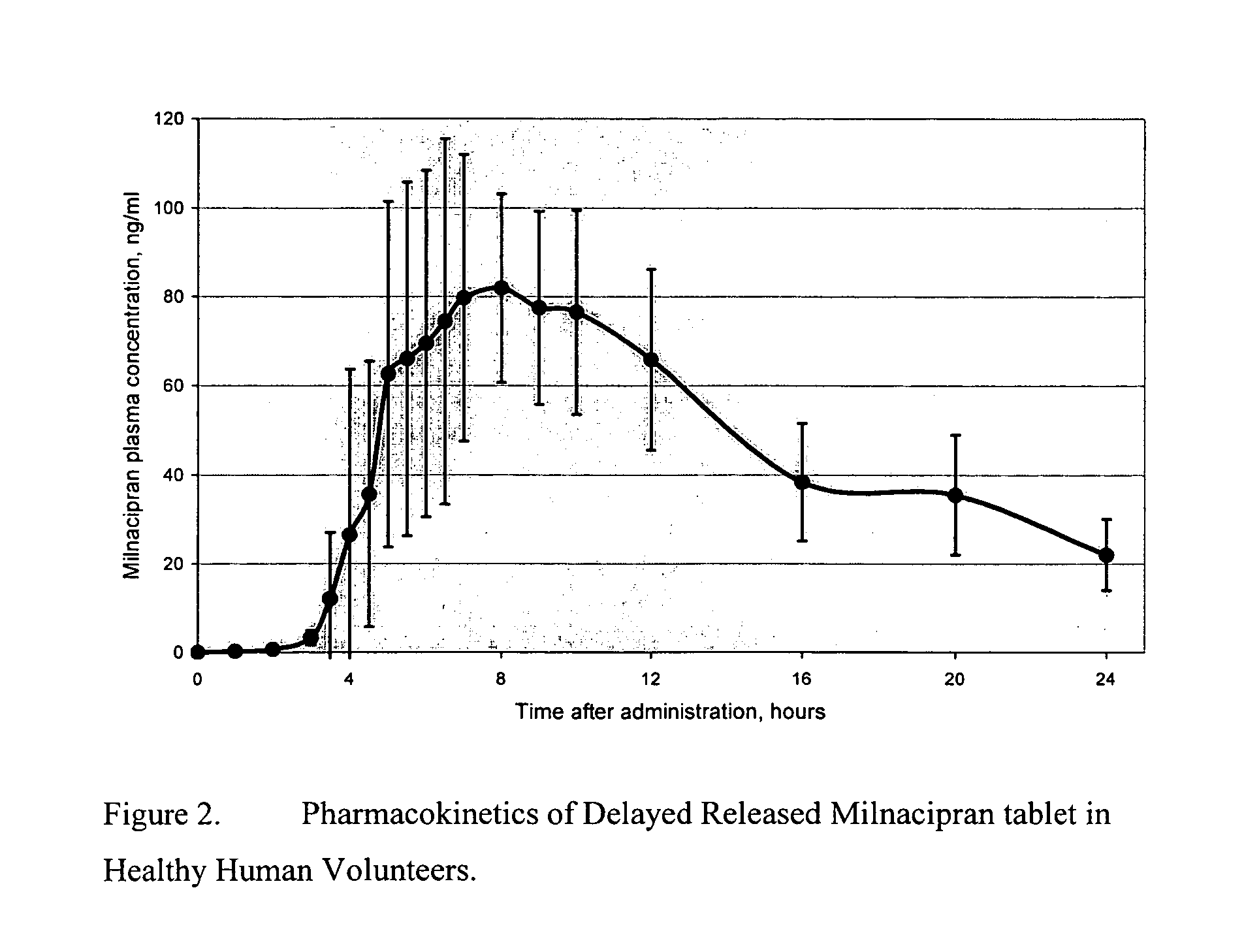
Modified release compositions of milnacipran
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition allows milnacipran to be delivered over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
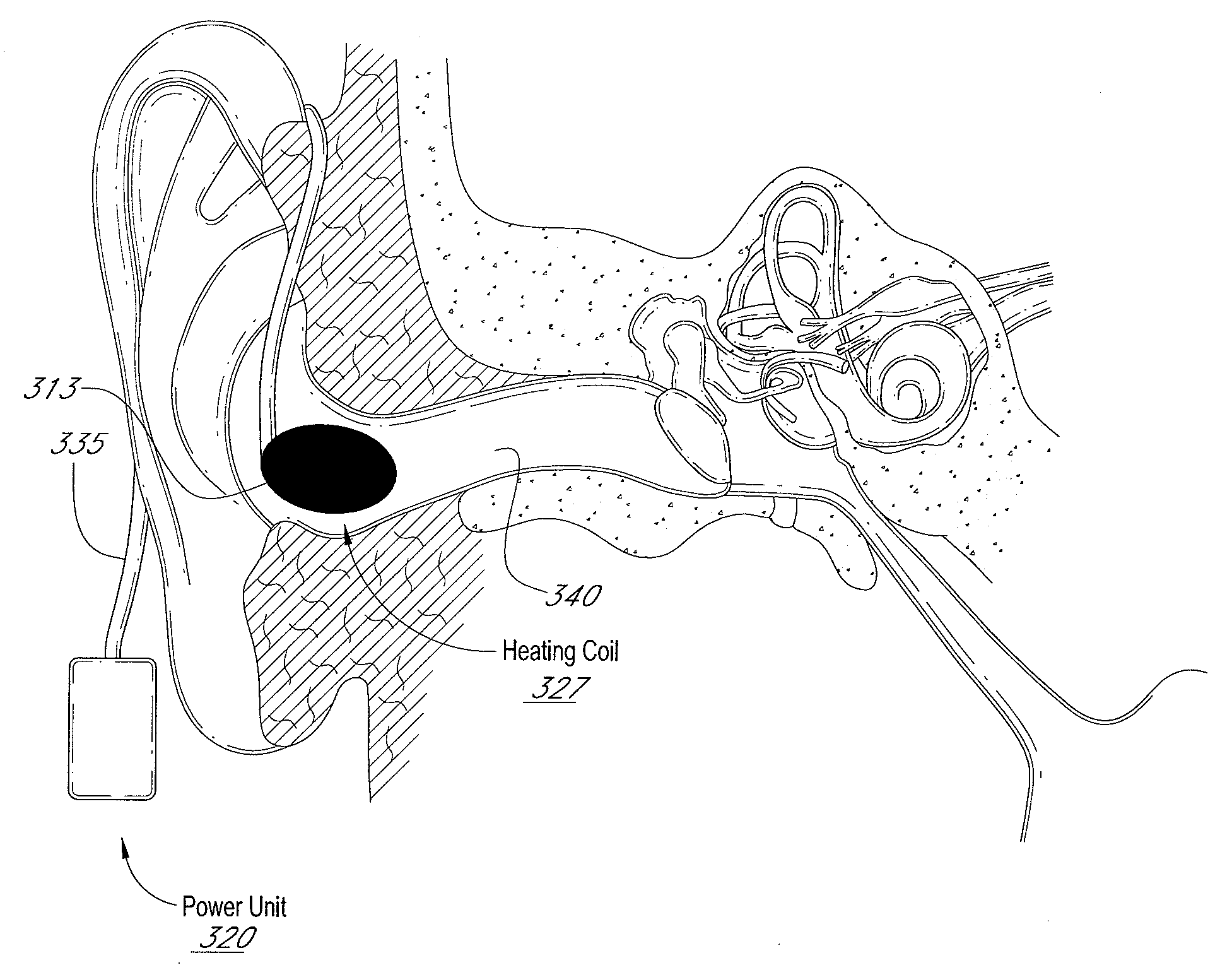
Auricular thermoregulation system for appetite suppression
InactiveUS20090182399A1Few calorieWeight controlTherapeutic coolingTherapeutic heatingSide effectVestibular system
Disclosed herein is a system to stimulate at least a part of the vestibular system of a patient to induce a sensation of anorexia to promote weight loss. The system can include a thermal element, a power source, a conduit, and a control unit configured to regulate the temperature of the thermal element, the duration in which the thermal element is applied, the duration in between thermal stimuli, and / or the number of stimulus cycles to promote a sensation of anorexia in a patient while avoiding or minimizing undesirable side effects such as nausea, vomiting, nystagmus, or vertigo. Methods of using the system are also disclosed.
Owner:SYLVESTRE DIANA
Tranexamic acid formulations with reduced adverse effects
InactiveUS20090017114A1Minimize and eliminate undesirable gastrointestinal side effectMinimize and prevent dissolutionBiocidePowder deliveryIntestinal structureSide effect
Tranexamic acid formulated in an oral dosage form with at least one agent that decreases tranexamic acid release in the stomach. Such formulations minimize nausea, vomiting, and other adverse gastric effects that may accompany tranexamic acid therapy, for example, to treat heavy menstrual bleeding. One embodiment is an extended release formulation with waxes, polymers, etc. that prevent a bolus release of tranexamic acid in the stomach. An alternative embodiment is a delayed release formulation with polymers that prevent release of tranexamic acid in the acid environment of the stomach and delay its release until the formulation reaches the less acid environment of the intestines. Such formulations enhance patient compliance with therapy because adverse effects of tranexamic acid therapy are reduced.
Owner:FERRING BV
Pulsatile release compositions of milnacipran
InactiveUS20060003004A1Minimize exposureReduces milnacipran gastrointestinal side effectCapsule deliveryCoatingsPalpitationsPanic
A once-a-day oral milnacipran pulsatile release composition has been developed that releases the drug in spaced apart “pulses”. The dosage forms are comprised of first, second and optional third dosage units, with each dosage unit having a different drug release profile. This dosage form provides in vivo drug plasma levels characterized by Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides pulsatile release of milnacipran to produce a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
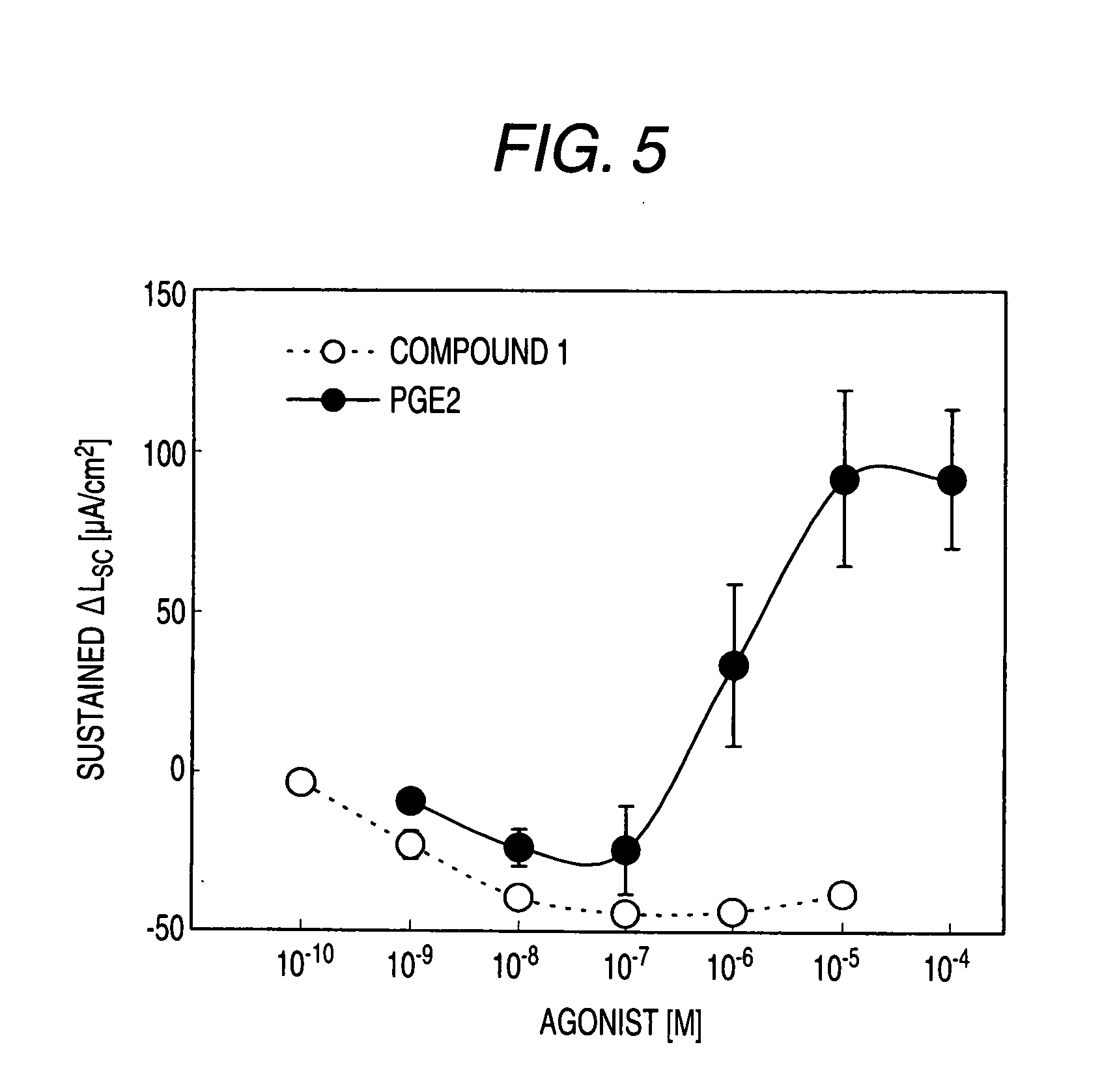
Preventive and/or Remedy for Hyperkalemia Containing Ep4 Agonist
InactiveUS20080234337A1Reduce functionImprove concentrationBiocideNervous disorderSide effectWeakness
The present invention relates to a preventive and / or therapeutic agent for hyperkalemia, and a potassium excretion promoter containing EP4 agonist. Since EP4 agonist promotes potassium excretion, it is useful as a preventive and / or therapeutic agent for hyperkalemia. In addition, if selective EP4 agonist uses, it is a preventive and / or therapeutic agent for hyperkalemia without side effects. Further, if EP4 agonist is used, it is useful as improving agent for various symptoms (e.g. paresthesia, error of perception, weakness, myoparalysis, nausea, vomit, abdominal pain, diarrhea, arrhythmia, atrioventricular block, ventricular fibrillation, atrial fibrillation, cardiac arrest, asphyxia and / or dyspnoea etc.).
