Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
492 results about "Drugs production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The term manufacturing encompasses a broad range of activities related to the production of drugs. While producing illegal substances in an in-house lab is clearly drug manufacturing, the crime also targets those who sell necessary precursor chemicals, specialized drug production equipment, or provide other operational support.
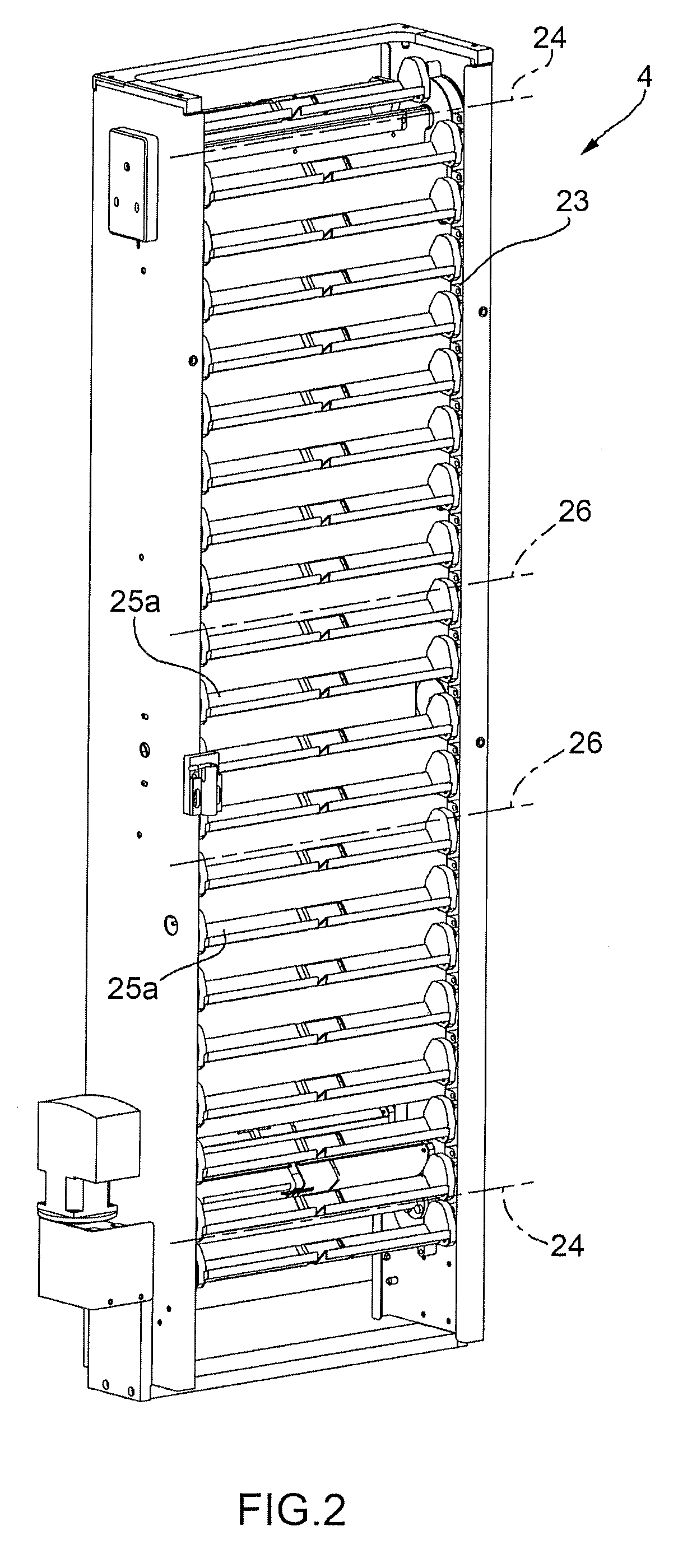
Full-automatic bottling liquor inspection machine
InactiveCN101403706AVolume measurement apparatus/methodsOptically investigating flaws/contaminationForeign matterImaging processing
The invention relates to a completely automatic bottled liquor testing machine, belonging to the field of medicine quality detection and control. The whole structure of the testing machine consists of a bottle feeding system, a detection system, a bottle outlet sorting system and a transmission system; medicine bottles to be tested are sequentially sent to a medicine clamp in the detection system by the bottle feeding system; the clamp drives the medicine bottles to rotate at high speed and stop suddenly; the medicine bottles stop rotating but the liquor continues rotating at high speed; a machine visible imaging and image processing system is used for obtaining the image and processing of the rotating liquor; and the unqualified medicine bottles are removed by the bottle outlet sorting system. The testing machine can check and sort various visible foreign matters in the bottled liquor of various specifications, the capacity of the liquor and the cracks of the bottle body by replacing the manpower, can be widely applied to bottled liquor checking link of medicine production enterprises and can be used for checking the foreign matters in various nutritional oral liquid, beverage and wine.
Owner:TSINGHUA UNIV
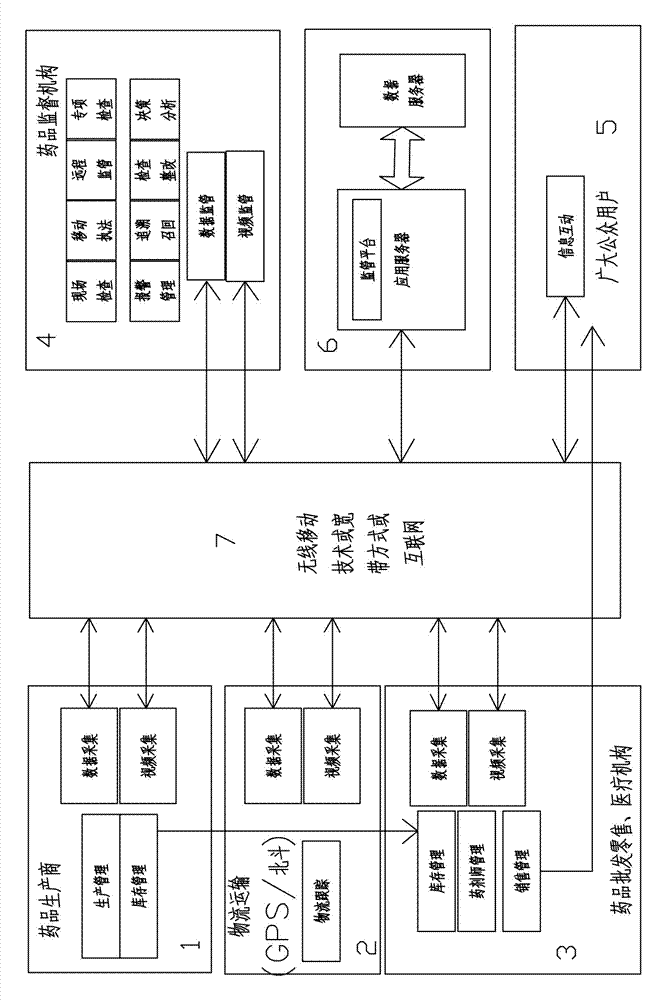
Medical logistic information processing system and method
InactiveCN102136124AAccurate inventoryReduce errorsData processing applicationsCo-operative working arrangementsInformation processingLogistics management
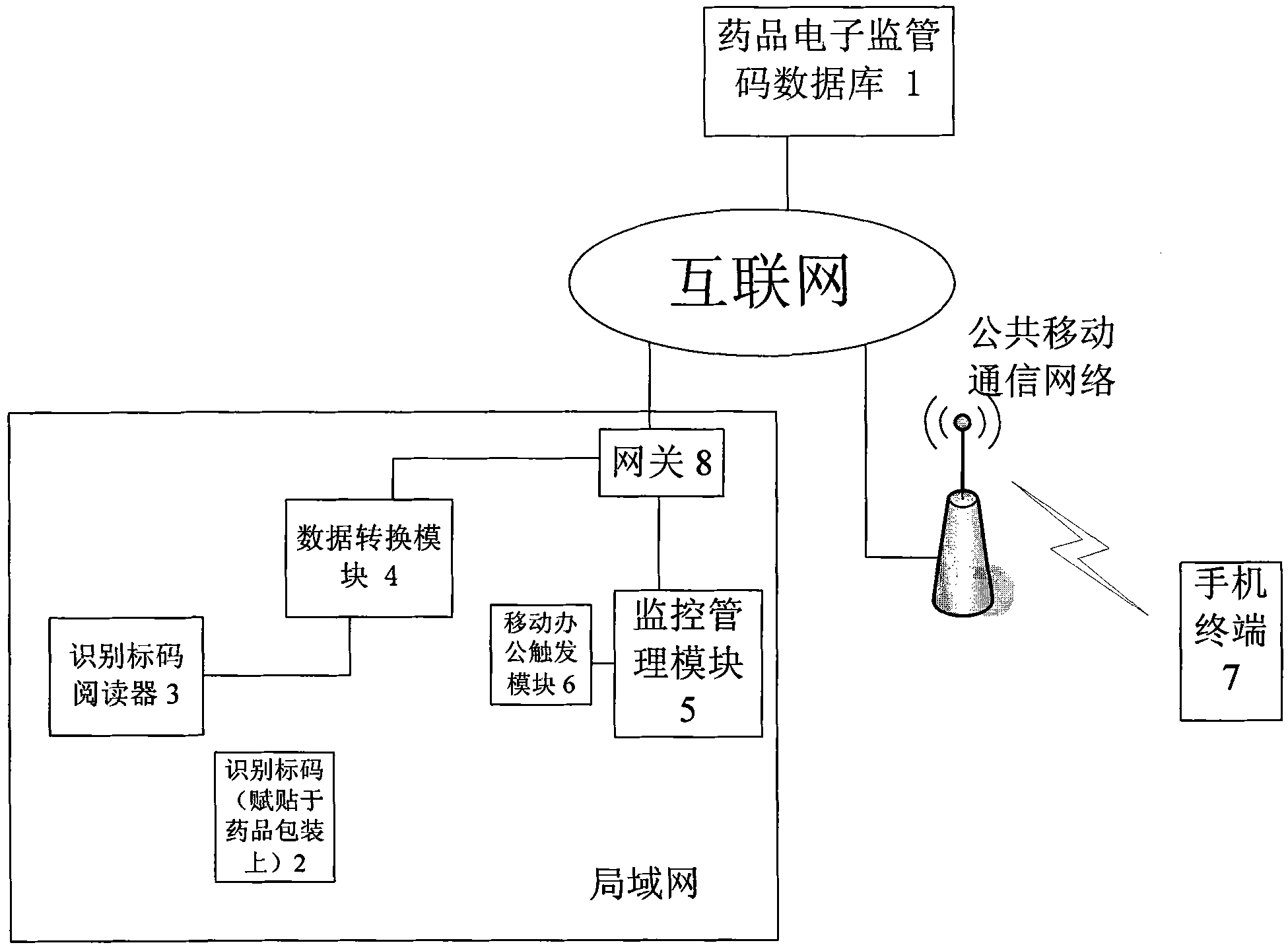
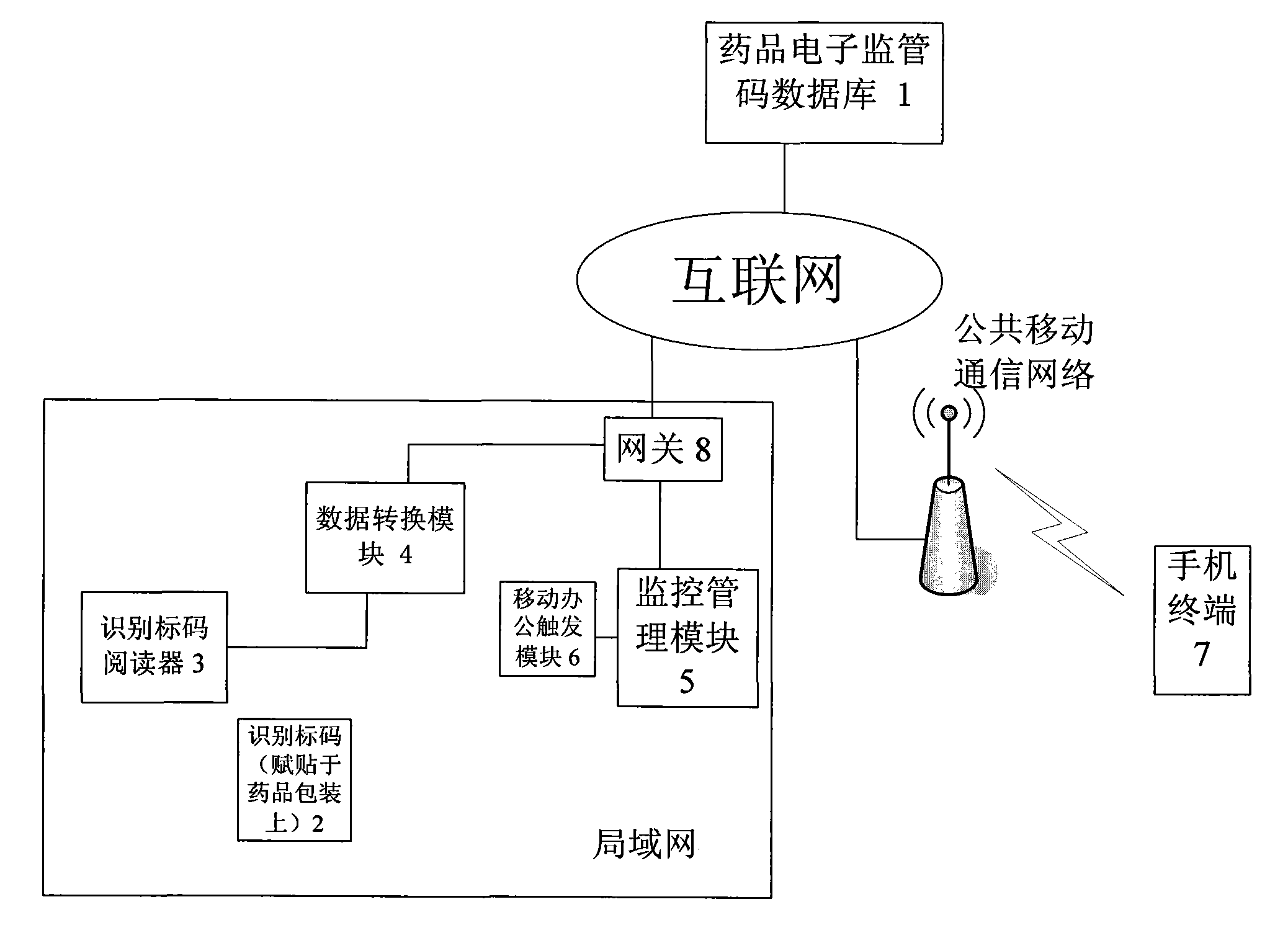
The invention provides an internet and mobile communication network based medical logistic information processing system and method. In the system, drug electronic supervision codes and recording information of corresponding drugs are stored in a drug electronic supervision database, and identification marks are adhered to all drug package boxes, so that the drugs can be supervised according to the identification marks in production, sales, transportation, storage, use, after-service and other logistic links of the drugs. By adopting the internet technology, the mobile communication technology and the identification mark technology, the system and the method perform real-time tracking management on production, sales and use of drugs, realizes seamless transition of all links of a medical supply chain, and perform full-process tracking of source, storage, transportation, sales, use and other links of the medical supplies.
