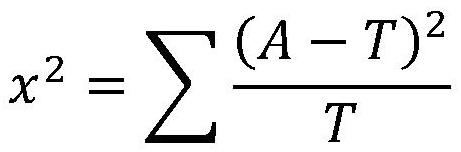Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "IV Immunoglobulins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunoglobulin therapy, also known as normal human immunoglobulin (NHIG), is the use of a mixture of antibodies (immunoglobulins) to treat a number of health conditions.
Affinity purified human polyclonal antibodies and methods of making and using them
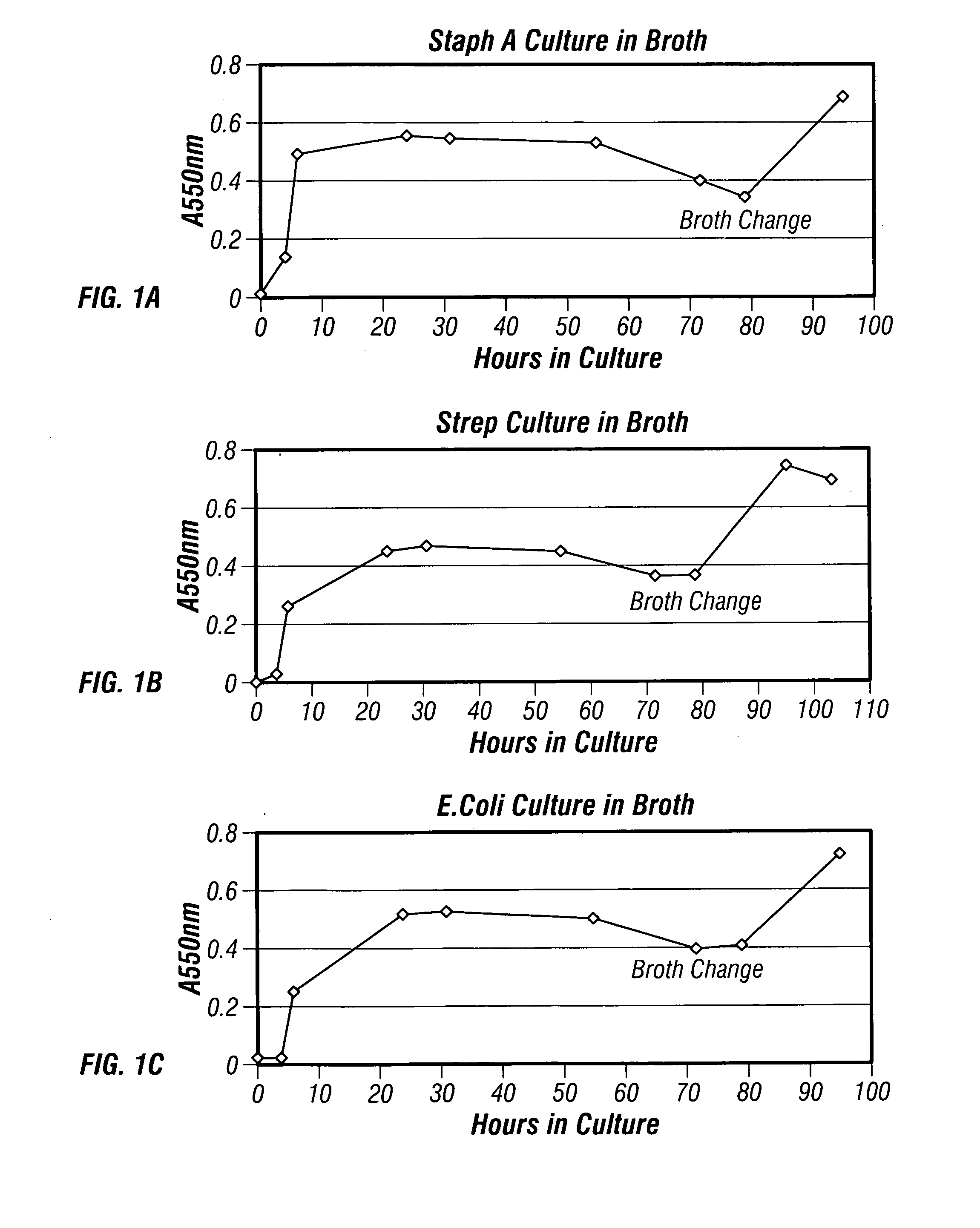
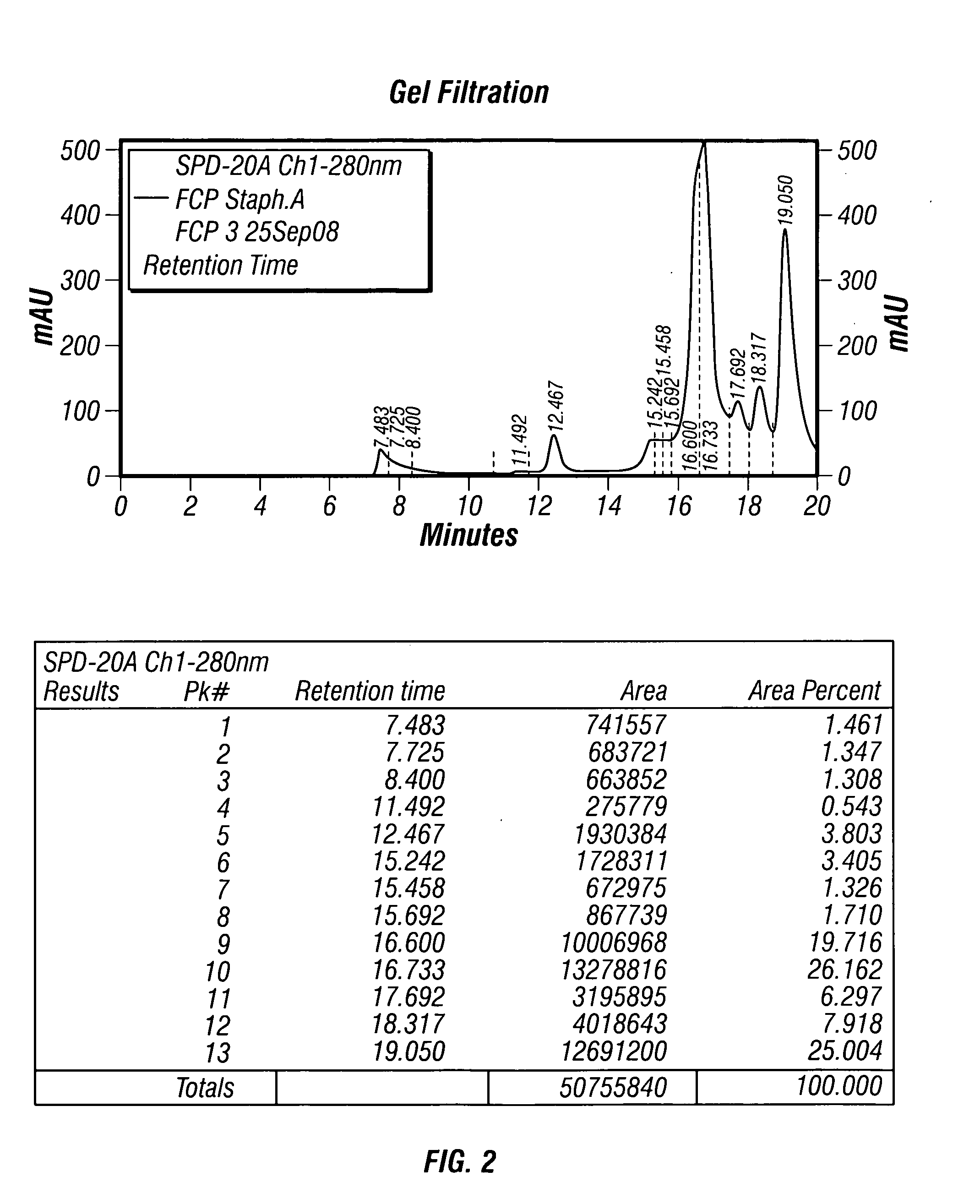
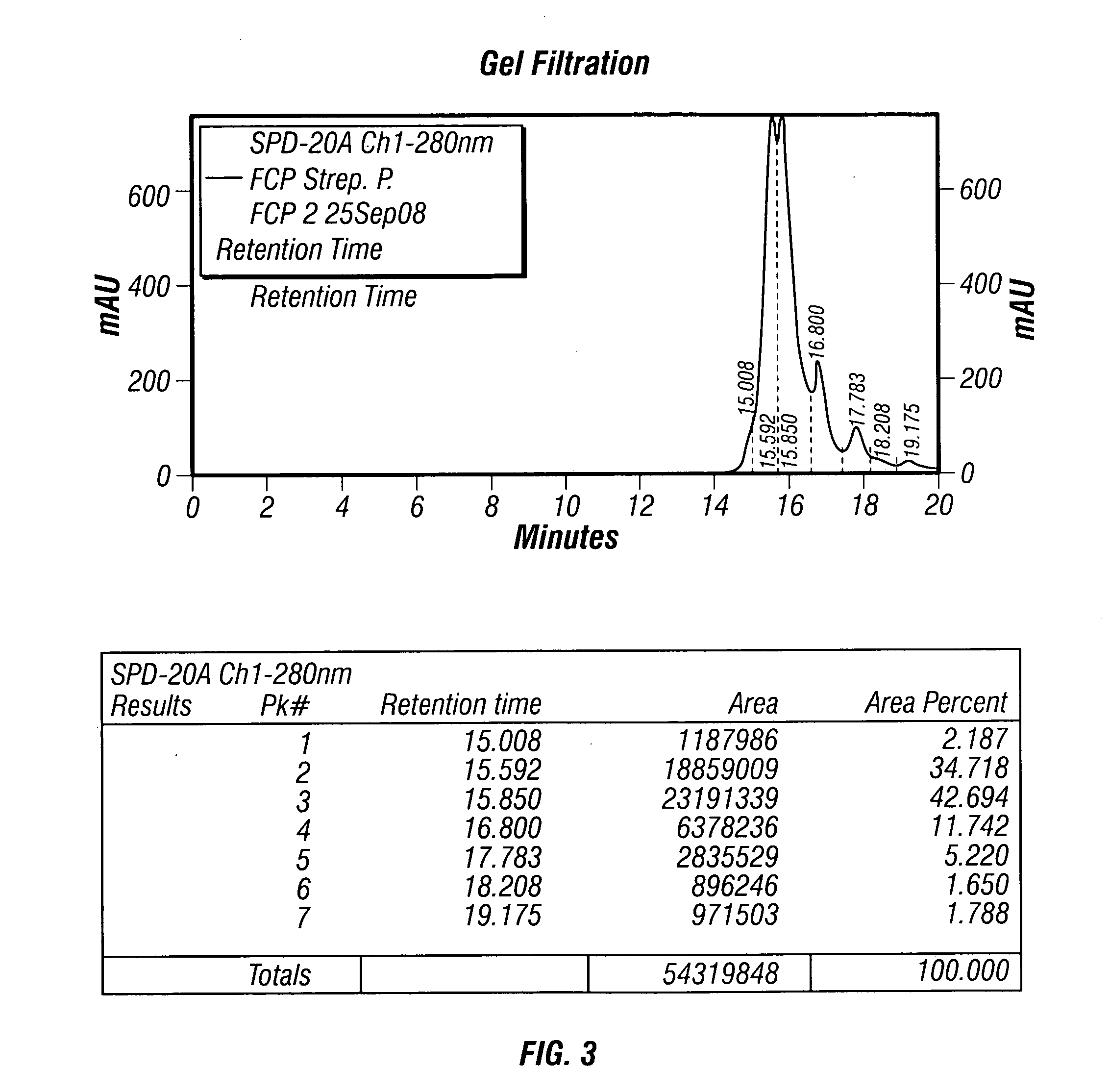
The present invention describes a method for treating, removing or preventing a bacterial infection, which method comprises administering to a human suffering, suspected of suffering or at risk of suffering from Staphylococcus aureus (S. aureus) infection, a Streptococcus infection, Escherichia coli (E. coli) infection, Pseudomonas aeruginosa (P. aeruginosa) infection, Acinetobacter baumannii (A. baumannii) infection, Enterococcus faecium (E. faecium) infection and / or Clostridium difficile (C. difficile) infection, an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from bacterial cells selected from S. aureus, a Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, and optionally, wherein said affinity purified human polyclonal antibodies are purified (e.g., as made more concentrated as compared to the starting or unpurified material) relative to the same human polyclonal antibodies in the unpurified or non-affinity-purified human blood sample, e.g., intravenous immunoglobulin (IVIG) sample, and / or also optionally, wherein said affinity purified human polyclonal antibodies are specific for the bacterial antigens used in the affinity purification, and / or further optionally wherein the affinity purified human polyclonal antibodies are substantially free of human antibodies that specifically bind to non-bacterial antigens in the human blood sample. Pharmaceutical compositions for treating bacterial infections, comprising an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from S. aureus, Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, are also provided.
Owner:SCANTIBODIES LAB
Intravenous immunoglobulin processing, diagnostic, and treatment systems and methods
InactiveUS20130108619A1Treatment safetyNervous disorderBiological material analysisImmunotherapyCancer research
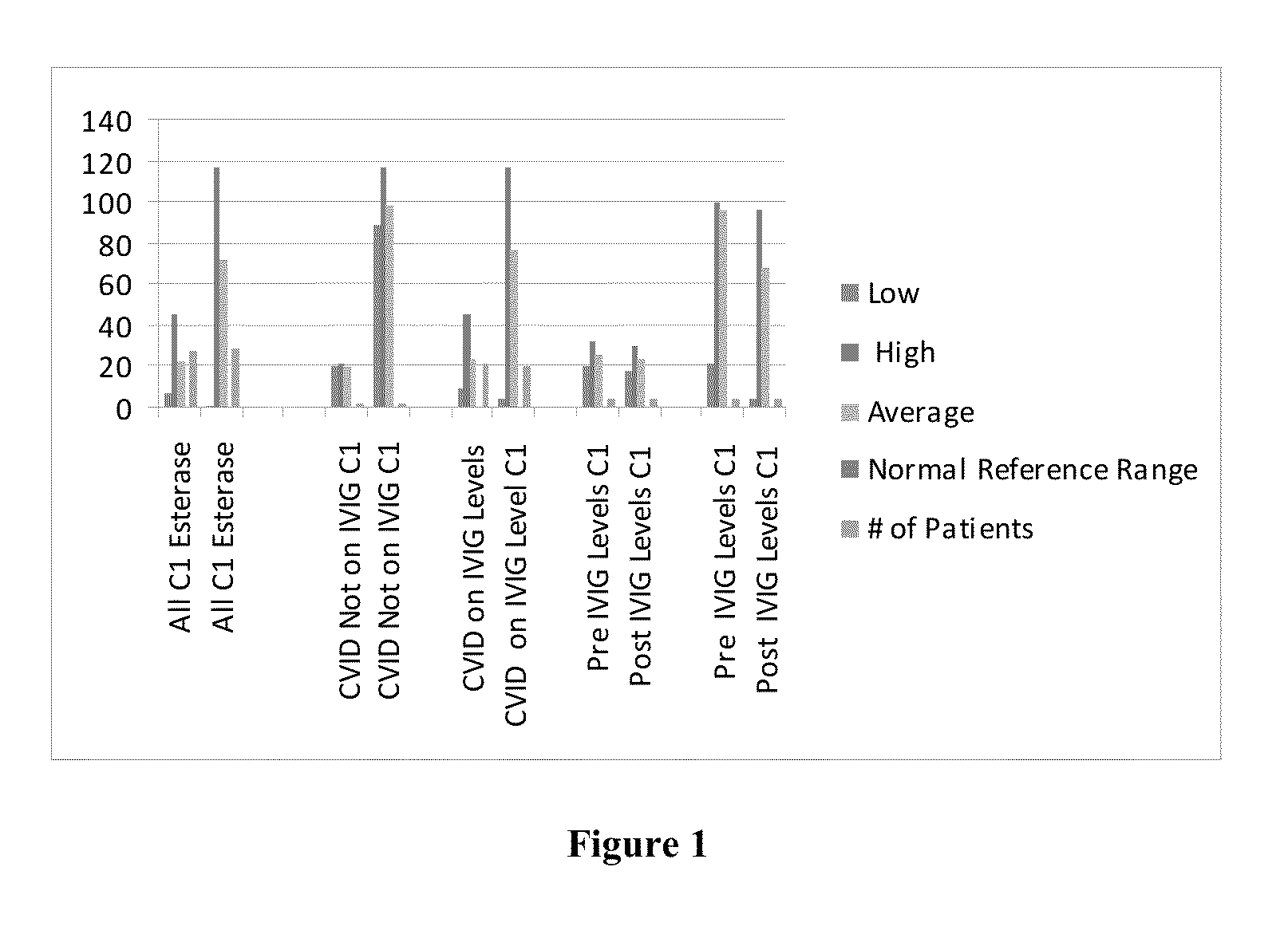
A method of providing an immunotherapy treatment to a patient includes determining a level of C1 esterase inhibitor or inhibitor activity in the patient, determining an intravenous immunoglobulin dosing protocol for the patient based on the level of C1 esterase inhibitor or inhibitor activity, and administering the immunotherapy treatment to the patient.
Owner:MELAMED ISAAC
Intravenous immunoglobulin composition
ActiveUS20130011388A1Reduce riskEasy to managePeptide/protein ingredientsAntibody mimetics/scaffoldsSpecific antibodyHigh titre
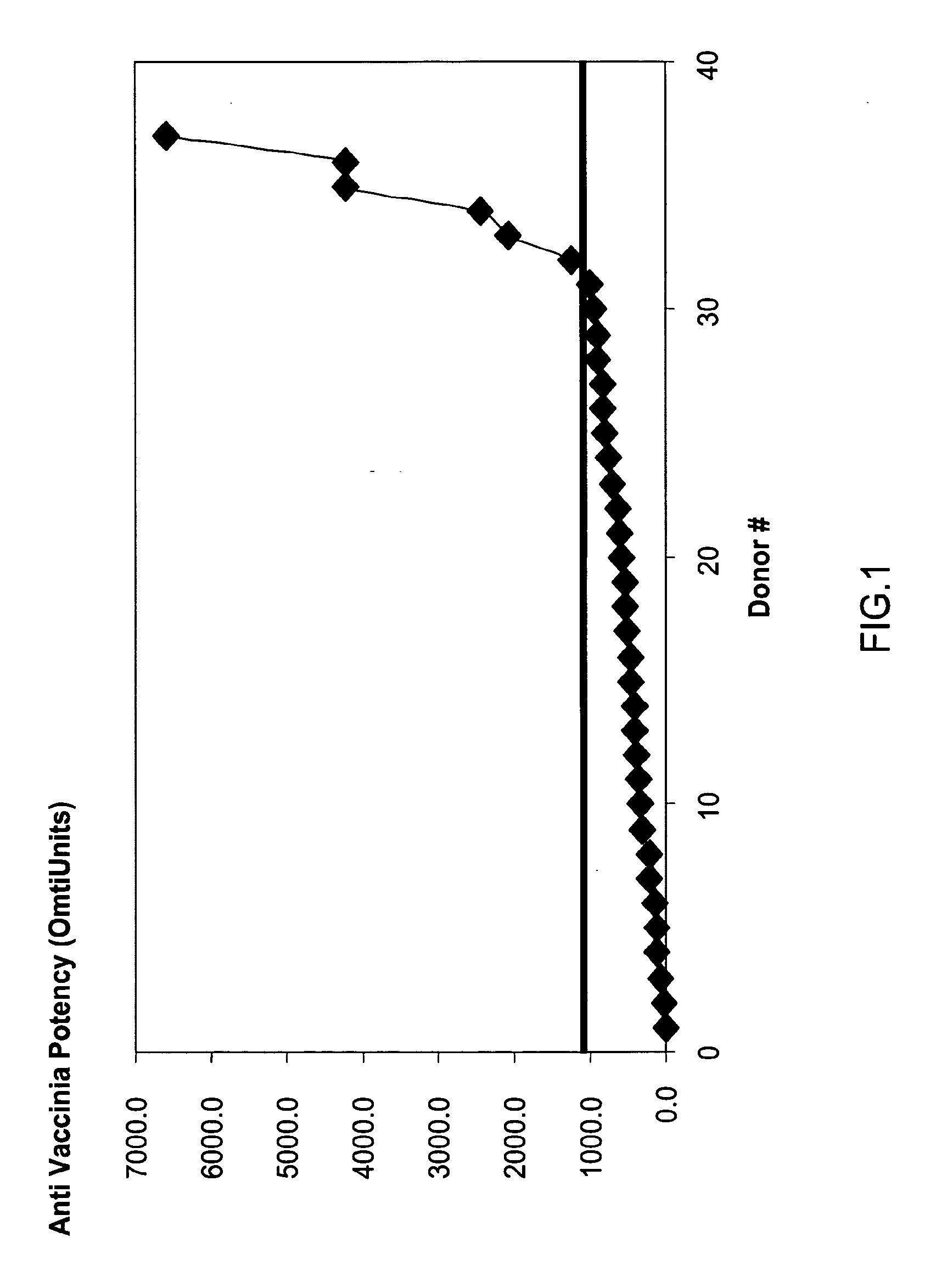
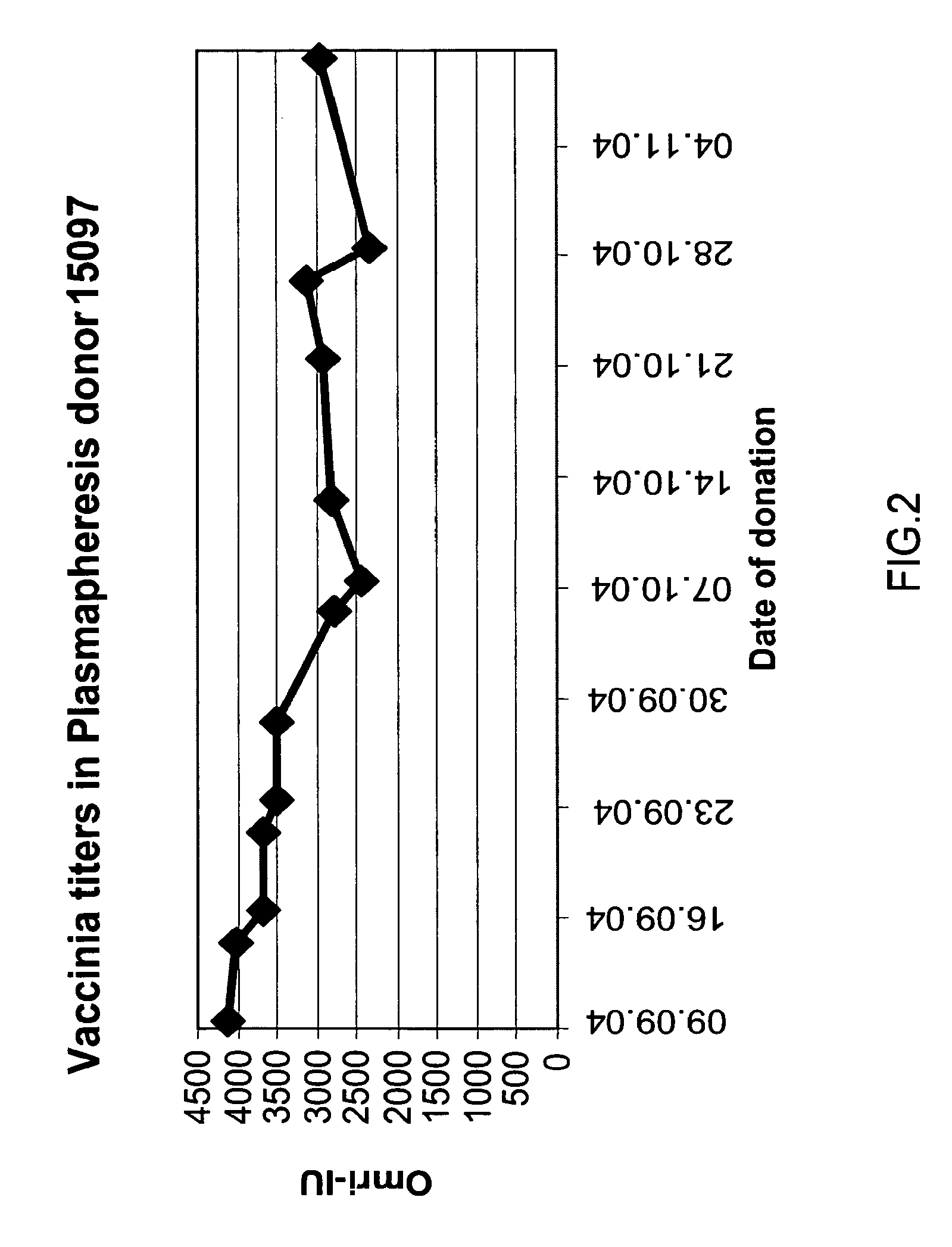
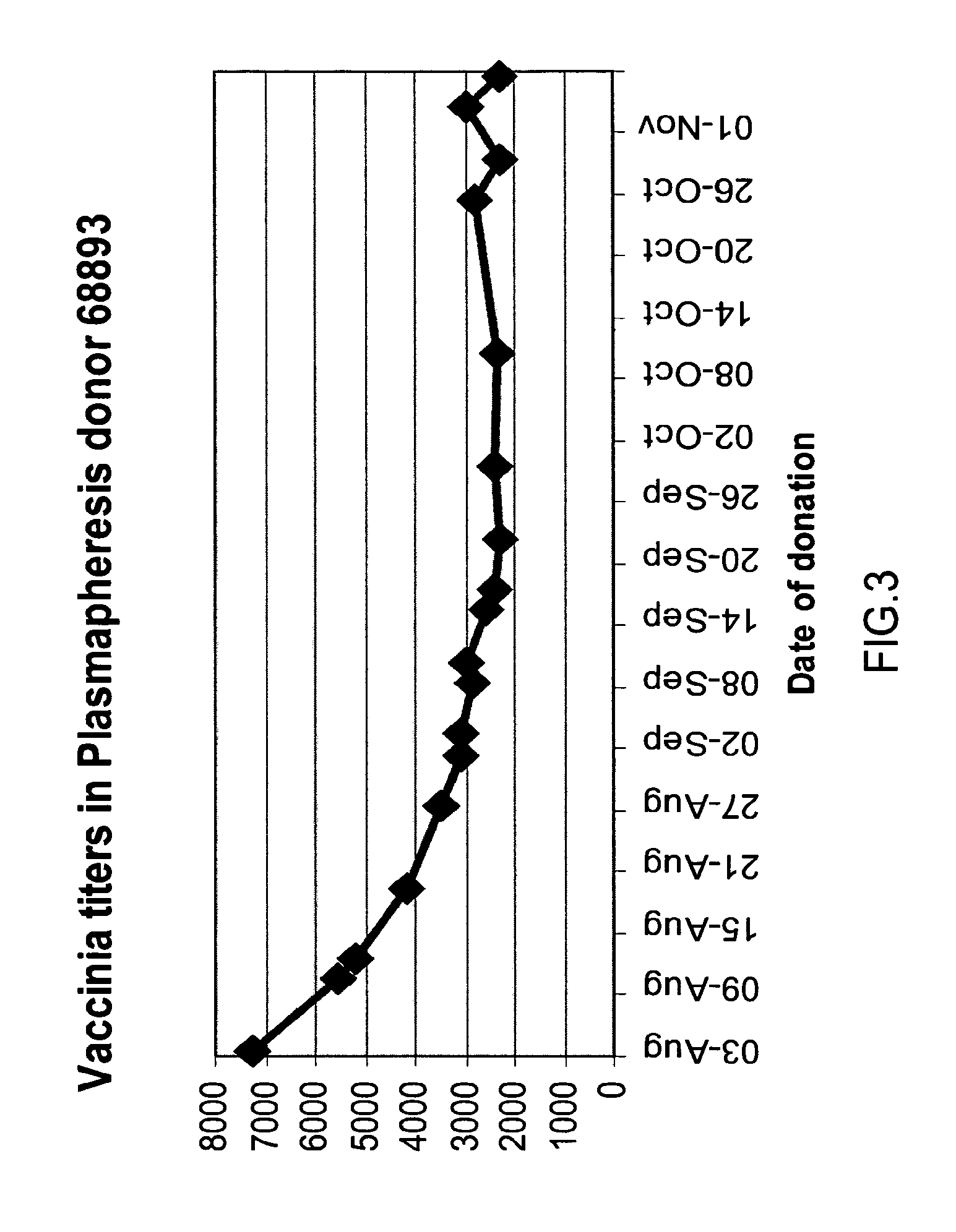
A concentrated, immunoglobulin composition for treating subjects vaccinated against or infected with a pathogenic microorganism, is made by (a) selecting a population of individuals previously vaccinated against antigens associated with the pathogenic microorganism; (b) identifying very high titre individuals by determining the level of specific antibodies immunoreactive with the pathogenic microorganism in the blood of the individuals; (c) combining blood from the very high titre individuals; and (d) purifying and / or concentrating the product of step (c). A concentrated immunoglobulin composition can include specific antibodies immunoreactive with a pathogenic microorganism, wherein the titre of specific antibodies is at least 5 times higher than the average titre of specific antibodies of a population of individuals previously vaccinated against antigens associated with the pathogenic microorganism. The composition has a relatively high protein concentration and a low percentage of protein aggregates. The pathogenic microorganism is preferably smallpox virus or vaccinia virus.