Owner:ONO PHARMA CO LTD
Compositions and methods for enhancing analgesic potency of covalently bound-compounds, attenuating its adverse side effects, and preventing their abuse
InactiveUS20100144645A1Lower potentialAmenable to synthesizing conjugatesBiocideNervous disorderChemical MoietyOpioid antagonist
The invention generally relates to compositions and methods with covalently bound compounds, such as controlled substances covalently attached to a chemical moiety, and opioid antagonists or covalently bound opioid antagonists to enhance analgesic potency and / or attenuate one or more adverse effects of covalently bound compounds, including adverse side effect(s) in humans such as nausea, vomiting, dizziness, headache, sedation (somnolence), physical dependence or pruritis. This invention relates to compositions and methods for selectively enhancing the analgesic potency of a covalently bound compound and simultaneously attenuating anti-analgesia, hyperalgesia, hyperexcitability, physical dependence and / or tolerance effects associated with the administration of a covalently bound compound. The methods of the invention comprise administering to a subject an analgesic or sub-analgesic amount of a covalently bound compound and an amount of excitatory opioid receptor antagonist such as naltrexone or nalmefene effective to enhance the analgesic potency of a covalently bound compound and attenuate the anti-analgesia, hyperalgesia, hyperexcitability, physical dependence and / or tolerance effects of covalently bound compound. The invention also relates to the addition of covalently-bound opioid antagonists to the compositions containing covalently bound compounds such that if the compositions are subjected to manipulation by illicit chemists, the opioid antagonist is released effectively reducing or eliminating the euphoric effect of the covalently bound compounds.
Owner:SHIRE PLC
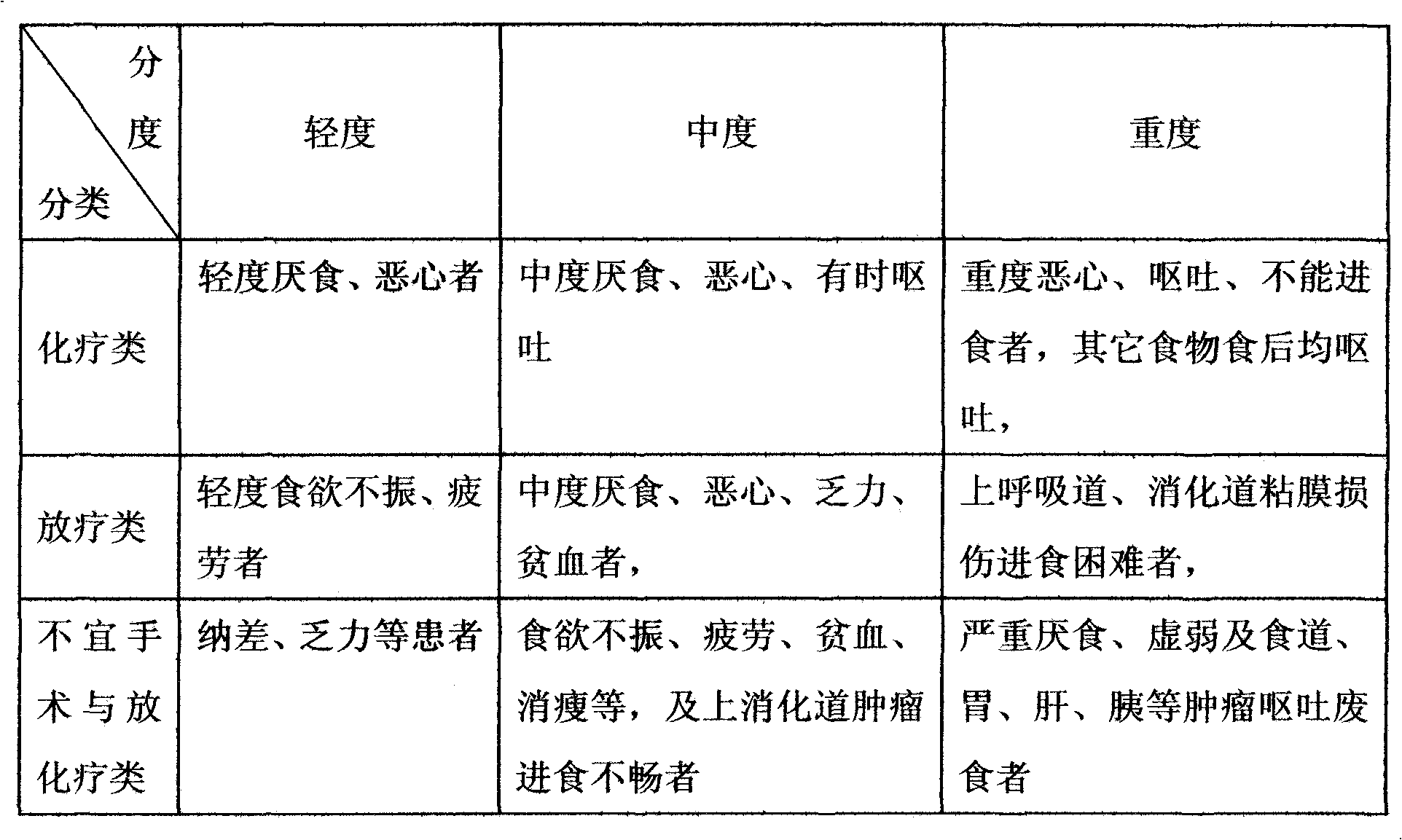
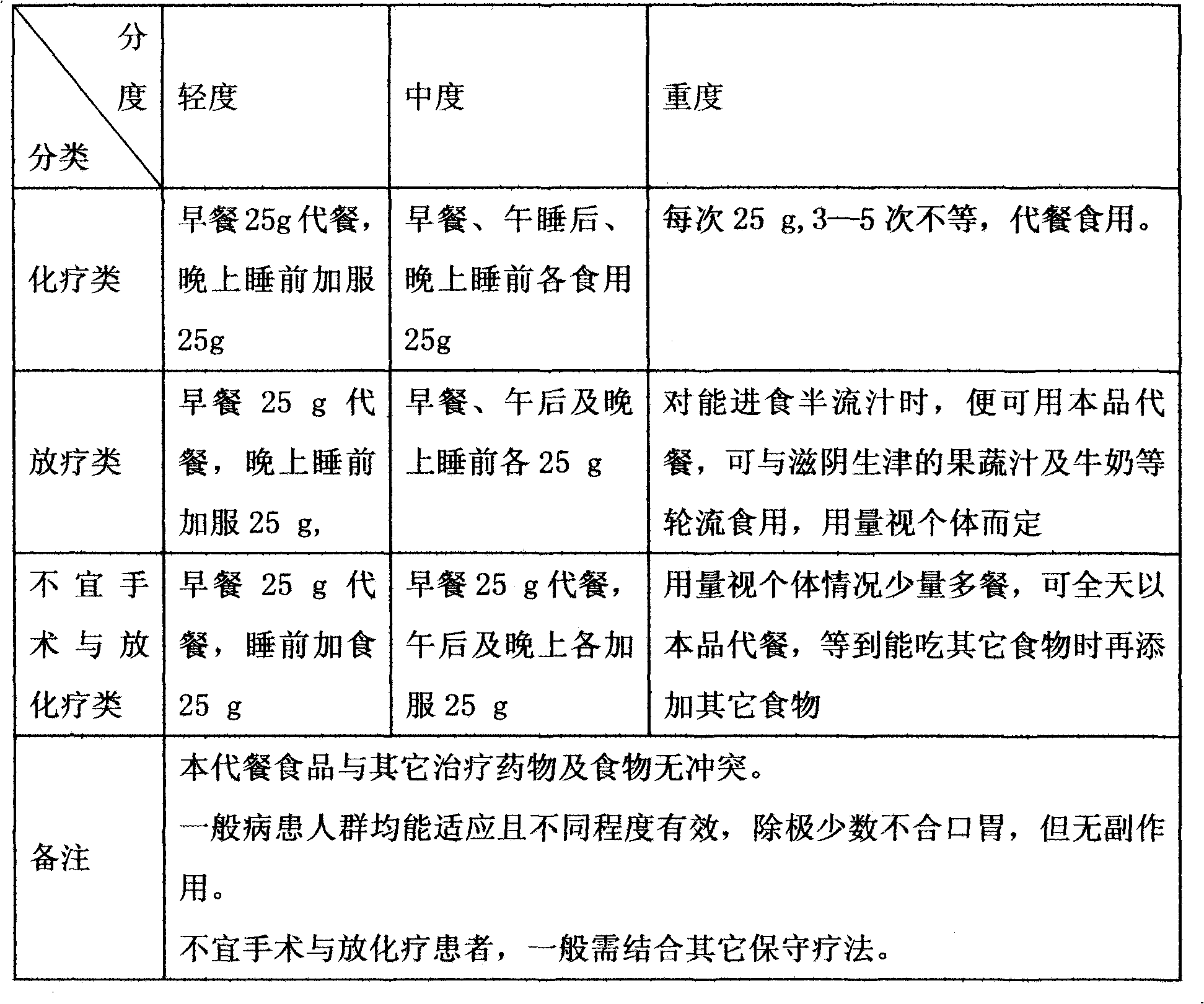
Functionality nutritional meal replacing food suitable for tumor patient and preparation method thereof
InactiveCN101822383AEnhance immune functionRegulate metabolismFood preparationSpirulina maximaMushroom
The invention relates to a functionality nutritional meal replacing food suitable for tumor patients. The food mainly comprises the components according to the mass percent: 10-30 parts of wheat seed, 4-10 parts of tuckahoe, 4-10 parts of Chinese yam, 4-10 parts of barbary wolfberry, 4-10 parts of fried white lablab bean, 1.5-4.5 parts of black sesame, 1.5-4.5 parts of mushroom extractive containing 20% of lentinan, 1.5-4.5 parts of dandelion extractive with 10:1 of herbal withdrawal ratio, 1.5-4.5 parts of bare peach kernel, 1-3 parts of spirulina platensis or spirulina maxima, 1-3 parts of pure fructus amomi and 6-66 parts of brown rice. The invention well solves the problems of the tumor patients (chemotherapy) such as poor appetite, poor eating, anorexia, nausea vomiting, being unableto eat anymore, has more comprehensive nutrition and is suitable for being used as the meal replacing food of the tumor patients, has auxiliary functions on body immunity enhancement, anti-tumor and relapse prevention and can obviously lighten the concurrent chemoradiotherapy gastrointestinal tract reaction. The invention also relates to a preparation method for the meal replacing food.
Owner:纪福黛
8'-hydroxy-dihydroergotamine compounds and compositions
InactiveUS20140179705A1Eliminate side effectsHigh activityBiocideLiquid surface applicatorsNausea sicknessDihydroergotamine
8′-Hydroxy-Dihydroergotamine (8′-OH DHE) medicinal compounds, compositions, and dosage forms containing such compositions are provided. Also provided herein are methods of treatment, prevention, or amelioration of diseases, conditions or disorders selected from amyotrophic lateral sclerosis (ALS), Parkinson's disease, stress / anxiety, nausea, emesis, aggression, pain, neuropathic pain, sleeplessness, insomnia, restless leg syndrome and depression using the compounds, compositions, dosage forms and administration techniques disclosed herein.