Owner:于佳辉
Preparation of medicinal D,L-2-hydroxy-4-methylthio calcium butyrate
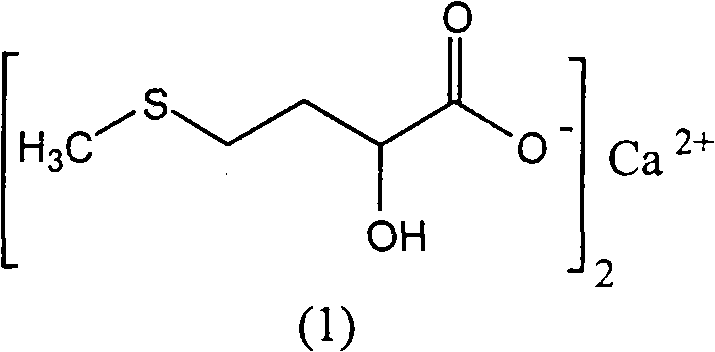
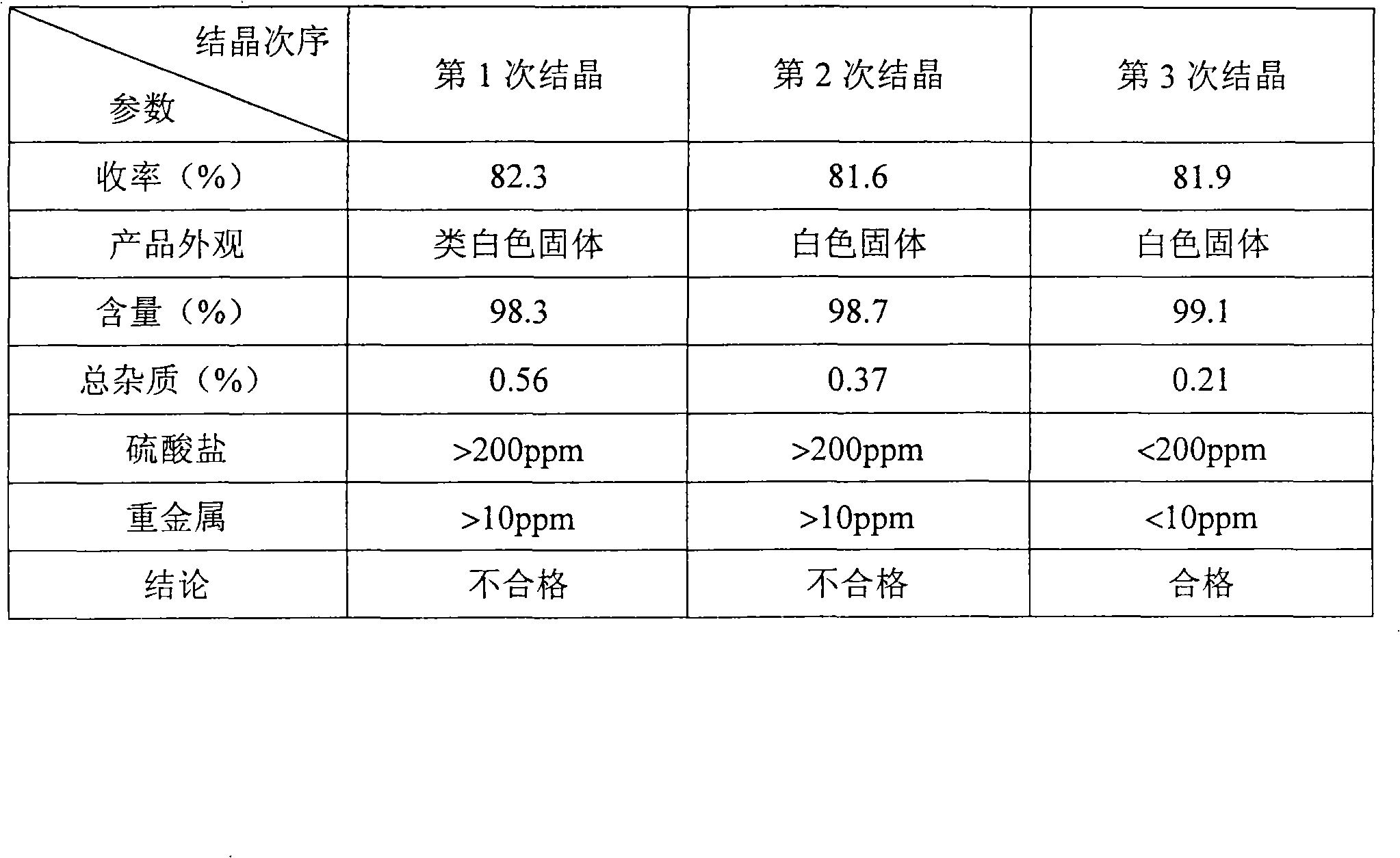
The invention discloses a method for preparing a D, L-2-hydroxyl-4-methylthio butanoic calcium salt for medicinal purpose. The method comprises following steps of: a. using a D,L-2-hydroxyl-4-methylthio butanoic acid and an alcohol with a general formula of ROH as the raw materials to carry out esterification to obtain a D, L-2-hydroxyl-4-methylthio butyrate, and; b. hydrolyzing the D, L-2-hydroxyl-4-methylthio butyrate in step a and calcium oxide in a solvent to produce the D, L-2-hydroxyl-4-methylthio butanoic calcium salt. The method provided by the invention for the production of the D, L-2-hydroxyl-4-methylthio butanoic calcium salt has the advantages of short course, readily available the raw materials, low cost, easy control over the quality of the product, and more importantly, the method can prepare high-purity D, L-2-hydroxyl-4-methylthio butanoic calcium salt for medicinal purpose, thereby satisfying the requirements of State Food and Drug Administration Bureau and Good Manufacturing Practice (GMP) for drug production, and facilitating the preparation of pharmaceutical preparations.
Owner:NANJING LIFENERGY R & D +1
Green extraction process for artemisinin
The invention discloses a green extraction process for artemisinin, and relates to the field of drug production. The process comprises the following steps: (1) drying of raw materials, that is, putting the raw materials into drying equipment for drying; (2) primary processing of artemisinin, that is, putting the dried raw materials into an extraction tank, adding petroleum ether, heating the extraction tank, carrying out countercurrent extraction, allowing the extract to flow out from the extraction tank, standing the extract to obtain a