Owner:OMRIX BIOPHARM
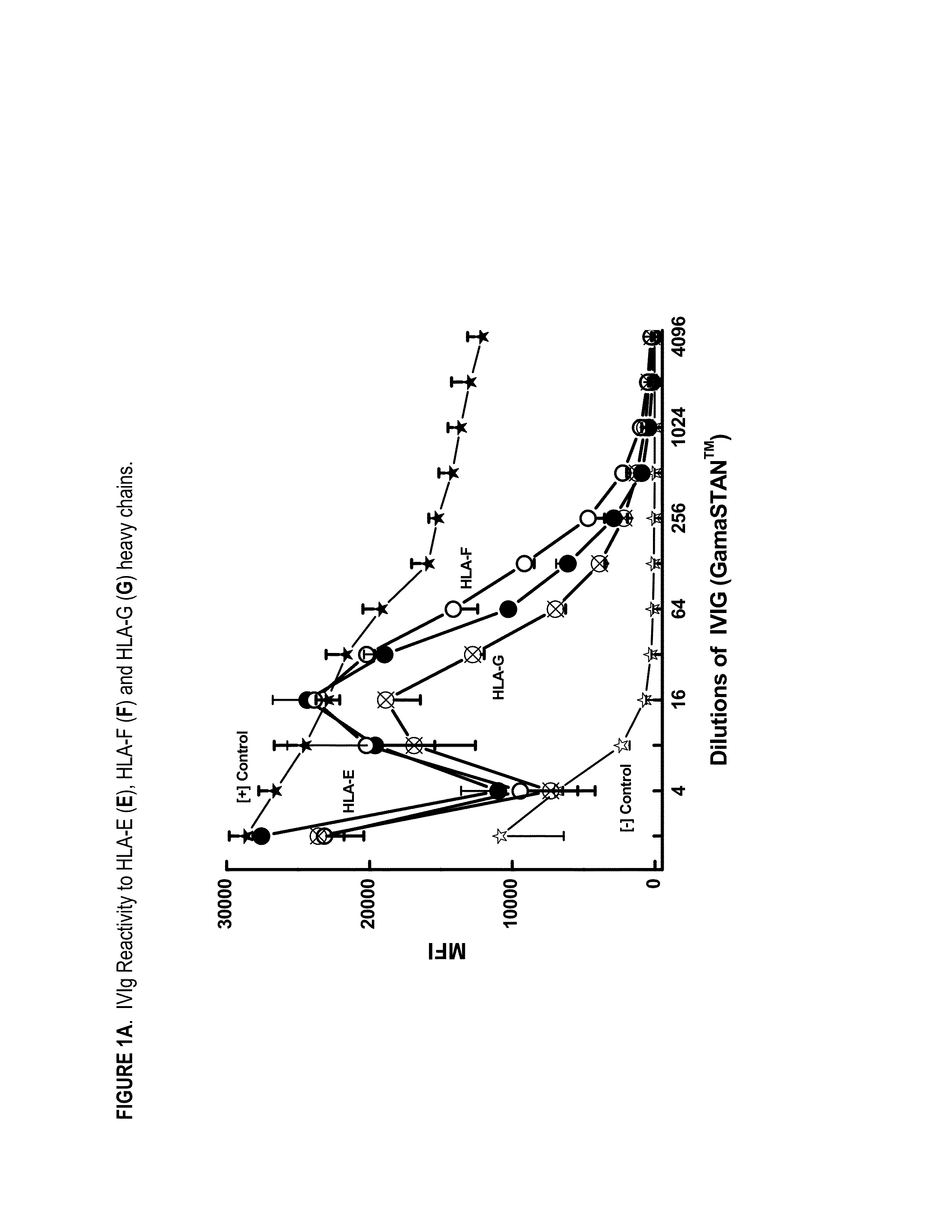
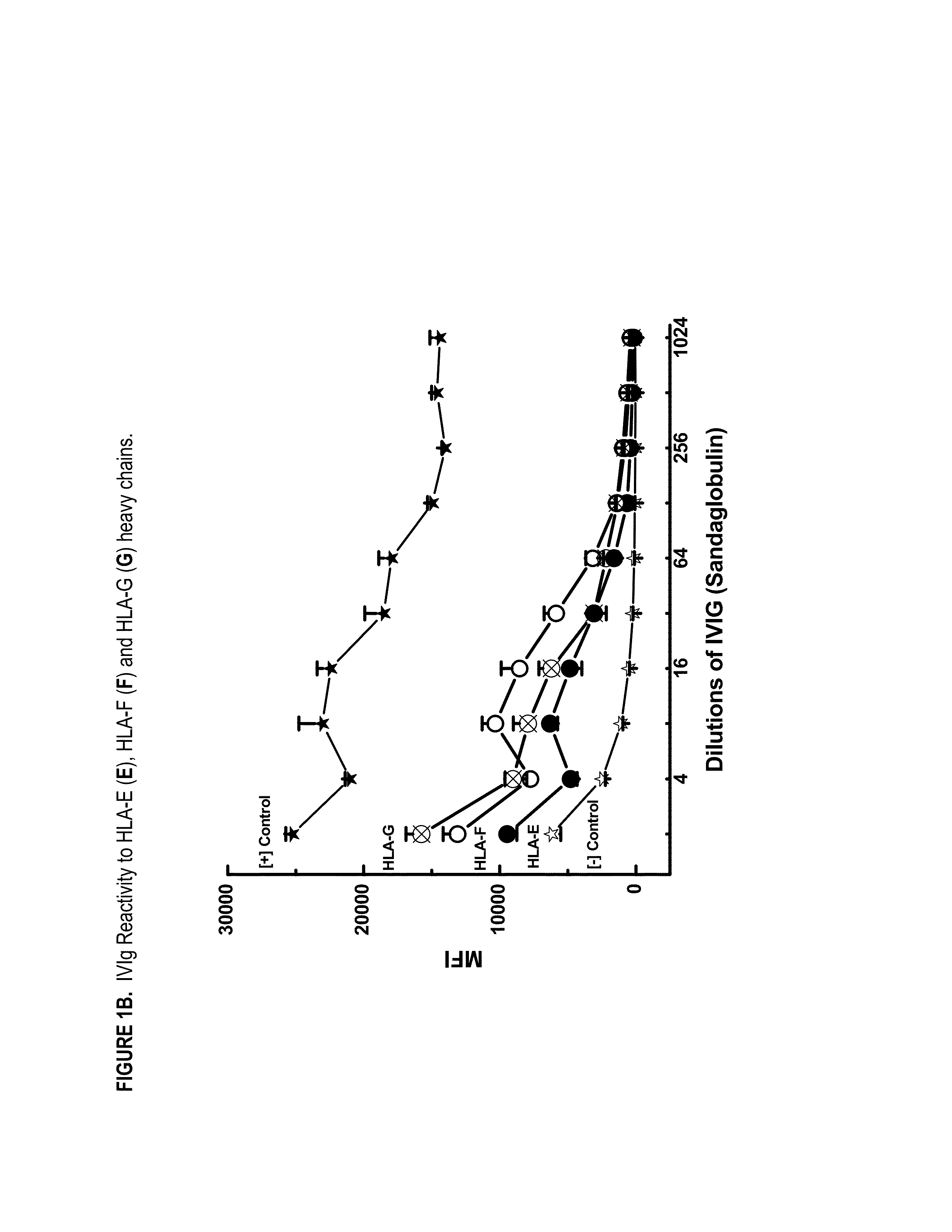
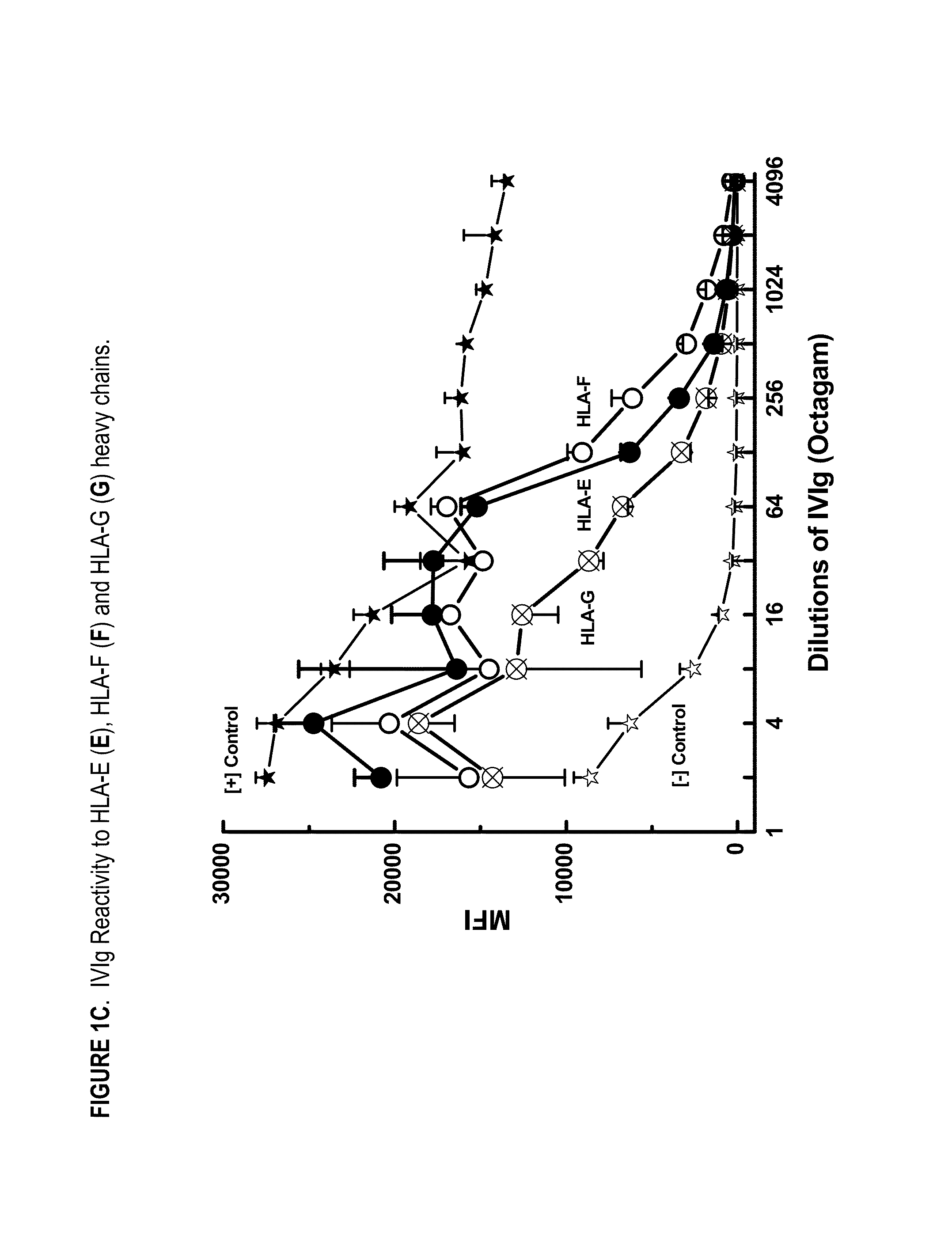
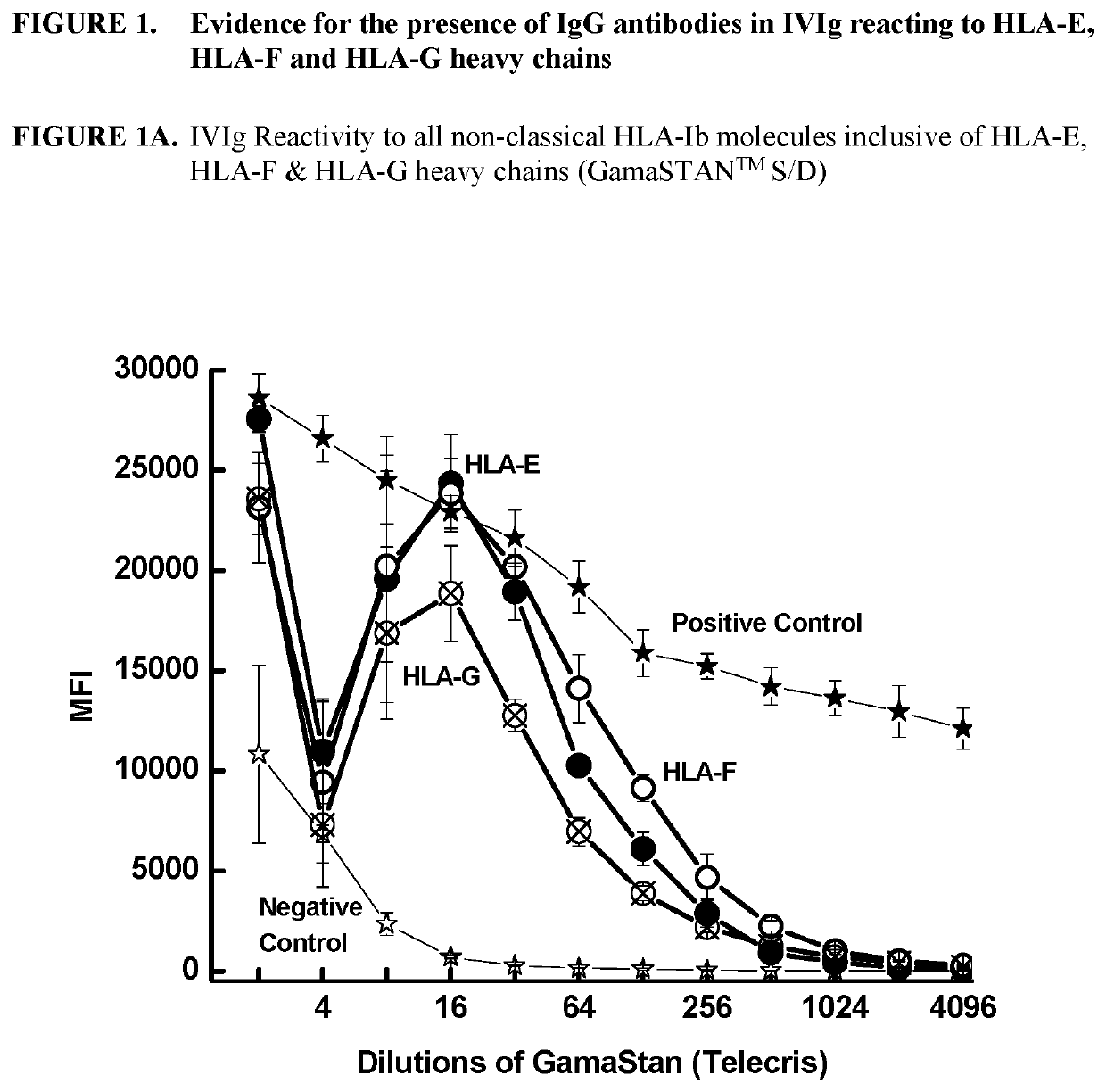
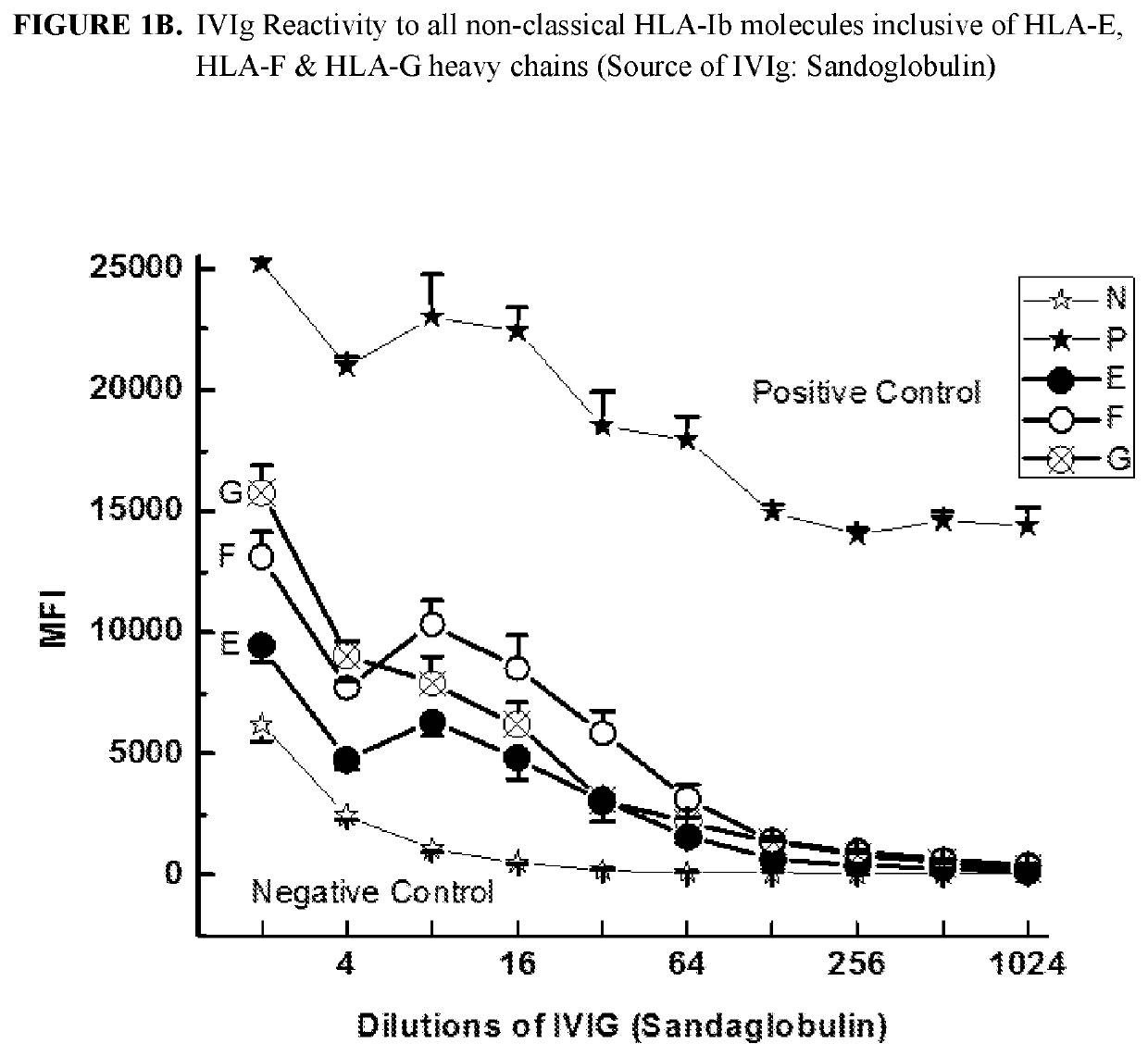
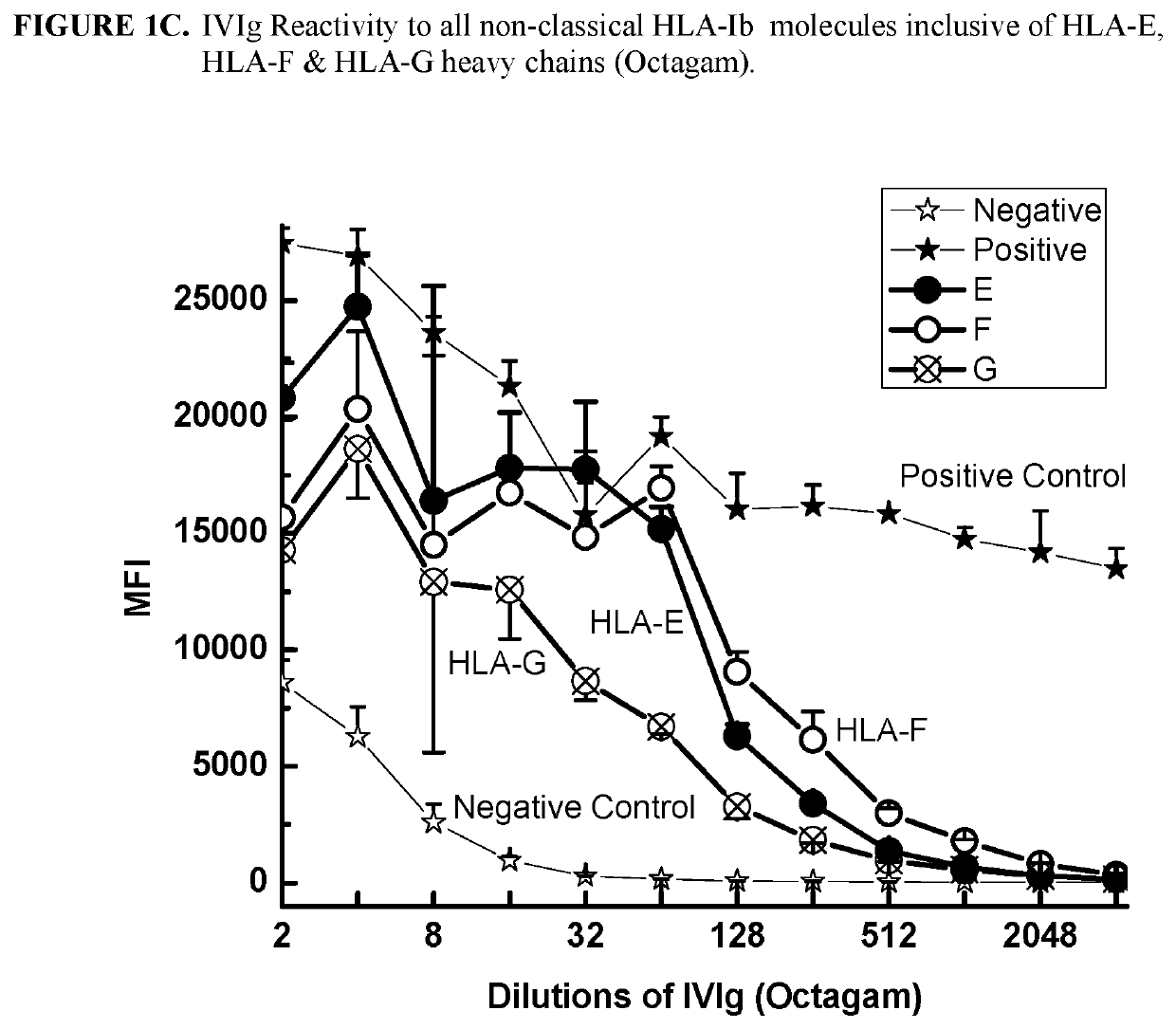
ANTI-HLA CLASS-Ib ANTIBODIES MIMIC IMMUNOREACTIVITY AND IMMUNOMODULATORY FUNCTIONS OF INTRAVENOUS IMMUNOGLOBULIN (IVIg) USEFUL AS THERAPEUTIC IVIg MIMETICS AND METHODS OF THEIR USE
InactiveUS20130177574A1Market price thereof has been risingMinimizing IVIg related side effectAntipyreticAnalgesicsDiseaseAntigen
Provided herein are compositions comprising anti-HLA-Ib antibodies as IVIg mimetics and methods for using the same for the prevention, treatment, therapy and / or amelioration of inflammation induced diseases and allograft rejection. In certain embodiments, the anti-HLA-Ib antibodies (monoclonal antibodies or mixed monoclonal antibodies, recombinant or chimeric or humanized or human antibodies) strongly mimic IVIg in immunoreactivity to HLA class Ia (HLA-A, HLA-B and HLA-Cw) and Ib antigens (HLA-E, HLA-F and HLA-G). In certain embodiments, the anti-HLA-Ib antibodies (monoclonal or mixed monoclonal antibodies; recombinant, chimeric, humanized or human antibodies) strongly mimic IVIg in immunomodulatory or immunosuppressive activities. While anti-HLA-Ib mAbs can be used to restore anti-tumor activities of CD8+ T cells and Natural killer cells by passive therapy in cancer patients, methods are also provided herein to induce production of polyclonal anti-HLA-Ib antibodies in cancer patients for restoring anti-tumor activities of CD8+ T cells and NK cells, by active specific immunotherapy.