Owner:MAP PHARMACEUTICAL INC
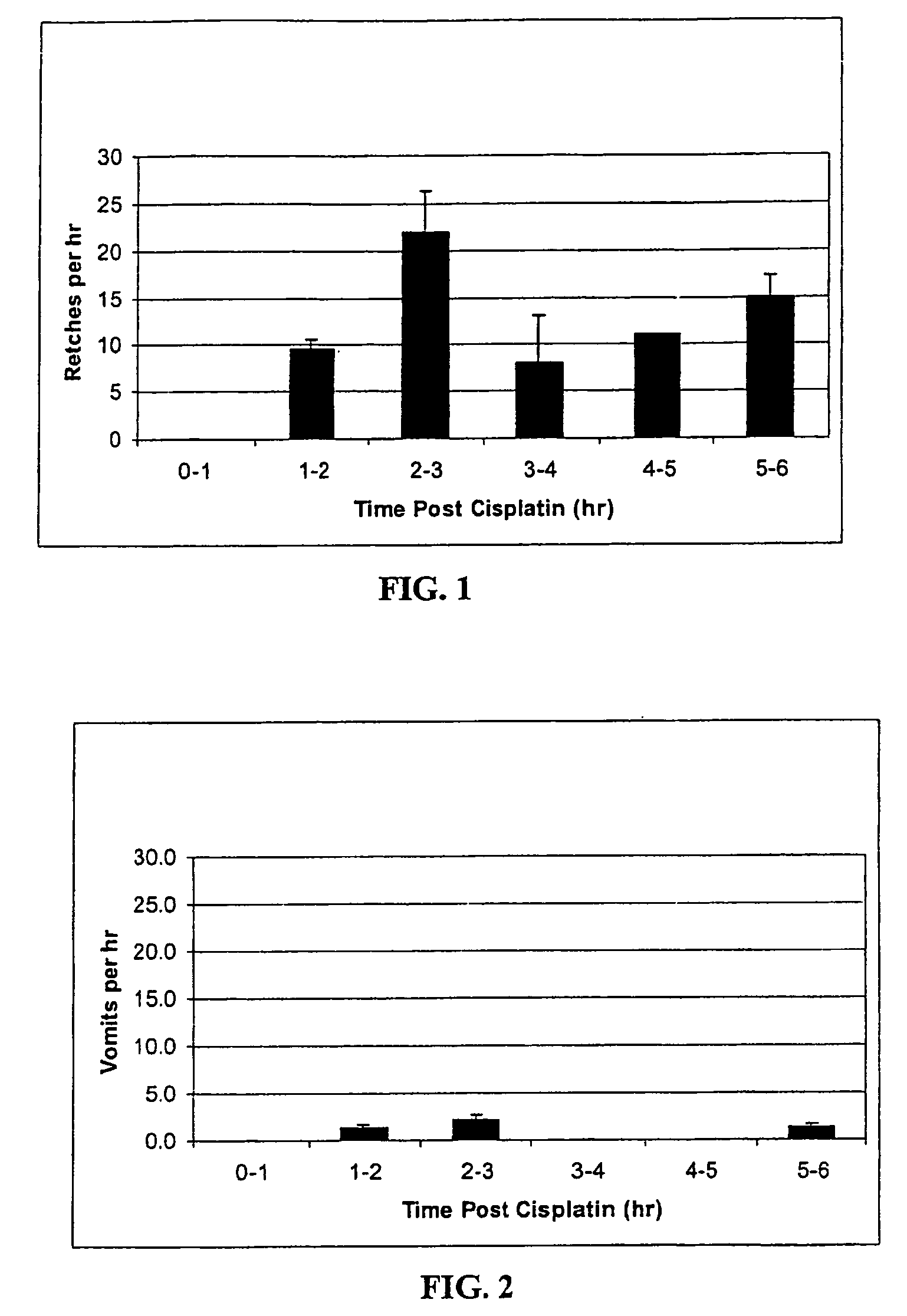
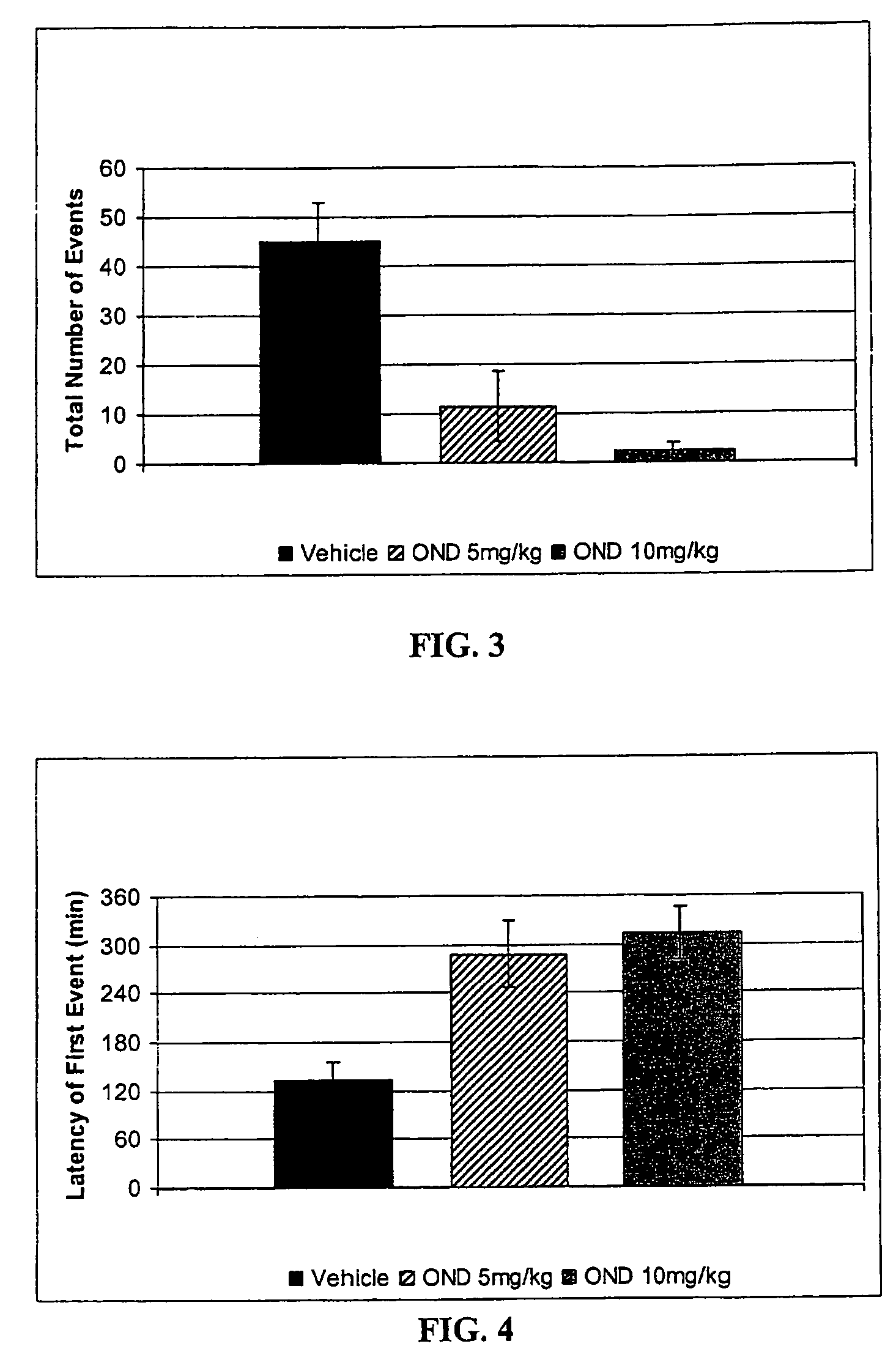
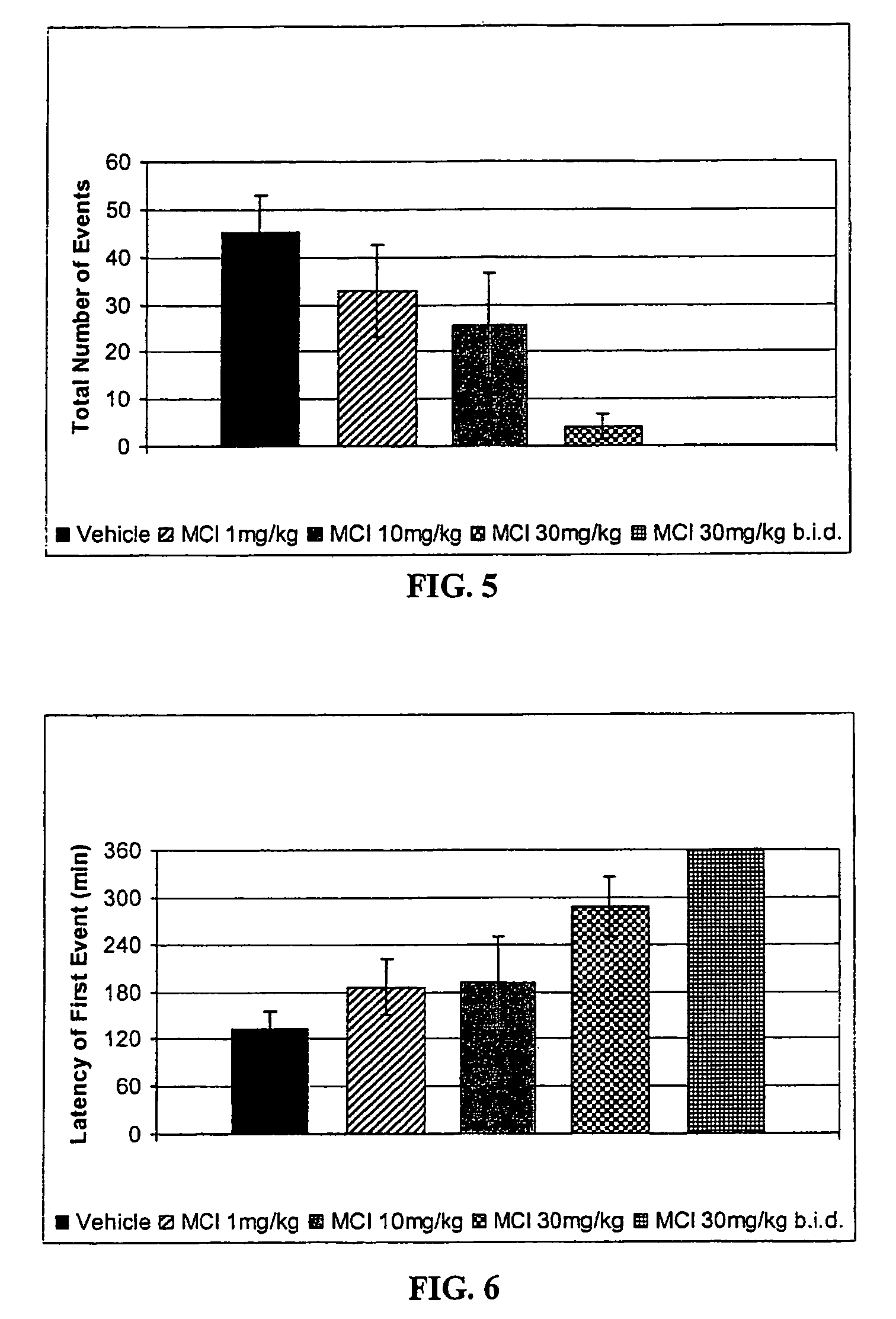
Method of treating nausea, vomiting, retching or any combination thereof
Methods are disclosed for the treatment of nausea and vomiting in a patient suffering therefrom comprising administering 4-(2-fluorophenyl)-6-methyl-2-(1-piperazinyl)thieno[2,3-D]pyrimidine.
Owner:EDUSA PHARMA
A composition for treating gastrointestinal distress
InactiveCN1843508AIntegrity guaranteedProtect the effect of tumor treatmentSalicyclic acid active ingredientsDigestive systemRegimenImmunodeficiency
A composition and method for treating and / or preventing acute and chronic gastrointestinal distress including nausea, vomiting, lactose intolerance, obstructive symptoms, diarrhea, mucositis, bleeding, weight loss, and malnutrition in a subject who is immunocompromised or receives a planned course of chemotherapy and / or radiotherapy. The method comprises administering a histone deacetylase inhibitor or in conjunction with a second agent to the subject. A composition and method using a histone deacetylase inhibitor for protecting normal tissues from chemotherapy and / or radiotherapy-induced injuries without the risk of tumor protection in cancer therapy is also provided. It is further provided a composition and method for treating and / or preventing cachexia, cancer-related fatigue, or chronic fatigue syndrome.