supernatant, allowing the supernatant to pass through asilica gel column, eluting the silica gel column with petroleum ether, collecting artemisinin fractions, putting the artemisinin fractions into a concentrating tank for concentration until crystals are precipitated, putting the obtained concentrate into a crystallizing tank for crystallization, carrying out filtering to obtain crystals, and drying the crystals so as to obtain crude products of artemisinin; (3) refining of artemisinin, that is, dissolving the crude products of artemisinin in an alcohol precipitating tank with ethanol, wherein the ethanol with a concentration of 93 to 95% is 65to 75 times the amount of the crude products, standing the solution, taking the supernatant of the solution for secondary filter, concentrating the filtrate in the concentrating tank, standing the concentrate for crystallization, carrying out filtering to obtain crystals, and drying the crystals under vacuum so as to obtain refined products of artemisinin.
Owner:GUANGXI XIANCAOTANG PHARMA
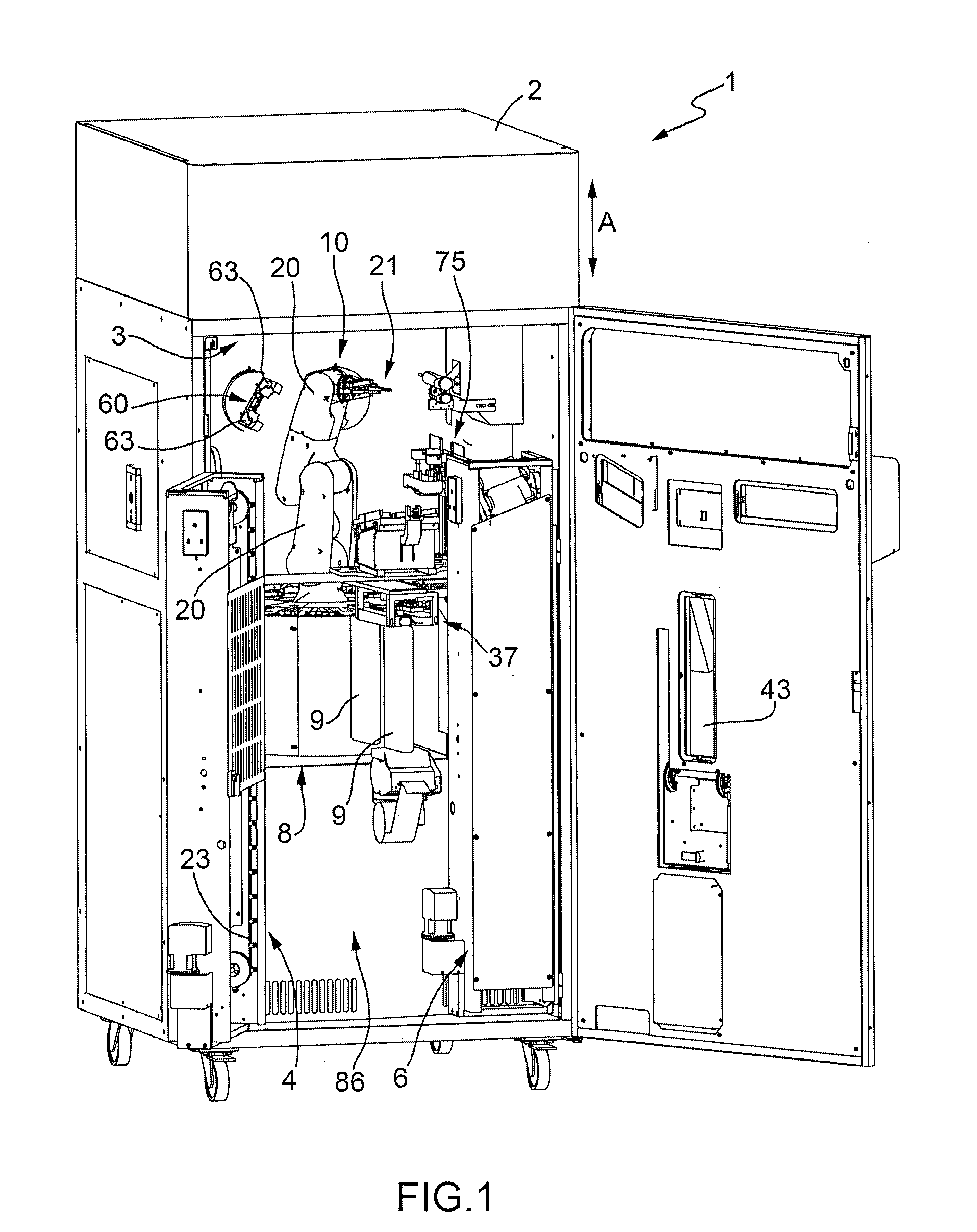
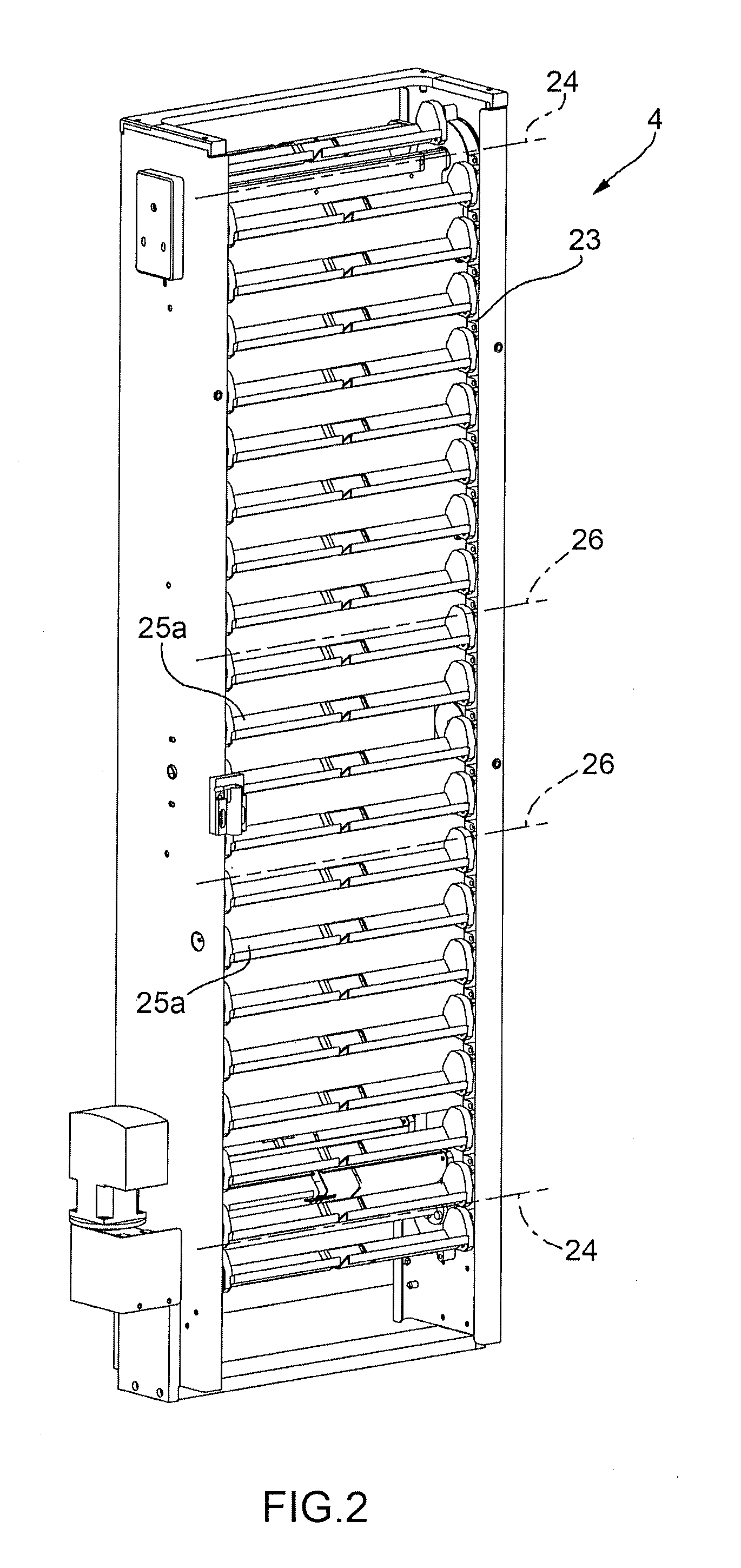
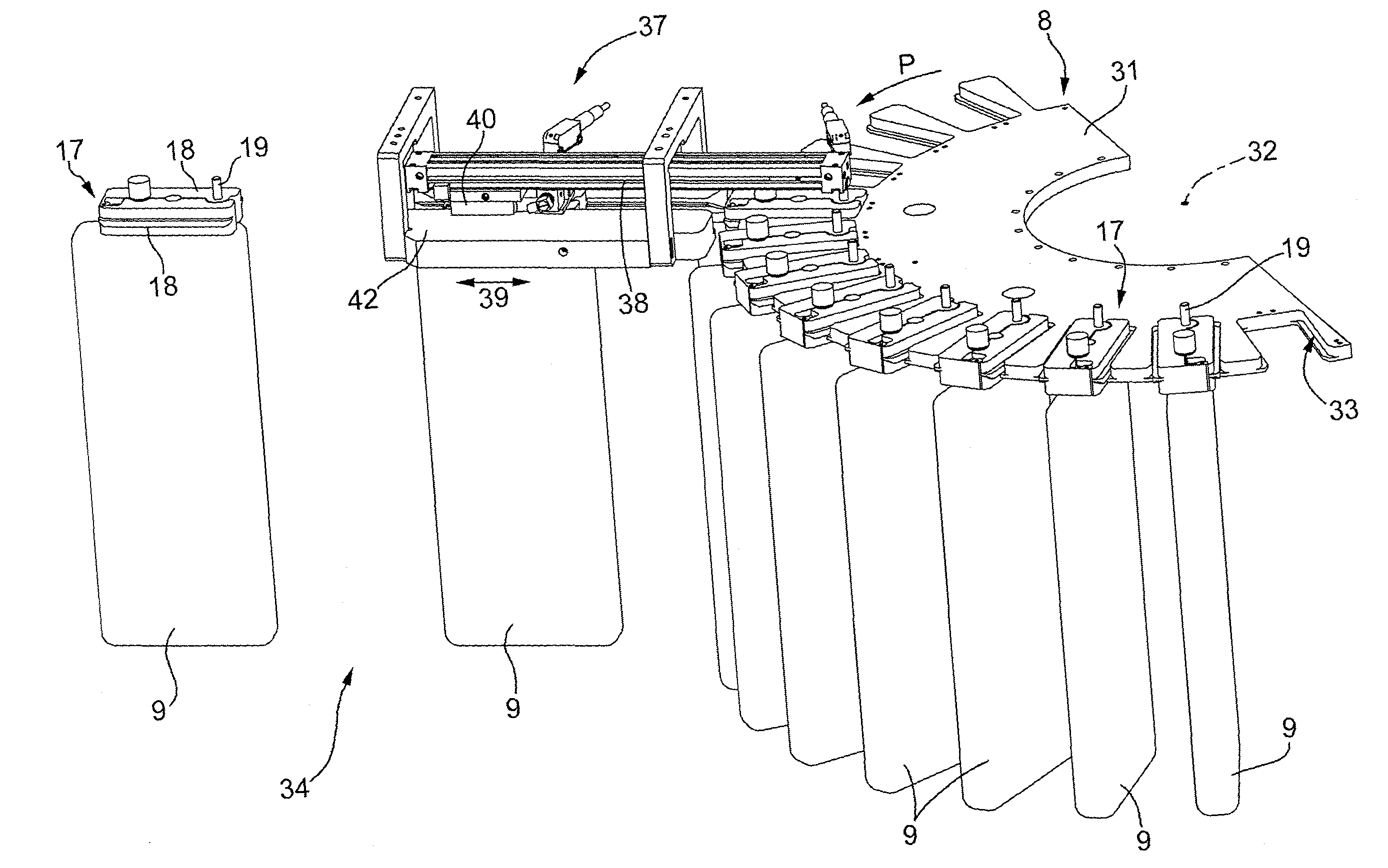
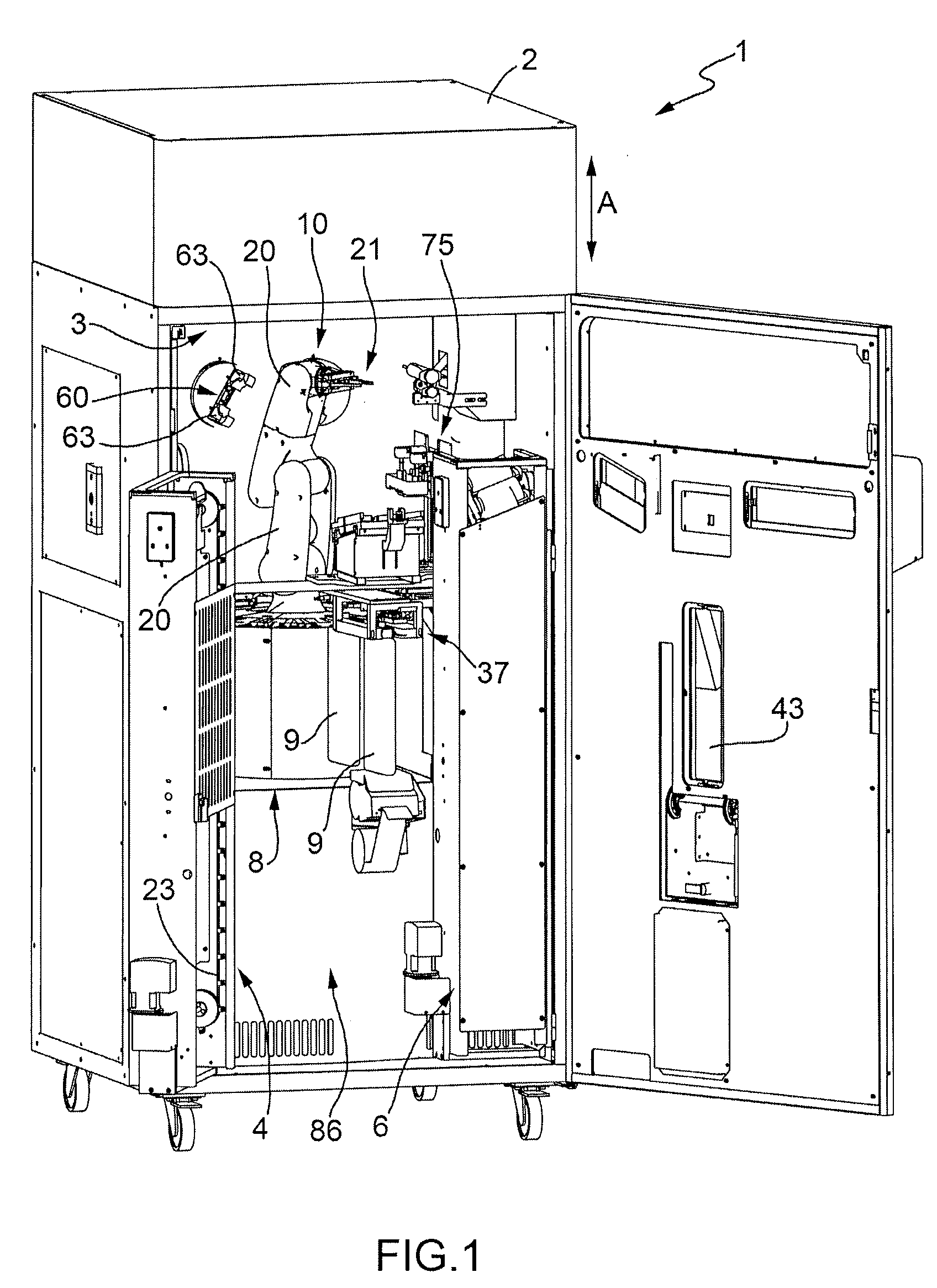
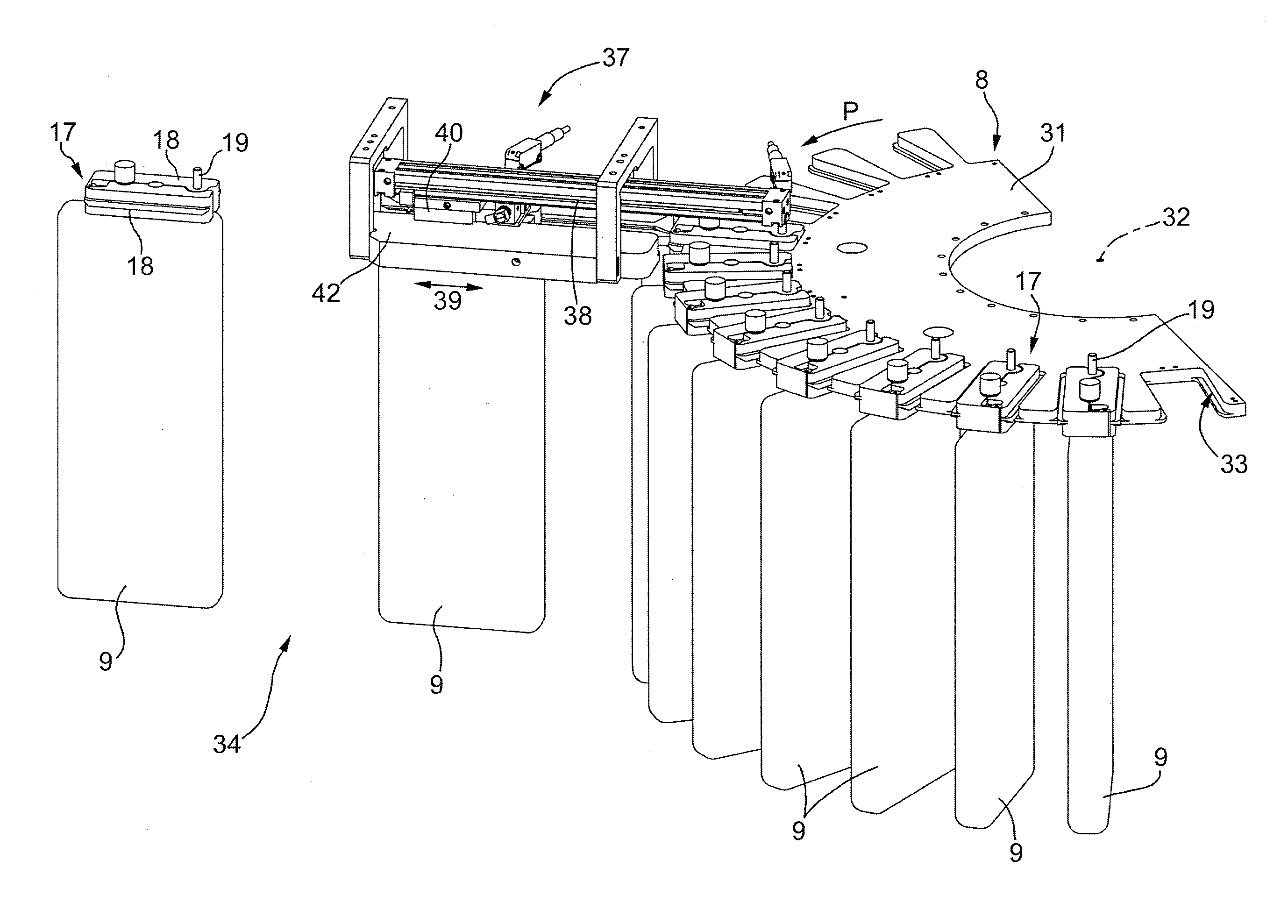
Machine for the Production of Pharmaceutical Products
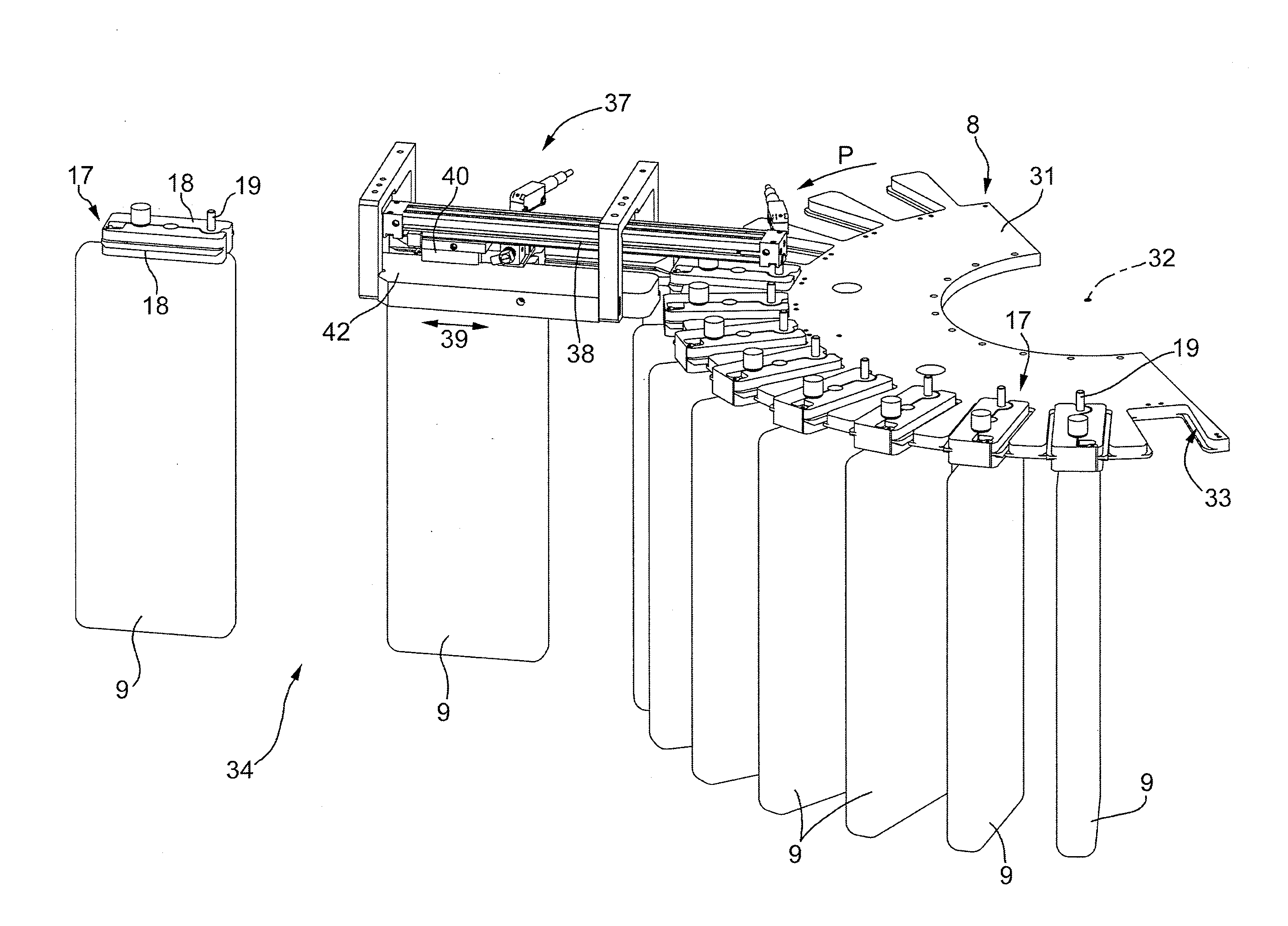
ActiveUS20120048676A1Simple and cost-effectivePharmaceutical containersPharmaceutical product form changeEngineeringMechanical engineering
A machine for the preparation of pharmaceutical products has a pocket conveyor, mobile along a loop-shaped path and provided with a plurality of pockets, each adapted to receive and withhold a respective container, and a transfer device for transferring the containers with a rectilinear motion between the respective pockets and at least one operating station for executing an operation on the containers themselves.