Owner:RAVINDRANATH MEPUR DR
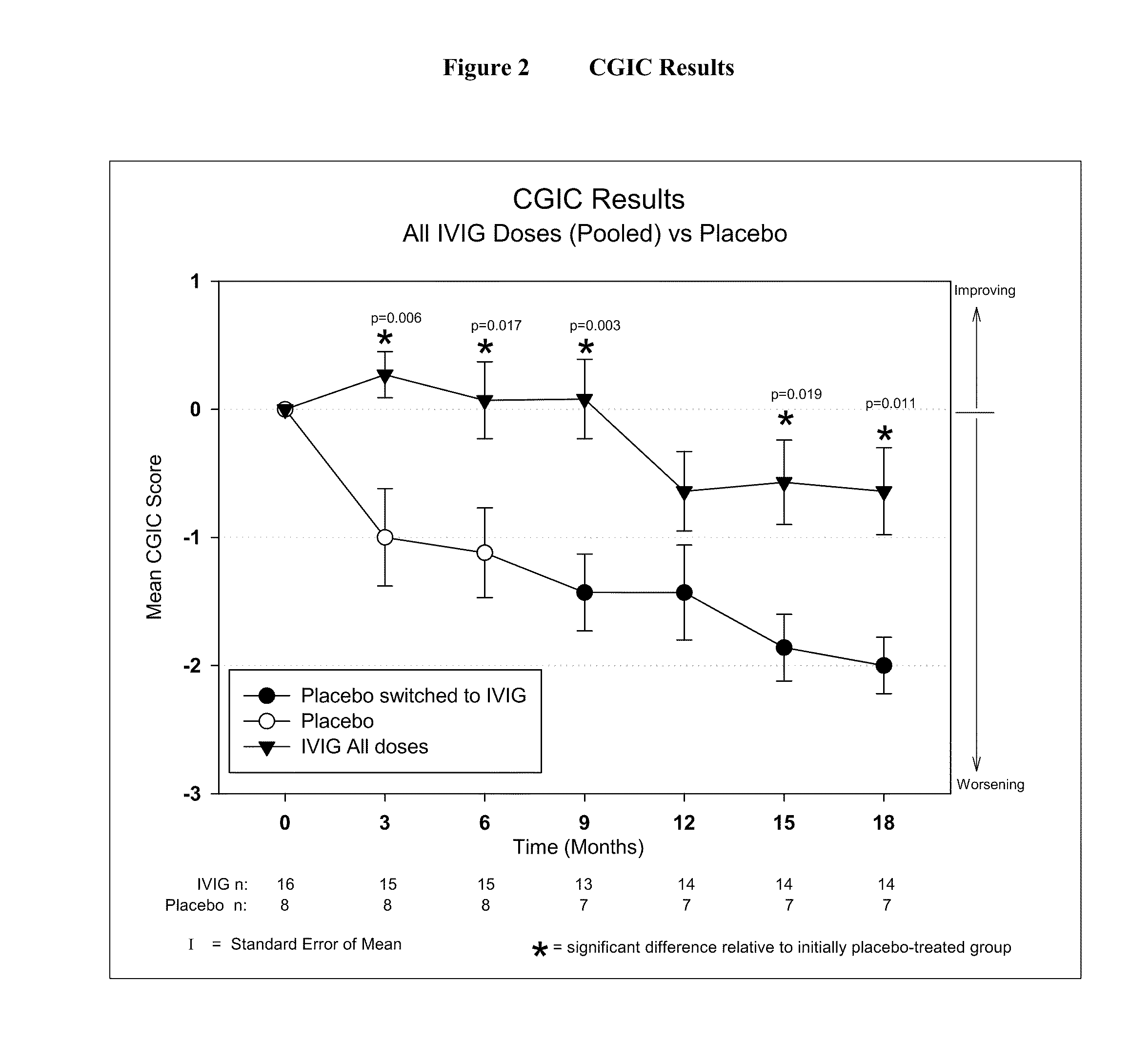
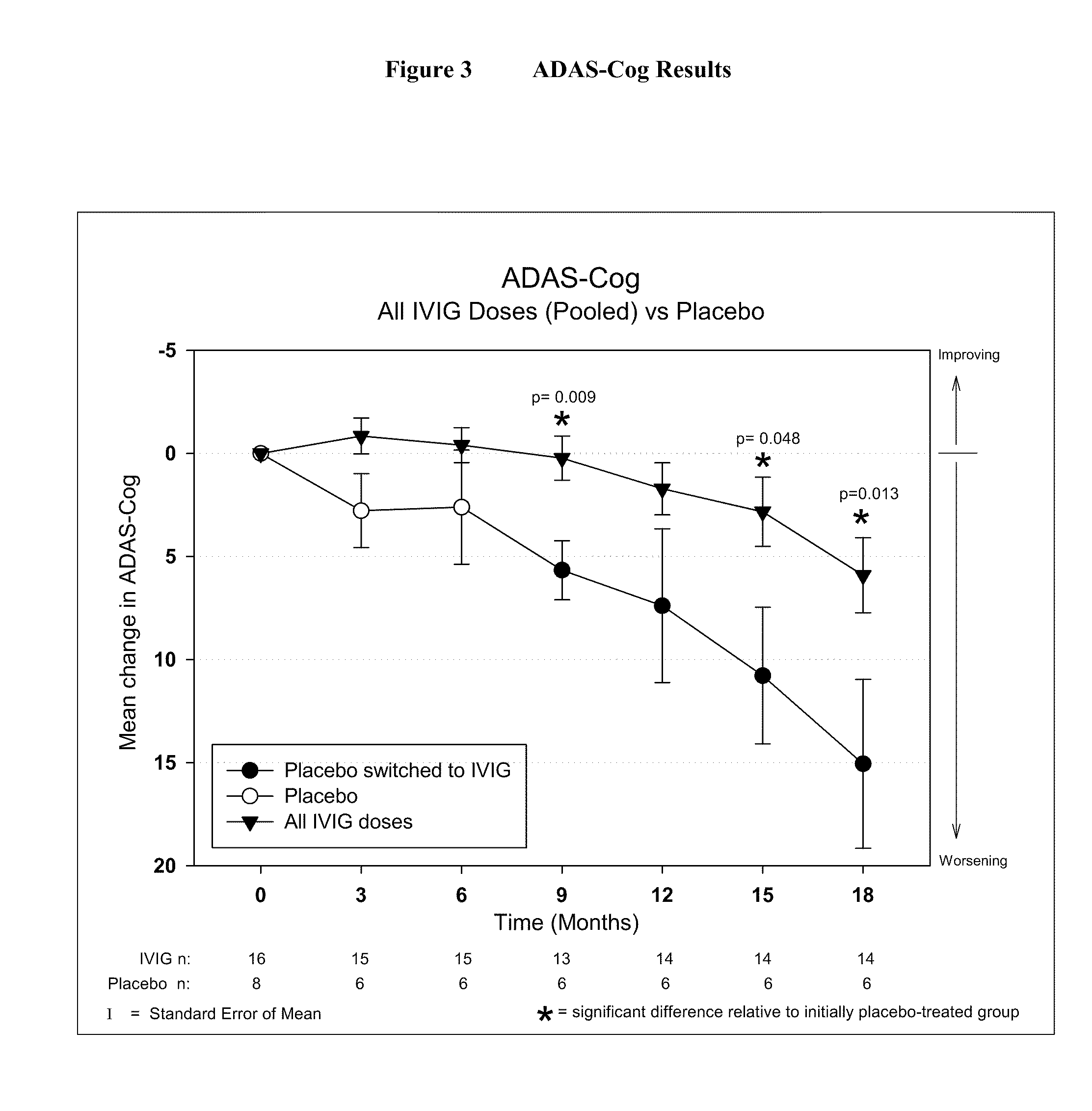
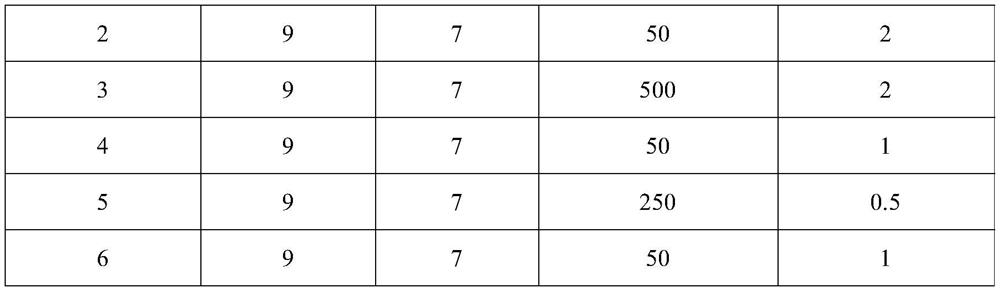
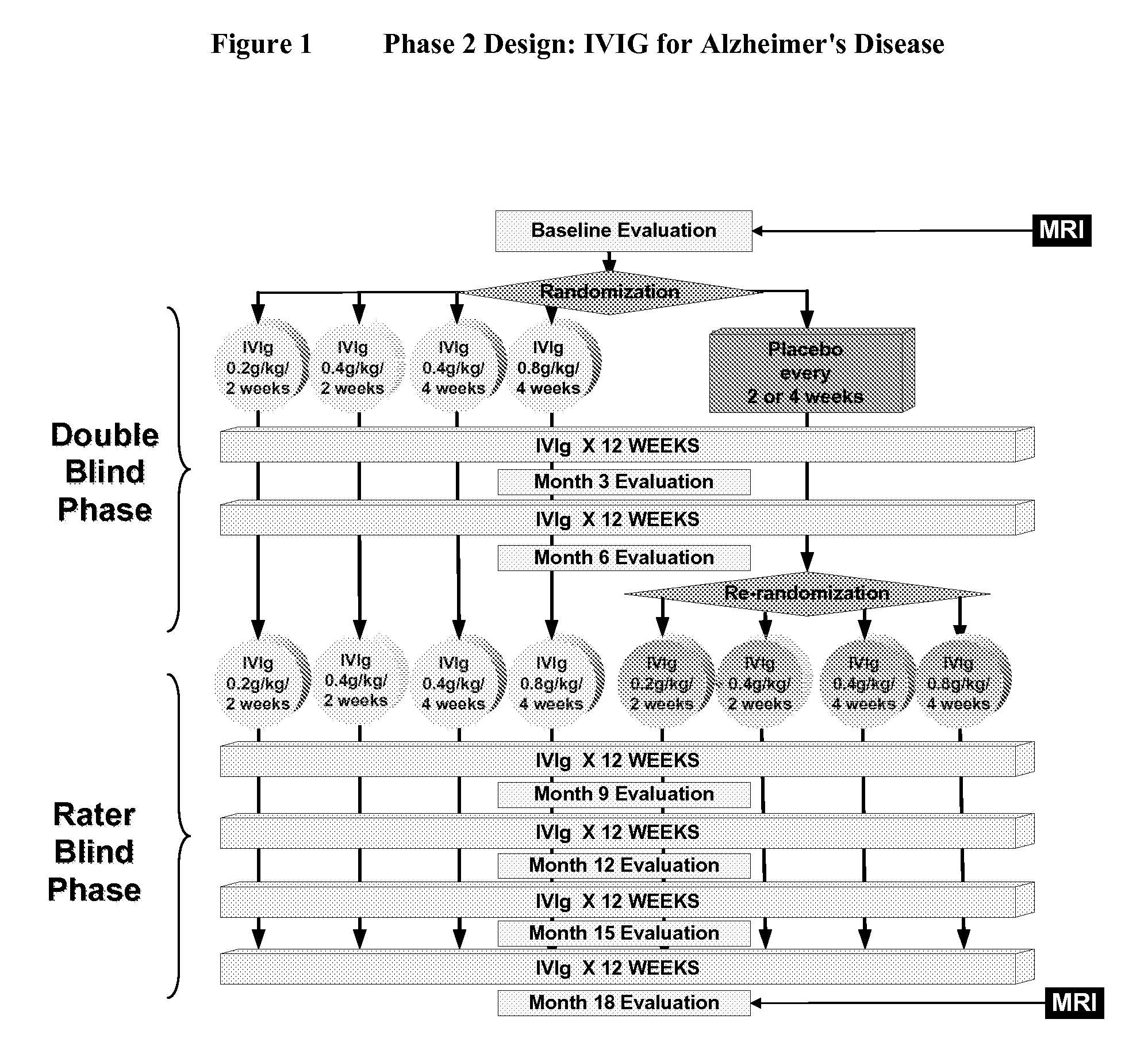
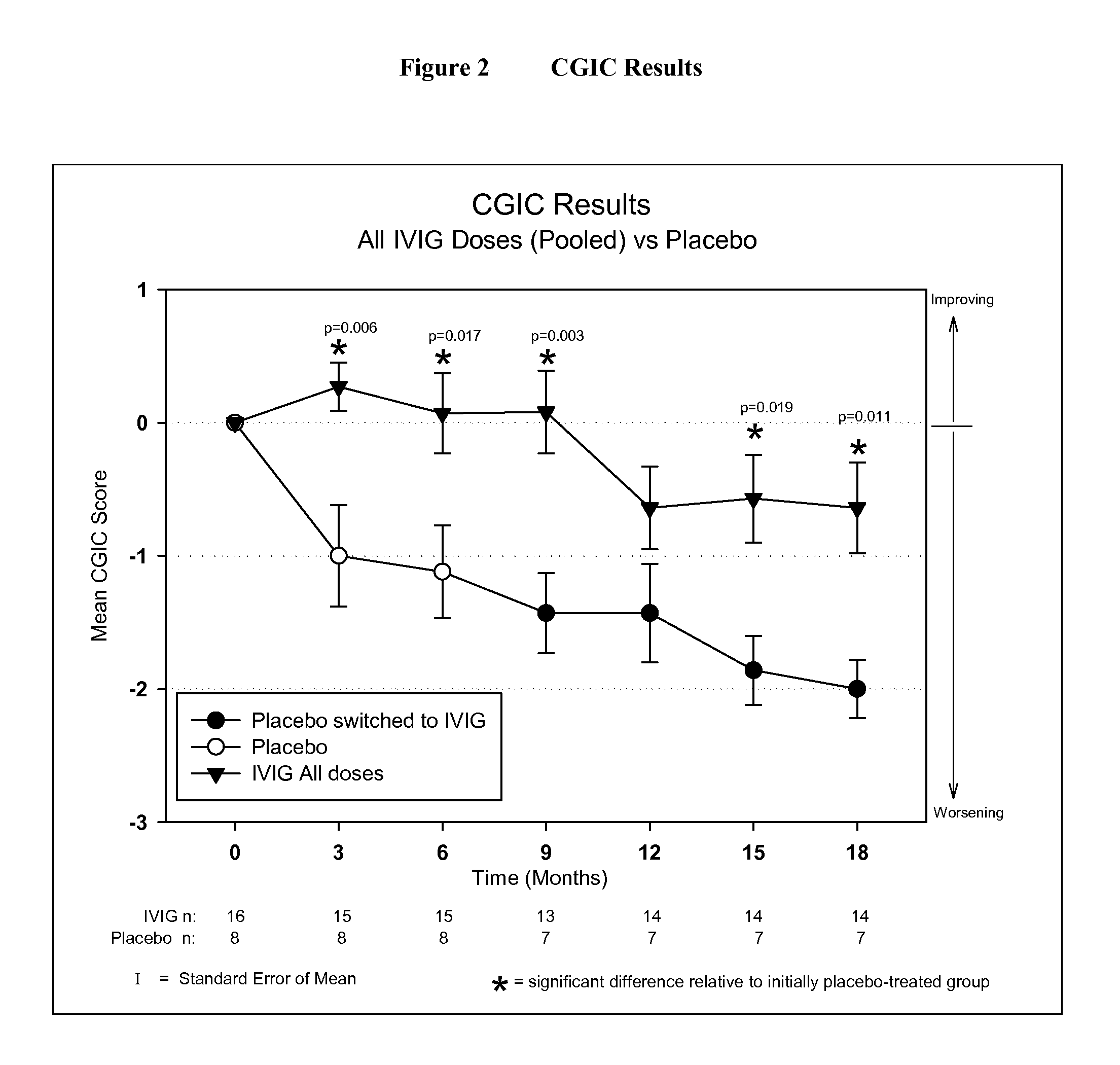
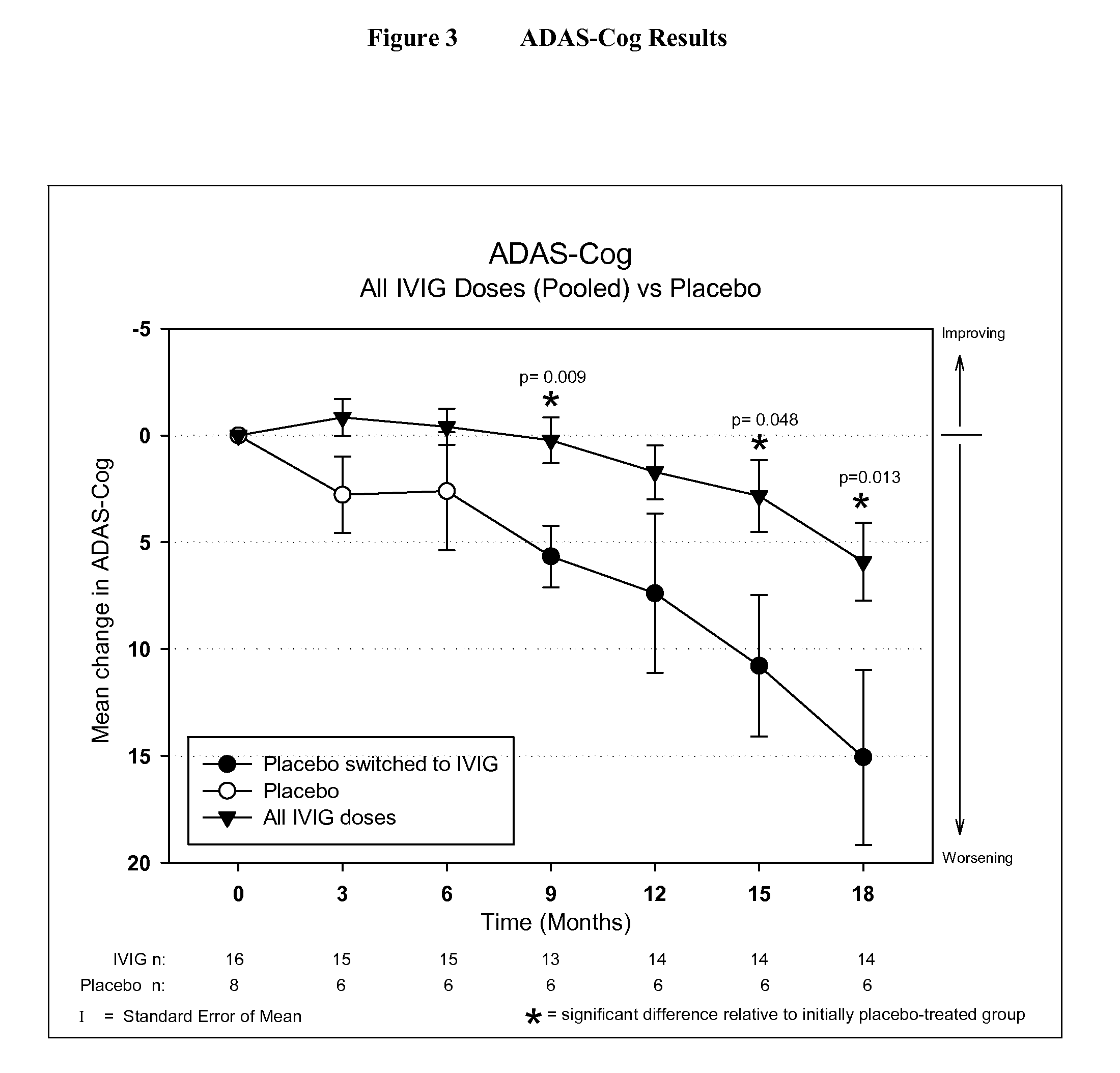
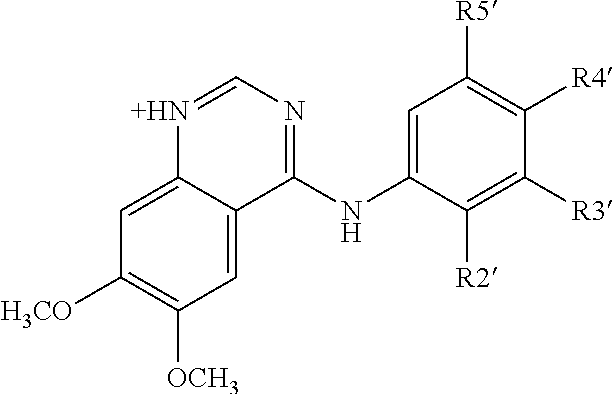
Use of ventricular enlargement rate in intravenous immunoglobulin treatment of alzheimer's disease
ActiveUS20110251479A1Guaranteed monitoring effectNervous disorderPeptide/protein ingredientsIntravenous IGDisease progression
The present invention relates to the use of MRI monitoring of ventricular enlargement rate as an objective measure for the purpose of assessing disease progression in patients suffering from Alzheimer's disease and for the purpose of determining therapeutic effectiveness of a treatment regimen for Alzheimer's patients. Methods for treating Alzheimer's Disease and monitoring therapeutic effectiveness are provided.
Owner:BAXALTA GMBH
Method for preparing intravenous immunoglobulin
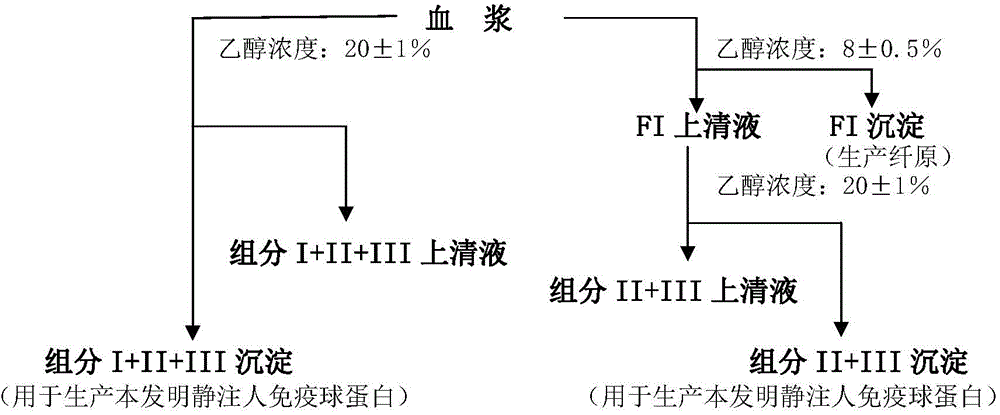
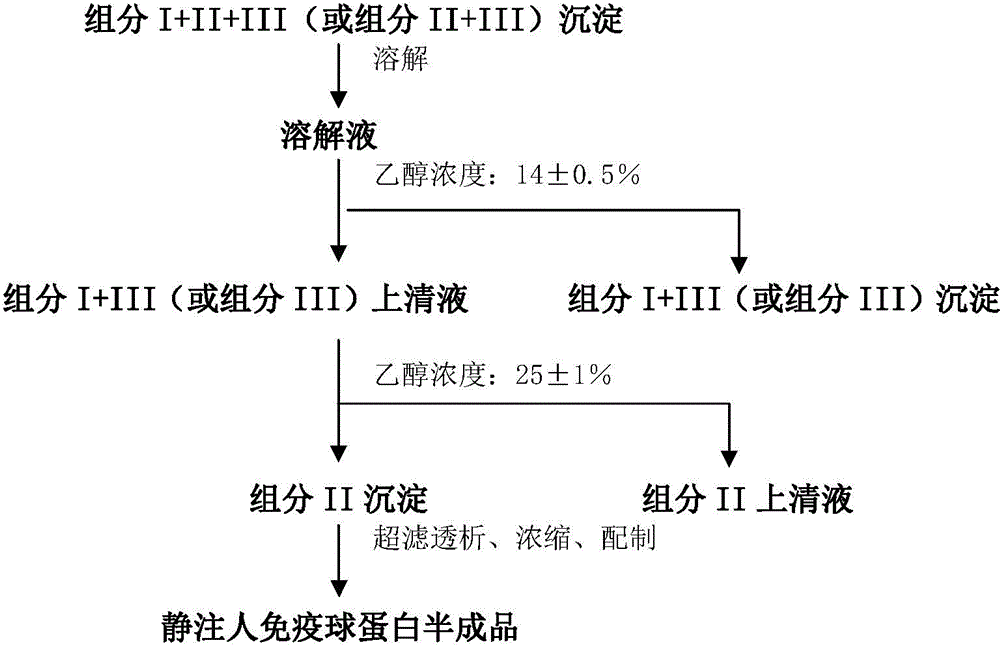
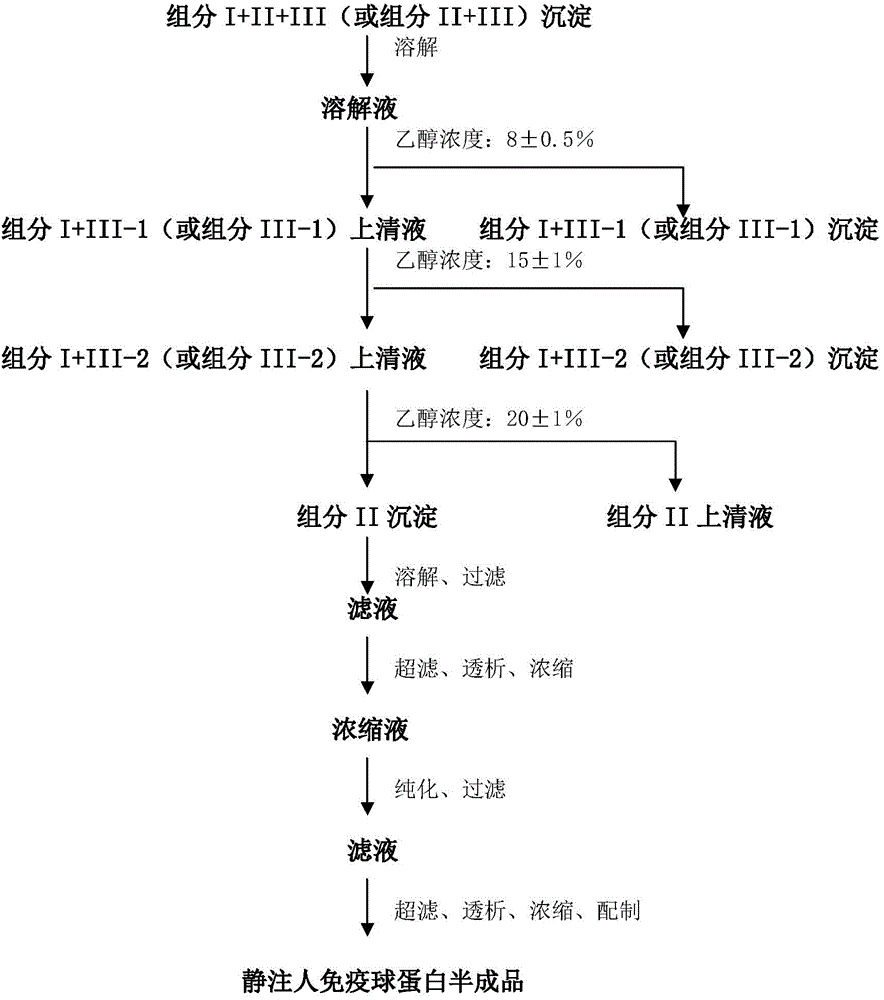
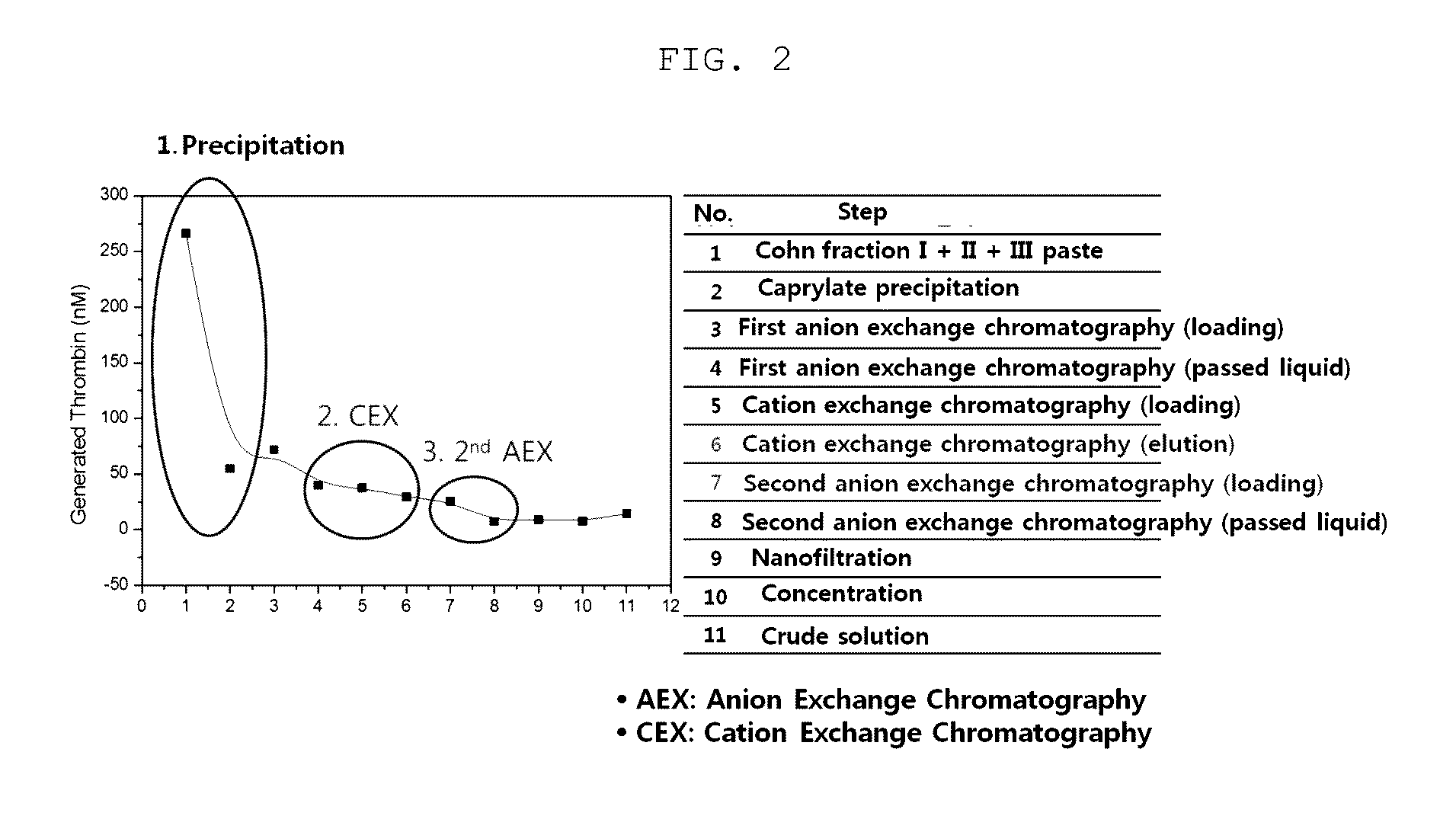
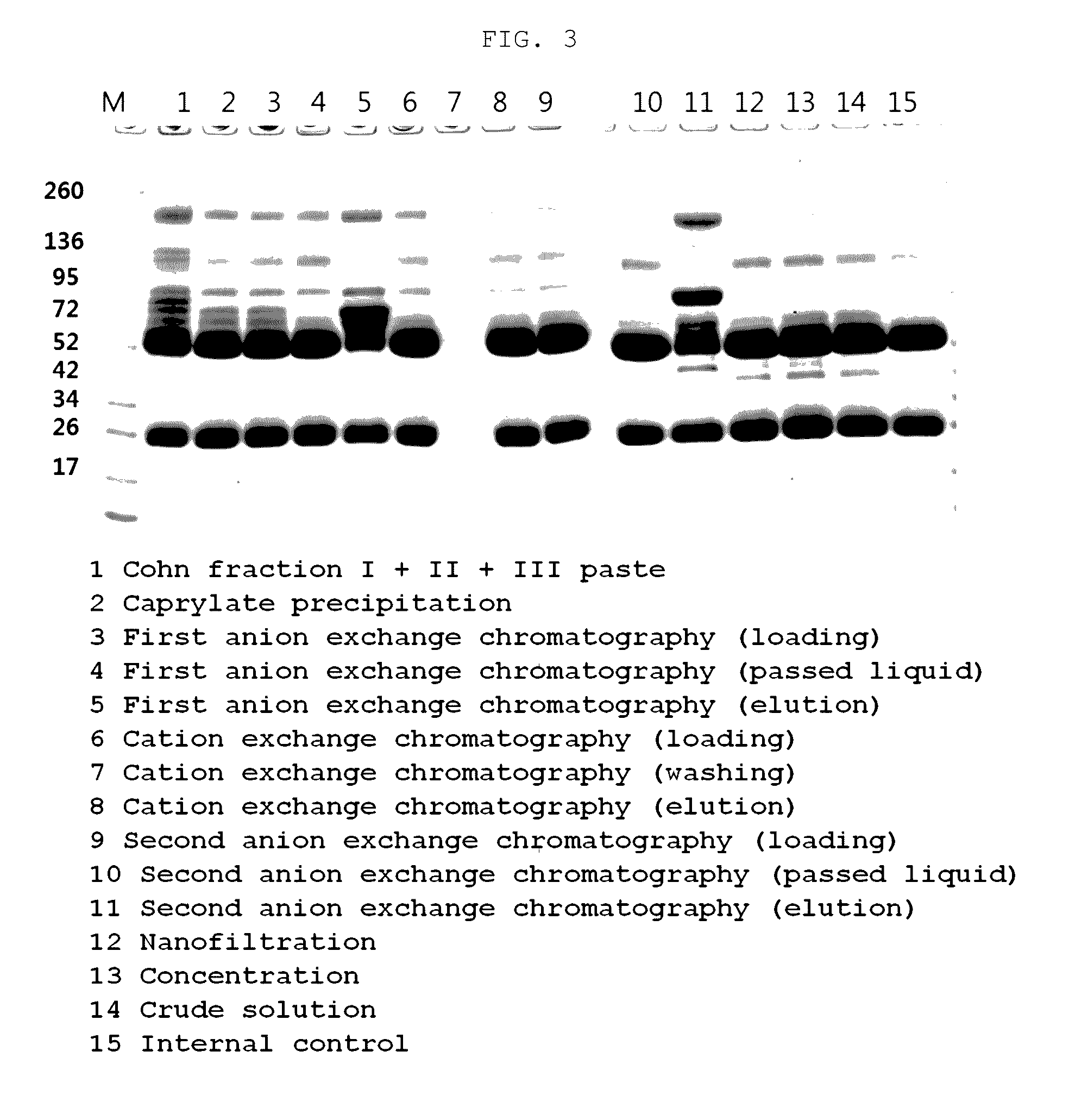
ActiveCN104479011ASolve the technical problems of low purity and high content of polymersReduce drug riskSerum immunoglobulinsPeptide preparation methodsPharmacyUltrafiltration
The invention provides a method for preparing the intravenous immunoglobulin. The method particularly includes the steps of dissolving component I+II+III or component II+III sediments; separating component I+III-1 or component III-1 sediments; separating component I+III-2 or component III-2 sediments; separating component II sediments; dissolving and filtering the component II sediments; conducting ultrafiltration, dialyzing and concentrating; conducting purification and filtering; conducting ultrafiltration dialysis, concentrating and preparation. By means of the method, steps and step parameters of the traditional process method are improved, specific combinations of the steps and the step parameters can be used for effectively extracting the human immunoglobulin for the intravenous injections from the component I+II+III or component II+III sediments, the technical problems that the human immunoglobulin for the intravenous injections is low in purity, and the content of polymer and the like is slightly high are solved, the pharmacy risk of people is reduced, and the pharmacy safety of people is guaranteed.