Owner:SUNNY PHARMTECH
Pharmaceutical composition containing flavonoids
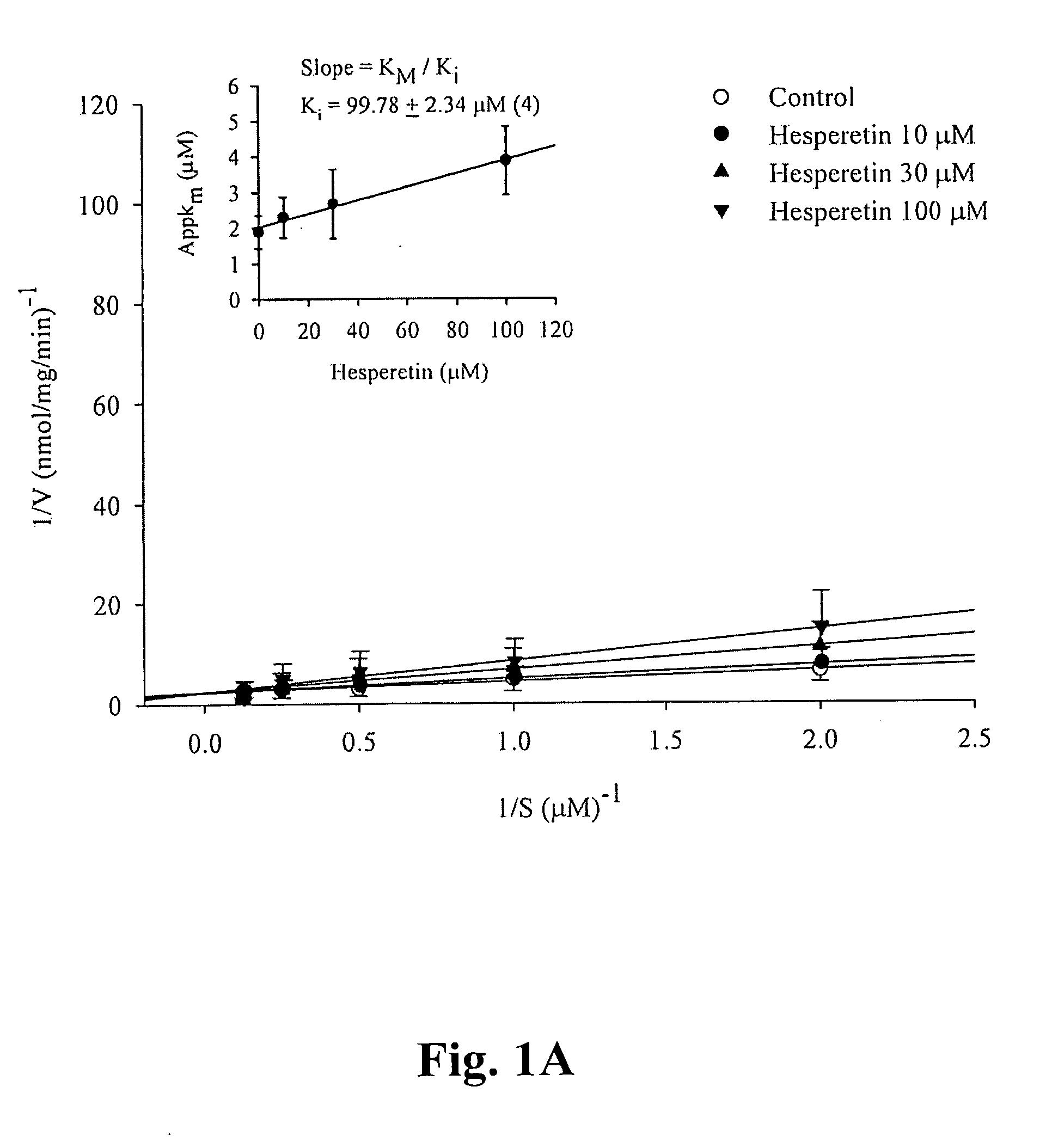
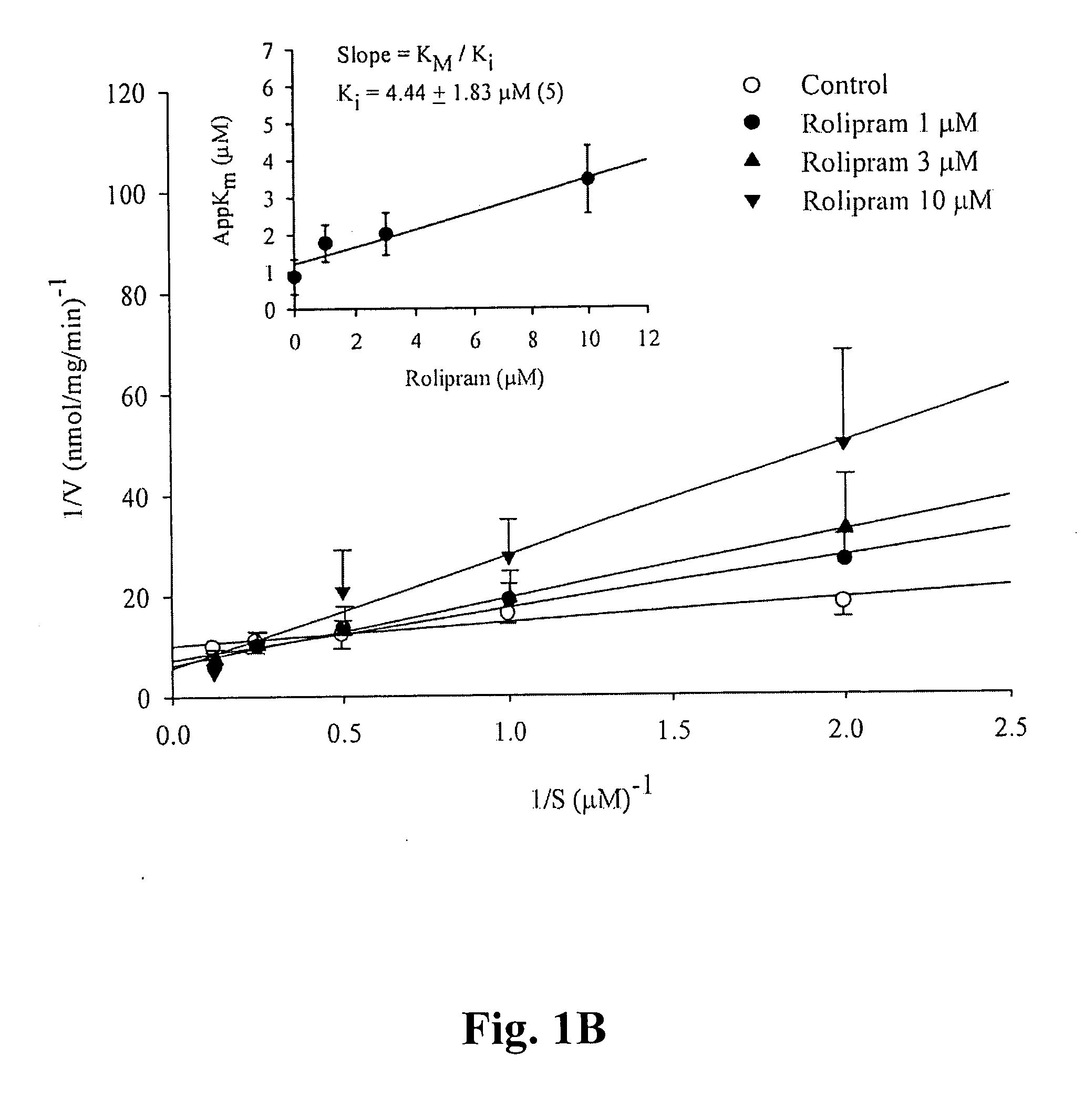
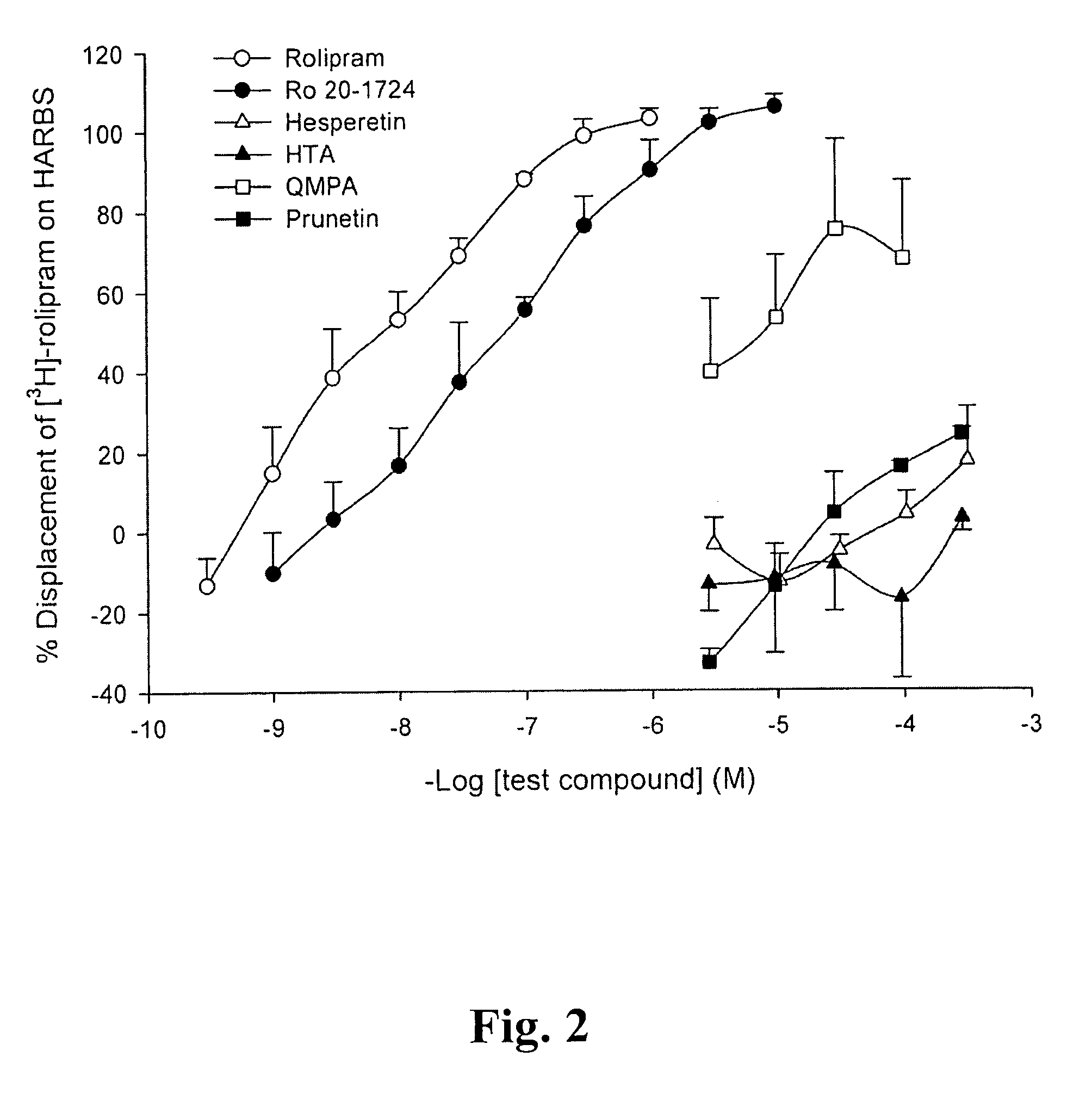
A pharmaceutical composition is provided, where the pharmaceutical composition contains formula (I), (II), or (III) flavonoids which possess selective phosphodiesterase 4 or 4 / 3 inhibition, as a main ingredient. Especially, this composition is used in the treatment of asthma, chronic obstructive pulmonary disease (COPD), or chronic inflammation, and has bronchodilatory effects. In addition, whether the above-mentioned flavonoids have side effects, such as nausea, vomiting, gastric hypersecretion, etc., in accordance with their binding to high affinity rolipram binding sites (HARBS) of particulates of brain cells are disclosed.
Owner:TAIPEI MEDICAL UNIV
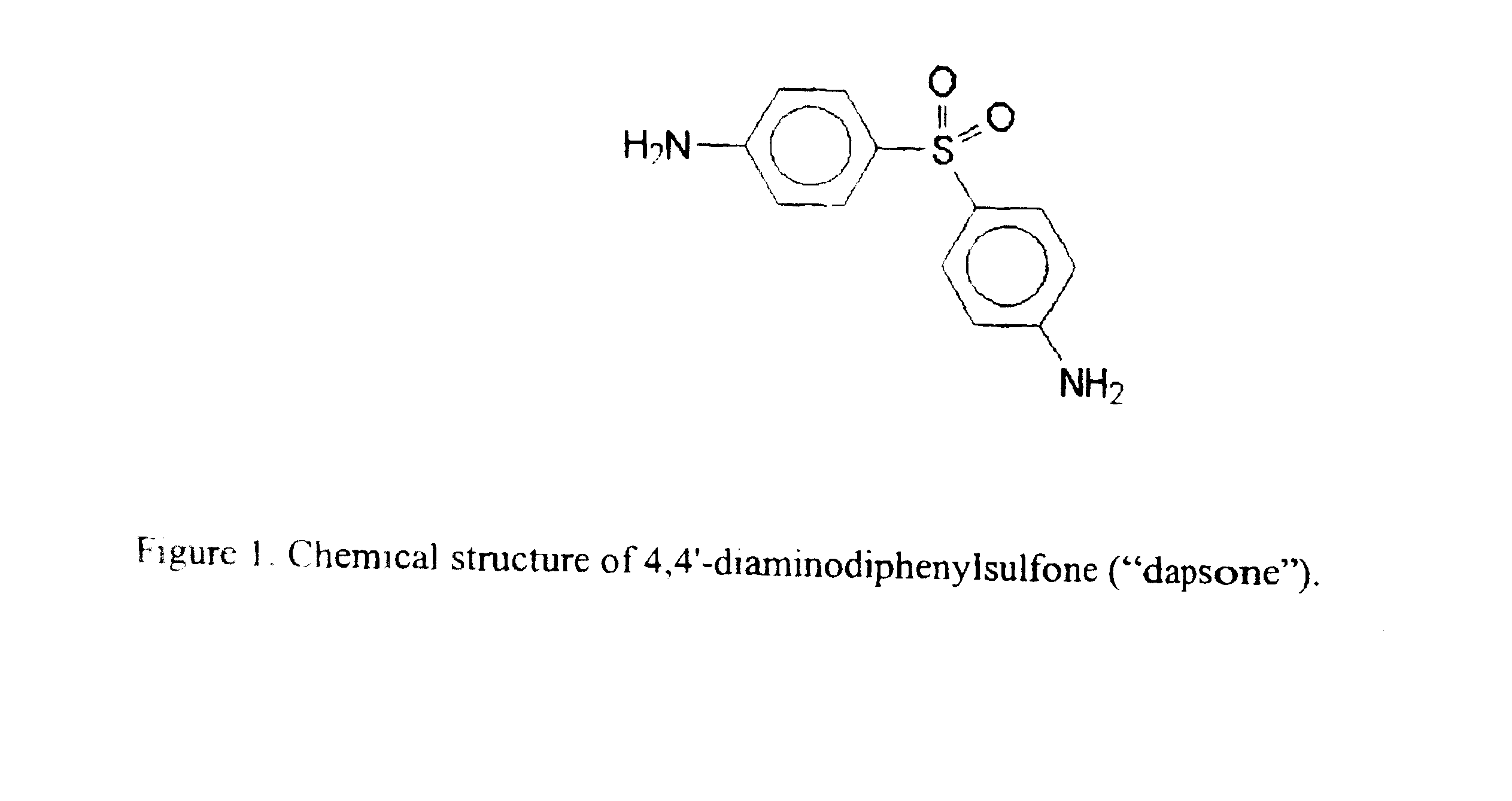
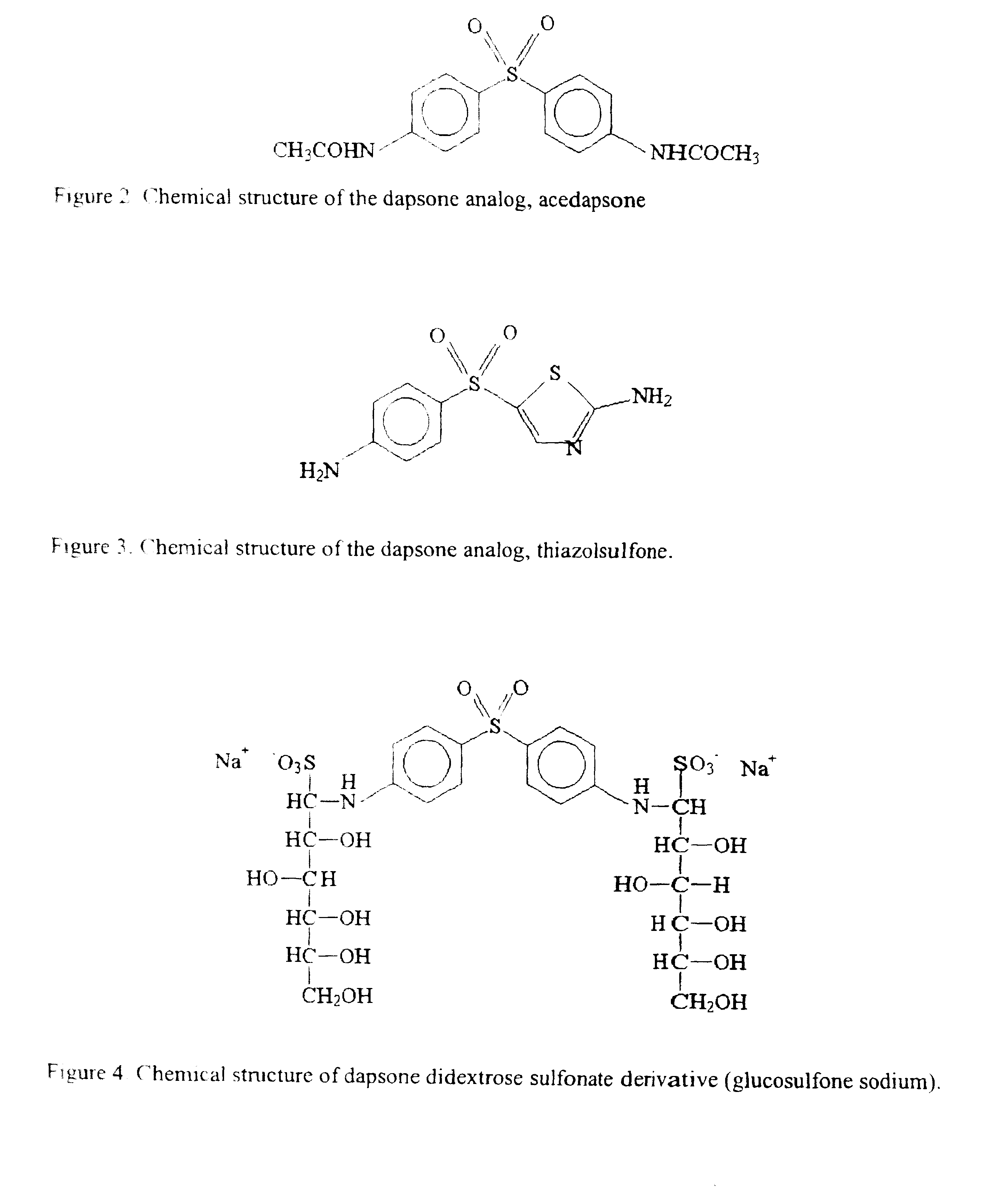
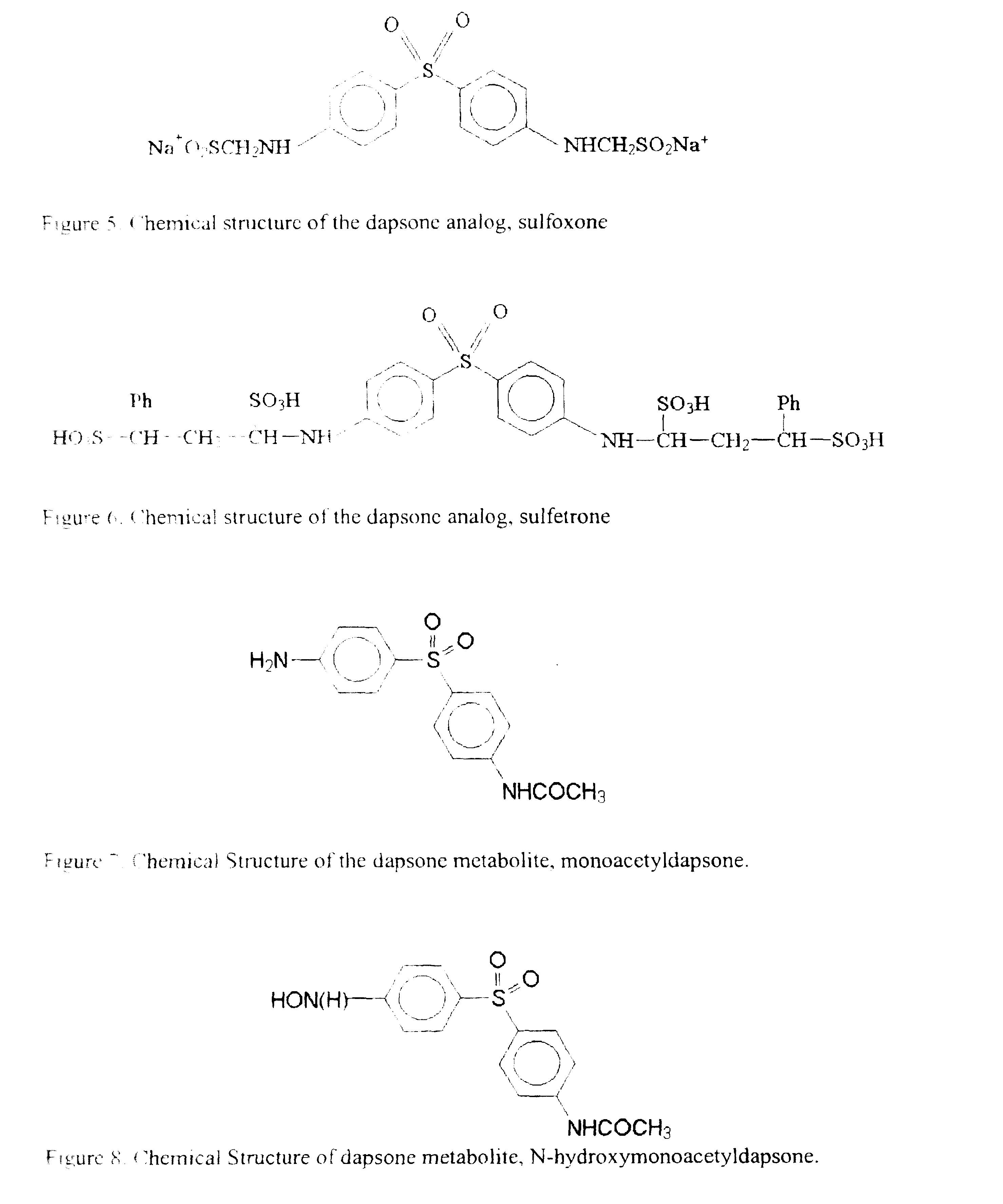
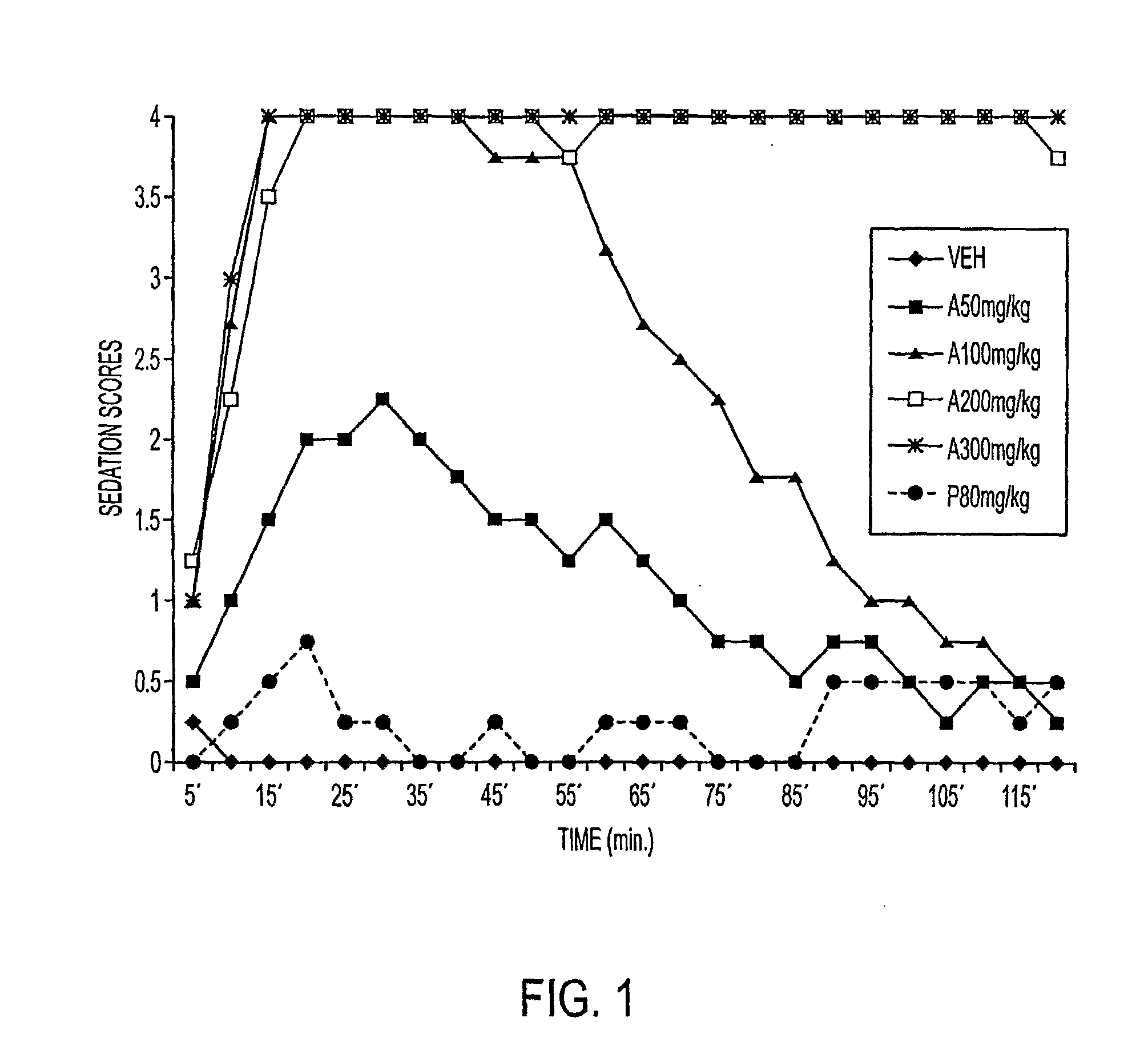
Galenical preparations of dapsone and related sulphones, and method of therapeutic and preventative treatment of disease
Dapsone and related sulfones are known to have therapeutic activity against leprosy, dermatitis herpetiformis, actinomycotic mycetoma, asthma, malaria, rheumatoid arthritis, Kaposiís sarcoma, pneumocystis carinií (pneumonia), subcorneal pustular dermatosis and cystic acne, in patients in need of such therapy. These sulfones are also known to have therapeutic activity against memory loss in patients in need of such therapy, including patients suffering from Alzheimer's disease and related neurodegenerative disorders. It has now been found that new, modified-release formulations of dapsone and related sulfones may also be used that decrease side effects and increase effectiveness of the drugs. New methods are disclosed utilizing certain formulations of dapsone and related sulfones that improve the therapeutic index of said drugs. Side effects of these drugs are known to those skilled in the art and include, but are not restricted to anorexia, psychosis, agranulocytosis, peripheral neuritis, hemolysis, methemoglobinemia, nausea, vomiting, headache, dizziness, tachycardia, nervousness, insomnia and skin disorders. Modified-release (as defined herein) formulations of dapsone have now been found to avoid some or all of these side effects, and to have more efficacy on potency.
