Application of intravenous immunoglobulins (IVIg) in inhibiting cholera toxin and galectin from being combined into ganglioside GM1
An immunoglobulin, ganglioside technology, applied in neurological diseases, drug combinations, antibodies, etc., can solve problems that have not been convincingly proven
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
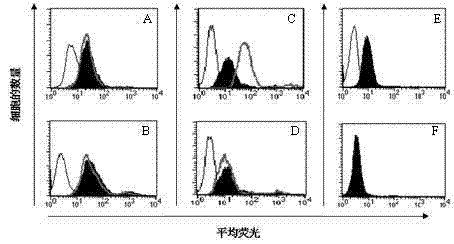
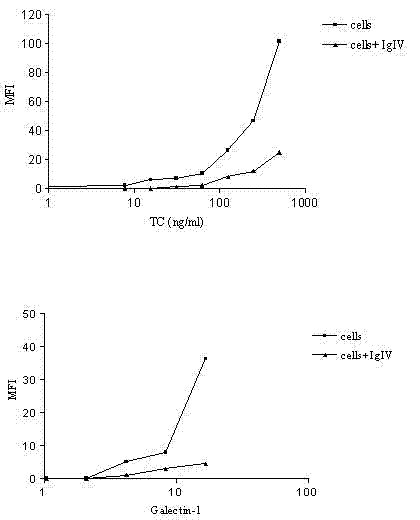
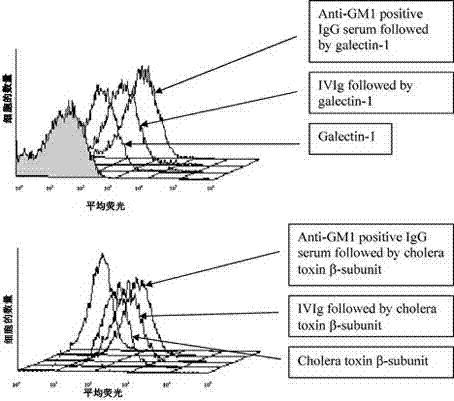
[0017] To explore the mechanism of action of IVIg in anti-GM1 antibody-associated diseases, we tested two hypotheses: (i) anti-idiotypic antibodies are present in different IVIg preparations; (ii) using flow cytometry, IVIg inhibits cholera toxin and semi Laclectin-1 binds to GM1-expressing cells.
[0018] Materials and methods
[0019] 1.1. Reagents and cell lines
[0020] Three different products of human intravenous immunoglobulin (IVIg) (50 mg / ml): Sandoglobuline (Novartis, Rueil Malmaison, France), Tegeline (LFB, Paris, France) and Endoglobuline (Baxter, Maurepas, France) were used. F(ab') 2 IVIg formulations were provided by Srini V. Kaveri (INSERM U430, Paris, France). Monosialylganglioside GM1 purified from bovine brain was purchased from Sigma (St Quentin Fallavier, France) and dissolved in methanol. The high-affinity GM1-specific ligand biotin-based cholera toxin β-subunit (0.5 mg / l) was purchased from Sigma (St Quentin Fallavier, France). Human biotinylated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com