Owner:SHENZHEN WEIGUANG BIOLOGICAL PROD
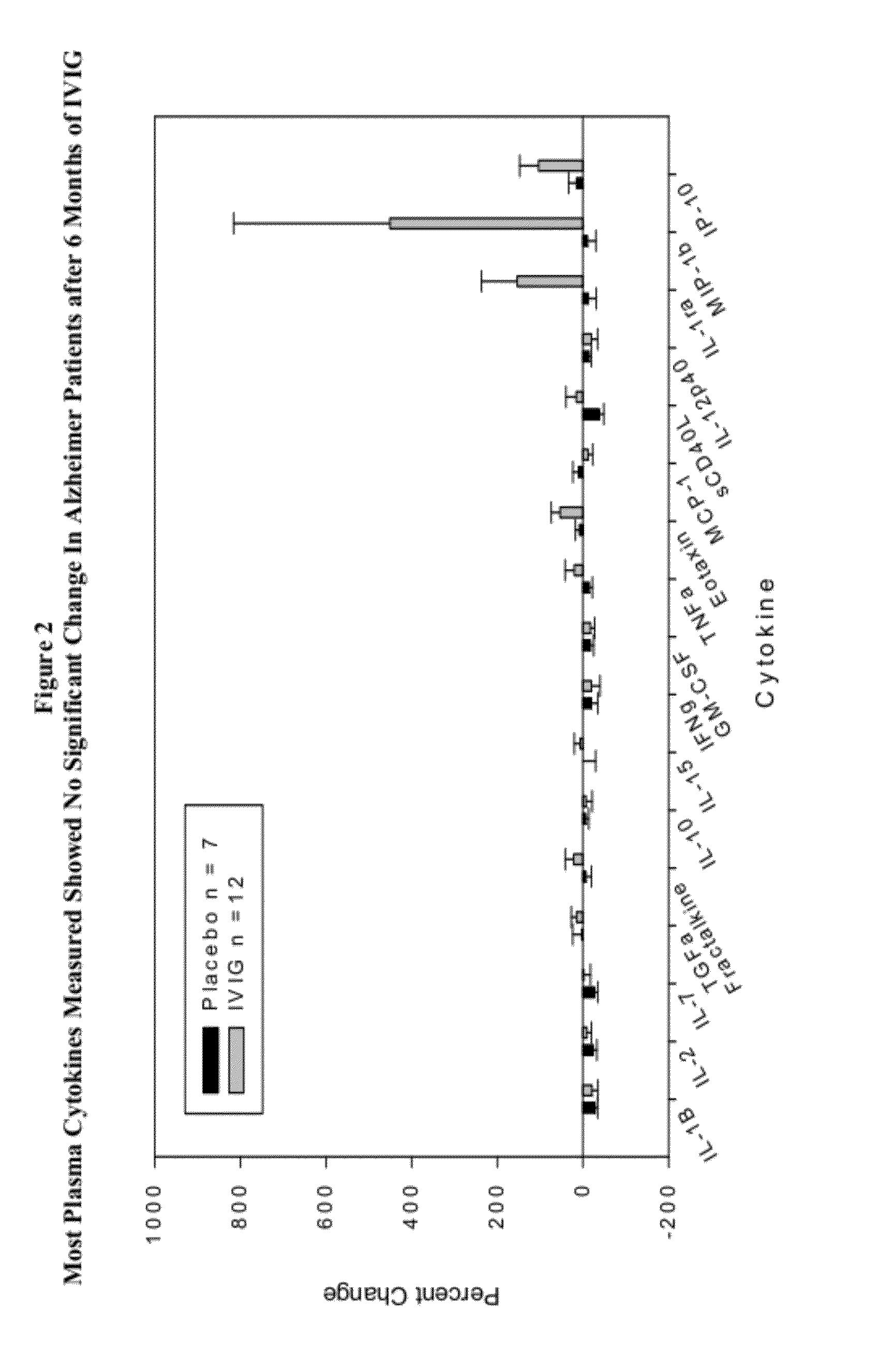
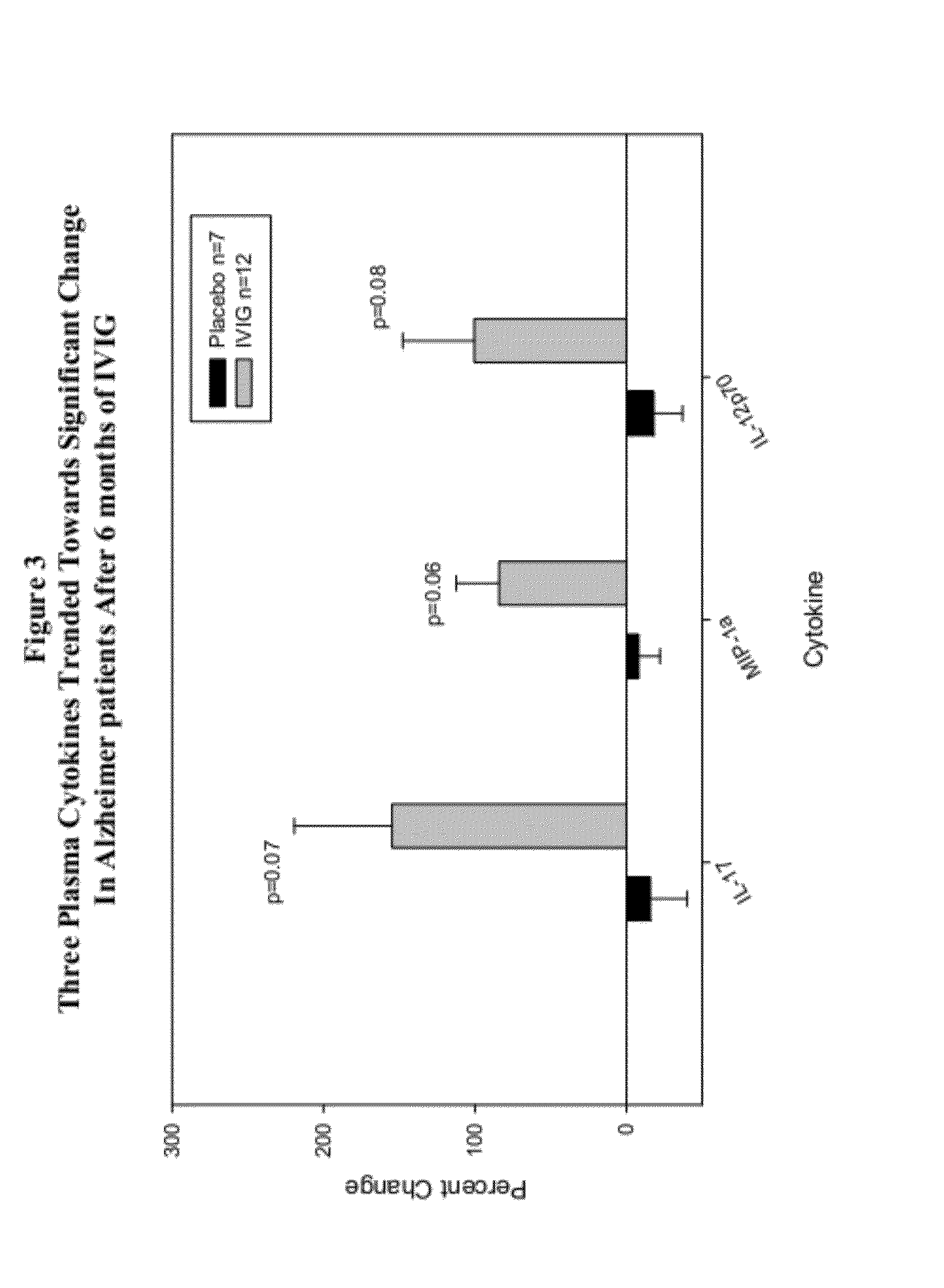
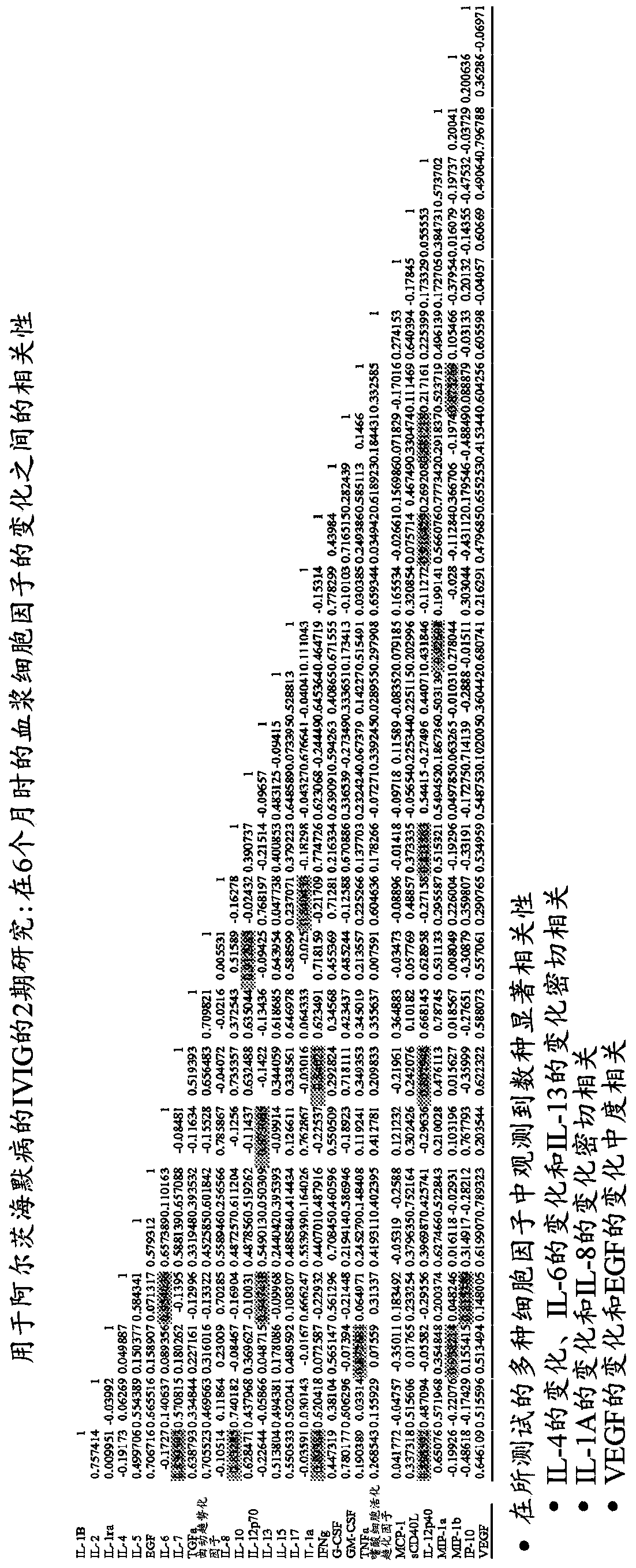
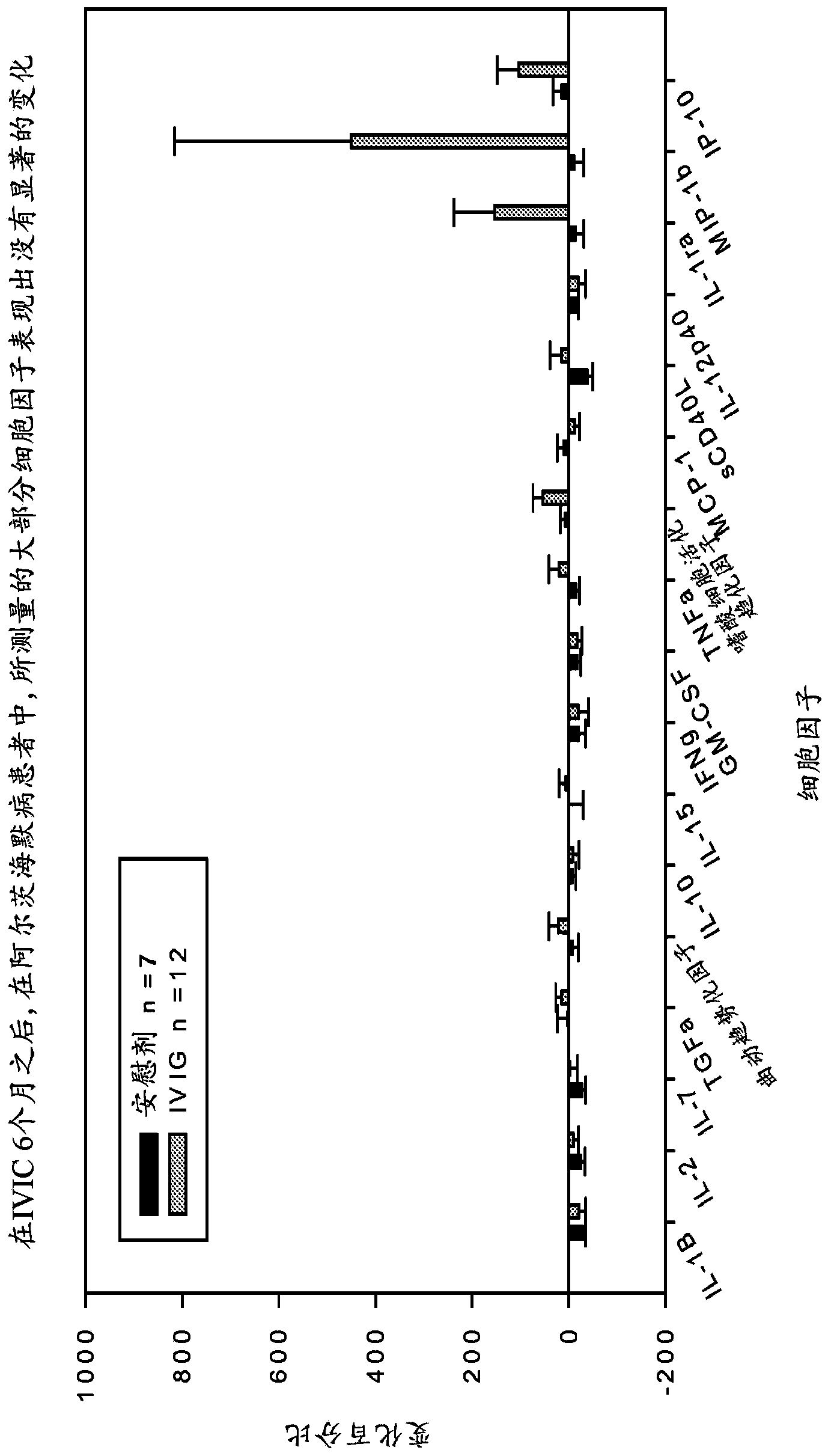
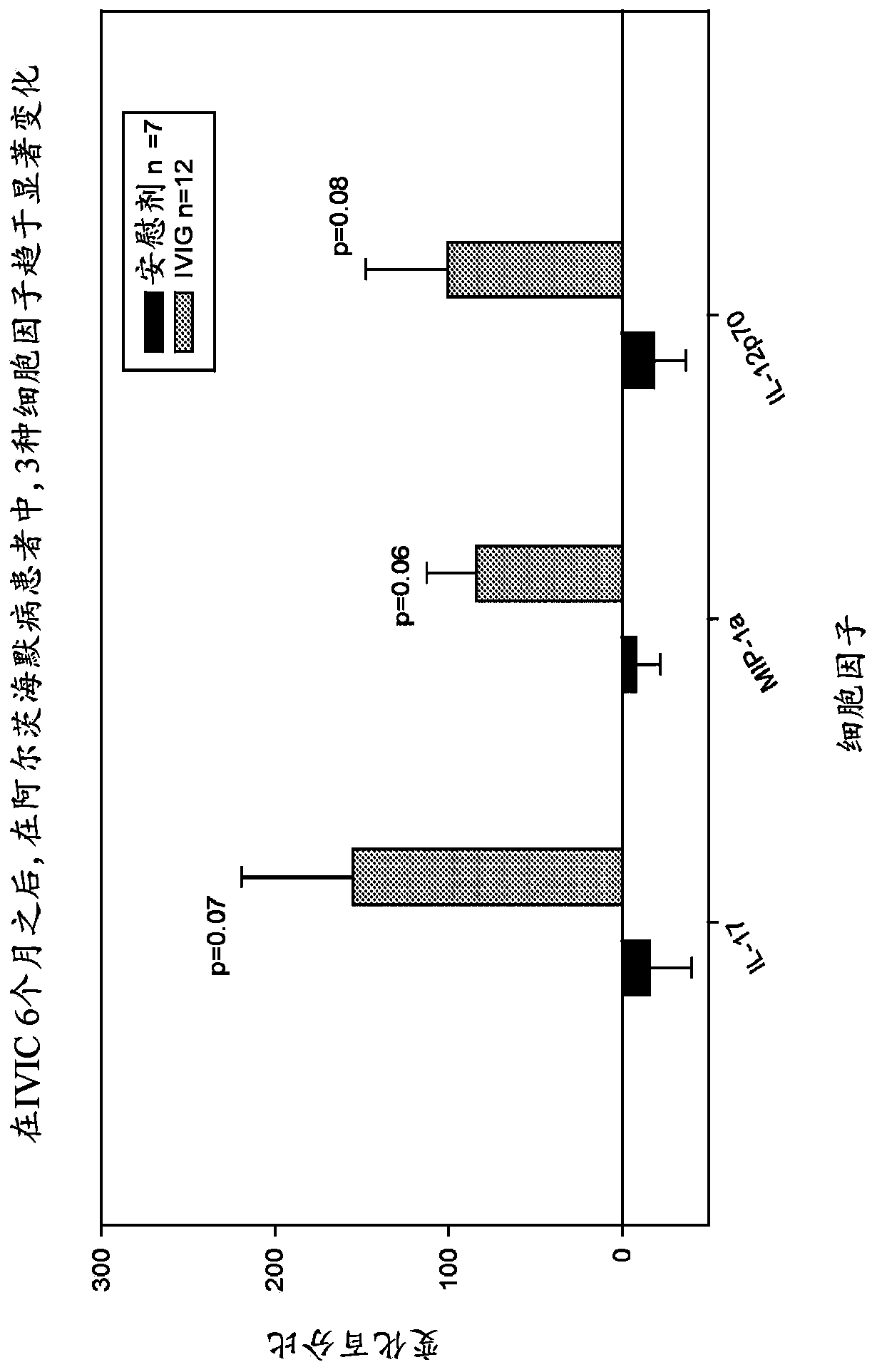
Use of cytokine levels in intravenous immunoglobulin treatment of alzheimer's disease
InactiveUS20120251524A1Guaranteed monitoring effectGood curative effectNervous disorderElectrolysis componentsCytokineDisease progression
The present invention relates to the use of the level of certain cytokines in a patient's blood as an objective measure for the purpose of assessing disease progression in patients suffering from Alzheimer's disease and for the purpose of determining therapeutic effectiveness of a treatment regimen. Methods for treating Alzheimer's disease and monitoring therapeutic effectiveness are provided.
Owner:BAXALTA GMBH
Products for prophylaxis and/or treatment of viral diseases and methods of making and using same
InactiveUS20100143377A1Improve the level ofHigh levelSerum immunoglobulinsImmunoglobulins against virusesDiseaseViral disease
The invention relates to an immunoglobulin composition suitable for prophylaxis and / or treatment of a viral mediated disease, disorder or condition and to methods of its preparation. In one embodiment, the invention provides a potent intravenous immunoglobulin composition useful for the treatment or prevention of a West Nile virus mediated disease, disorder or condition.
Owner:OMRIX BIOPHARM
Method for purifying immunoglobulin
ActiveUS20170015732A1Reduce impurityRemove substanceSerum immunoglobulinsPeptide preparation methodsPurification methodsPolyethylene glycol
The present invention relates to a method for purifying an immunoglobulin, and more particularly, to a method for purifying an immunoglobulin, which comprises: dissolving immunoglobulin-containing plasma protein fraction I+II+III or fraction II+III; adding caprylate to the solution to cause precipitation; performing dialysis and concentration after removal of the precipitate; performing anion exchange resin and ceramic cation exchange resin purification processes to effectively remove a solvent and detergent added to inactivate viruses; and performing elution while maintaining salt concentration at a constant level to maintain the immunoglobulin polymer content at a low level. According to the method for preparing the intravenous immunoglobulin according to the present invention, a precipitation step of preparing fraction II from fraction I+II+III or fraction II+III as a starting material can be omitted, and problems, including a complicated process and a low yield, which occur in the conventional preparation process employing the polyethylene glycol treatment process, can be solved by use of first sodium caprylate precipitation, anion exchange chromatography and cation exchange chromatography. In addition, when the immunoglobulin purification method according to the present invention is used, the efficiency with which impurities and thrombotic substances are removed can be increased and the immunoglobulin polymer content can be maintained, and thus a stable immunoglobulin with increased quality can be produced.