Owner:IMMUNE NETWORK
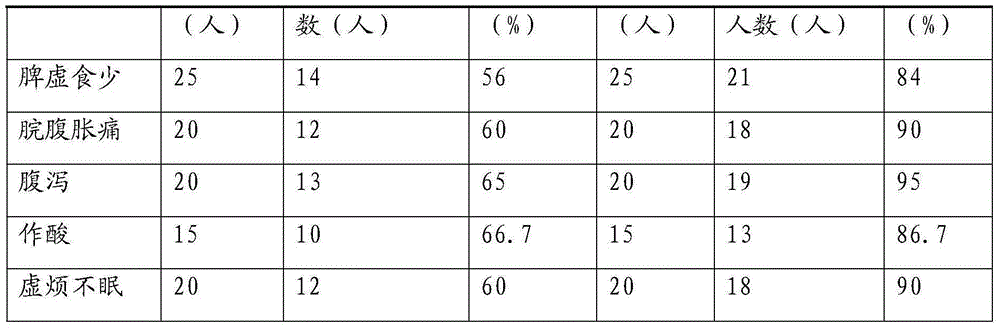
Chinese medicinal preparation for treating pernicious vomiting
InactiveCN102895591ASmall dosageGood curative effectHeavy metal active ingredientsDigestive systemMiscarriageSide effect
The invention relates to the technical field of Chinese medicines, in particular to a pure Chinese medicinal preparation for treating pernicious vomiting. The pure Chinese medicinal preparation is prepared from the following Chinese herbal medicines in parts by weight: 10 to 30 parts of codonopsis pilosula, 10 to 30 parts of large-head atractylodes rhizome, 10 to 30 parts of poria, 10 to 30 parts of south dodder seed, 8 to 20 parts of pinellia tuber, 10 to 30 parts of dried orange peel, 10 to 30 parts of wrinkled gianthyssop herb, 15 to 35 parts of red ochre, 8 to 20 parts of costus root, 10 to 30 parts of bitter orange, 8 to 20 parts of amomum fruit, 10 to 30 parts of perilla, 10 to 30 parts of bamboo shavings and 5 to 10 parts of liquorice root; the pure Chinese medicinal preparation can be prepared into pharmaceutically acceptable medicines such as pills, powder, decoction and the like; the pure Chinese medicinal preparation is prepared from pure Chinese medicines, and can tonify the spleen, soothe the liver, promote the circulation of qi, harmonize the stomach, lower adverse qi, control nausea and vomiting, tonify the kidney and prevent miscarriage; the pure Chinese medicinal preparation is suitable for symptoms of early trimester of pregnancy, nausea vomiting, non-eating, vomiting immediately after ingestion, acid water or bitter water vomiting, dislike of smelling greasy food, tastelessness, drool vomiting, dizziness, fatigue of a body, flatulence, thoracic fullness, hypochondriac pain and the like; and the Chinese medicinal preparation is small in dosage, good in curative effect, quick in response and obvious in pharmacological effect, and does not have toxic or side effects.