Owner:OMNICELL
Tissue culture method of huperizia serrata
InactiveCN101889551AReduce pollutionReduce browning ratePlant tissue cultureHorticulture methodsPteridophyteFungal endophyte
The invention discloses a method for tissue culture method of huperizia serrata. The method comprises the following steps of: selecting an explant; killing surface bacteria; performing inoculation and cultivation; killing endophyte; performing propagation cultivation; and performing rooting cultivation. The huperizia serrata is a precious medicinal pteridophyte which has a tough requirement on a habitat, grows slowly under a natural condition and has a long production period. In a conventional method, propagation coefficient and biomass are low and requirements on medicament production cannot be met. Due to the adoption of the method, the differentiation rate of a huperizia serrata test tube plantlet is up to 90 percent, monthly multiplication multiple is up to 2.1 to 3.5, rooting rate is up to 100 percent, a root system grows quickly, root number is up to 1.6 to 3.4 per plant, a regeneration seedling grows healthily and the root system is developed. The method has the advantages of effectively restraining the pollution and browning of a huperizia serrata explant, promoting the survival and growth of the explant and providing important technical support for the realization of manual mass planting of the huperizia serrata along with simpleness, convenience, practicability, high efficiency and low cost.
Owner:HEFEI UNIV OF TECH
Method for purifying sodium aescinate
InactiveCN102532241AReduce dissolutionSolve the cumbersome extractionSugar derivativesSteroidsHydrogenSodium aescinate
The invention relates to a method for purifying sodium aescinate. The method comprises the steps of crushing Chinese buckeye seed, adding 80-95% ethanol with amount of 5-10 times than that of the Chinese buckeye seed to carry out circumfluence extraction for 2 to 3 times, pressure-reducing the extraction liquid, recycling the ethanol, dispersing and filtering mother liquor by adding right amount of water, adding macroporous resin columns for absorption, eluting by using 50-70% ethanol solution with 4-6 BV, collecting the eluting liquid, pressure-reducing and recycling the ethanol, adding cation exchange resin columns into concentrated solution, adjusting the pH (potential of hydrogen) value of the lower resin column liquid to be 7.5-8.5, adding the macroporous resin columns again for absorption, adding acetone solution into the lower column liquid, mixing fully to obtain precipitation, dissolving the precipitate by using the ethanol solution, shortening the column by using peroxide aluminum, pressure-reducing the column filtering liquid, recycling the ethanol to be in a small volume, placing for crystallization, carrying out circumfluence dissolving on crystallization products by using the ethanol solution and re-crystallizing for 1-3 times, and drying the crystallization products to obtain sodium aescinate products with content not less than 95%. By using the method for purifying sodium aescinate to produce the sodium aescinate, the process is simple, the operation is easy, the content of obtained products is high, and the method can be directly used on medicine production.
Owner:苏州宝泽堂医药科技有限公司
Short bifidobacteria with functions of anti-gastrointestinal tract pathogen, oxidation resistance and blood pressure reduction
ActiveCN101314763AStrong resistanceImprove antioxidant capacityAntibacterial agentsBacteriaBiotechnologyFeces
The invention discloses a novel Bifidobacterium breve with excellent probiotic function. The Bifidobacterium breve strains are obtained by screening the feces of the long-lived elders in Hetian, XinJiang, and the obtained strains have outstanding resistance to acid and bile acid salts, adhesion characteristics to intestinal tract epidermic cells, excellent antibacterial performance and good oxidization resistance; and the strains have an effect on reducing the blood pressure as well as the edible safety, therefore, the Bifidobacterium breve can be widely applied in the fields such as functional additives, dairy products, beverage, functional food, drug production, etc.
Owner:PRESIDENT ENTERPRISES (CHINA) INVESTMENT CO LTD +1
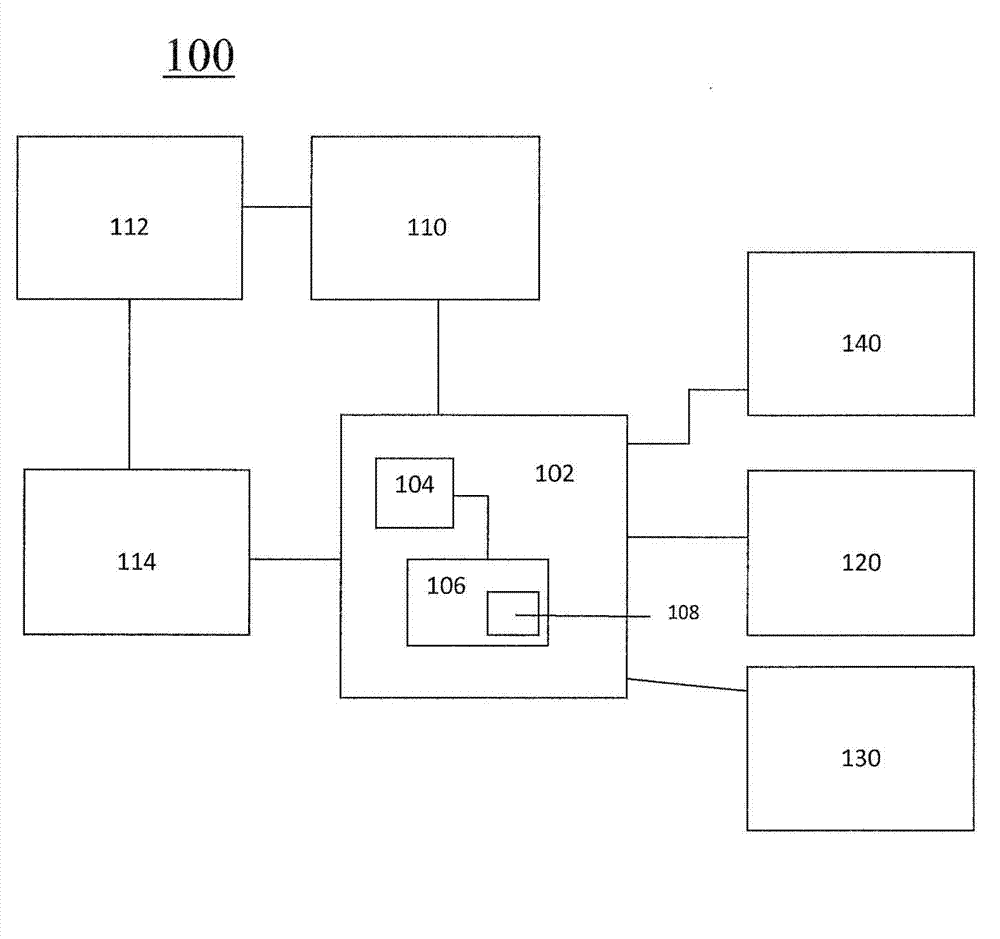
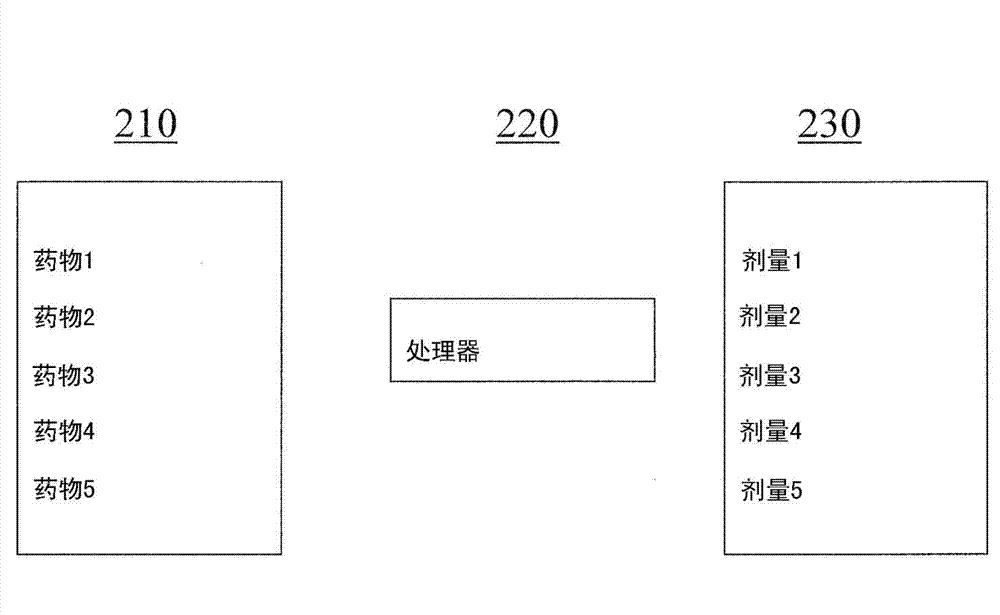
System and methods for the production of personalized drug products
InactiveCN103250176AData processing applicationsDrug and medicationsTransdermal patchDrug dispensing
A system and method for determining an optimal combination drug product for a particular patient includes a processor that receives patient information and determines an optimal combination drug product based on the received information. A system which can provide information regarding predicted events or pathologies based on received patient information and guidance on subsequent steps to ameliorate, treat or intervent. A drug production device includes a plurality of drug containers, each of which are coupled to a drug dispensing channel. A controller controls the dispensing of drug through each channel, and a combination drug product is produced from the dispensed drugs. A combination drug product includes a plurality of discrete units of a first drug, and a plurality of discrete units of a second drug.; A transdermal patch includes a plurality of drug compartments, each containing a quantity of drug product, and a controller for controlling the release of drugs from each compartment. Feedback loop elements can enable iterations to optimized personalized doses.