Owner:GREEN CROSS HLDG
Method for detecting activated blood coagulation factor XI in human intravenous immunoglobulin
ActiveCN103185710AAvoid preactivationIndirectly reflect changes in production contentFluorescence/phosphorescenceInitiation factorFactor ii
The invention discloses a method for detecting an activated blood coagulation factor XI in human intravenous immunoglobulin. The method comprises the steps as follows: (1) mixing an IVIG (intravenous immunoglobulin) sample with specially-preprocessed platelet-poor plasma (PPP); (2) adding a thrombin specific fluorogenic substrate with a phospholipid agent into a reaction system; (3) adding an initiation factor for starting a TGT (thrombin generation test), wherein the initiation factor comprises a calcium ion source and the phospholipid agent; (4) obtaining a thrombin generation curve providing parameters by the optimized reaction system; and (5) negatively correlating the peak time of thrombin (TTP) of the detected sample with the FXIa (activated blood coagulation factor XI) level in the sample. The FXIa level in the detected sample can be indirectly obtained by comparing the TTP of the detected sample with that of an FXIa standard sample. The method is suitable for detecting residual micro FXIa in human intravenous immunoglobulin (IVIG) products and is used for extracorporal evaluation of thrombosis risk of related products.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +1
Method for preparing immunoglobulin G by rapidly extracting plasma of COVID-19 patient in rehabilitation period
ActiveCN112010968AHigh antibody titerFast access to plasmaSerum immunoglobulinsImmunoglobulins against virusesIntravenous gammaglobulinErythroid cell
The invention relates to a method for preparing immunoglobulin G by rapidly extracting plasma of a COVID-19 patient in a rehabilitation period, and more particularly, to a method for preparing intravenous injection COVIDI-19 immunoglobulin G by rapidly separating component plasma from whole blood by using a closed multicellular component automatic separation system (MACSS), for example, to prepareintravenous injection superimmunoglobulin. The intravenous immunoglobulin can be used for treating patients infected by novel coronavirus. The method comprises the following steps: separating human peripheral blood into three component layers, namely an erythrocyte layer, a cell concentration layer and a plasma layer, by using a closed multi-cell component automatic separation system, and then carrying out virus inactivation on the obtained plasma to obtain plasma capable of being used for preparing intravenous immunoglobulin G. The invention also relates to a method for preparing an intravenous immunoglobulin G preparation by using the plasma component obtained by the above method. According to the method, the plasma obtaining speed is high, and the antibody titer of the prepared plasmaand intravenous immunoglobulin G is high.
Owner:深圳博雅感知药业有限公司
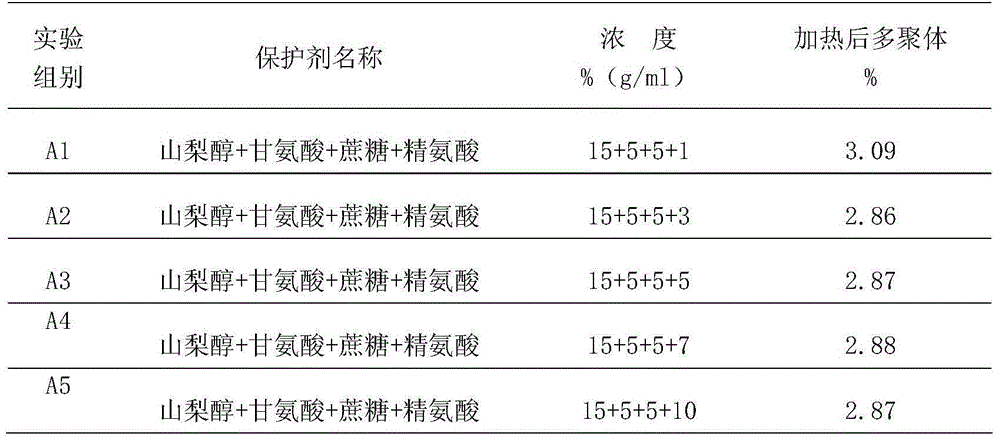
Protein protective agent for Pasteur inactivating human intravenous immunoglobulin and inactivation method of protein protective agent
ActiveCN103550780AExtensive inactivationProtect proteinAntibody ingredientsPharmaceutical non-active ingredientsWater bathsGlycine
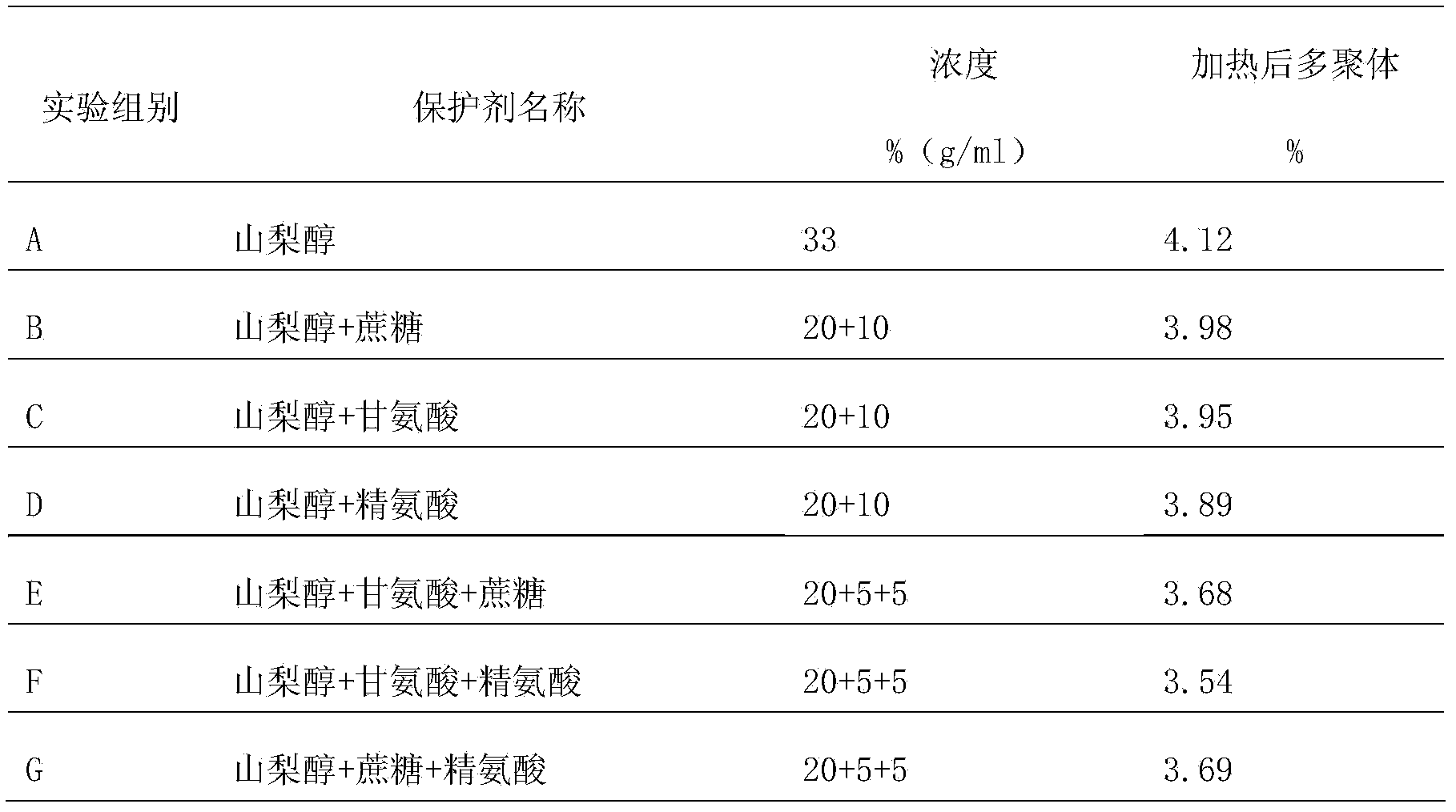
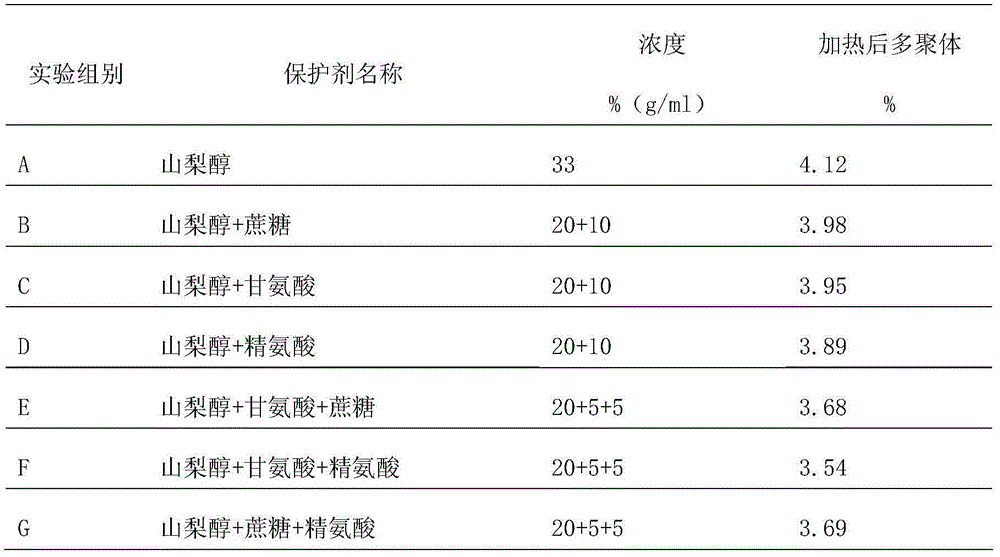
The invention provides a protein protective agent for Pasteur inactivating human intravenous immunoglobulin and an inactivation method of the protein protective agent. A Pasteur inactivation method of human intravenous immunoglobulin comprises the following steps of adding the protein protective agent to a human intravenous immunoglobulin solution before inactivating; adjusting the content of sampled protein to be 40 to 60g / L, and adjusting pH (Potential of Hydrogen) to be 4.7 to 5.3; agitating for 30 minutes; transferring into a water bath under 60+ / - 1 DEG C; maintaining the solution at temperature of 60+ / -0.5 DEG C for 10 hours after the solution temperature reaches 60 DEG C, thus accomplishing the inactivation of human intravenous immunoglobulin, wherein the protein protective agent is saccharose, arginine, glycine and sorbitol which are respective 50 to 70g / L, 20 to 40g / L, 30 to 60g / L and 240 to 260g / L in final content. According to the protein protective agent, simple sorbitol is used as the protective agent, the content of a polymer is decreased from 4.12% to 1.96%, thus the protein can be protected well, and the product quality can be improved.
Owner:BANGHE PHARMA CO LTD
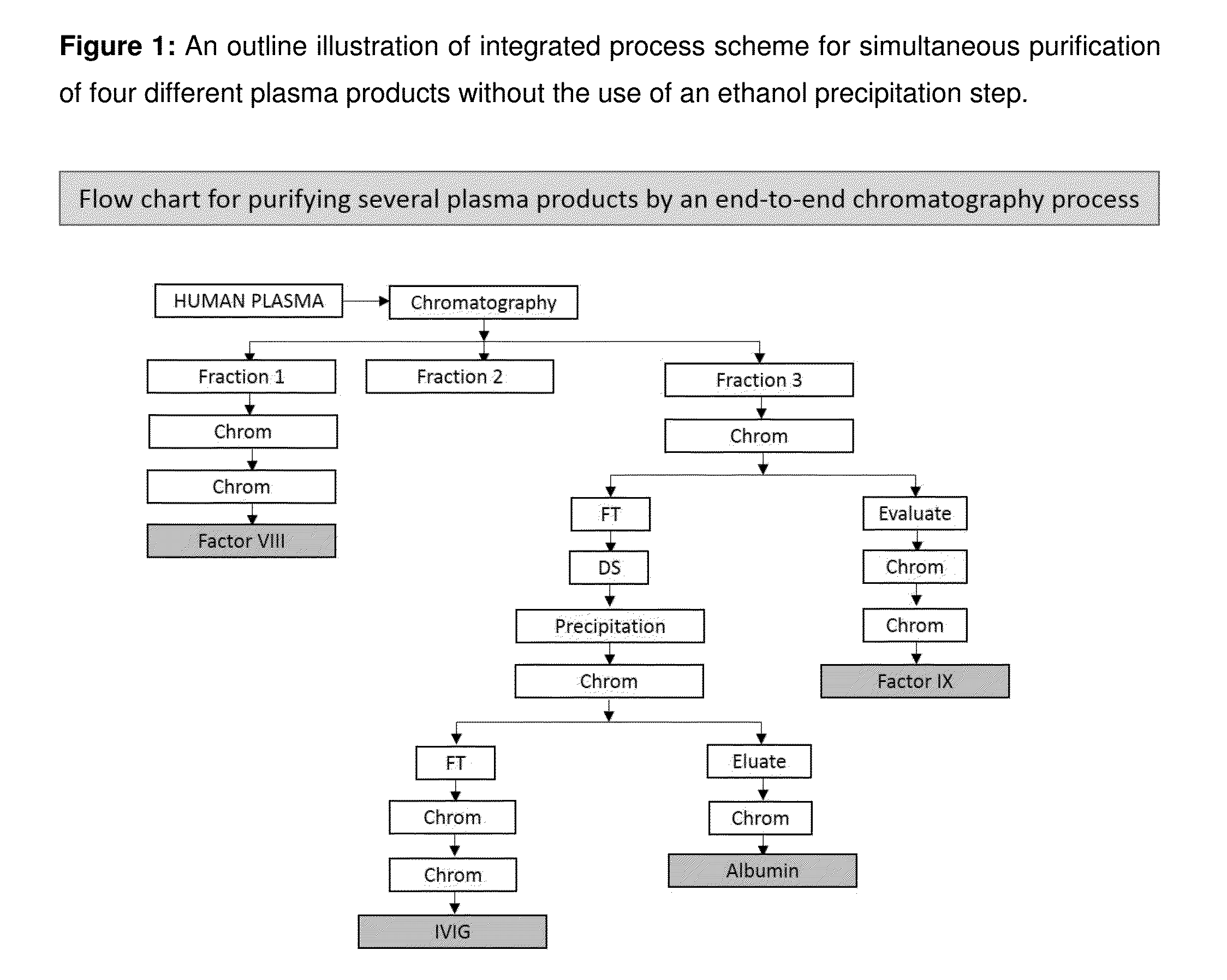
Integrated process for the production of therapeutics (human albumin, intravenous immunoglobulins, clotting factor viii and clotting factor ix) from human plasma
ActiveUS20150210737A1Low costImprove affordabilityHydrolasesChemical industryFractionationHuman albumin
The invention relates to an integrated scheme for fractionation and purification of plasma products (human albumin, intravenous immunoglobulin (IVIG), clotting factor VIII and clotting factor IX) by sequential chromatography and virus reduction steps. The therapeutically administrable protein IVIG has purity levels exceeding 98%, aggregates and dimers at less than 0.2%, Fc function of >90% and anti-complementary activity of less than 0.5 CH50 per mg of Ig. The distribution of IgG isomers is comparable to the ranges seen in normal plasma. Human albumin for therapeutic use, purified by this integrated scheme has an electrophoretic purity of close to 100%, with monomers exceeding 98%. The levels of aluminium and pre-kallikrein activator are below the detection limit for the respective tests. The Factor IX preparations have a specific activity of ≧200 IU / mg. The impurity levels of Factor-II, Factor VII, Factor X are at least 10-fold lesser (≦0.5% instead of 5%) and the heparin impurity of ≦0.01 IU (against 0.5 IU limit for this impurity) is 50-fold lesser the specified pharmacopoeial limits.The purification carried out by an all-chromatography scheme, avoids the use of ethanol precipitation in the entire manufacturing process of the said four plasma products. The invention describes an integrated process for purifying four different proteins from human plasma to high therapeutic grade purity levels, with a potential to purify more therapeutic proteins from a given plasma sample by incorporating additional chromatography steps in the sequence.
Owner:ICHOR BIOLOGICS PTE LTD
Treatment of diabetes mellitus
ActiveUS20190062447A1Organic active ingredientsMetabolism disorderInsulin dependent diabetesLymphocyte
The present invention provides a method of treating insulin-dependent diabetes mellitus in a subject, comprising administering to the subject a therapeutically effective amount of a Janus kinase inhibitor, or a pharmaceutically acceptable salt or ester thereof, or a therapeutically effective amount of intravenous immunoglobulin, or a therapeutically effective amount of a therapeutic agent that destroys B lymphocytes, or a combination thereof. The present invention also provides kits containing the same.
Owner:LEVIT EYAL
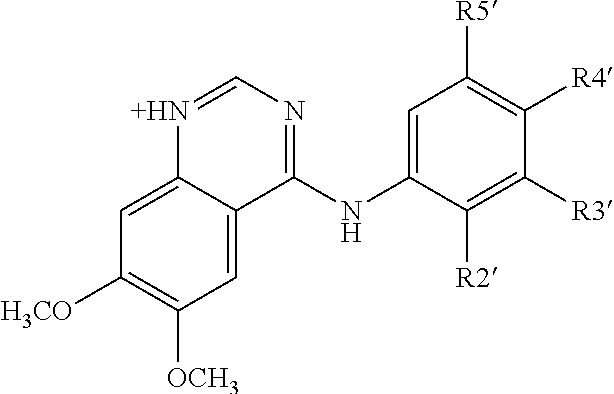
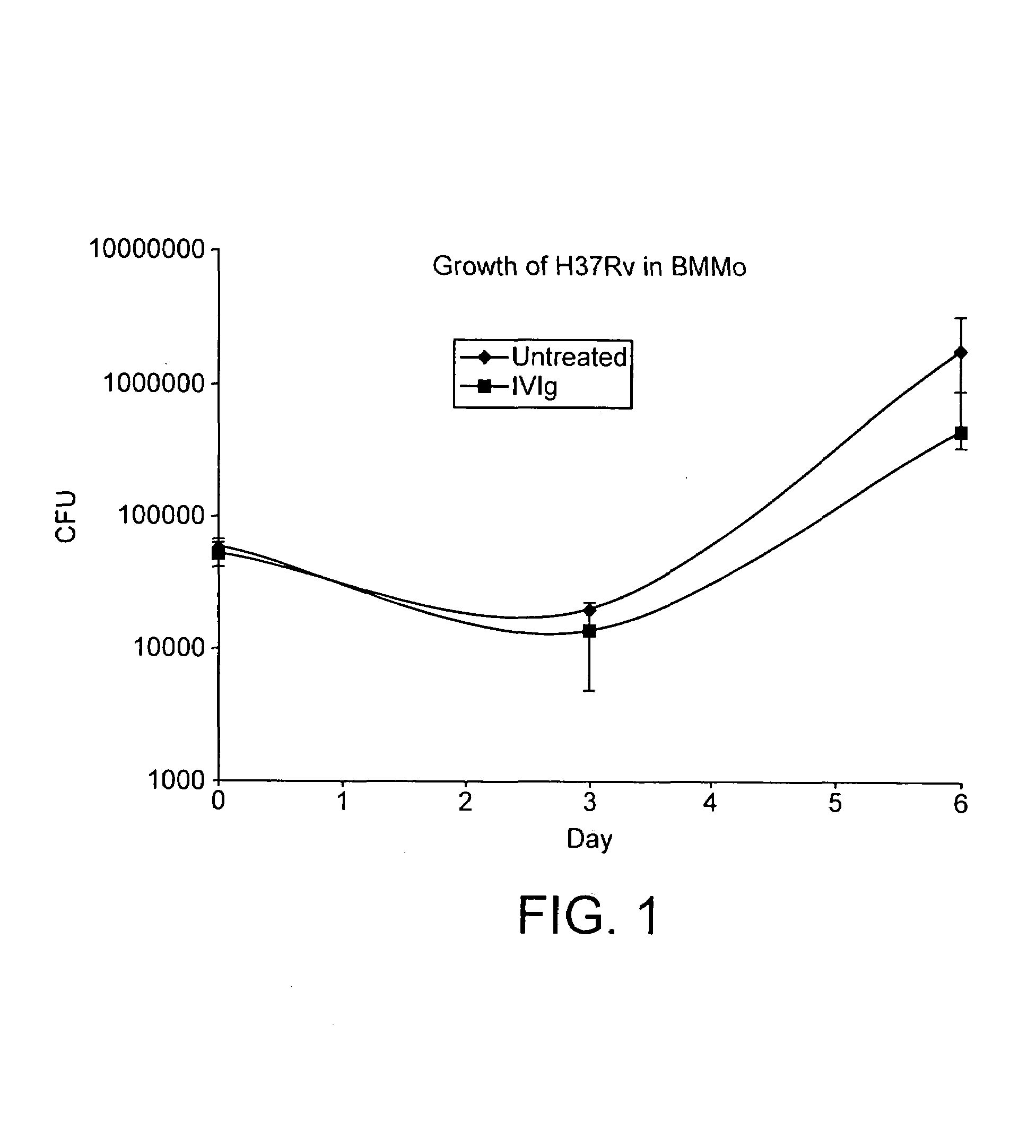
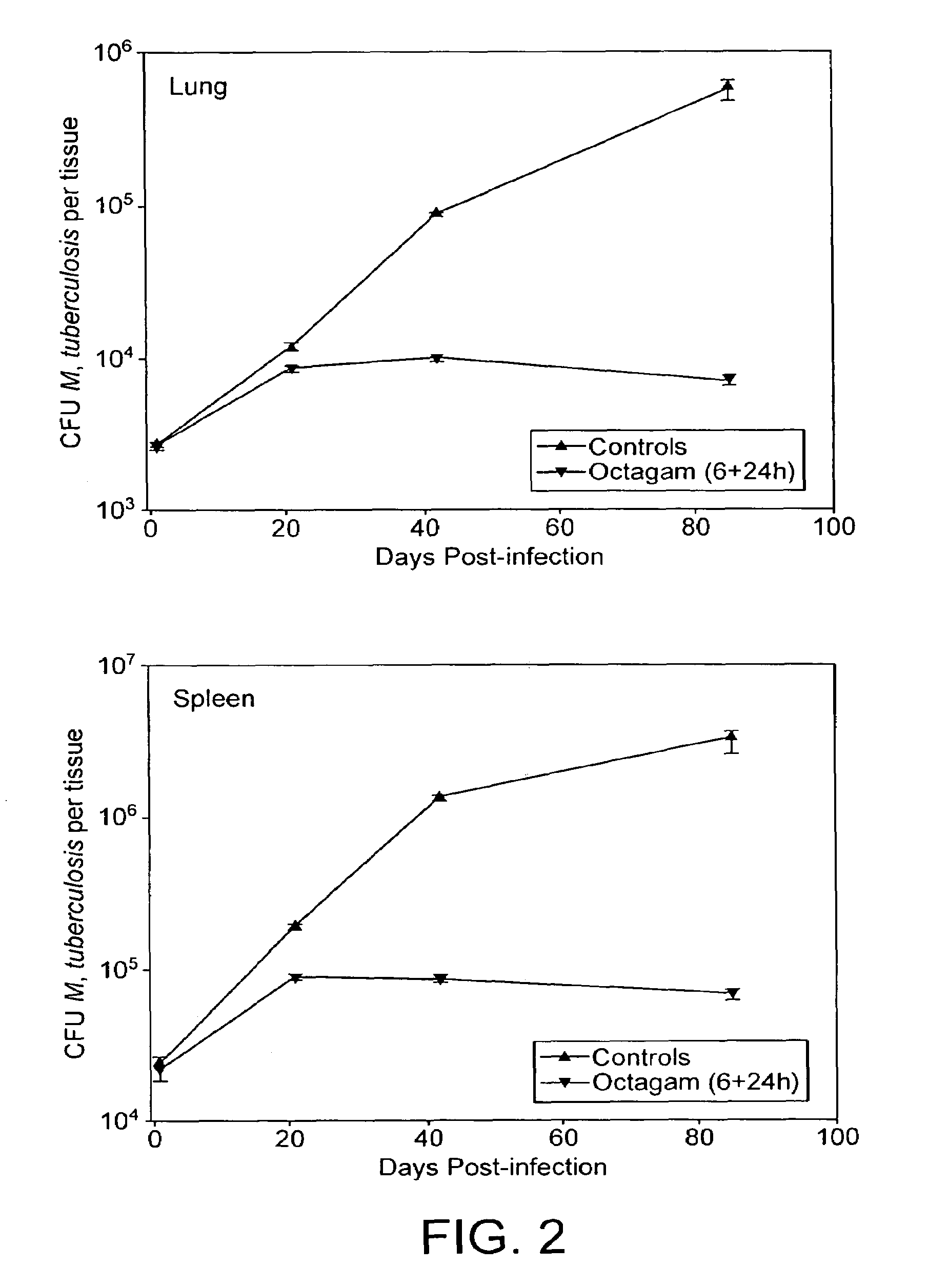
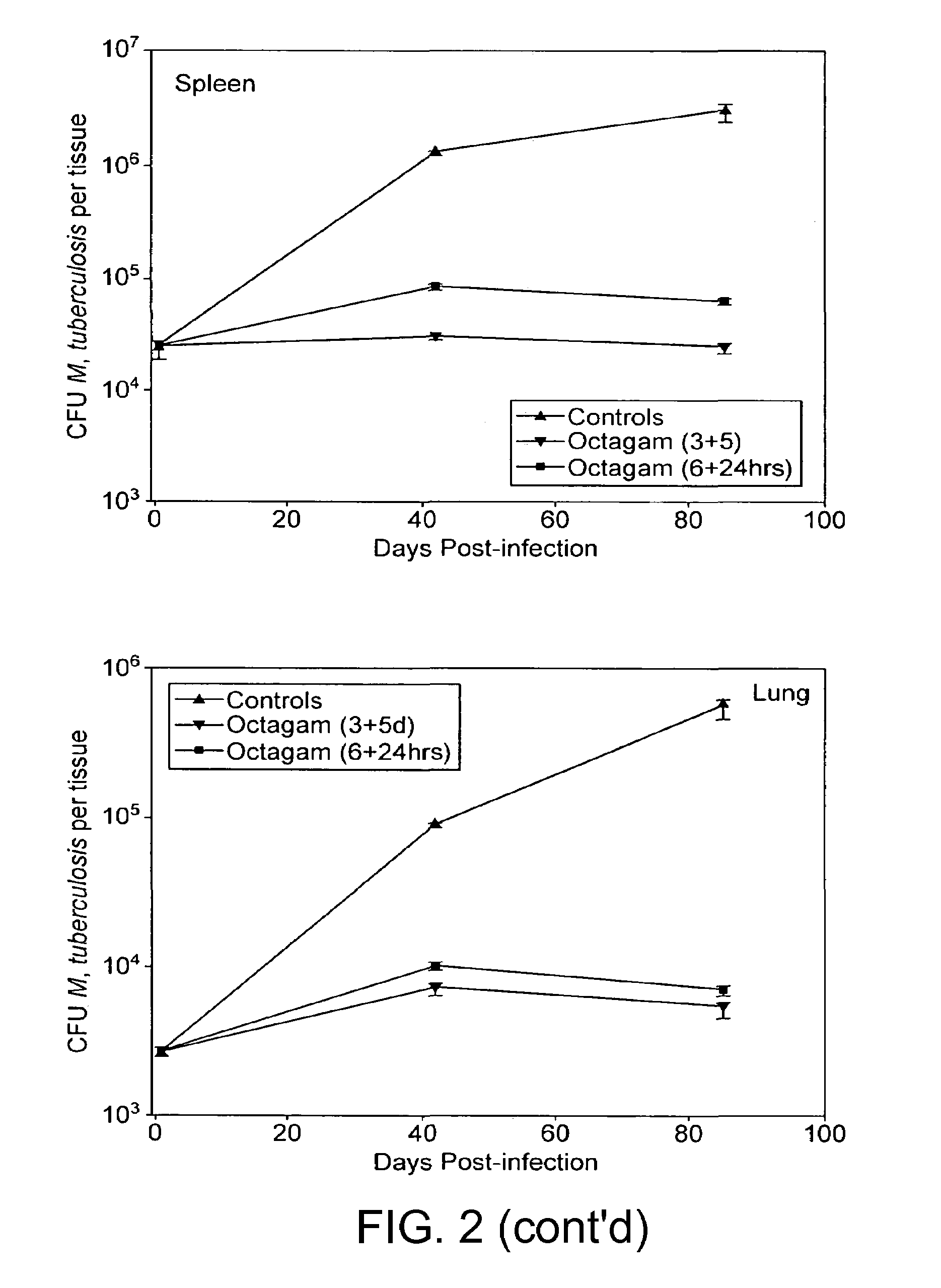
Method for the treatment or prophylaxis of tuberculosis
InactiveUS7687060B2Colony countStimulate immune responseSerum immunoglobulinsImmunoglobulins against bacteriaTuberculosisTuberculosis prophylaxis
Owner:MEDICAL RESEARCH COUNCIL
Method for rapidly extracting plasma of COVID-19 patient in rehabilitation period by automatic separation system
ActiveCN112225799AHigh speedHigh titerSerum immunoglobulinsInorganic non-active ingredientsIntravenous gammaglobulinEngineering
The invention relates to a method for rapidly extracting plasma of a COVED-19 patient in a rehabilitation period by an automatic separation system. The method comprises the following steps of: separating peripheral blood taken from a human body into three component layers, namely an erythrocyte layer, a cell concentration layer and a plasma layer, by using a closed multi-cell component automatic separation system, performing virus inactivation on the obtained plasma, and optionally cryopreserving the plasma to obtain the plasma which can be used for preparing intravenous immunoglobulin G. Theinvention also comprises a method for preparing an intravenous immunoglobulin G preparation. The method comprises the following steps: obtaining a plasma component by the method, precipitating the plasma by a low-temperature ethanol method to obtain components I + II + III, separating a component II from a component I + III, respectively preparing an immunoglobulin G semi-finished product 1 and animmunoglobulin G semi-finished product 2, performing sterilization and virus inactivation to obtain an immunoglobulin G stock solution. and further preparing the intravenous immunoglobulin G preparation for intravenous injection. The method provided by the invention achieves excellent technical effects as described in the specification.