Owner:赵自强
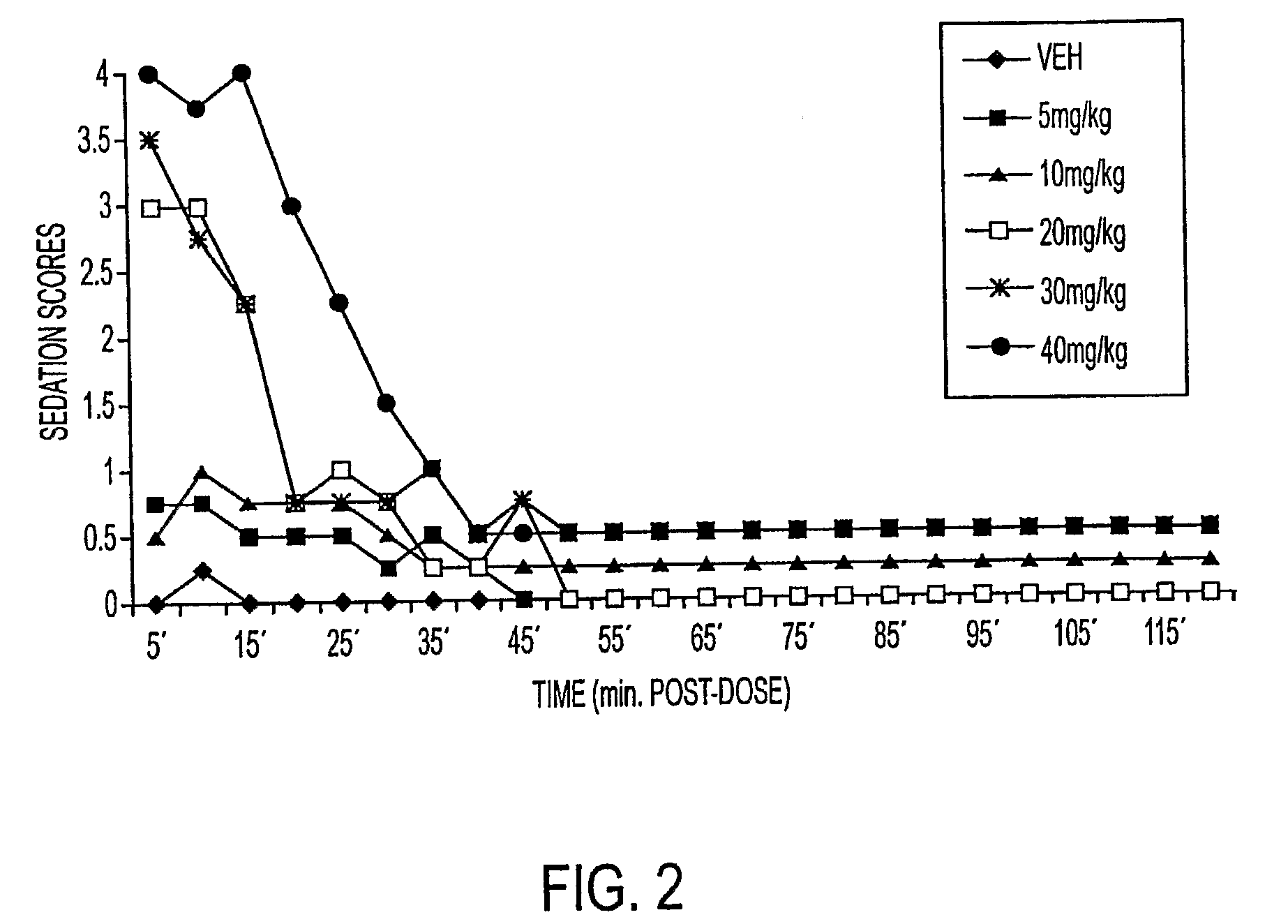
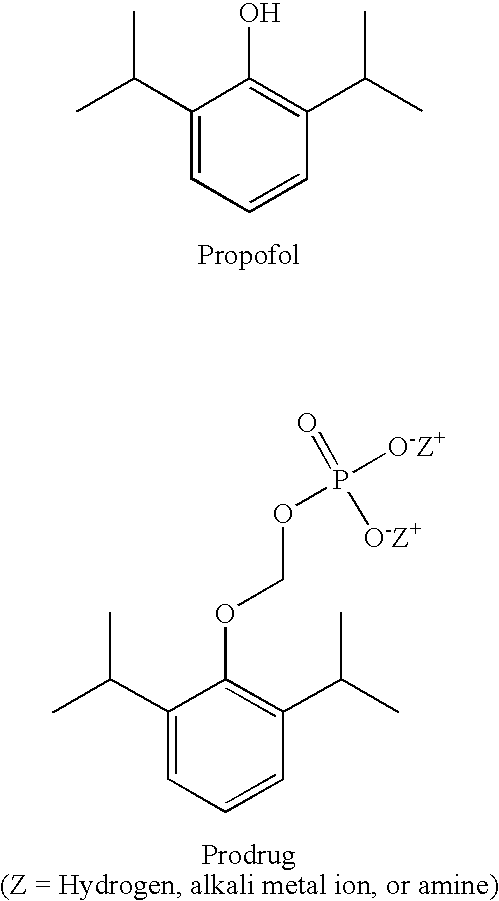
Methods of Administering Water-Soluble Prodrugs of Propofol
A method of administering a prodrug of propofol, preferably O-phosphonooxymethyl propofol disodium salt, comprises the subcutaneous or rectal administration of the prodrug in amounts sufficient to induce or maintain a generalized anesthetized state, a conscious sedated state, or to treat insomnia, anxiety, nausea, vomiting, pruritus, epilepsy, and a range of pain syndromes, including migraine pain, and other medical conditions.
Owner:EISAI INC
Functionality nutritional meal replacing food suitable for tumor patient and preparation method thereof
InactiveCN101822383BEnhance functions such as stomach protection and detoxificationReasonable food formulaFood preparationSpirulina maximaMushroom
Owner:纪福黛
Natural tea for protecting liver, relieving or neutralizing the effect of alcohol
InactiveCN101366423ARelieves alcohol and protects the liverNo side effectsNervous disorderPre-extraction tea treatmentAlcoholismsSide effect
The invention relates to a natural liver-protecting antialcoholic tea which consists of pueraria flower, hovenia acerba, chitosan, ginseng leave and tea leave. The natural liver-protecting antialcoholic tea is drunk before drinking alcohol and can greatly increase alcohol capacity; the natural liver-protecting antialcoholic tea is drunk after drinking the alcohol and can rapidly relieve the unwell symptoms of dizziness, nausea, emesis and the like after the drinking, thereby achieving the effects of protecting liver, relieving alcoholism and preventing ebriety, having no toxic and side effects and having strong effects of antialcoholism, liver protection and health care.
Owner:许子良
Micro-powder dom peridone maleate medicinal composition and its preparing method
The present invention relates to a medicine composition with domperidone maleate. It is characterized by that said medicine composition is micropowdered, and can be used for invigorating function of gastrointestinal tract and curing the diseases of dyspepsia and nausea-vomiting, etc. Said micropowdered domperidone maleate preparation can raise its bioavailability. Besides, said invention also provides its preparation method.
Owner:NANJING CHANGAO PHARM CO LTD
Novel material composition capable of regulating activity of intestinal flora and preparation method of novel material composition
The invention discloses a novel material composition capable of regulating activity of intestinal flora and a preparation method of the novel material composition. The novel material composition consists of the following components in parts by weight: 15-30 parts of strain, 10-20 parts of small molecular organic acid, 1-2 parts of fruit oligose, 1-10 parts of Radix Et Caulis Acanthopanacis Senticosi, 3-10 parts of spirulina, 10-20 parts of roasted malt, 1-2 parts of Phlomis mongolica Turcz., 1-2 parts of Allium schoenoprasum and 3-10 parts of nutlet. The novel material composition capable of regulating the activity of the intestinal flora can regulate the intestinal flora, growth of beneficial bacteria is promoted, growth of harmful bacteria is inhibited, and repeatedly relapsed intestinal diseases such as diarrhea, abdominal pain, abdominal distention, inappetence, nausea, emesis, constipation and the like can be cured. The preparation method is simple and practicable, and is low in preparation cost and suitable for large-scale industrial production.
Owner:天津温茂科技有限公司
Novel material composition for activity regulation of intestinal flora and preparation method of novel material composition
The invention discloses a novel material composition for activity regulation of intestinal flora and a preparation method of the novel material composition. The novel material composition is prepared from the following components in parts by weight: 15-30 parts of strains, 10-20 parts of small molecular organic acid, 10-20 parts of agaro-oligo saccharide, 1-2 parts of fructooligosaccharide, 1-10 parts of ulothrix flacca, 3-10 parts of spirulina platensis, 10-20 parts of monostroma arcticum, 1-2 parts of kelps, 1-2 parts of chondracanthus intermedius and 3-10 parts of sargassum pallidum. According to the novel material composition for the activity regulation of the intestinal flora, the intestinal flora can be regulated, the growth of beneficial bacteria is promoted, the growth of harmful bacteria is inhibited, and intestinal diseases, such as diarrhea, abdominal pain, abdominal distension, inappetence, nausea, vomit and constipation which are repetitively recrudescent, can be cured. The preparation method is simple and feasible, the preparation cost is low and the novel material composition is suitable for large-scale industrial production.
Owner:天津迎南科技有限公司
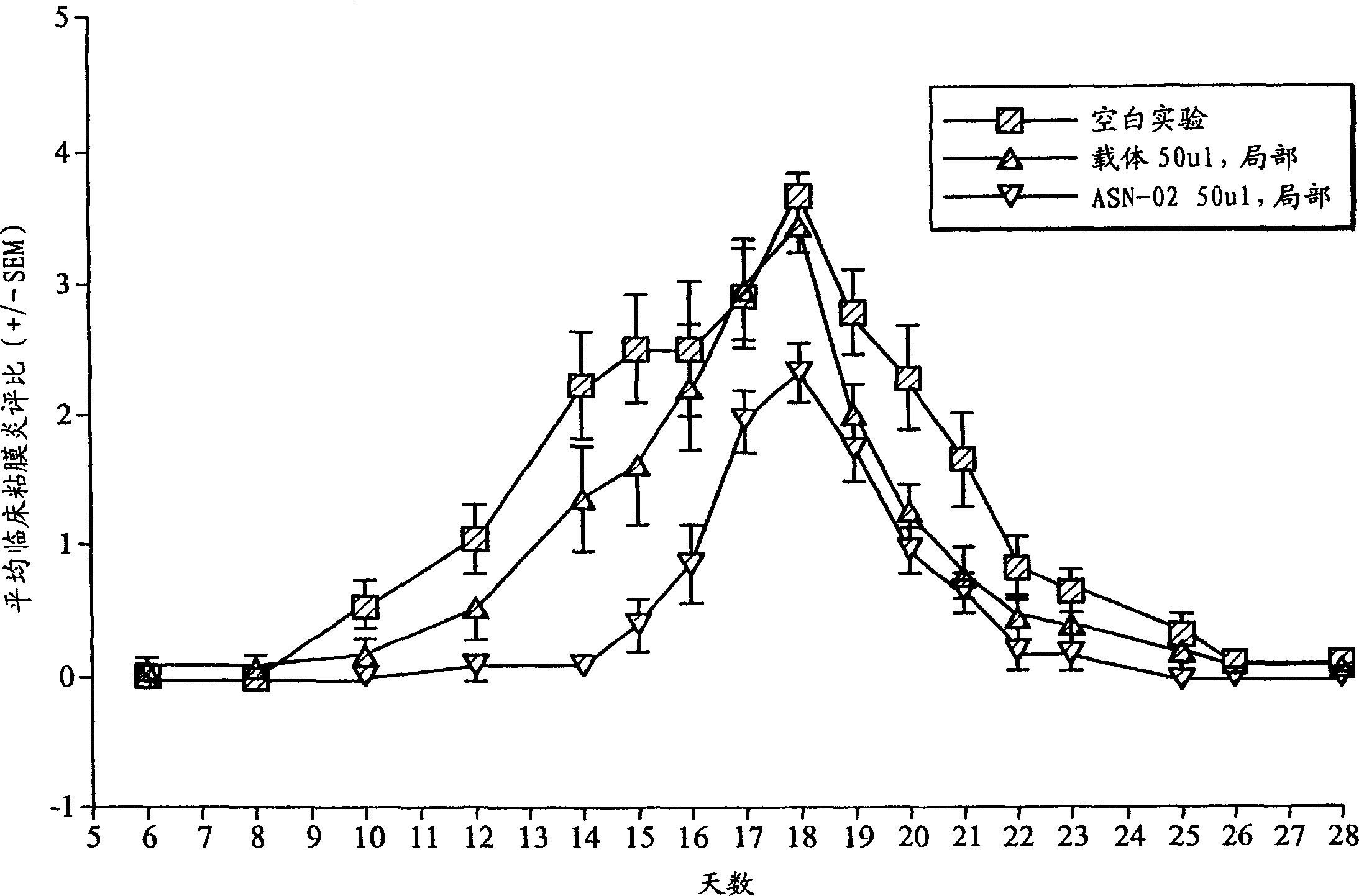
Use of a standardised dry extract of leaves of buddleja globosa hope, bg-126, for the treatment and prevention of gastrointestinal disorders caused by treatment with nitrofurantoin and other antimicrobials
The invention relates to the use of a composition comprising extract of Buddleja globosa Hope and pharmacologically accepted excipients for the preparation of a phyto-pharmaceutical agent, a drug or a nutraceutical for the prevention of gastrointestinal disorders, particularly those associated with treatment with nitrofurantoin or gastric disorders produced by ulcers, gastric diseases or damage to the gastrointestinal tract. The aforementioned disorders can correspond to abdominal pain, dyspepsia, nausea, vomiting, diarrhea, constipation, inflammation and cephalalgia resulting from treatment with nitrofurantoin and other antimicrobials. In addition, the extract and the compositions thereof can be used to enhance the antibacterial action of nitrofurantoin, particularly against Escherichia coli.