Owner:INTELLIMEDICINE +1
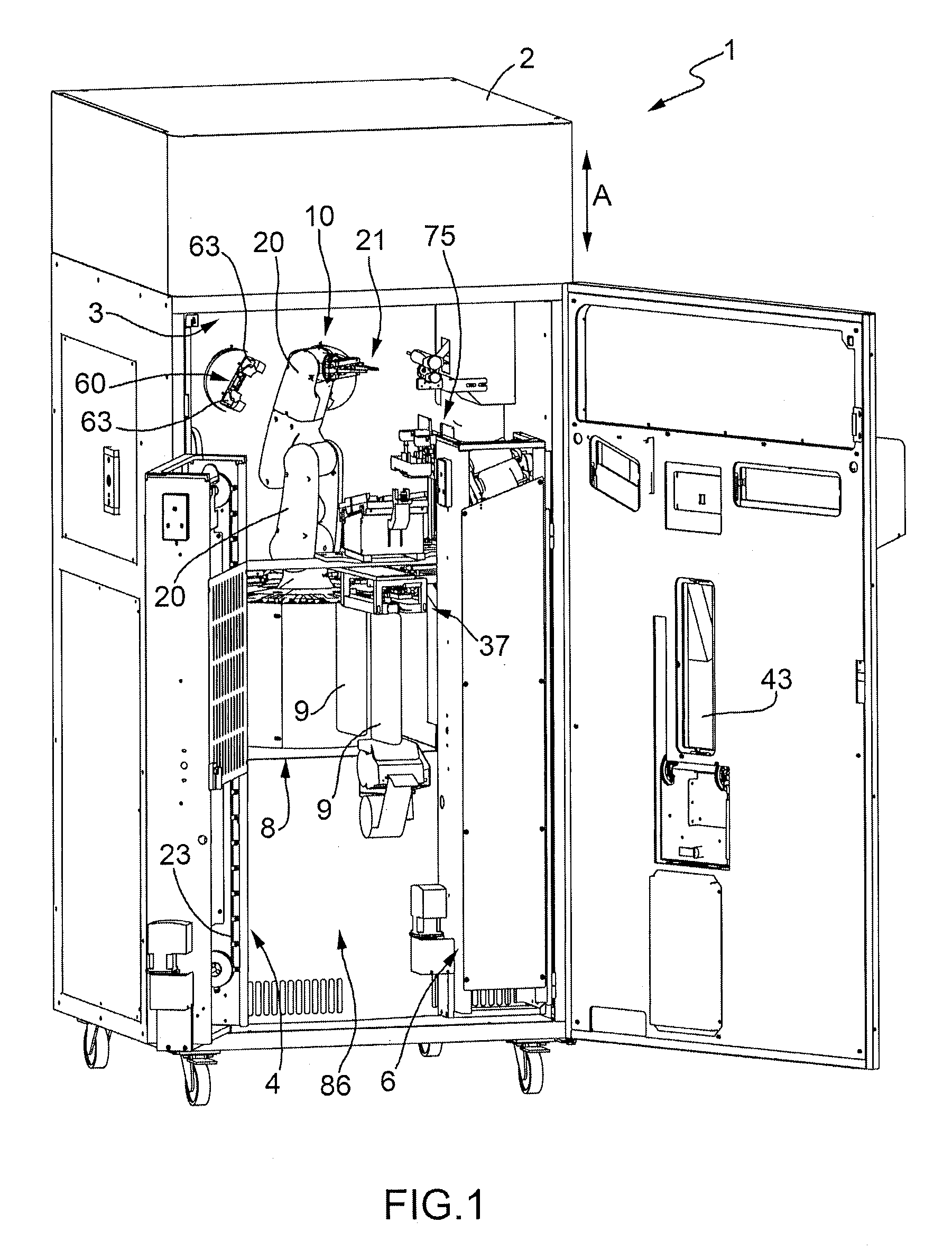
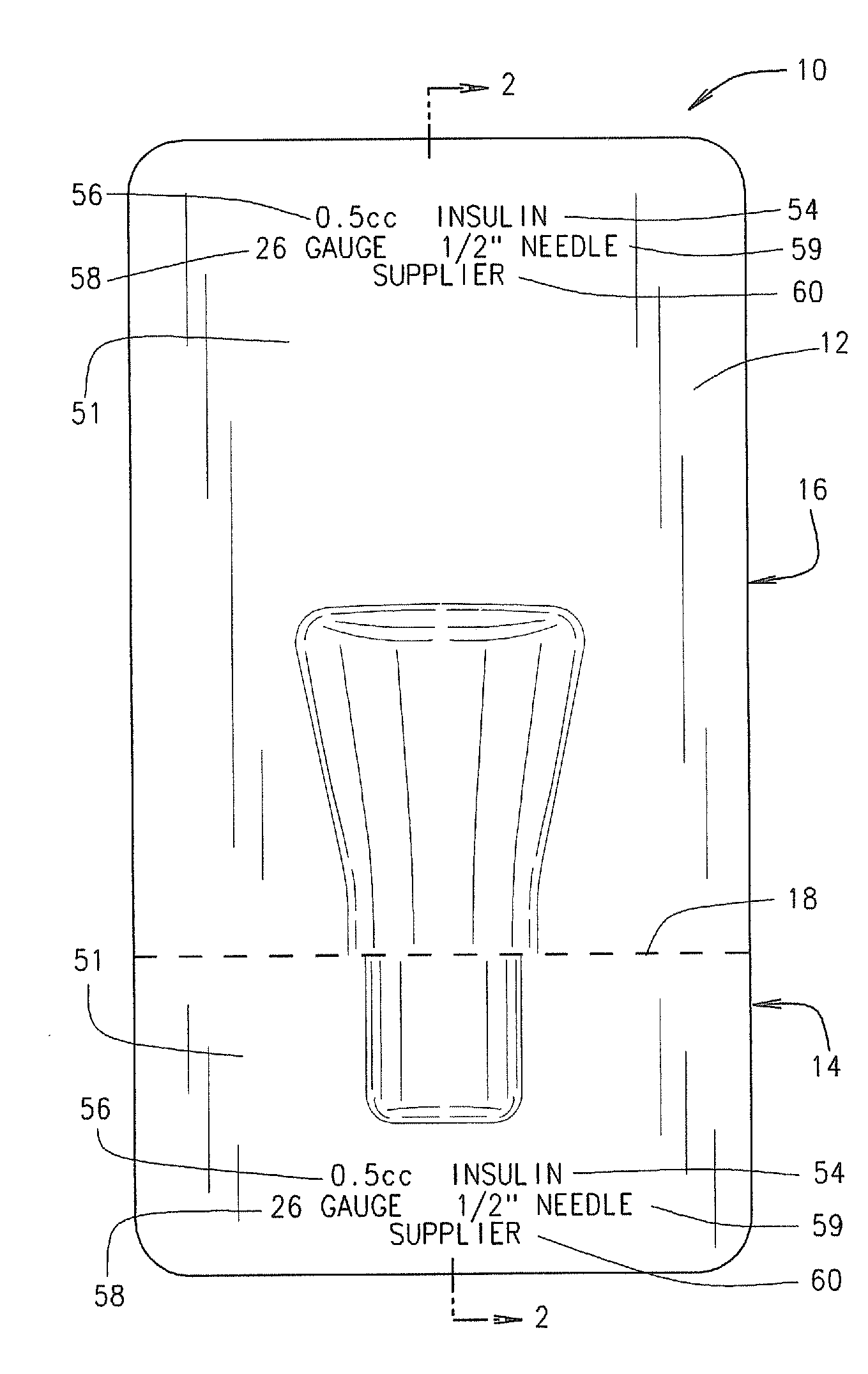
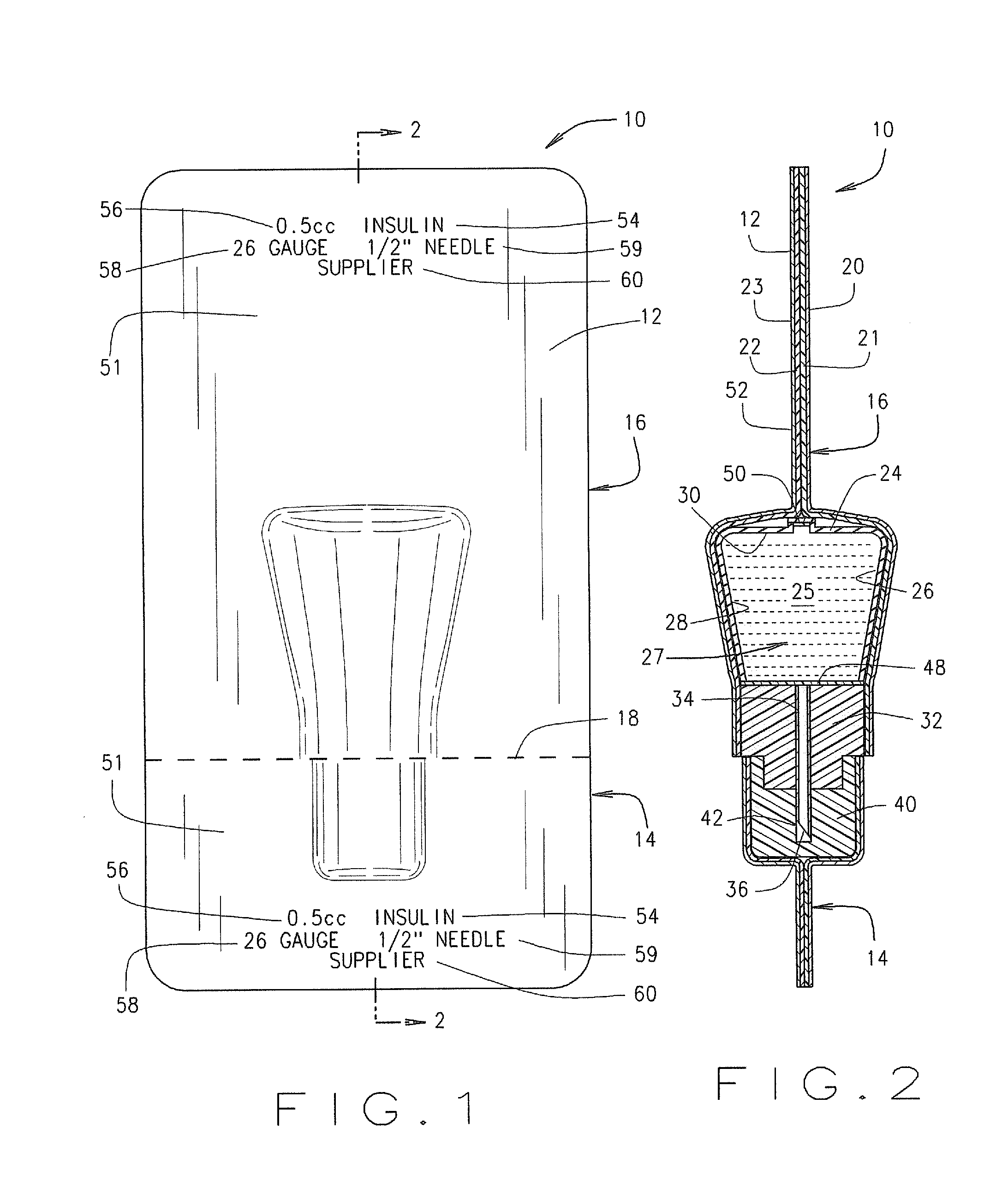
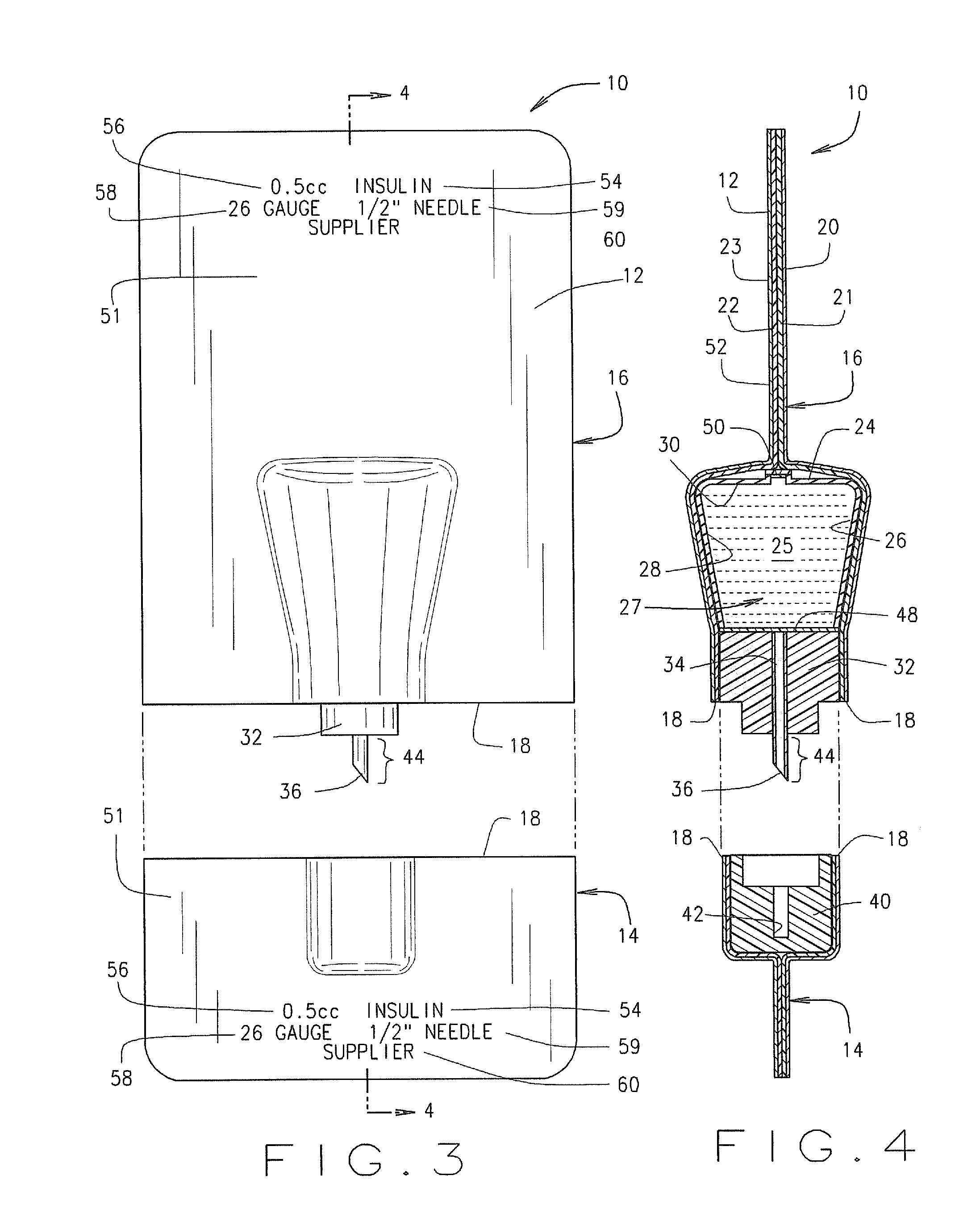
Method for the Production of Pharmaceutical Products
ActiveUS20120048419A1Simple and cost-effectiveLiquid fillingDiagnosticsBiomedical engineeringDrugs production
A method for the preparation of pharmaceutical products according to which a diluent is fed into a container containing a lyophilized or powdered pharmaceutical by means of a needle, which is then extracted from the container, inserted in its protective cap, and rinsed by feeding the diluent through the needle and into the protective cap itself.