Owner:深圳博雅感知药业有限公司
Use of ventricular enlargement rate in intravenous immunoglobulin treatment of Alzheimer's disease
The present invention relates to the use of MRI monitoring of ventricular enlargement rate as an objective measure for the purpose of assessing disease progression in patients suffering from Alzheimer's disease and for the purpose of determining therapeutic effectiveness of a treatment regimen for Alzheimer's patients. Methods for treating Alzheimer's Disease and monitoring therapeutic effectiveness are provided.
Owner:BAXALTA GMBH
Anti-HLA class-IB antibodies mimic immunoreactivity and immunomodulatory functions of intravenous immunoglobulin (IVIG) useful as therapeutic IVIG mimetics and methods of their use
ActiveUS10800847B2Easy to understandMinimize side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAntiendomysial antibodies
Owner:RAVINDRANATH MEPUR DR
Treatment of diabetes mellitus
The present invention provides a method of treating insulin-dependent diabetes mellitus in a subject, comprising administering to the subject a therapeutically effective amount of a Janus kinase inhibitor, or a pharmaceutically acceptable salt or ester thereof, or a therapeutically effective amount of intravenous immunoglobulin, or a therapeutically effective amount of a therapeutic agent that destroys B lymphocytes, or a combination thereof. The present invention also provides kits containing the same.
Owner:LEVIT EYAL
Use of cytokine levels in intravenous immunoglobulin treatment of alzheimer's disease
The present invention relates to the use of the level of certain cytokines in a patient's blood as an objective measure for the purpose of assessing disease progression in patients suffering from Alzheimer's disease and for the purpose of determining therapeutic effectiveness of a treatment regimen. Methods for treating Alzheimer's disease and monitoring therapeutic effectiveness are provided.
Owner:BAXTER INT INC +1
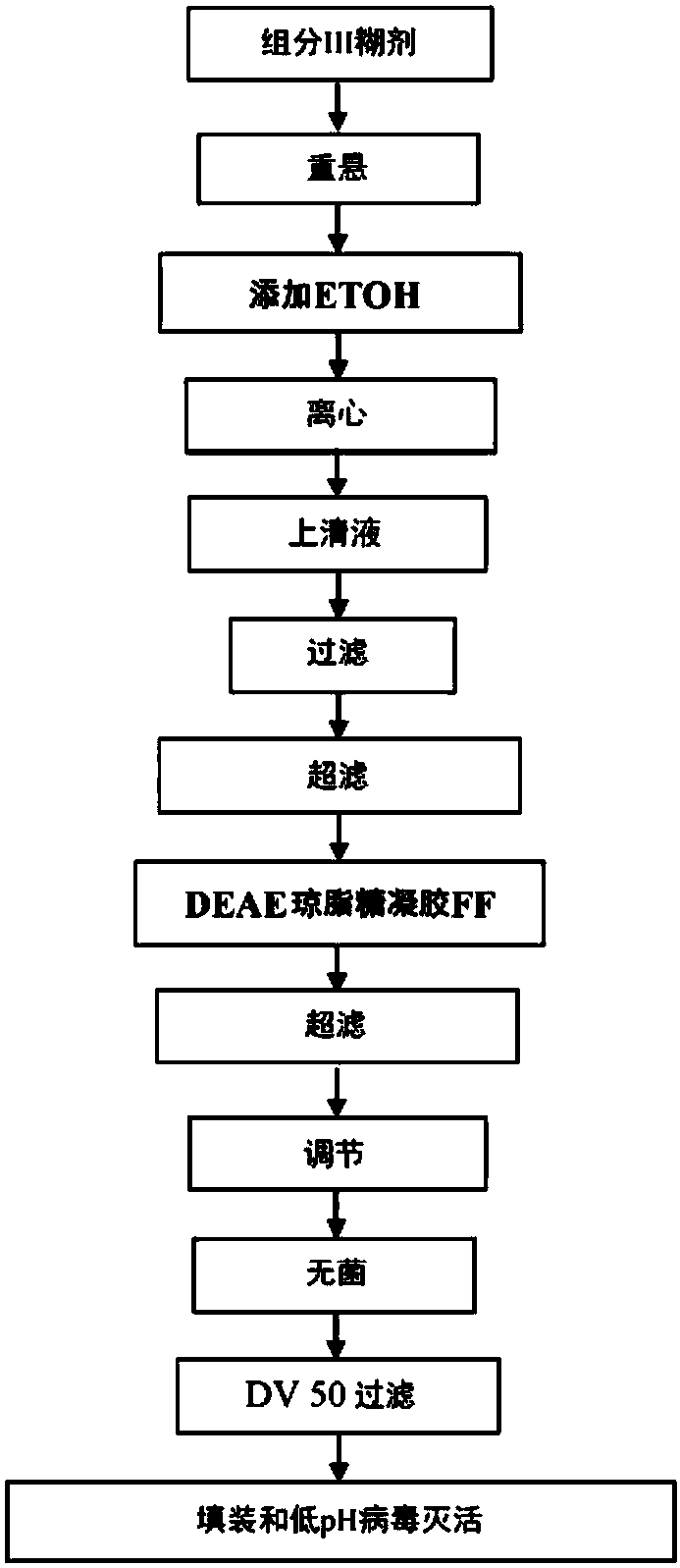
A method of manufacturing intravenous immunoglobulin from fraction iii
InactiveCN107921079APeptide/protein ingredientsImmunoglobulinsAnion-exchange chromatographyBlood plasma
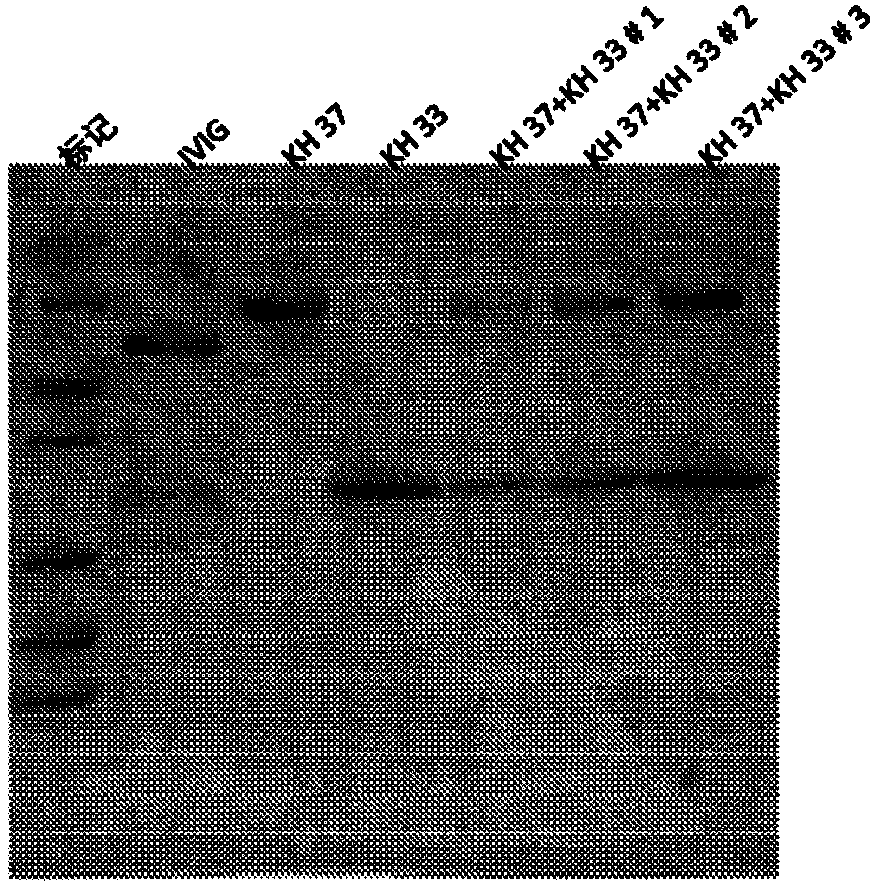
The present subject matter is directed to a method of manufacturing purified IVIG from Fraction III of plasma, comprising re-constituting a Fraction III paste in a buffer; adjusting the pH and temperature; adding ethanol and then gradually lowering the temperature; centrifuging and filtering the supernatant; ultra-filtrating to remove alcohol; undergoing weak anion exchange chromatography; ultra-filtrating to reach a desired protein concentration; aseptic filtrating; nano filtrating for virus removal; and incubating at low pH for virus inactivation to obtain a resulting Fraction III suspensioncomprising purified IVIG. The present subject matter is directed to IVIG having 14 newly-found proteins, namely KH 26, KH 27, KH 28, KH 29, KH 30, KH 31, KH 32, KH 33, KH 39, KH 40, KH 41, KH 42, KH43, and KH 44 for both liquid and lyophilized form.
Owner:K 黄
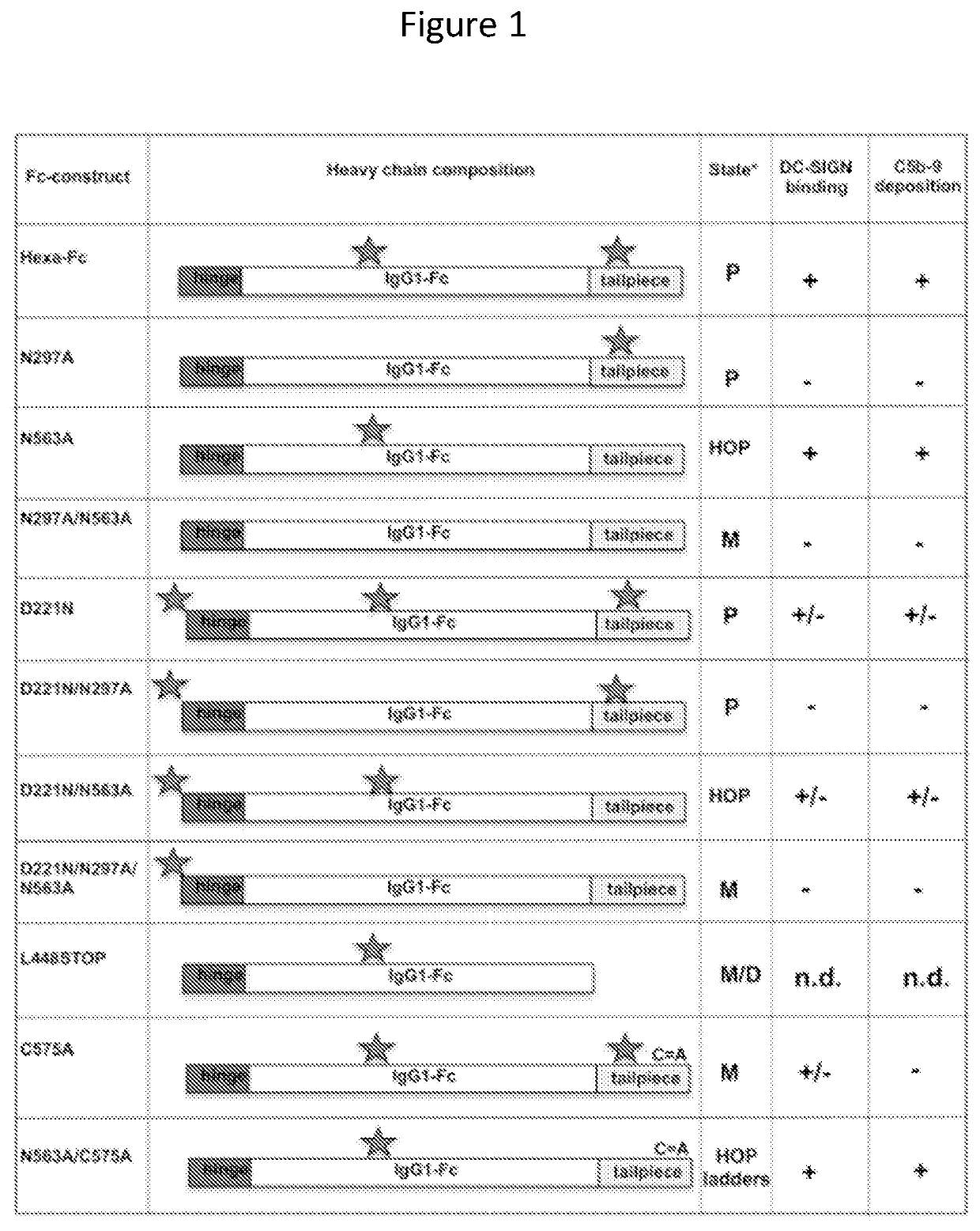
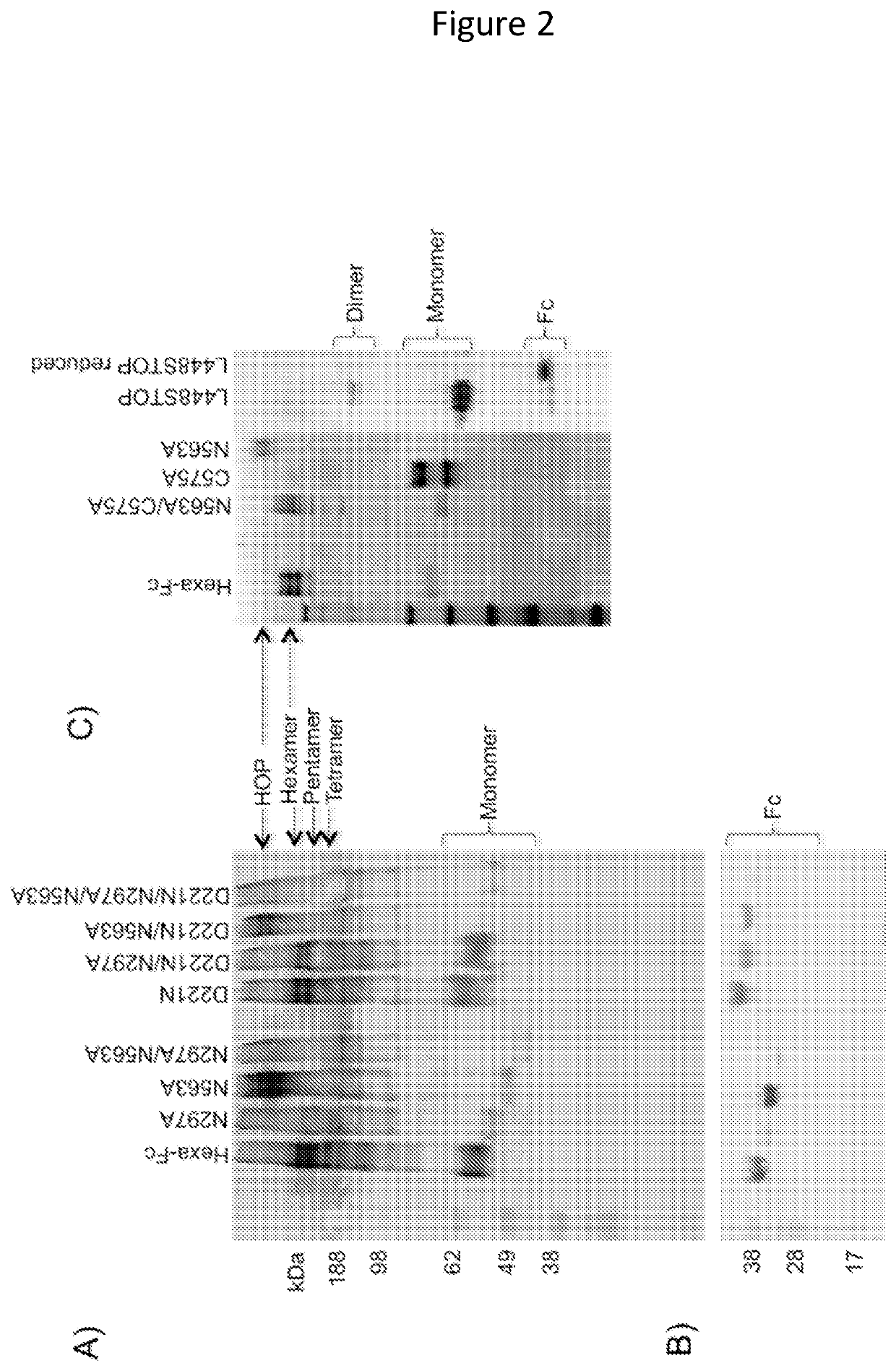
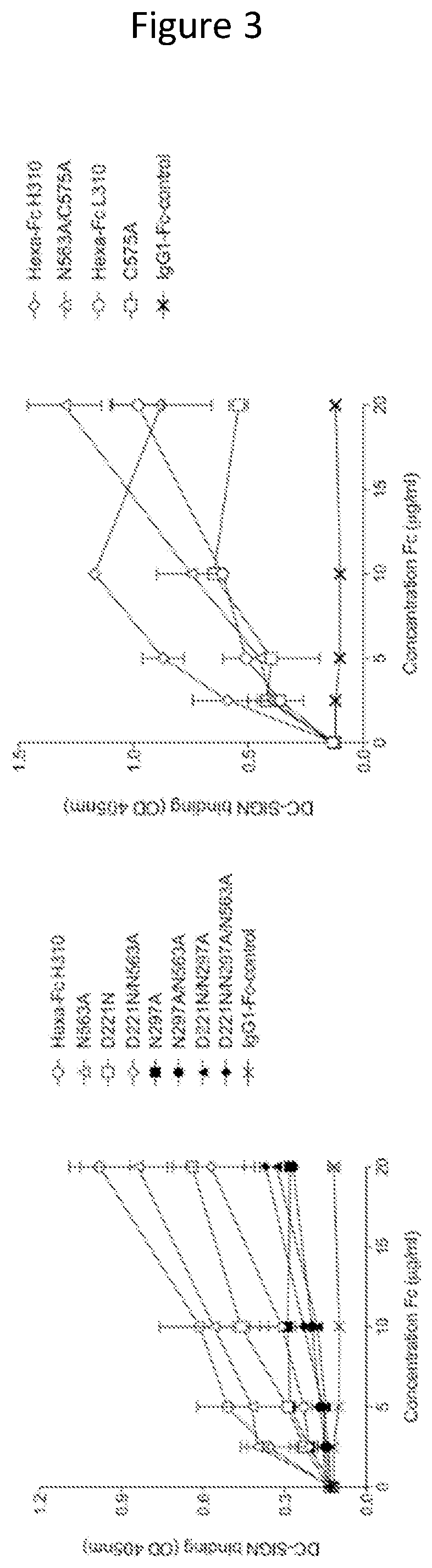
Monomeric proteins and uses thereof
ActiveUS11090382B2Improve efficiencyUseful in therapyHybrid immunoglobulinsAntibody mimetics/scaffoldsDiseaseAmino acid
Provided are proteins comprising two chimeric polypeptide chains; wherein each chimeric polypeptide chain comprises an Fc receptor binding portion comprising two immunoglobulin G heavy chain constant regions; and an immunoglobulin tailpiece region. The amino acid sequence and glycosylation of the tailpiece region of the proteins is adapted, as compared to the sequence and glycosylation of wild-type immunoglobulin, to inhibit polymerisation of the protein. The adaptation of the amino acid sequence may be the loss of a cysteine residue, for example the cysteine residue corresponding to residue 248 of SEQ ID NO: 1. The proteins may be used in intravenous immunoglobulin (IVIG) or subcutaneous immunoglobulin (SCIG) therapy. They may be used in the prevention or treatment of a disease mediated through binding of sialic acid-dependent receptors. Proteins of the invention may be used in the prevention and / or treatment of autoimmune or inflammatory diseases. The proteins may be conjugated to an immune modulator, and in such cases are suitable for vaccine use.