Owner:GONZALEZ LAURA XIMENA POLANCO
NK1 receptor-targeting antagonist and application of same to chemotherapy-induced nausea and vomiting
ActiveCN109384712AExcellent NK1 receptor antagonist activityExcellent receptor antagonist activityOrganic chemistryDigestive systemAdjuvantMedicine
The invention relates to a NK1 receptor-targeting antagonist and application of the same to chemotherapy-induced nausea and vomiting, belonging to the technical field of adjuvant therapeutics for tumor chemotherapy. The invention provides a compound as shown in a formula I which is described in the specification, or a racemate, stereoisomer, tautomer, isotopic label, oxynitride or pharmaceutically-acceptable salt thereof. The invention also provides a preparation method for the compound, a pharmaceutical compositions and the application of the compound or the racemate, stereoisomer, tautomer,isotopic label, oxynitride or pharmaceutically-acceptable salt thereof.
Owner:北京宽厚医药科技有限公司
Pharmaceutical composition for the treatment of premature ejaculation
The present invention provides an oral pharmaceutical composition for the treatment of premature ejaculation, and the composition provides the effective and excellent treatment of premature ejaculation as well as reduced side effects like nausea, vomiting, drowsiness, sedation effect, awakening effect, and weight-loss etc.
Owner:YUHAN +1
Dendrobium officinale soup stock for strengthening stomach and helping digestion and a production method thereof
InactiveCN104921205ANourishing yin and nourishing kidneyWith eyesight and strong bodyFood ingredient functionsFood preparationDolichorisVerbena
The invention discloses a dendrobium officinale soup stock for strengthening stomach and helping digestion and a production method thereof, and belongs to the technical field of food and preparation technology. The dendrobium officinale soup stock for strengthening stomach and helping digestion is composed of the following raw materials by weight: 5-10 parts of Dendrobium officinale, 8-15 parts of chicken's gizzard membrane powder, 8-15 parts of coix seed, 10-15 parts of semen dolichoris, 10-15 parts of hawthorn, 1-3 parts of verbena, 1-3 parts of Faeces Togopteri, 1-3 parts of dry reed rhizome, 1-3 parts of rhizoma imperatae, 1-3 parts of corydalis tuber, 1-2 parts of mint, 10-15 parts of smoked plum sauce, 20-30 parts of mutton, 8-10 parts of honey, 8-10 parts of rice vinegar, 3-8 parts of olive oil, 2-3 parts of yellow rice wine, 1-2 parts of ginger and 1-8 parts of salt. The soup stock for cooking soup is not only delicious, but also has significant effect of health care; and the soup stock is particularly suitable for eating by people with anorexia with abdominal distention after eating, distending pain in stomach duct and abdomen or diarrhea, belching, nausea and vomiting.
Owner:GUANGXI JIANBAO SHIHU
Non-pharmaceutical methods of mitigating addiction withdrawal symptoms
Non-pharmaceuticals method of treating the effects of addiction withdrawal are described. The method includes providing a person with stimuli include visual and / or auditory stimuli which are pulsed at the rate of various types of brain waves. The use of the method lessens various withdrawal symptoms, such as anxiety, sleepiness, sweating, tearing of the eyes, running of the nose, goosebumps, shaking, hot flushes, cold flushes, bones aching, muscles aching, restlessness, nausea, vomiting, muscle twitching, stomach cramps, pain, the need to use an opioid, the desire to use an opioid, sleep disturbances. and the use of rescue medications.
Owner:SANA HEALTH INC
Urea compounds and their use as enzyme inhibitors
ActiveUS20170101381A1Quality improvementHigh yieldOrganic active ingredientsSenses disorderNausea sicknessArthritis
A compound having the following structure:or a pharmaceutically acceptable salt or derivative thereof. The compound may be used in the treatment or prevention of a disorder selected from appetite regulation, obesity, metabolic disorders, cachexia, anorexia, pain, inflammation, neurotoxicity, neurotrauma, stroke, multiple sclerosis, spinal cord injury, Parkinson's disease, levodopa-induced dyskinesia, Huntington's disease, Gilles de la Tourette's syndrome, tardive dyskinesia, dystonia, amyotrophic lateral sclerosis, Alzheimer's disease, epilepsy, schizophrenia, anxiety, depression, insomnia, nausea, emesis, alcohol disorders, drug addictions such as opiates, nicotine, cocaine, alcohol and psychostimulants, hypertension, circulatory shock, myocardial reperfusion injury, atherosclerosis, asthma, glaucoma, retinopathy, cancer, inflammatory bowel disease, acute and chronic liver disease such as hepatitis and liver cirrhosis, arthritis and osteoporosis.
Owner:BIAL PORTELA & CA SA
Processing method of snow frog compound nutrient preparation for pregnant women
InactiveCN102309524AReduce morning sicknessEasy to useAmphibian material medical ingredientsMetabolism disorderHigh pressureImpurity
