Protein protective agent for Pasteur inactivating human intravenous immunoglobulin and inactivation method of protein protective agent
A human immunoglobulin and protein technology, applied in pharmaceutical formulations, inactive medical preparations, antibodies, etc., can solve problems such as the increase of multimers, and achieve the effect of protecting proteins and improving product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A pasteurized inactivation method for intravenous injection of human immunoglobulin, comprising the following steps:
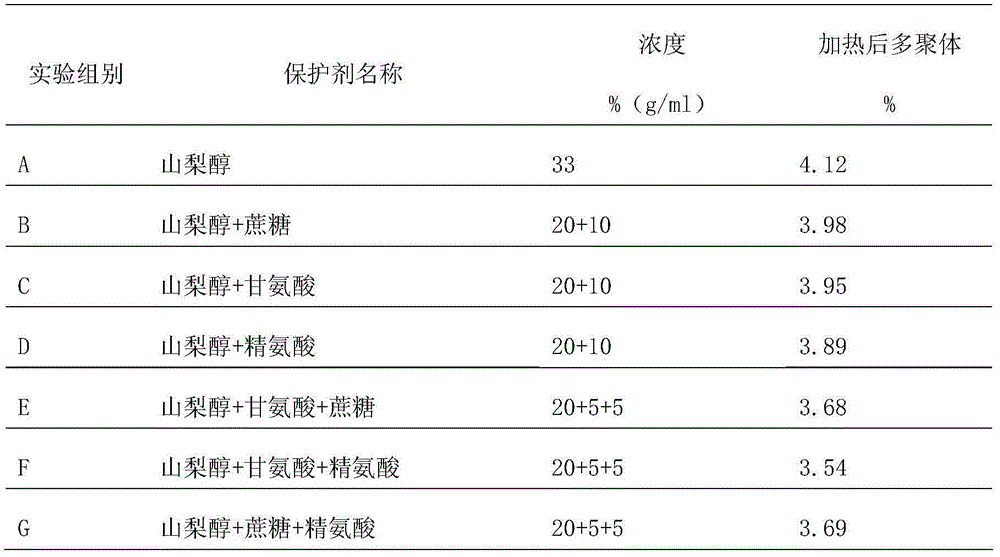
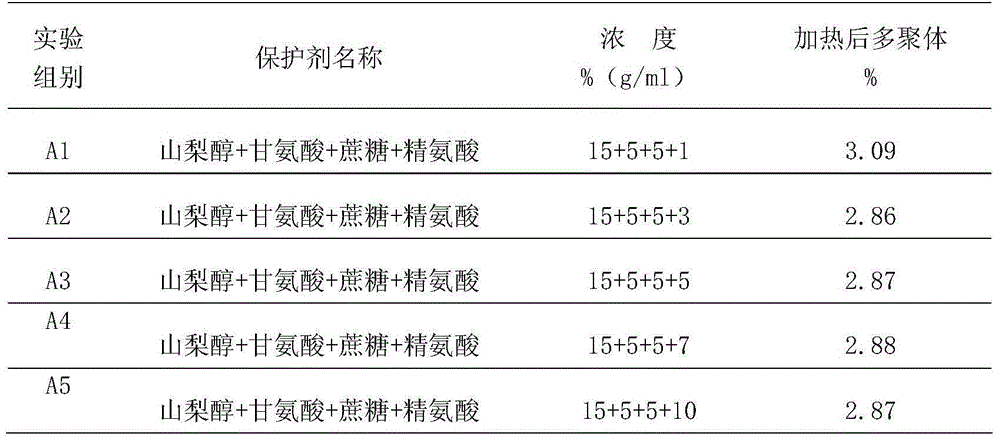
[0046] First, add protein protectant to the intravenous human immunoglobulin solution before inactivation, then adjust the protein content of the sample to 40g / L-60g / L, pH value 4.7-5.3, stir for 30 minutes, and put it in a water bath at 60°C±1°C When the temperature of the solution reaches 60°C, keep the solution temperature at 60°C±0.5°C for 10 hours to complete the inactivation of the intravenous injection of human immunoglobulin. The protein protectant includes 60g / L sucrose and 30g / L arginine L, glycine 40g / L and sorbitol 250g / L. After inactivation, take it out and measure the change of polymers before and after heating. The determination method is determined according to the "Chinese Pharmacopoeia" 2010 edition three appendix VI R "Human immunoglobulin products IgG monomer plus dimer determination method". The measured multimer change content was...
Embodiment 2
[0048] A pasteurized inactivation method for intravenous injection of human immunoglobulin, comprising the following steps:
[0049] First, add protein protectant to the intravenous human immunoglobulin solution before inactivation, then adjust the protein content of the sample to 40g / L-60g / L, pH value 4.7-5.3, stir for 30 minutes, and put it in a water bath at 60°C±1°C In the process, when the temperature of the solution reaches 60°C, keep the solution temperature at 60°C±0.5°C for 10 hours to complete the inactivation of the intravenous injection of human immunoglobulin. The protein protectant includes 70g / L sucrose, 20g / L arginine L, glycine 30g / L and sorbitol 260g / L. After inactivation, take it out and measure the change of polymers before and after heating. The determination method is determined according to the "Chinese Pharmacopoeia" 2010 Edition Three Appendix VI R "Human Immunoglobulin Products IgG Monomer Plus Dimer Determination Method"; The multimer change content...
Embodiment 3
[0051] A pasteurized inactivation method for intravenous injection of human immunoglobulin, comprising the following steps:
[0052]First, add protein protectant to the intravenous human immunoglobulin solution before inactivation, then adjust the protein content of the sample to 40g / L-60g / L, pH value 4.7-5.3, stir for 30 minutes, and put it in a water bath at 60°C±1°C In the process, when the temperature of the solution reaches 60°C, keep the solution temperature at 60°C±0.5°C for 10 hours to complete the inactivation of the intravenous injection of human immunoglobulin. L, glycine 20g / L 1 and sorbitol 240g / L. After inactivation, take it out and measure the change of polymers before and after heating. The determination method is determined according to the "Chinese Pharmacopoeia" 2010 Edition Three Appendix VI R "Human Immunoglobulin Products IgG Monomer Plus Dimer Determination Method"; The multimer change content was 1.98%, and the multimer change content before inactivati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com