Owner:UNIV OF LIVERPOOL +1
A kind of production technology of intravenous immunoglobulin
ActiveCN108101981BImmunoglobulins against blood group antigensSerum immunoglobulinsOctanoic AcidsFiltration
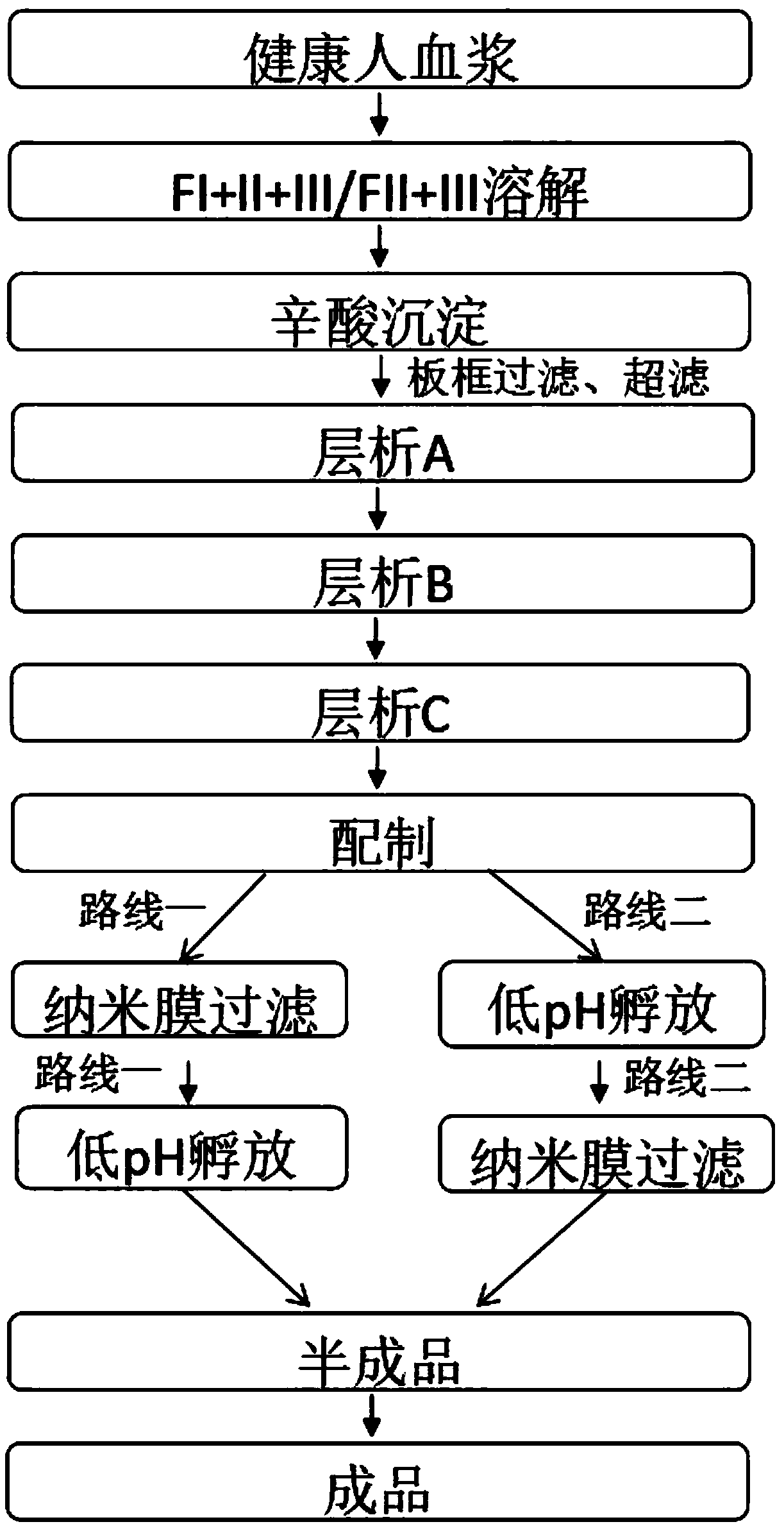
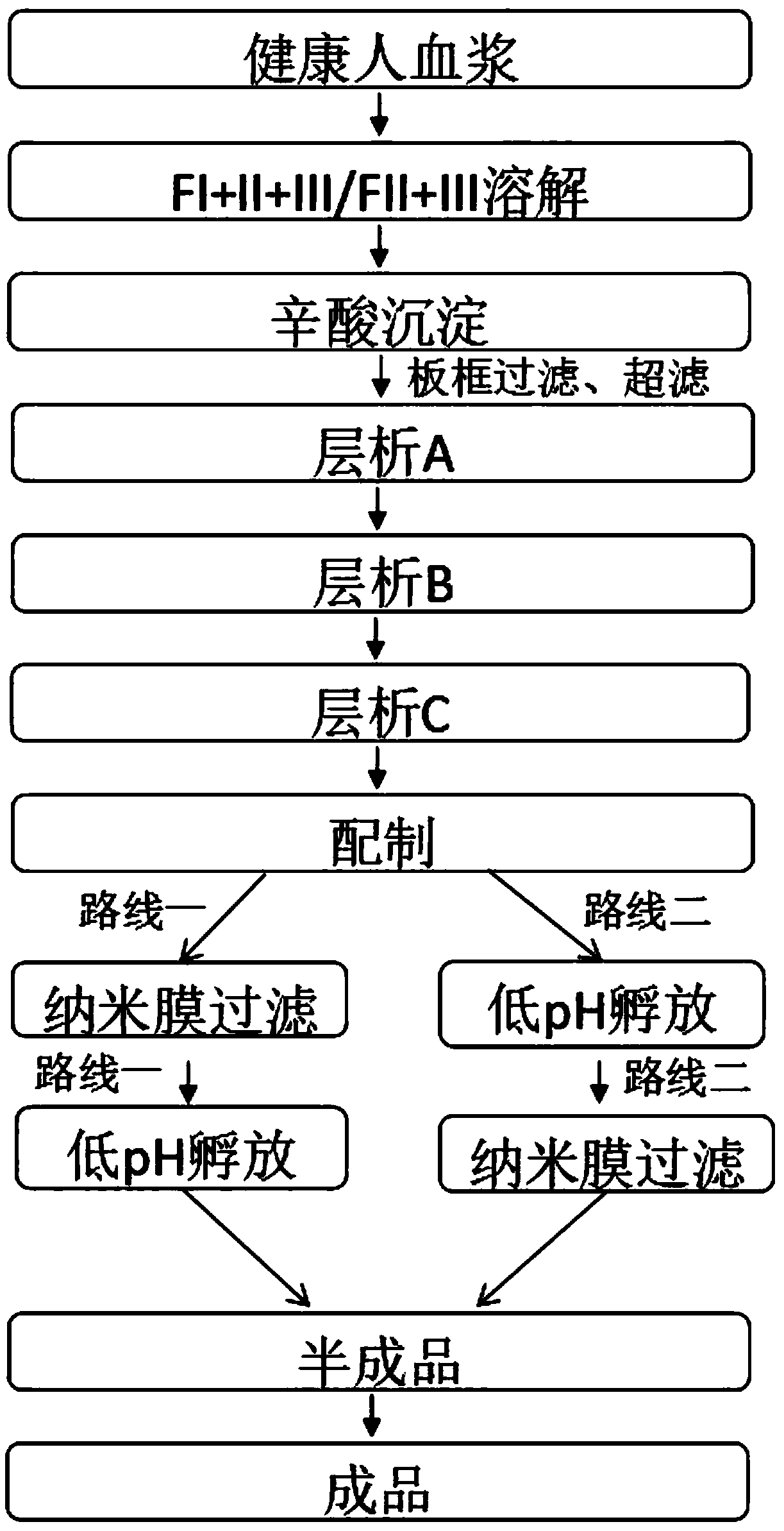
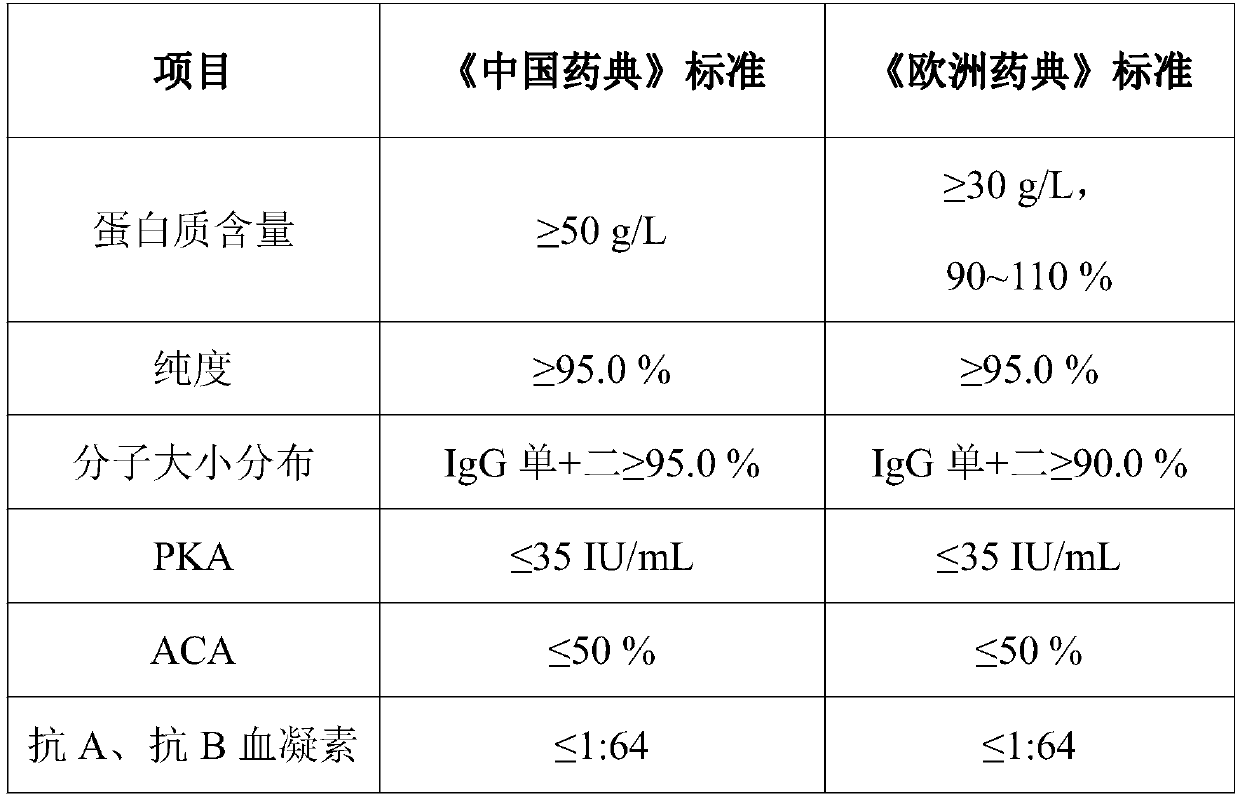
The invention provides a production process for intravenous immunoglobulin, which comprises separating the components I+II+III / II+III from healthy human plasma; Through the following chromatography: anion chromatography A, anion chromatography B, affinity chromatography C; after chromatography, the washing solution is prepared by ultrafiltration, concentration or dilution, and then filtered through a nano-membrane, incubated at a low pH value, and incubated After release, the solution is mixed, prepared and sterilized to obtain immunoglobulin for intravenous injection. The production process provided by the present invention can obtain IgG products with high yield and purity, and more importantly, the anti-A, anti-B indexes and ACA indexes of the obtained products are all greatly reduced, which meets the requirements of "Chinese Pharmacopoeia" well , and the osmolarity of the sample also meets the requirements very well.
Owner:SICHUAN YUANDASHUYANG PHARM CO LTD
Protein protective agent for Pasteur inactivating human intravenous immunoglobulin and inactivation method of protein protective agent
ActiveCN103550780BProtect proteinReduce aggregationAntibody ingredientsPharmaceutical non-active ingredientsWater bathsGlycine
The invention provides a protein protective agent for Pasteur inactivating human intravenous immunoglobulin and an inactivation method of the protein protective agent. A Pasteur inactivation method of human intravenous immunoglobulin comprises the following steps of adding the protein protective agent to a human intravenous immunoglobulin solution before inactivating; adjusting the content of sampled protein to be 40 to 60g / L, and adjusting pH (Potential of Hydrogen) to be 4.7 to 5.3; agitating for 30 minutes; transferring into a water bath under 60+ / - 1 DEG C; maintaining the solution at temperature of 60+ / -0.5 DEG C for 10 hours after the solution temperature reaches 60 DEG C, thus accomplishing the inactivation of human intravenous immunoglobulin, wherein the protein protective agent is saccharose, arginine, glycine and sorbitol which are respective 50 to 70g / L, 20 to 40g / L, 30 to 60g / L and 240 to 260g / L in final content. According to the protein protective agent, simple sorbitol is used as the protective agent, the content of a polymer is decreased from 4.12% to 1.96%, thus the protein can be protected well, and the product quality can be improved.
Owner:BANGHE PHARMA CO LTD
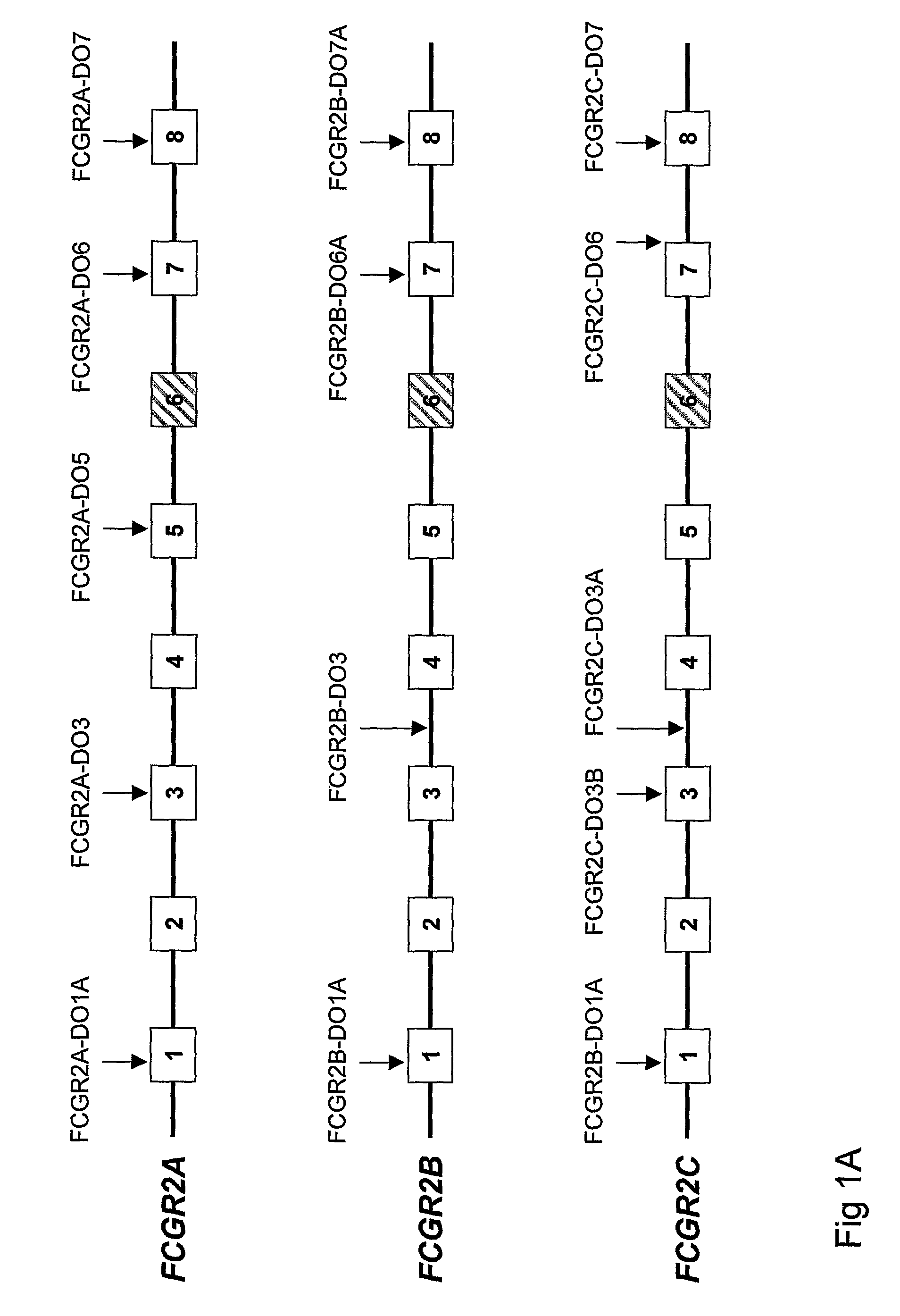
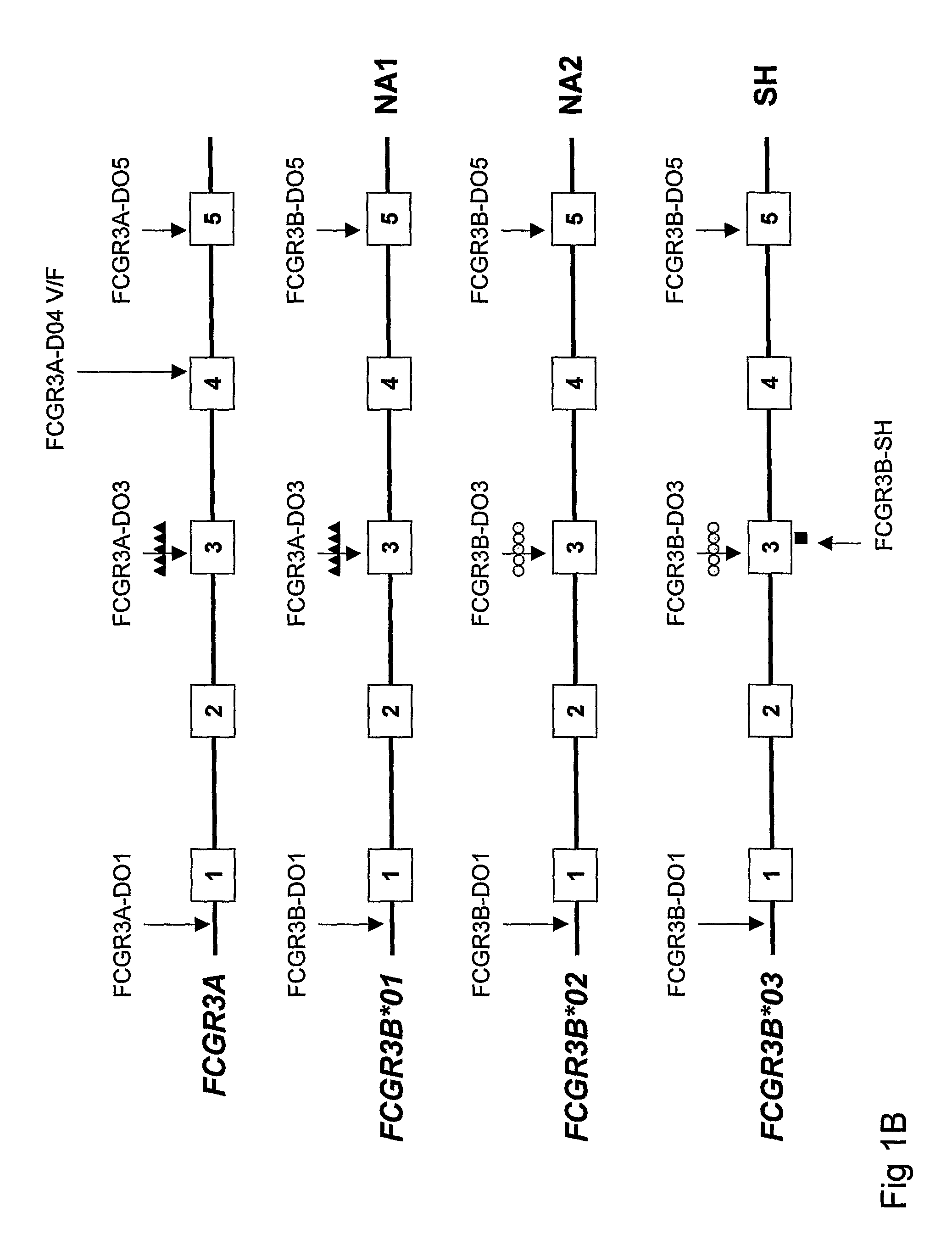
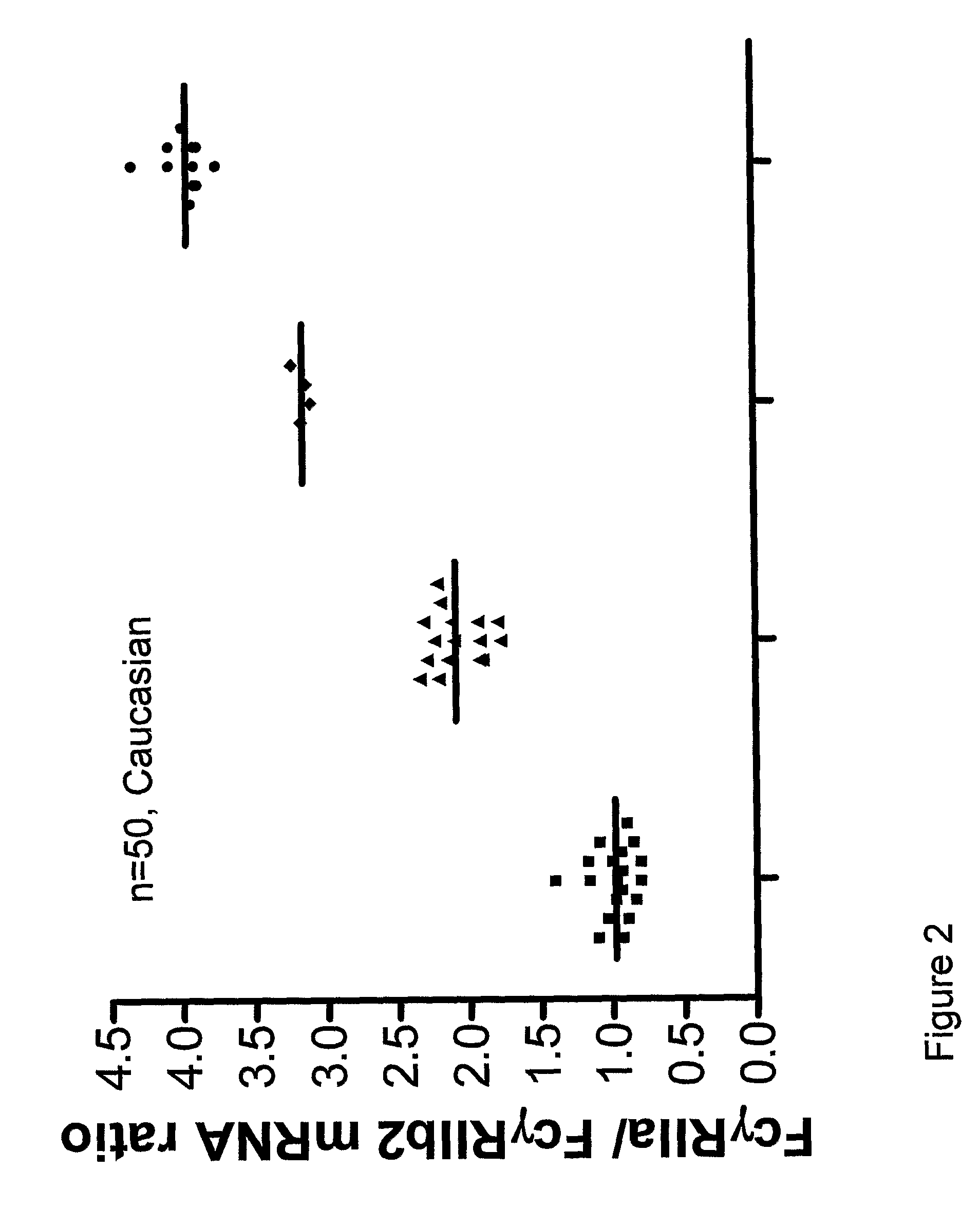
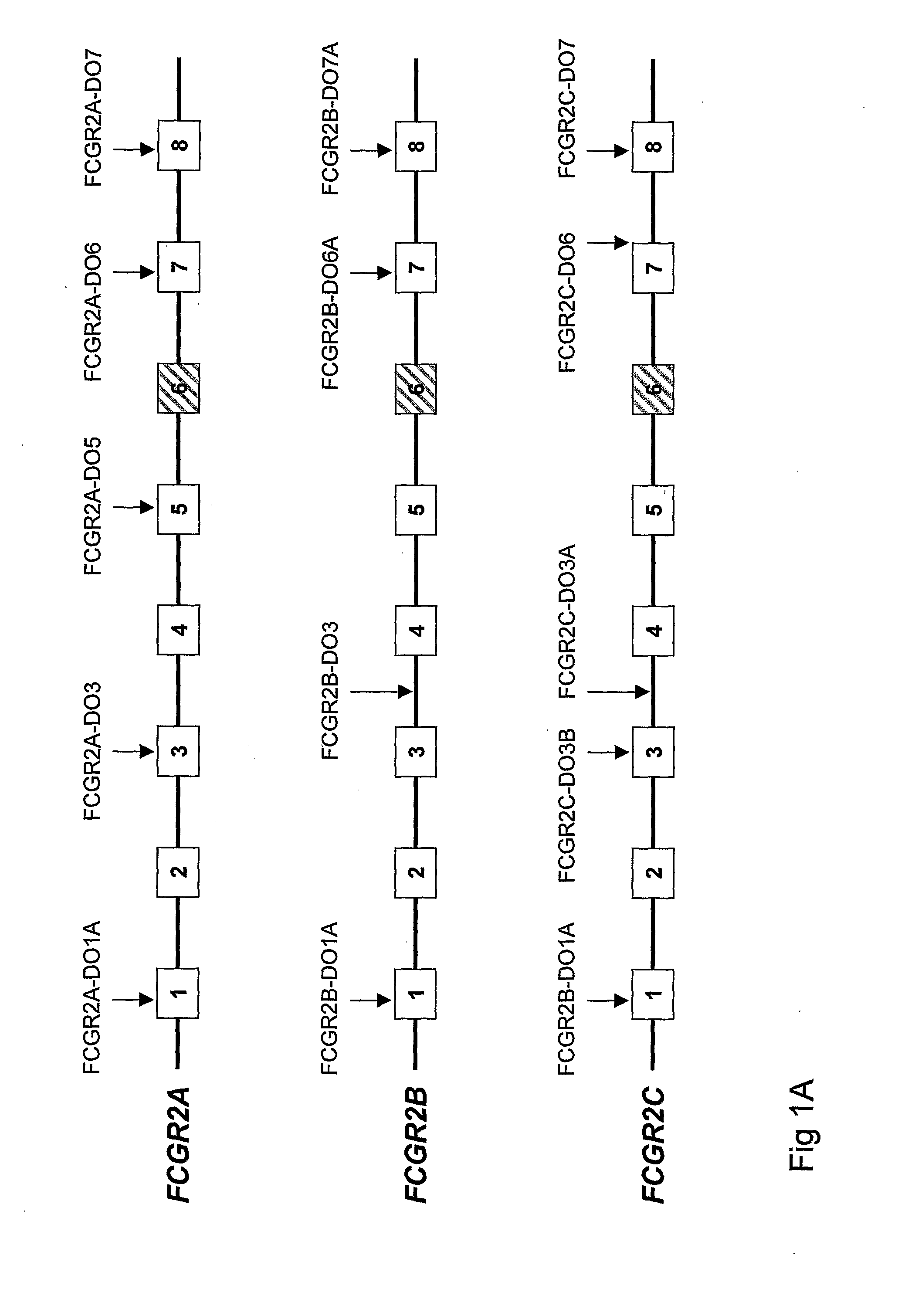
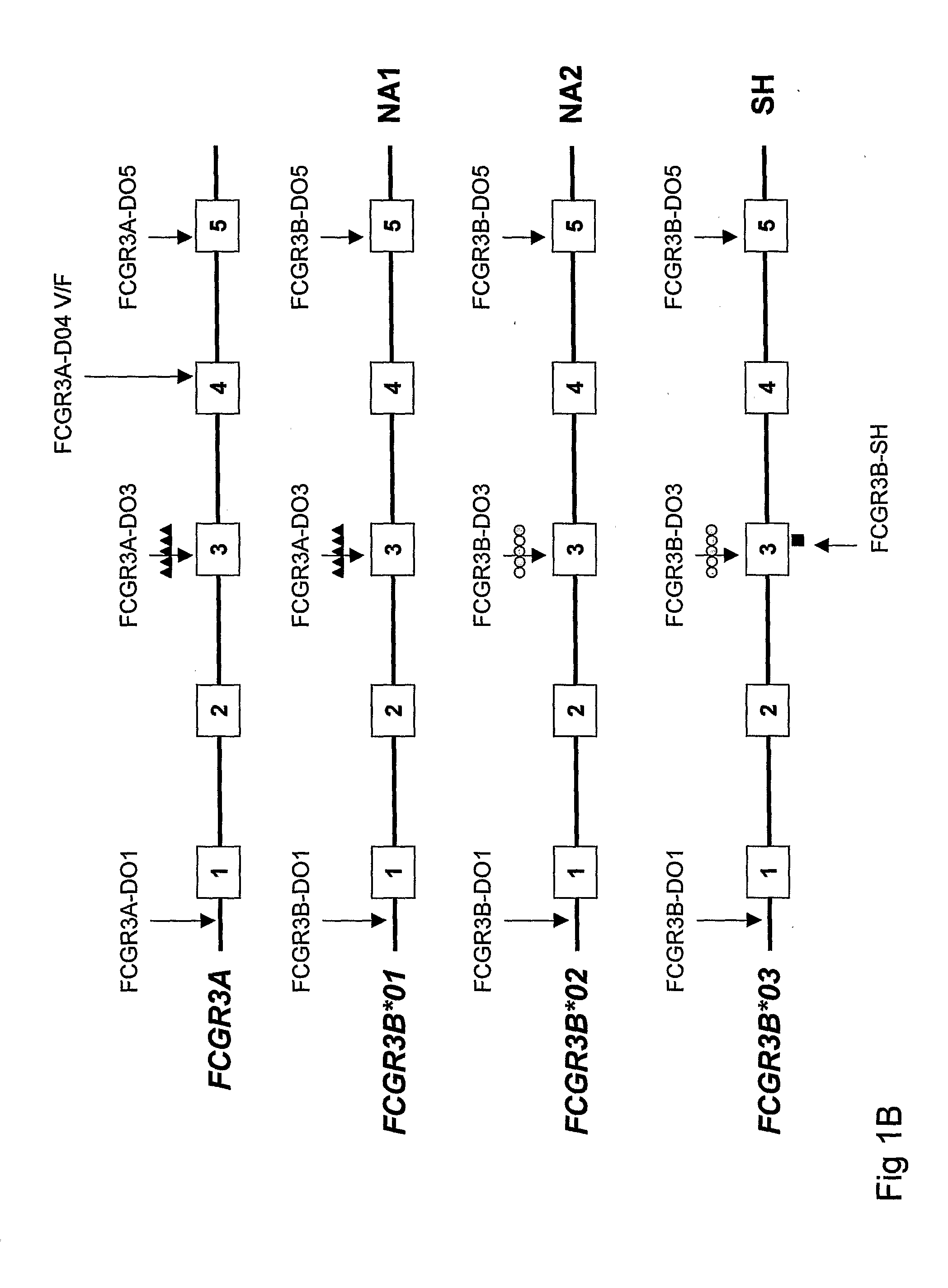
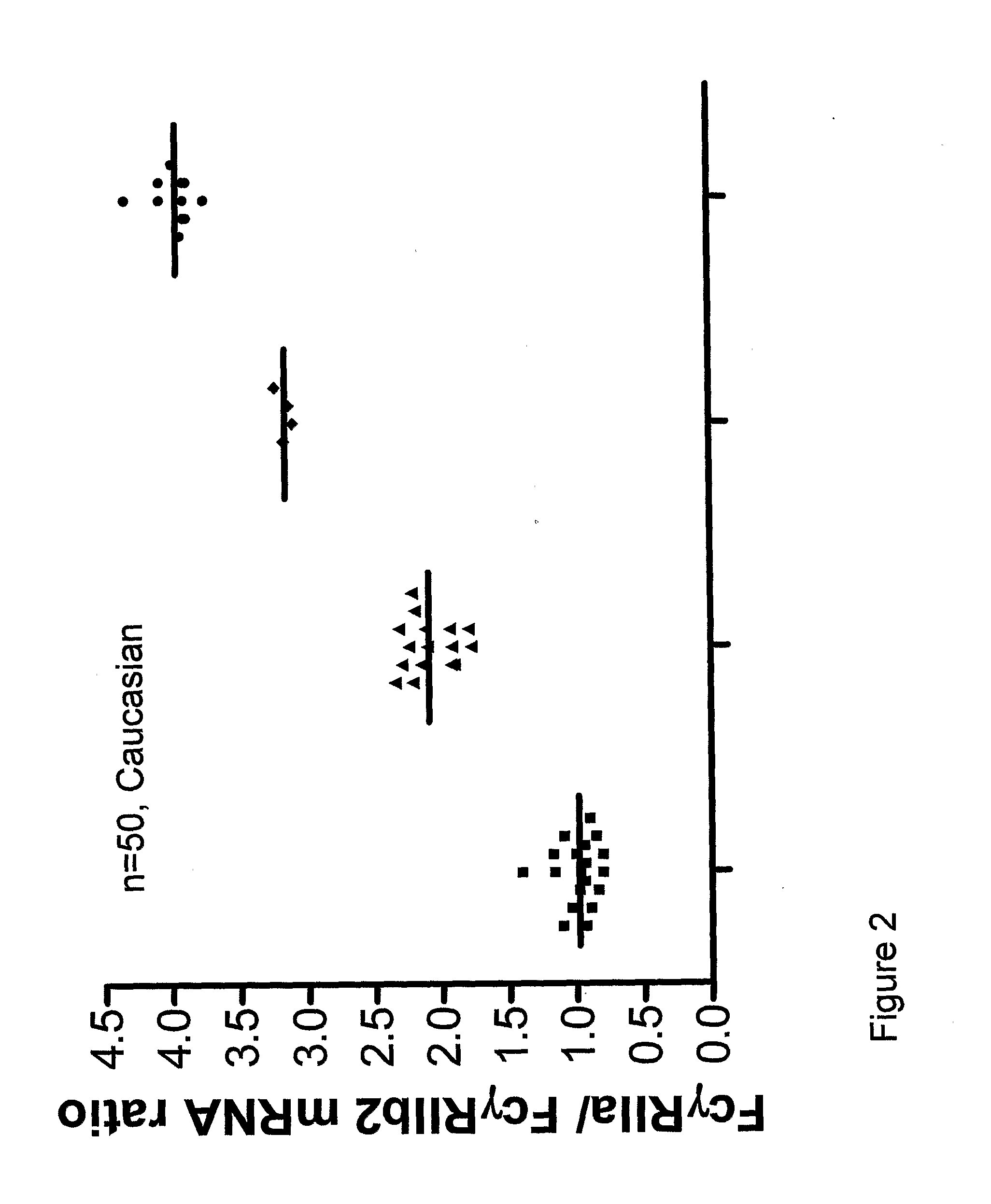
Diagnostic methods involving determining gene copy numbers and SNPs in the FcyRII/FcyRIII gene cluster, and probes for use in such methods to detect susceptibility to and treatment efficacy in autoimmune diseases
InactiveUS7994300B2Strong specificitySolution value is not highSugar derivativesMicrobiological testing/measurementAutoimmune conditionAutoimmune disease
The invention relates to diagnostic methods to predict whether a subject is predisposed for acquiring a disease or to predict the therapy responsiveness of an individual patient. Provided is a method for determining whether a subject is predisposed for developing an autoimmune disease, comprising determining in a sample isolated from said subject the amount of intact genes, or gene products thereof, of the FcγRII / FcγRIII gene cluster, said gene cluster comprising the FCGR2C, FCGR3A, FCGR2A and FCGR3B genes encoding an activating FcγR, and FCGR2B encoding an inhibitory Fcγ R; and correlating said amount to the amount observed in a healthy population. Also provided is a method to predict the responsiveness of a subject to therapy with intravenous immunoglobulin (IVIg) therapy or a monospecific biological, such as a humanized or human monoclonal antibody or a chimeric molecule, comprising the C-terminal Fc-tail of IgG.