The invention relates to a processing method of a snow frog compound nutrient preparation for pregnant women. The processing method is characterized by comprising the following steps of: 1, removing dirt from fresh frog oil; 2, airing and drying the frog oil to dryness, crushing into 40-60 meshes, placing the crushed frog oil and edible gum into crystal sugar water in proportion, and mixing by matching with dextrin and other auxiliaries; 3, processing obtained mixed feed liquid by a colloid mill (into 200-300 meshes), then homogenizing in a homogenizer (at an average-value temperature of 40 DEG C and under 20Pa at a low-pressure region and 40Pa at a high-pressure region); 4, removing impurities by adopting a centrifugal machine (at the speed of 4000 turns per 15 minutes); and 5, blending, tabletting and drying, sterilizing and packaging. A product produced in the invention is simple to use, good for absorption, all-round in nutrients and reasonable in proportioning, and has the effect of effectively alleviating morning sicknesses, such as nausea, vomit and giddiness, of the pregnant women, as well as other pregnancy complications, such as cramps, anemia, abortion, premature birth, preeclampsia and the like.
Owner:吉林博大农林生物科技有限公司
Pharmaceutical composition for adjunctively treating cancer
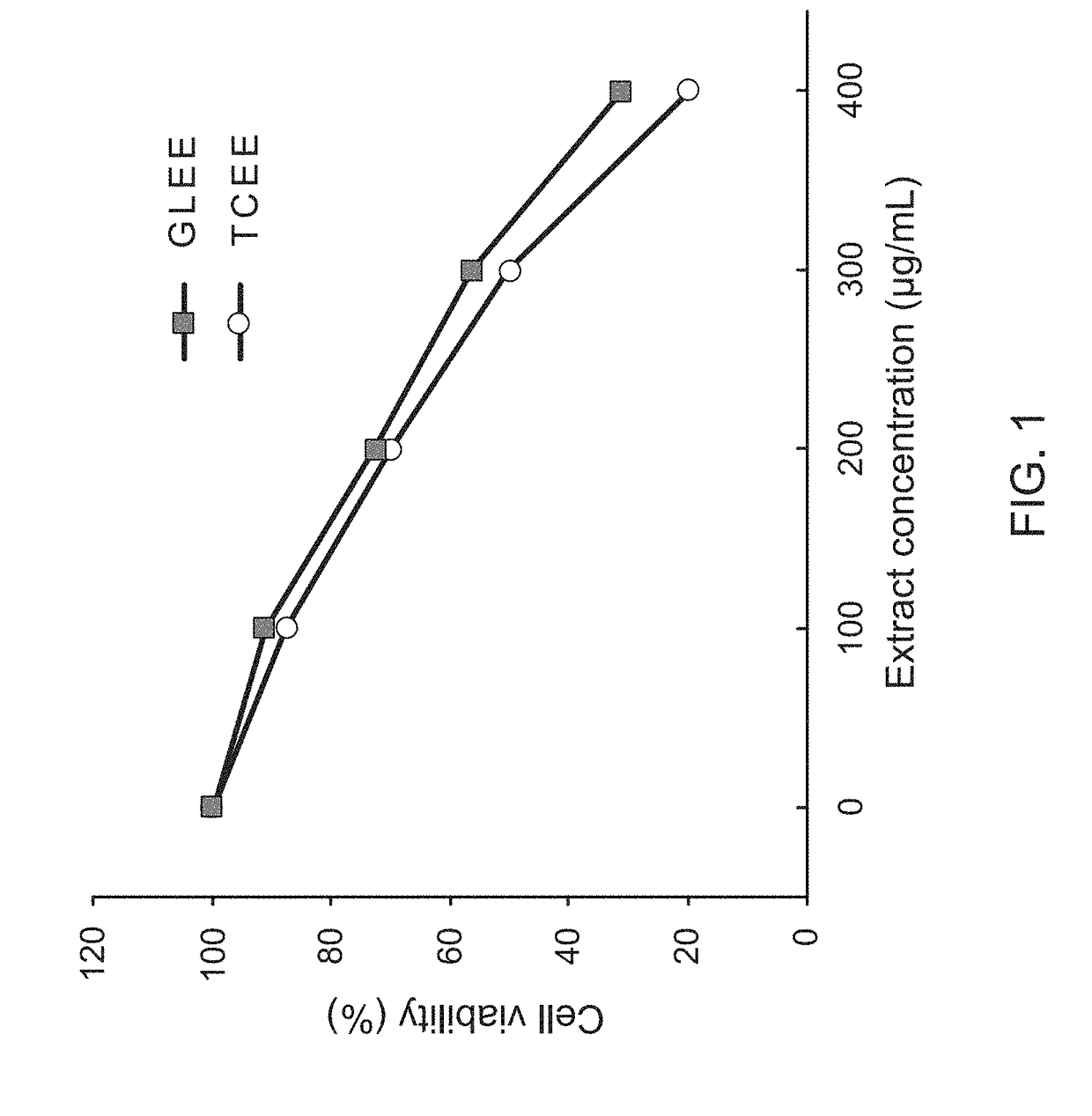
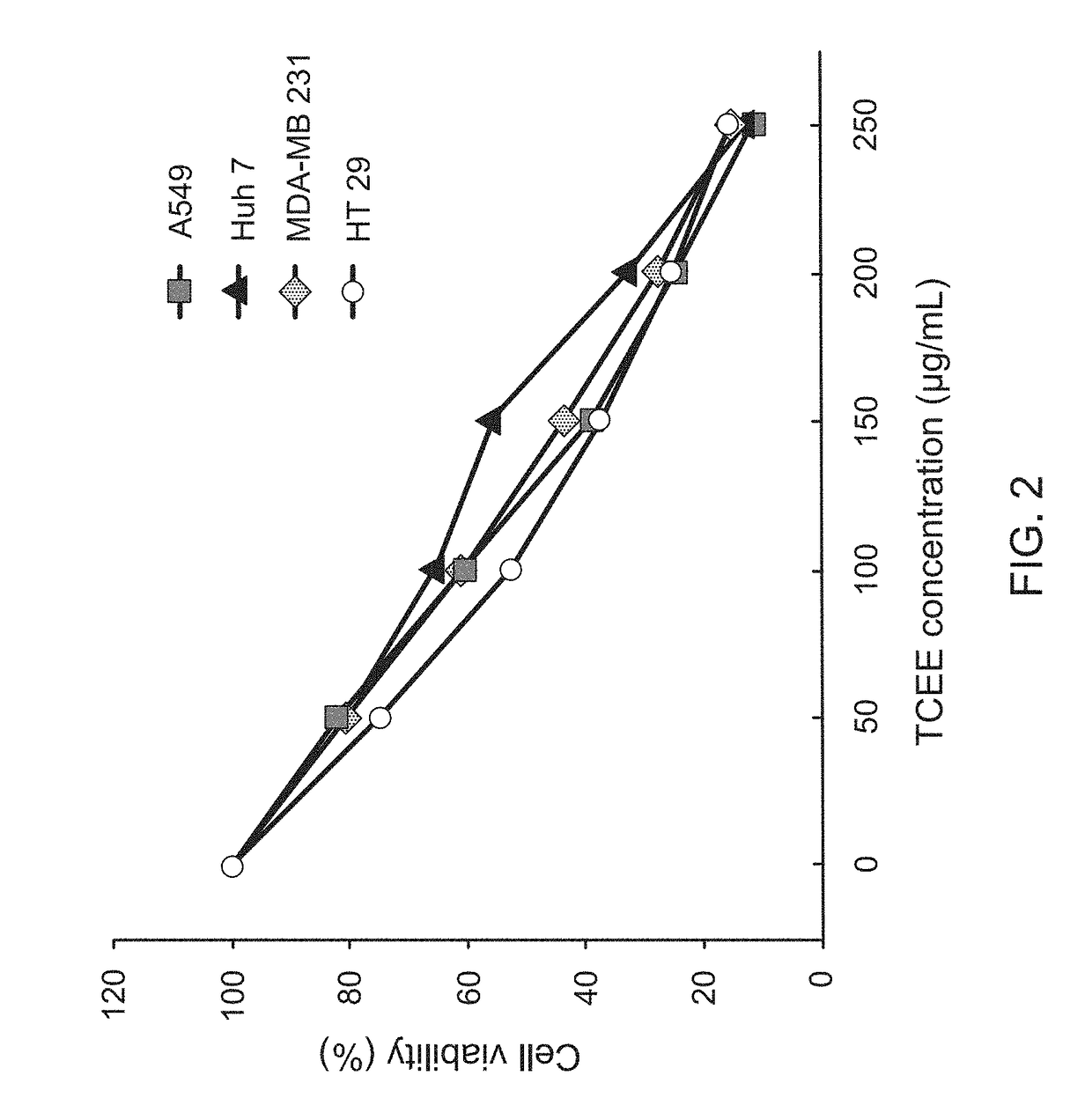
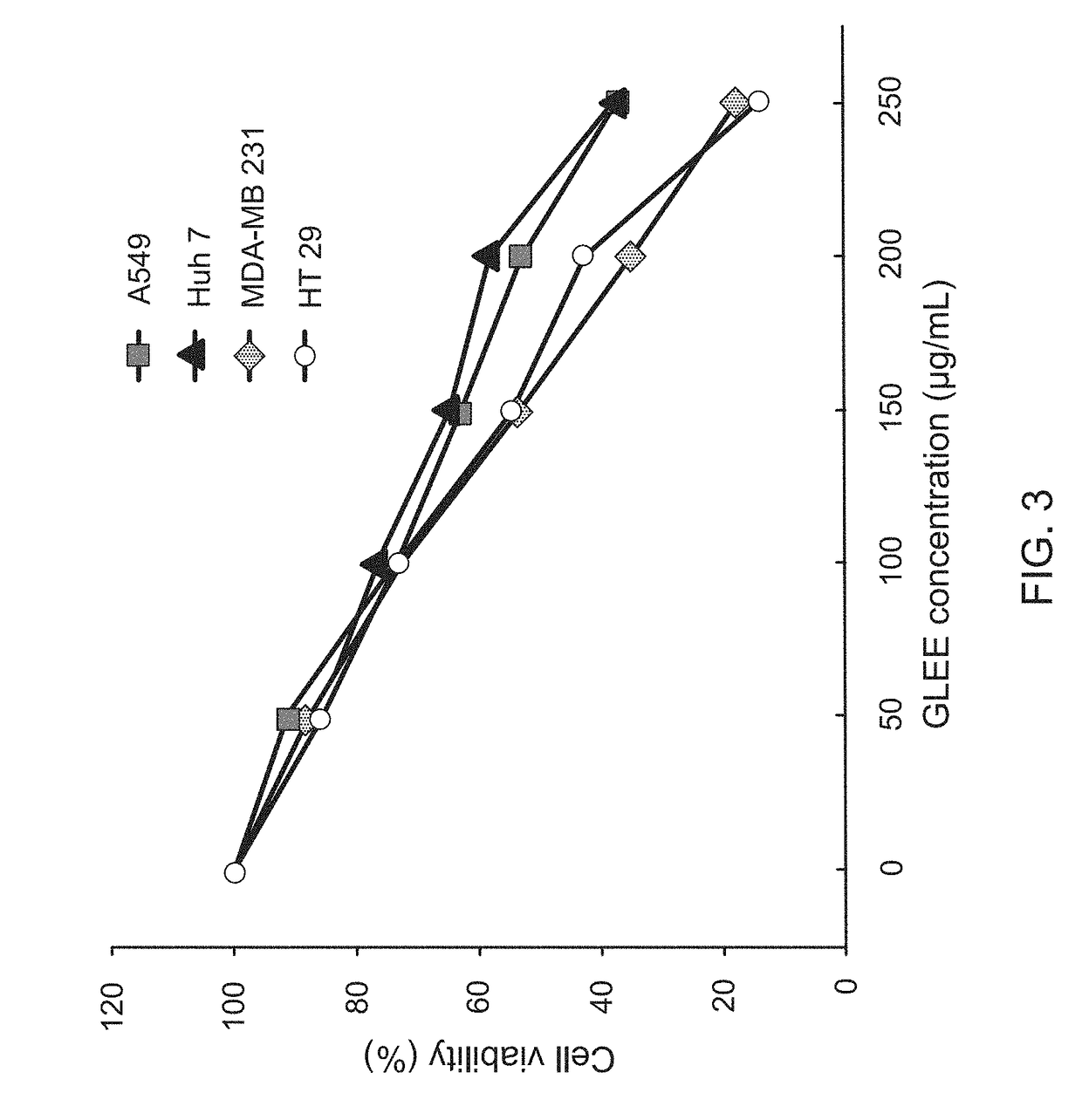
InactiveUS20170333502A1Reduce cell viabilityPoor appetiteFungi medical ingredientsAntineoplastic agentsSide effectApoptosis
The present invention provides a pharmaceutical composition for adjunctively treating cancer. Differing from conventional therapies commonly using anti-cancer drugs and / or radiation substances to cause the apoptosis of cancer cells, the present invention particularly adopts an ethanol extract of Taiwanofungus camphorata (TCEE), an ethanol extract of Ganoderma lucidum (GLEE) or a combination of the TCEE and the GLEE as the a novel pharmaceutical composition for causing the apoptosis of lung cancer cells A594, hepatoma cells Huh 7, breast cancer cells MDA-MB 231, and colorectal cancer cells HT 29. Moreover, differing from conventionally-used anti-cancer drugs and radiation substances always causing some side-effects such as nausea, vomit, and poor appetite, this novel pharmaceutical composition can not only be used for adjunctively treating cancer, but also possesses many healthy effects, including: detoxification, enhancing immunity, and increasing appetite.
Owner:TAIWAN INDIGENA BOTANICA CO LTD +1
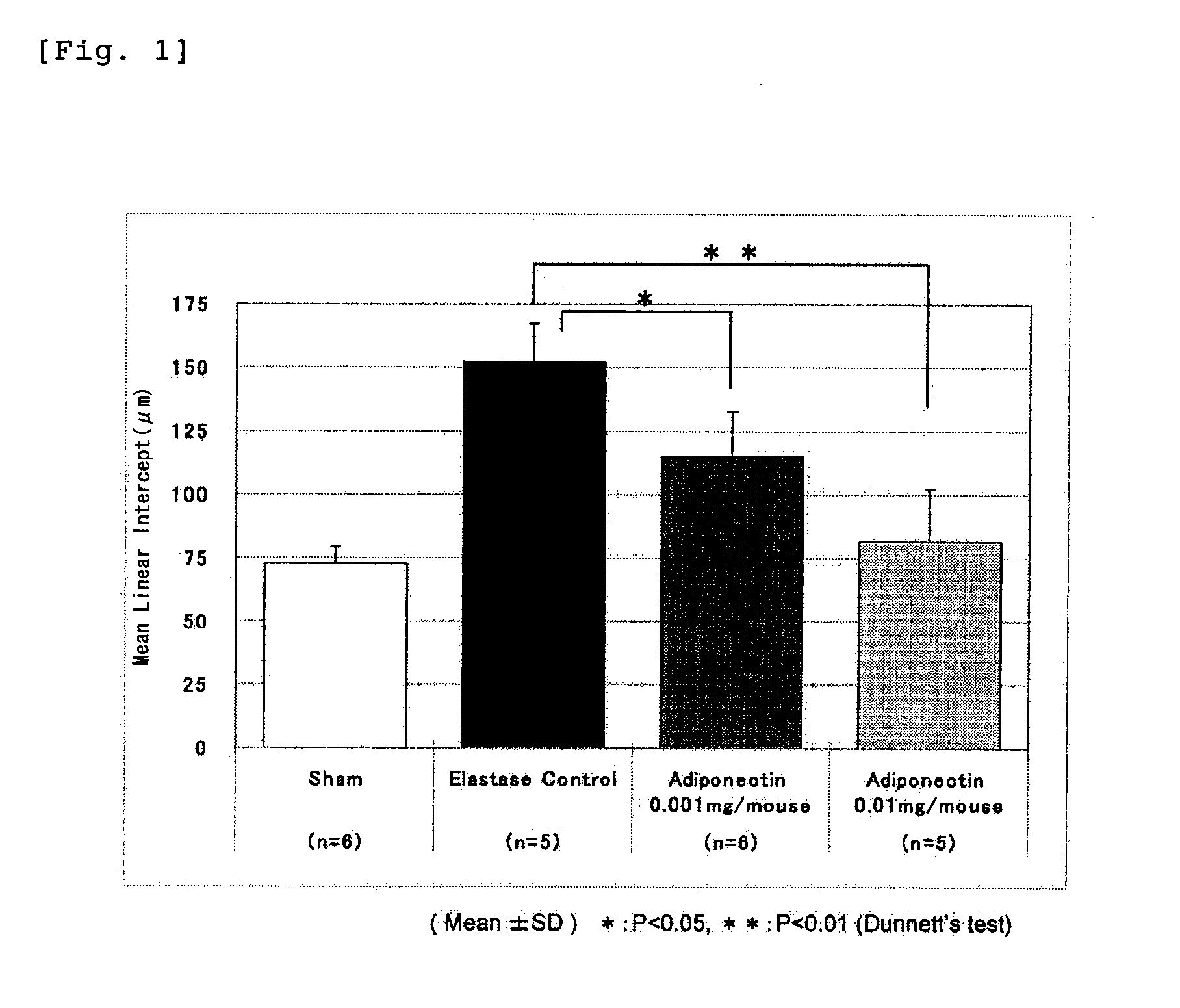
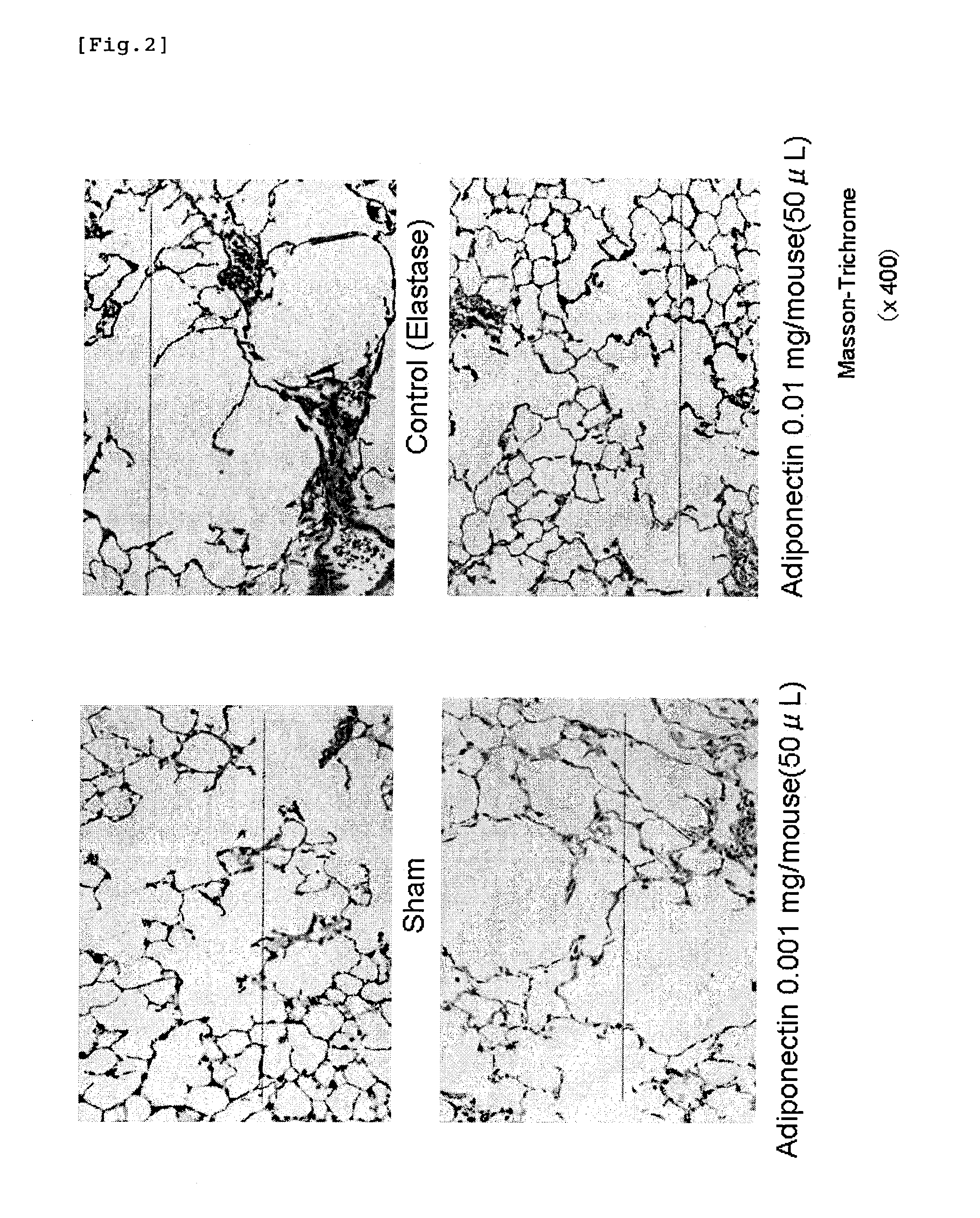
Adiponectin for treating pulmonary disease
InactiveUS20110218146A1Reduce deteriorationHighly effectiveHormone peptidesPeptide/protein ingredientsDiseaseNausea sickness
The present invention provides an agent for inhibiting alveolar airspace enlargement containing adiponectin or an agent for inhibiting alveolar wall destruction containing adiponectin. The pulmonary disease therapeutic agents of the present invention are highly safe drugs that possess an excellent effect of decreasing the deterioration of the pulmonary function, such as airflow limitation, and exhibit an extremely high effect of treating pulmonary disease accompanied by irreversible deterioration in pulmonary function, with fewer adverse side effects such as nausea, vomiting and gastric acid secretion.
Owner:OTSUKA PHARM CO LTD
Use of oligosaccharides molecule in preparing contraceptive medicament
InactiveCN101352447ASmall molecular weightSimple structureOrganic active ingredientsSexual disorderSolubilityHealth index
The invention belongs to the field of medicine, and more particularly relates to the new usage of oligosaccharide molecule in the preparation of combined oral contraceptive pill. Although various kinds of combined oral contraceptive pills exist, the patients who have serious angiocardiopathy, hepatonephritis, endocrine disease, tumour or hematonosis are not fit for taking the combined oral contraceptive pills, and the side effects such as dizziness, nauseation, vomit and irregular menstrual period can be generated along with the unsuitable dose or the sorts of medicine. The invention provides the new usage of oligosaccharide molecule in the preparation of combined oral contraceptive pill, the degree of polymerization of oligosaccharide which is the sugar component of C4-C20 is 1-1000. The oligopolymerization micromolecule sugar is native compound, has low molecular weight and simple structure, and the combined oral contraceptive pill prepared by the oligosaccharide molecule has high efficiency, good water-solubility, low cost and convenient (external) use; furthermore, the hormone or physiological structure is not changed, the side effects for the human body are low, and the living quality and health index of people are further improved.
Owner:FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com