Owner:OMNICELL
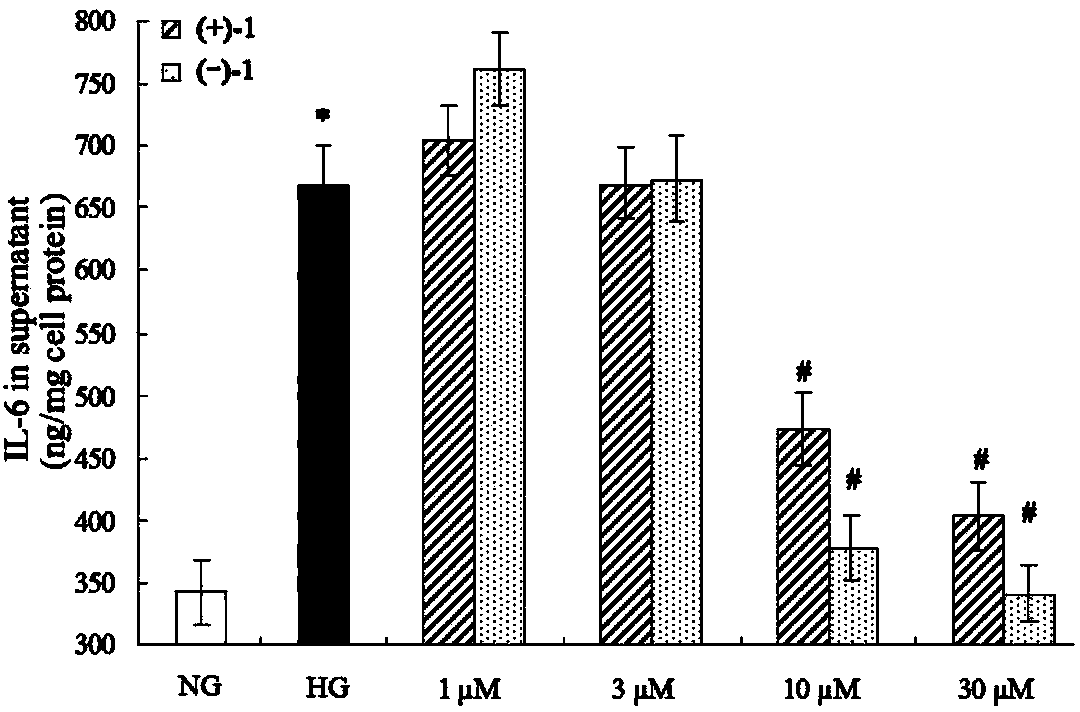
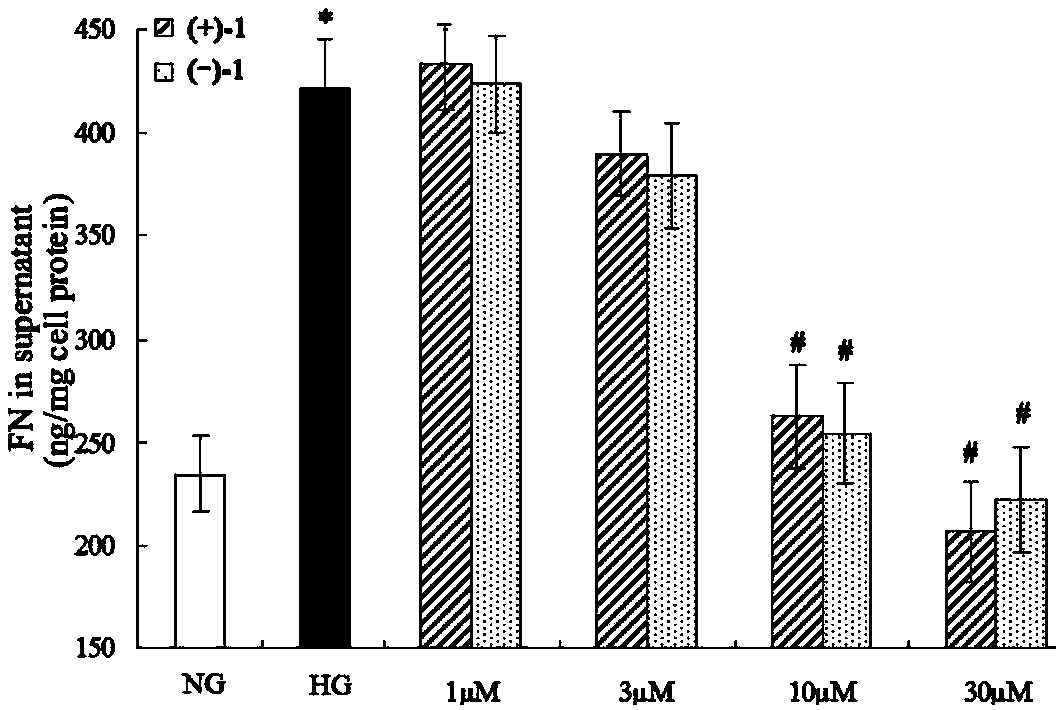
Lingzhiol A and application of lingzhiol A in drug production and foods
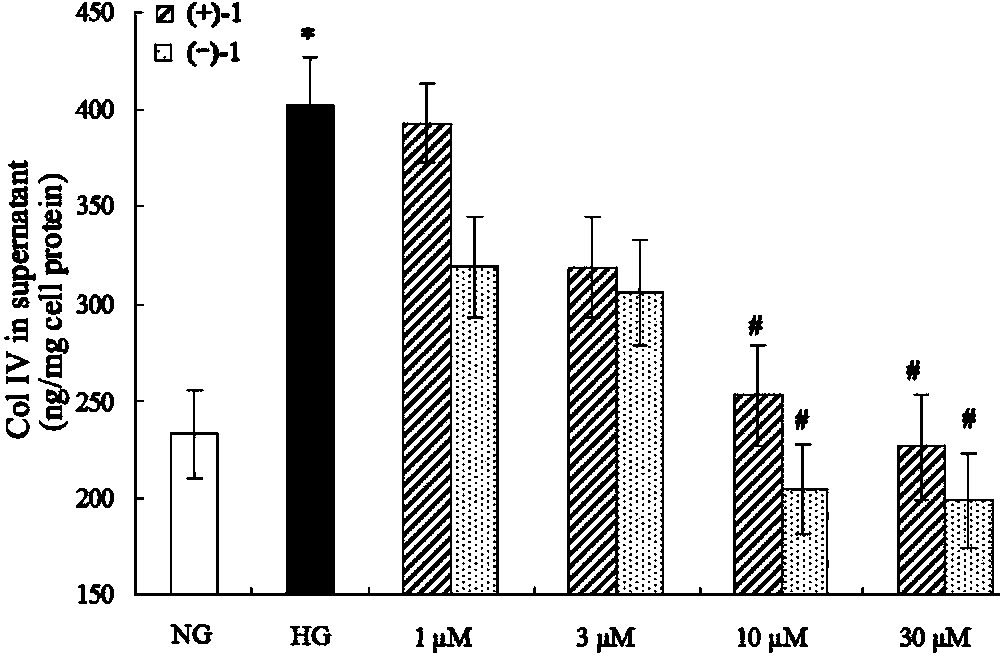
Glossy ganoderma as a traditional Chinese medicine is called immortal grass since ancient times and known as the ability to treat various diseases; a pair of lingzhiol A optical enantiomers is purified from ganoderma lucidum, and the lingzhiol A optical enantiomers have obvious effects on inhibiting rat renal mesangial cell strains induced by high glucose to generate reactive oxide species, IL-6, fibronectin and IV type collagen, and also can obviously inhibit the phosphorylation of the renal tubular epithelial cell Smad3 induced by TGF-beta1, so that the application prospect of the compound in preparation of medicines for treating diabetic nephropathy and chronic nephropathy is shown.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
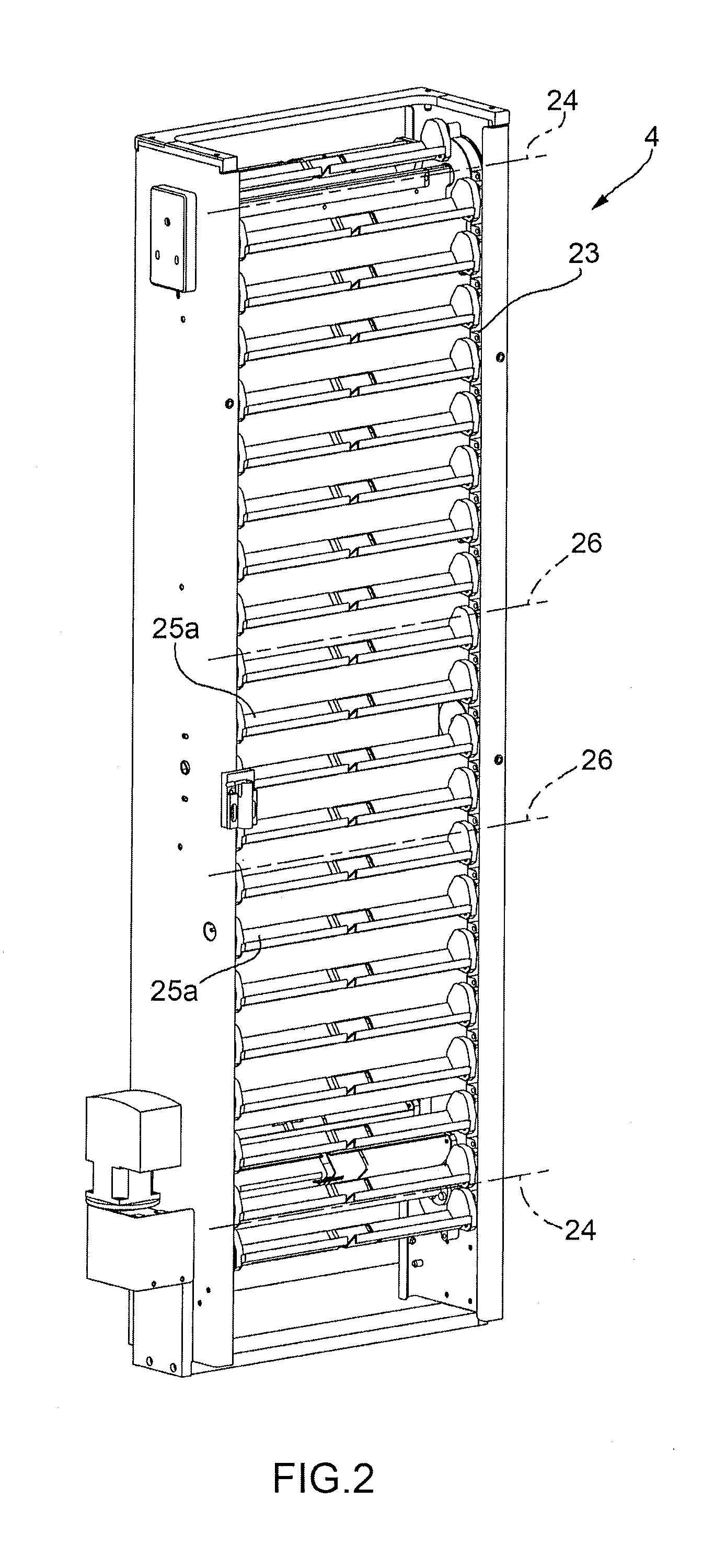
Machine for the Production of Pharmaceutical Products
ActiveUS20120048675A1Simple and cost-effectivePharmaceutical containersPharmaceutical product form changeMechanical engineeringLoop shaping
A machine for the preparation of pharmaceutical products has a pocket conveyor, mobile along a loop-shaped path and provided with a plurality of pockets, each adapted to receive and withhold a respective container, and a transfer device for transferring the containers with a rectilinear motion between the respective pockets and at least one operating station for executing an operation on the containers themselves.