Owner:STICHTING SANQUIN BLOEDVOORZIENING
DIAGNOSTIC METHODS INVOLVING DETERMINING GENE COPY NUMBERS AND SNPs IN THE FcyRll/FcyRlll GENE CLUSTER, AND PROBES FOR USE IN SUCH METHODS TO DETECT SUSCEPTIBILITY TO AND TREATMENT EFFICACY IN AUTOIMMUNE DISEASES
InactiveUS20110189672A1Strong specificitySolution value is not highSugar derivativesMicrobiological testing/measurementAutoimmune conditionTreatment effect
The invention relates to diagnostic methods to predict whether a subject is predisposed for acquiring a disease or to predict the therapy responsiveness of an individual patient. Provided is a method for determining whether a subject is predisposed for developing an autoimmune disease, comprising determining in a sample isolated from said subject the amount of intact genes, or gene products thereof, of the FcγRII / FcγRIII gene cluster, said gene cluster comprising the FCGR2C, FCGR3A, FCGR2A and FCGR3B genes encoding an activating FcγR, and FCGR2B encoding an inhibitory FcγR; and correlating said amount to the amount observed in a healthy population. Also provided is a method to predict the responsiveness of a subject to therapy with intravenous immunoglobulin (IVIg) therapy or a monospecific biological, such as a humanized or human monoclonal antibody or a chimeric molecule, comprising the C-terminal Fc-tail of IgG.
Owner:STICHTING SANQUIN BLOEDVOORZIENING
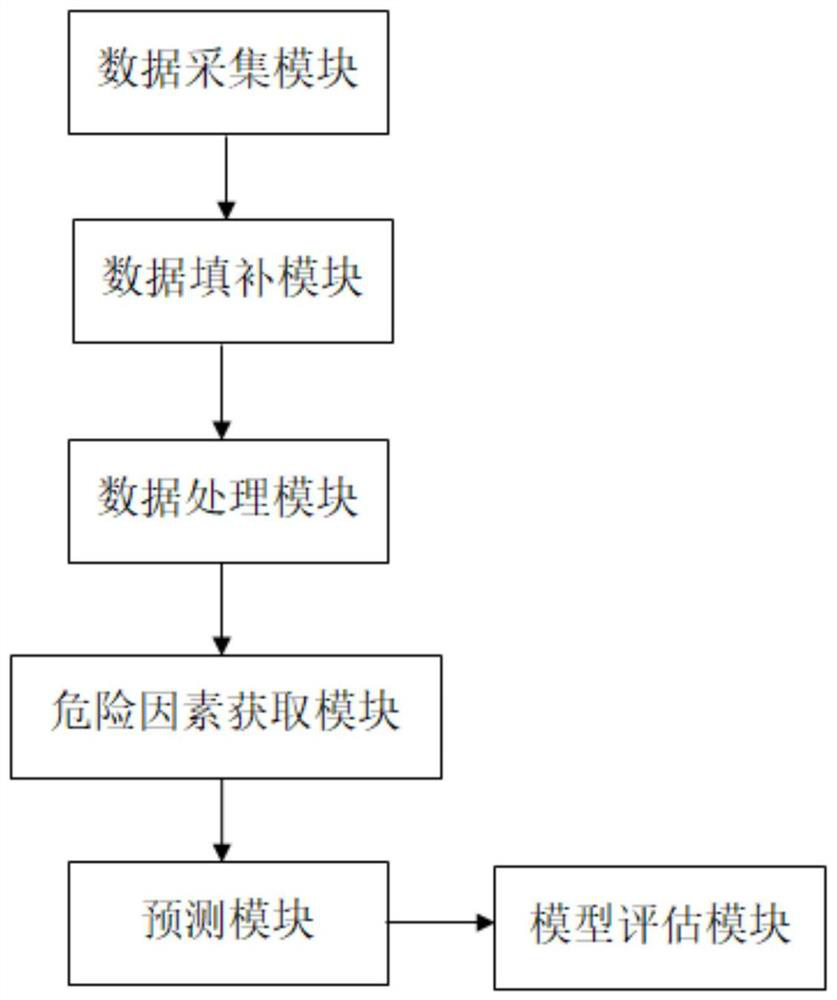
System and device for predicting resistance of child suffering Kawasaki disease after first injection of intravenous immunoglobulin
InactiveCN113380329AAvoid collectingHealth-index calculationBiostatisticsBaseline dataMedical equipment
The invention belongs to the technical field of Kawasaki disease medical equipment, and particularly discloses a system and device for predicting the resistance of child suffering Kawasaki disease after first injection of intravenous immunoglobulin, the prediction system comprises: a data acquisition module which is used for acquiring baseline data, image data and laboratory data of a sample before first IVIG treatment; a data processing module which is used for receiving and processing the data information acquired by the data acquisition module and acquiring variable parameters; a risk factor acquisition module which is used for receiving the variable parameters and screening to obtain IVIG resistance risk factors; and the prediction module which is used for bringing the IVIG resistance risk factors into a machine learning model and establishing a prediction model. According to the technical scheme, through cooperation of all the modules, collection and screening of sample information are achieved, and whether a child patient has first dose IVIG resistance or not is predicted according to the acquired information.
Owner:CHONGQING MEDICAL UNIVERSITY
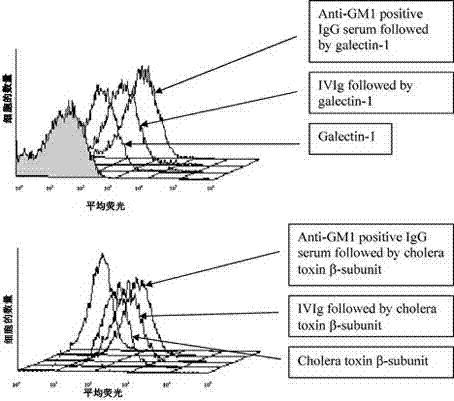
Application of intravenous immunoglobulins (IVIg) in inhibiting cholera toxin and galectin from being combined into ganglioside GM1
InactiveCN102949719ADownregulation of inflammationNervous disorderAntibody ingredientsImmunologic disordersAutoimmune condition
The invention relates to an application of intravenous immunoglobulins (IVIg) in preparing a medicament for treating autoimmune diseases of a peripheral nervous system, the autoimmune diseases are related ganglioside GM1 antibody diseases, and the IVIg is used for treating the related ganglioside GM1 antibody diseases by inhibiting cholera toxin and galectin from being combined into ganglioside GM1. The advantages are as follows: the research finds that the IVIg can not inhibit the combination of the GM1 antibody with the antigen thereof and the GM1 antibody titer is not reduced, the inhibition of the IVIg on the combination of the cholera toxin and galectin into the GM1 can be displayed; and put forward in a first time, galactosyl / non-galactosyl immune globulin with correction rate in the IVIg are mutually acted on a macrophage receptor, so that the inflammation functions of autoimmune disease of macrophage and a peripheral nervous system can be reduced.
Owner:CENT HOSPITAL XUHUI DISTRICT SHANGHAI CITY
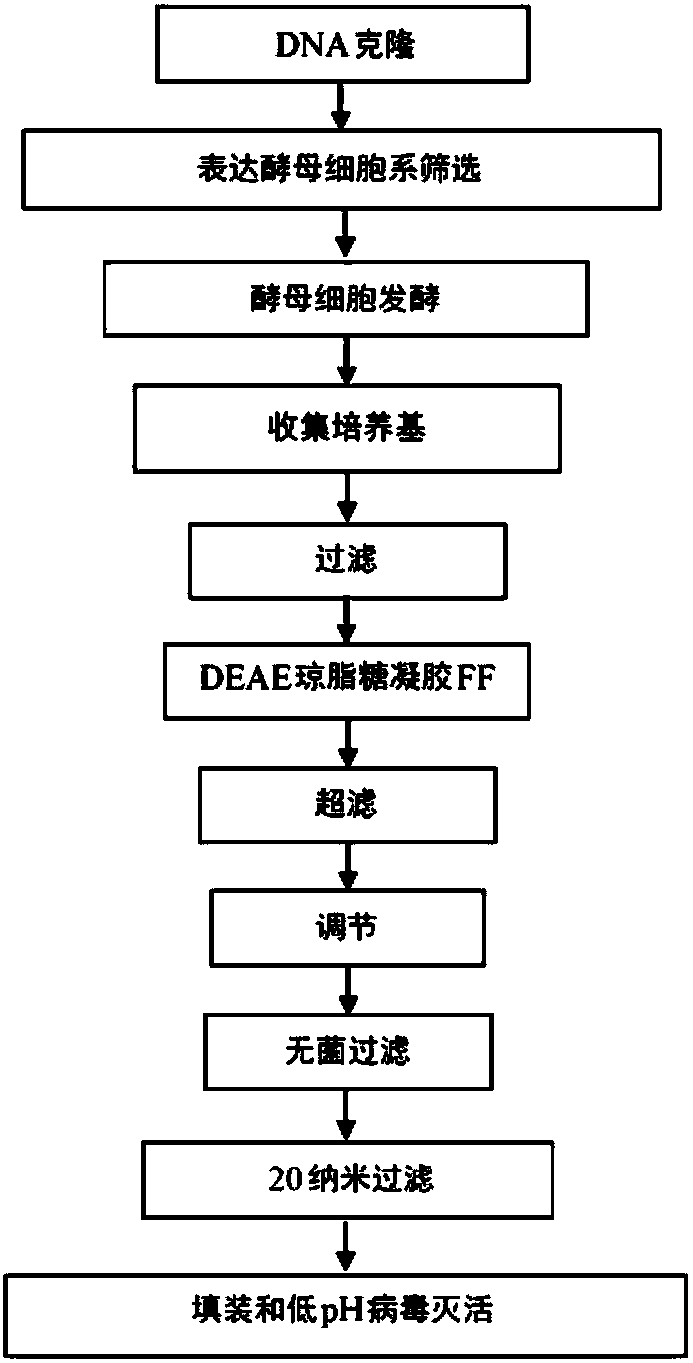
Process of cloning and further purification to make a recombinant intravenous immunoglobulin
The present subject matter is directed to a process of cloning and purifying recombinant intravenous immunoglobulin (IVIG), comprising cloning a target gene of human immunoglobulin; in vitro screeningof a yeast cell expressing the target gene of human immunoglobulin to create a yeast cell line; fermenting the yeast cell line and collecting a resulting culture medium; filtering the culture medium;undergoing weak anion exchange chromatography to collect a flow-through solution; ultra-filtrating the flow-through solution to reach a desired protein concentration; aseptic filtrating the flow-through solution; nano-filtrating the flow-through solution for virus removal; and filling and incubating the flow-through solution at low pH for virus inactivation to obtain a purified recombinant IVIG.The present subject matter is directed to purified recombinant IVIG having five newly-found proteins, namely KH 33, KH 34, KH 35, KH 36, and KH 37 for both liquid and lyophilized forms.
Owner:K 黄
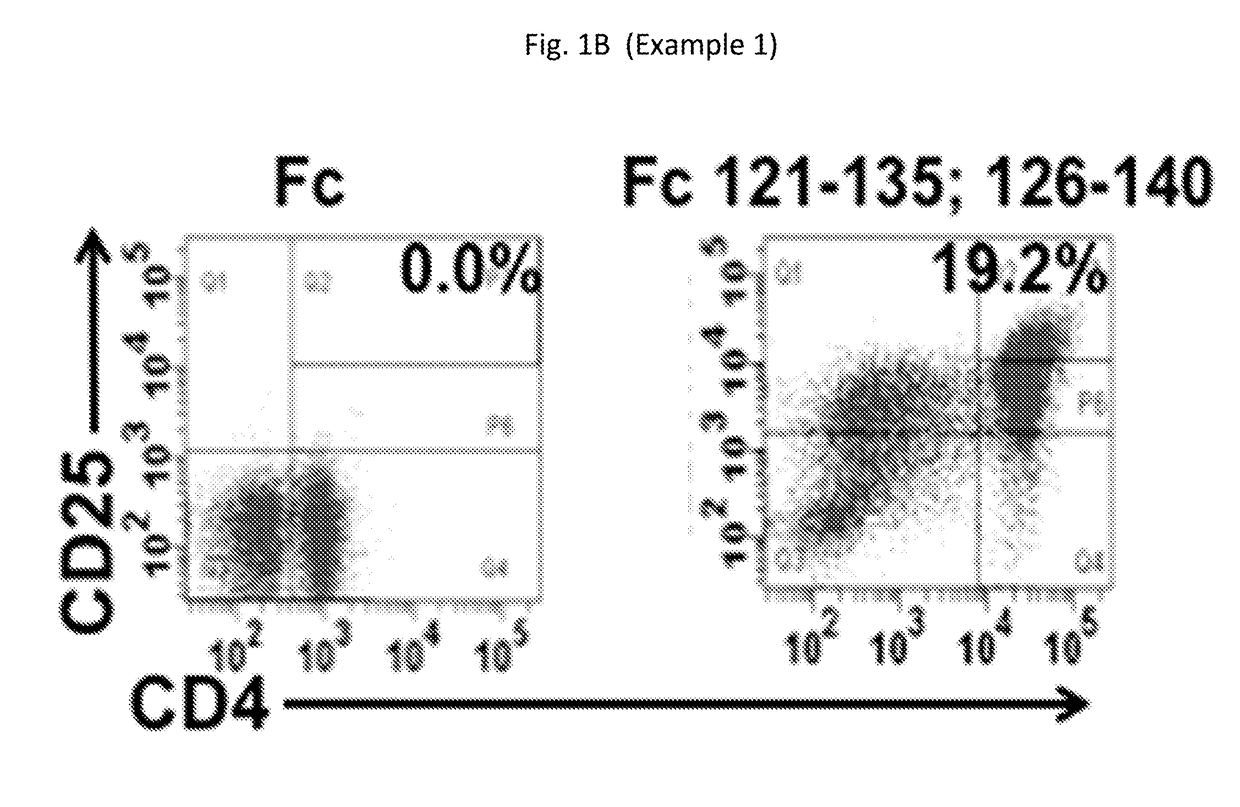
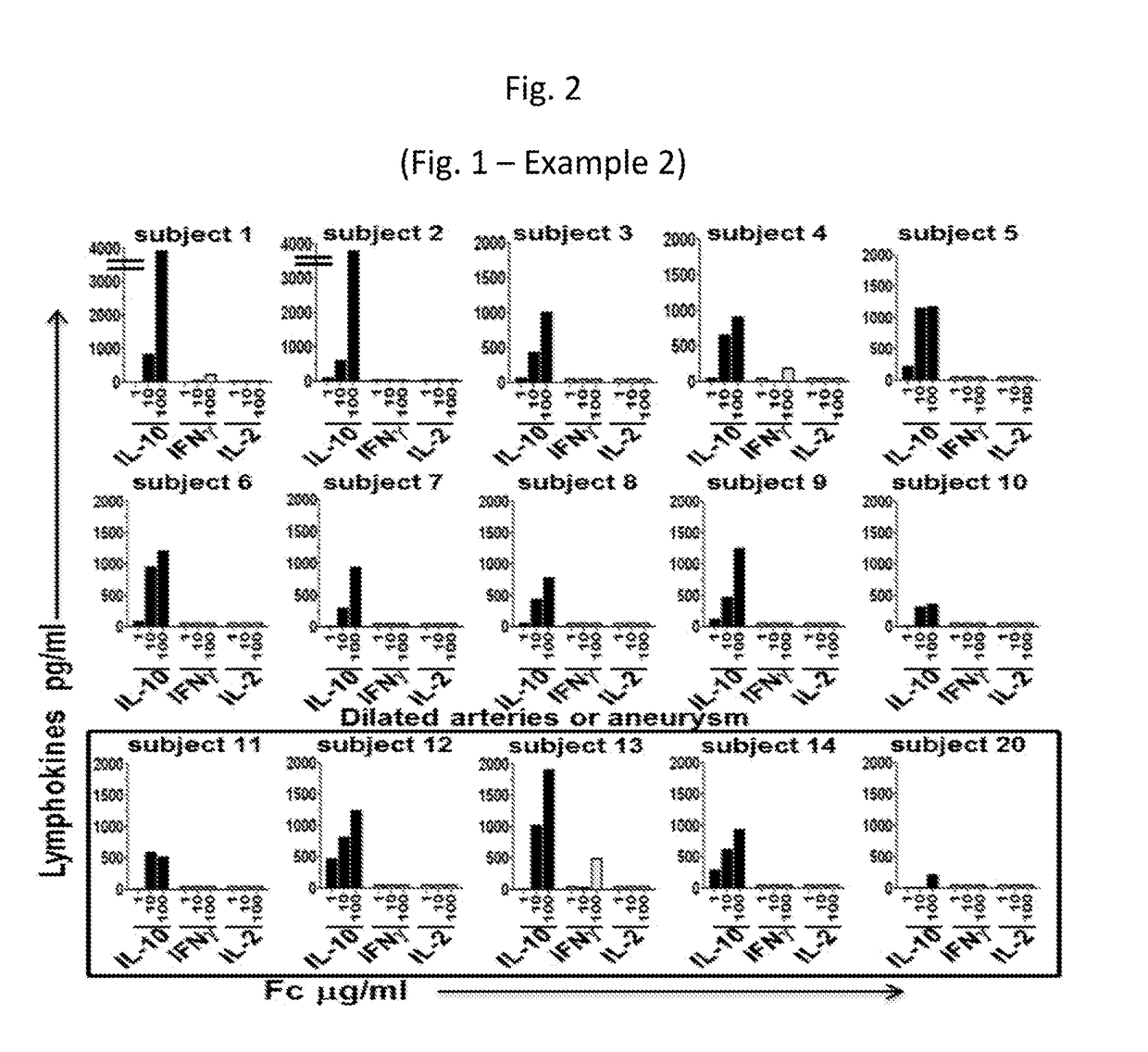
Compositions for expanding regulatory t cells (TREG), and treating autoimmune and inflammatory diseases and conditions
ActiveUS20170260236A1Suppression problemPowder deliveryPeptide/protein ingredientsAutoimmune conditionVascular inflammation
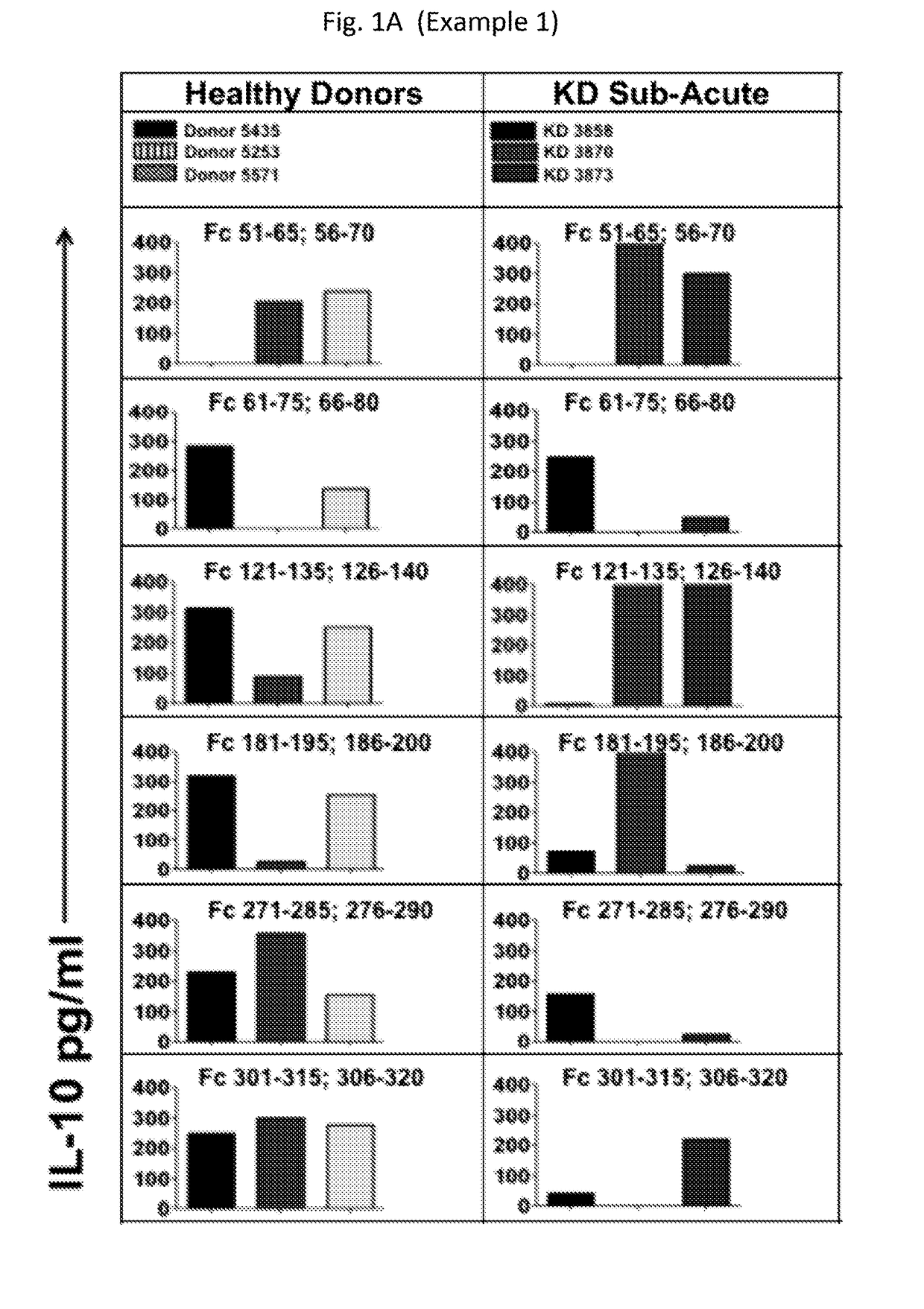
Provided are compositions, including isolated, synthetic or recombinant peptides for: expanding regulatory T cells (Treg) populations; for treating or ameliorating a vascular inflammation, and Kawasaki disease (KD) or a pediatric acute vasculitis of the coronary arteries, including vascular coronary abnormalities, and acute or chronic vascular inflammatory abnormalities, and methods for making and using them. Provided are immunotherapies for promoting expansion of natural, Treg to establish, or re-establish, vascular homeostasis; or, for ameliorating: a disease or condition associated with an autoimmune disease or condition; an immune-mediated vascular disorder; a disease or condition treated with intravenous immunoglobulin (WIG) therapy; a vascular coronary abnormality; an acute or a chronic vasculitis; an autoimmune inflammatory vasculitis; a T cell mediated pediatric vasculitis; Kawasaki disease (KD) or a pediatric acute vasculitis of the coronary arteries; atherosclerosis; preventing miscarriage in autoimmune women; rheumatoid arthritis or Juvenile Idiopathic Arthritis; a neoplastic hematological disorder, or a leukemia.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com