Owner:OMNICELL
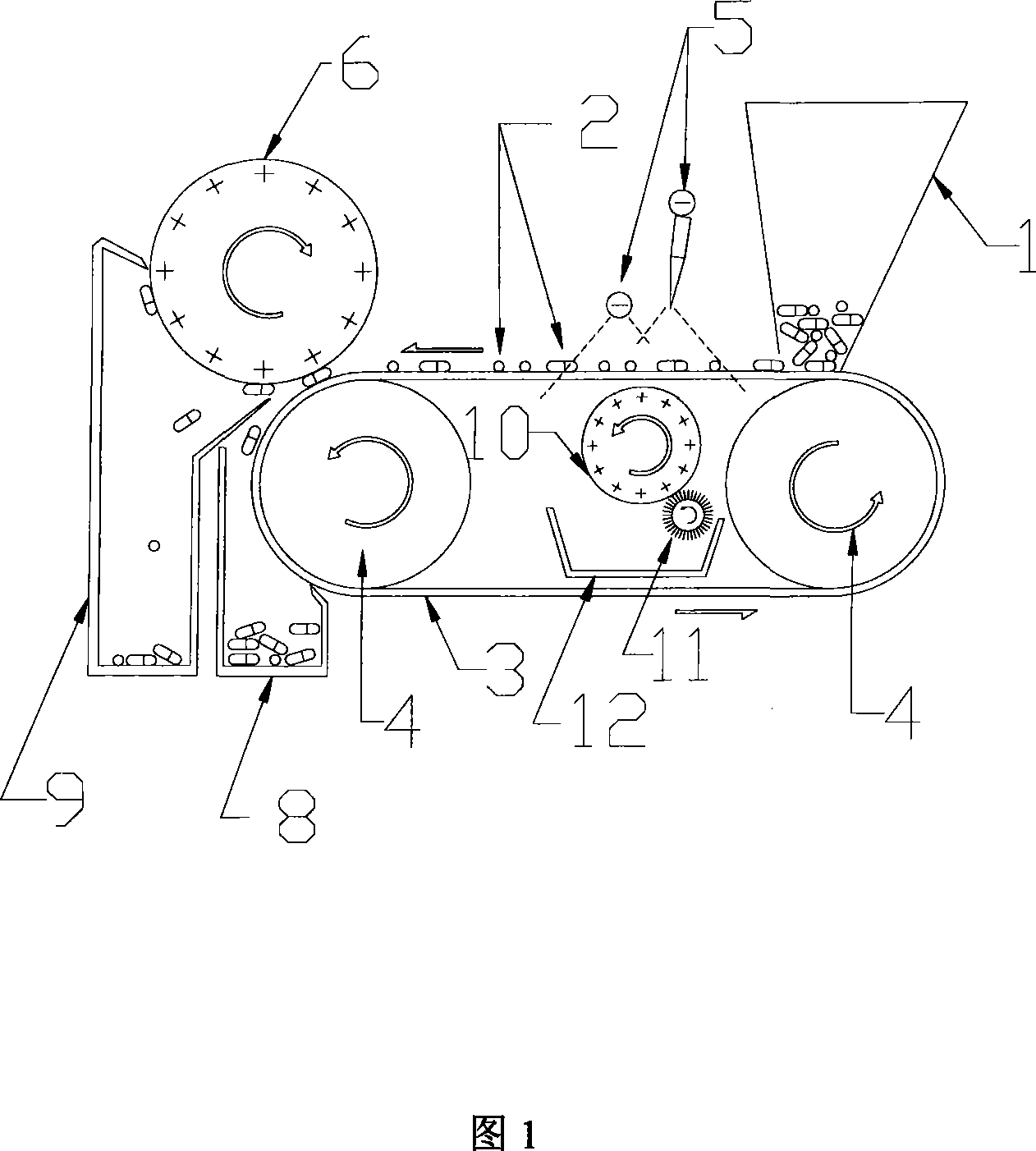
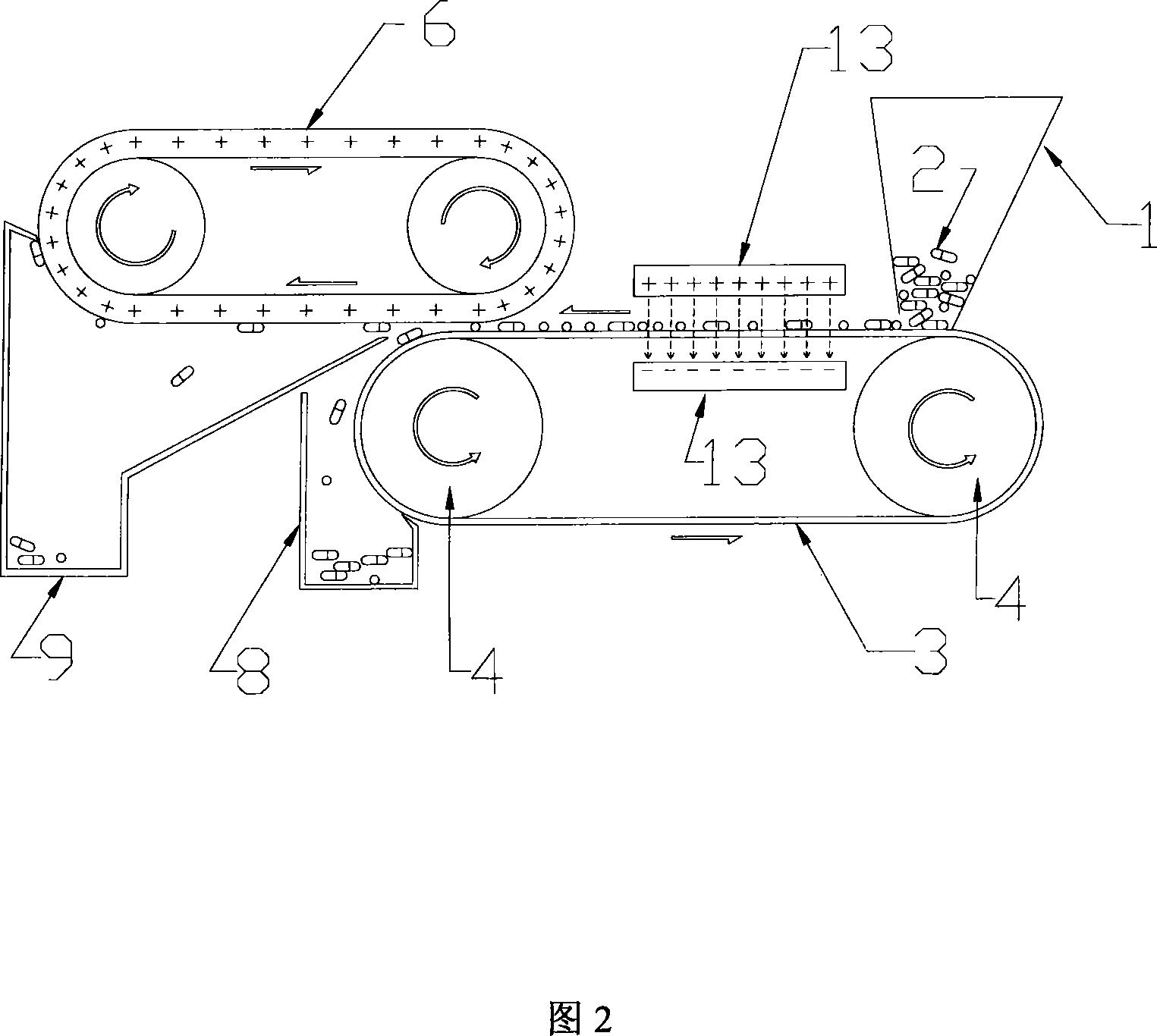
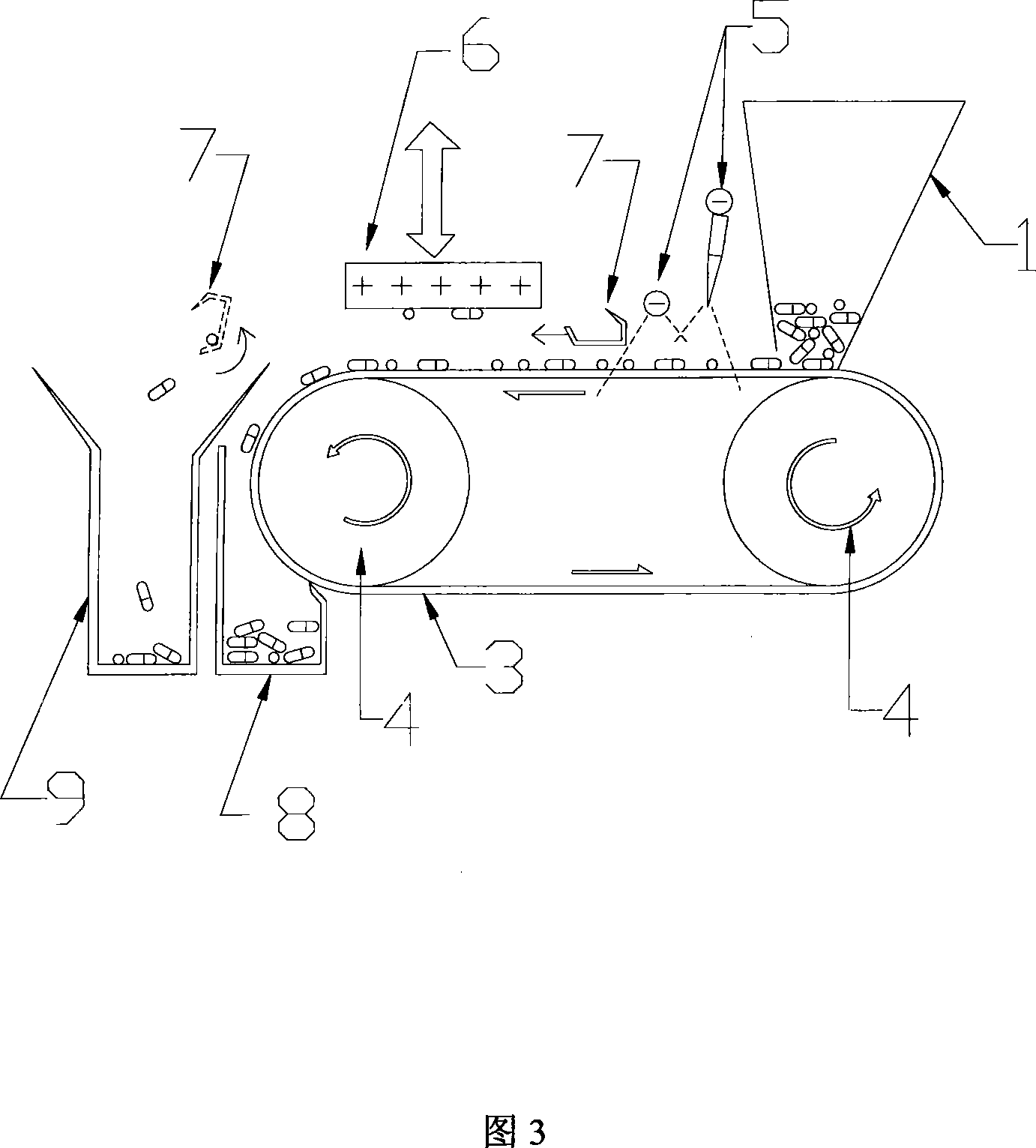
Method and apparatus for sorting electrostatic attraction type weight
InactiveCN101219414AImprove sorting efficiencyQuick sortingElectrostatic separationEngineeringElectrostatic attraction
The invention relates to an electrostatic attracted weight sorting method and equipment, which can electrify the to-be-sorted objects with a charge, the sorting mechanism with another charge. The attracting force between the to-be-sorted objects and the sorting mechanism can reach an expected value through regulating the distance between the to-be-sorted objects and the sorting mechanism. Therefore the to-be-sorted objects with a weight smaller than the sorting standard are attracted by the sorting mechanism; the to-be-sorted objects with a weight larger than the sorting standard can not be attracted by the sorting mechanism, while the to-be-sorted objects with a weight equal to the sorting standard maintain the original state. The to-be-sorted objects being attracted and the to-be-sorted objects not being attracted are collected respectively, thus the sorting process is completed. Compared with the existing weight sorting method, the method has the advantages of high efficiency and sorting precision, convenient in popularization, and is particularly suitable for the weight sorting of solid preparations such as capsules, tablets and pills during the production process of medicine so as to remove non-conforming products and enhance the overall quality of medicines.
Owner:韩杰
Off-site regulation method used for medicine circulation
InactiveCN103198409AImprove the level of scientific managementSolve problemsCommerceInformation processingThe Internet
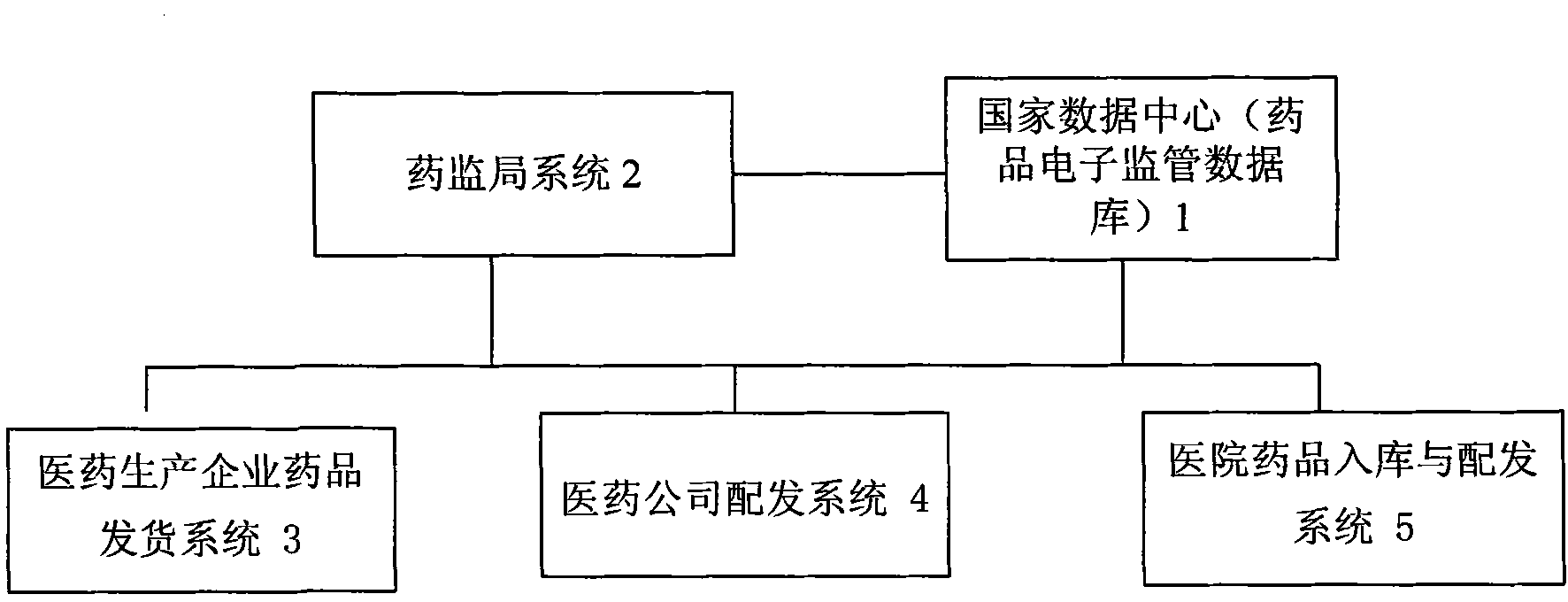
The invention discloses an off-site regulation method used for medicine circulation. The method includes: by application of the computer information processing technology and the internet technology, enterprise related information of medicine manufacturers is collected, and the collected information is transmitted and stored to a data server; in the logistics transport link of medicines, transport condition information of the medicines is collected in real time, and the information is transmitted and stored to the data server; medicine wholesale and retail mechanisms or medical mechanisms carry out data and video collection and storage on goods examination information of the medicines; a medicine supervisory organization carries out real-time tracking and early warning on the above information; the medicine supervisory organization establishes a corresponding medicine enterprise credit or credit standing platform; and general public users can carry out inquiry and complaint operation. The off-site regulation method used for the medicine circulation effectively solves the problems that medicine supervisors are shorthanded, and investigation and evidence collection are difficult, provides related information and rigorous evidences and bases for medicine tracking and plays an active role in ensuring medication safety of the general public masses.
Owner:福州天虹电脑科技有限公司
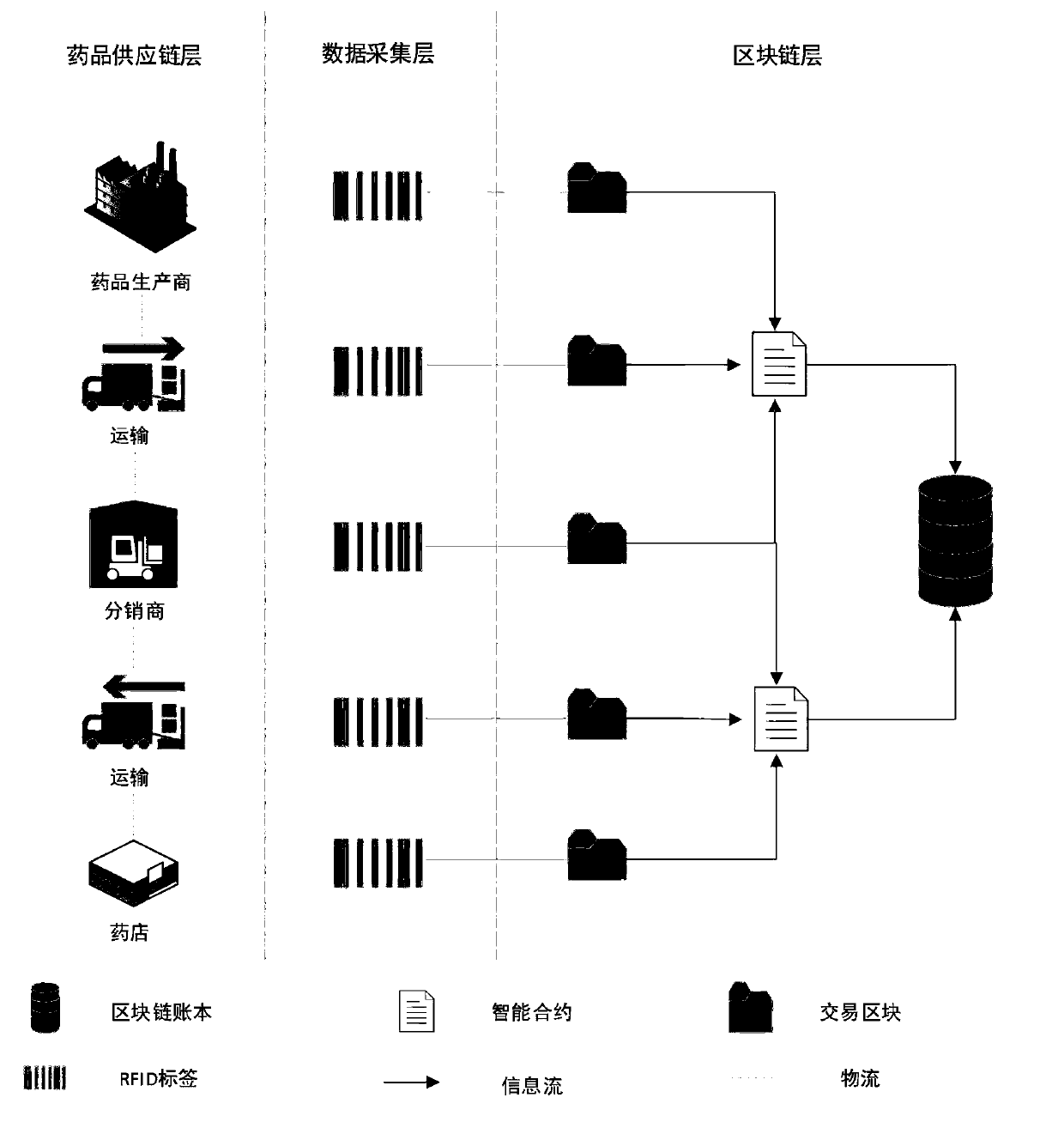
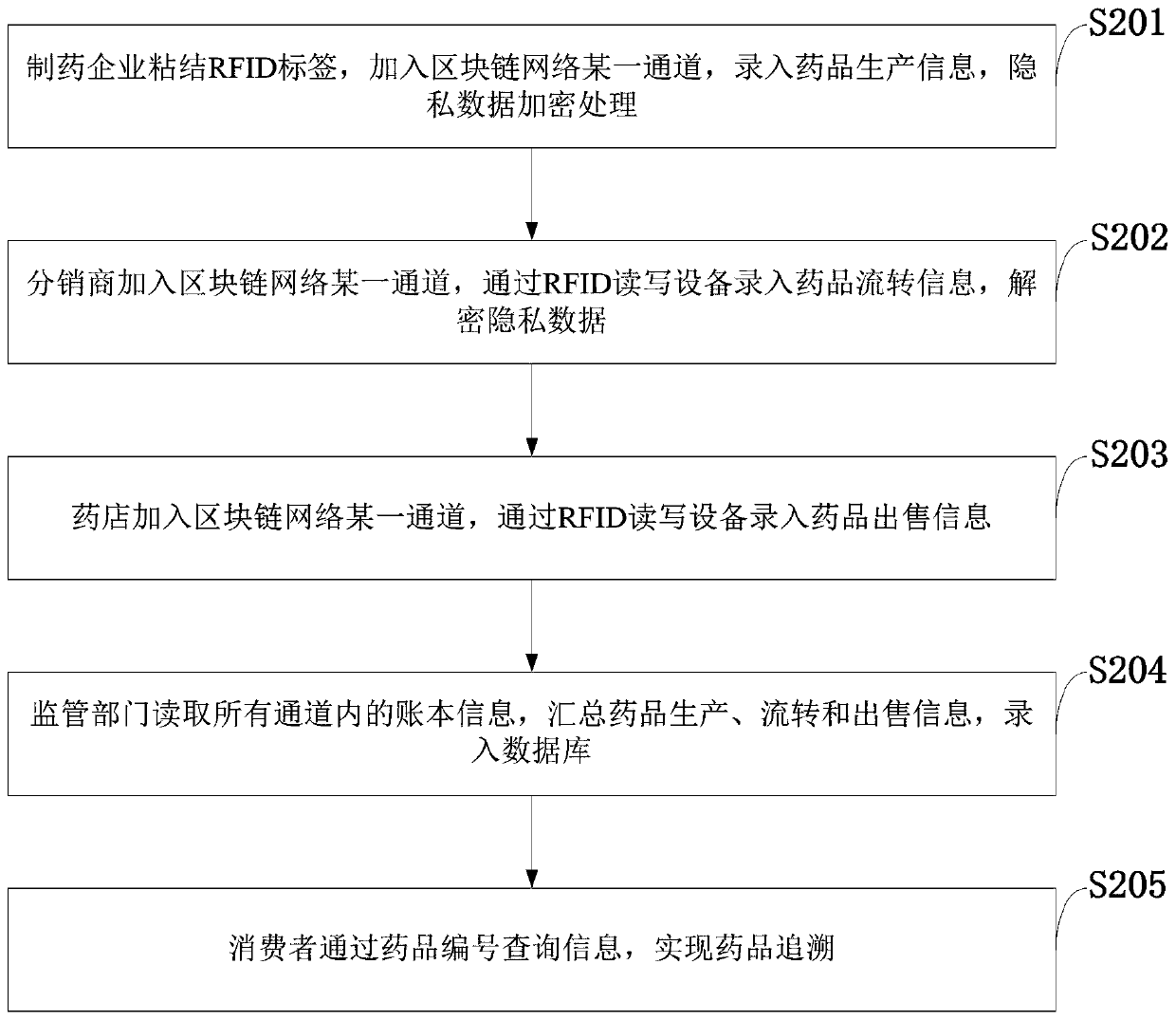
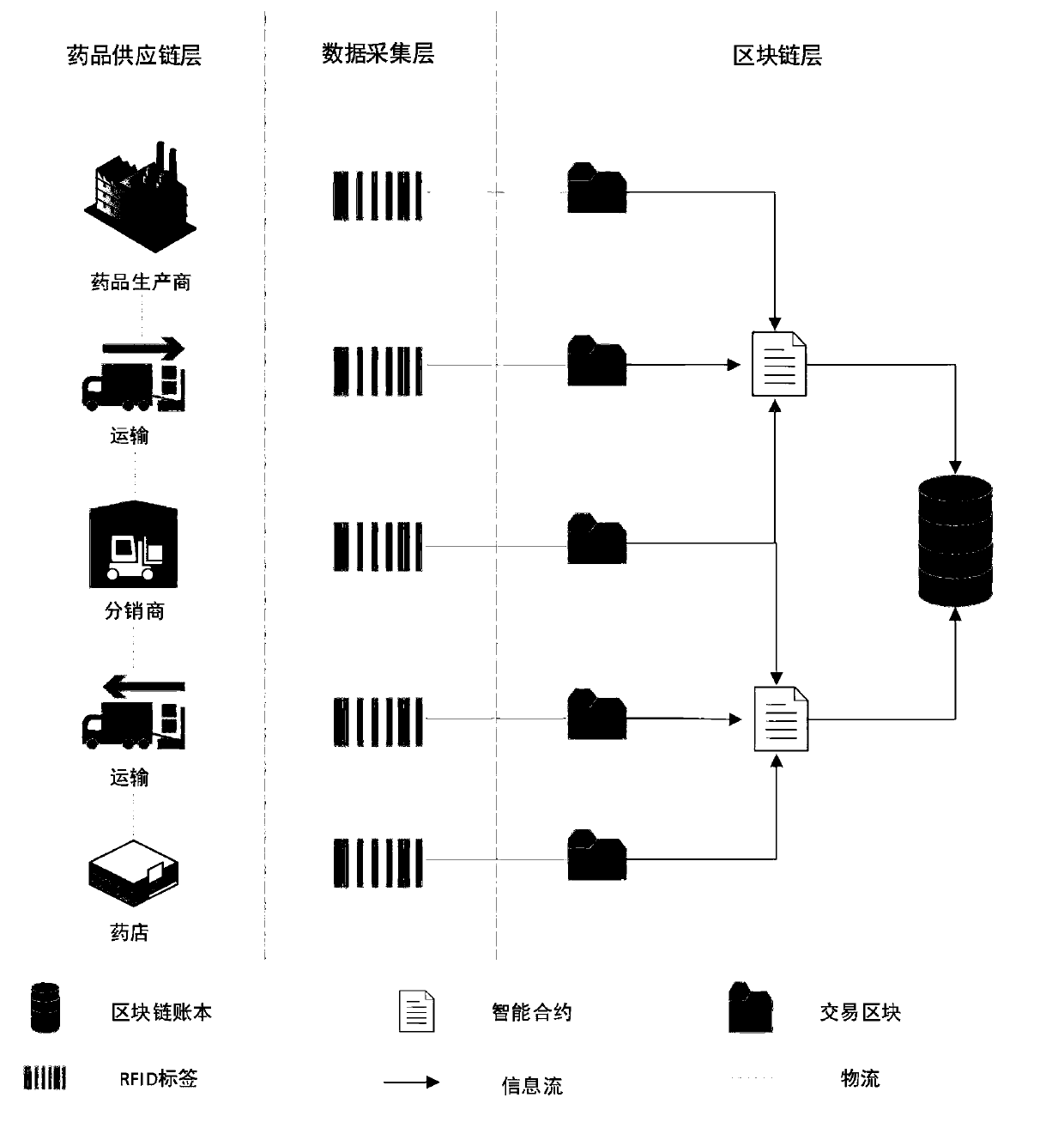
Medicine tracing system based on RFID and block chain and implementation method
PendingCN109993546APossibility of cost reductionDecentralizedMemory record carrier reading problemsDigital data protectionData centerPrivacy protection
The invention belongs to the technical field of commerce, such as shopping or electronic commerce, and discloses a medicine tracing system based on RFID and a block chain and an implementation methodthereof, and the system comprises the steps: automatically inputting data of a medicine production, transportation and sales process into the block chain through RFID read-write equipment, and storingthe data; enabling the blockchain platform to adopt the form of an alliance chain, and allow a plurality of participants to jointly participate in the establishment and maintenance of an account book. The block chain network adopts a multi-channel architecture to isolate account book information in different channels to realize coarse-grained privacy protection, and further adopts an encryption algorithm and a permission management strategy in the channels to realize fine-grained privacy protection. The drug tracing system based on the RFID and the block chain is used for tracing drugs. The defects of data source reliability and data centralized storage of an existing drug enterprise self-built tracing system or a third-party tracing platform are overcome, and publicity and transparency of the drug tracing system are achieved.
Owner:XIAN XIDIAN BLOCKCHAIN TECH CO LTD +1
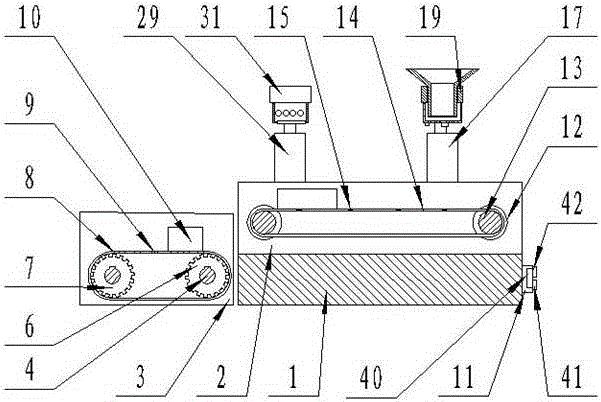
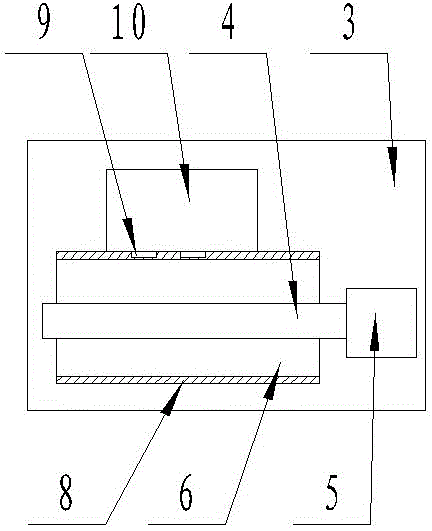
Medicine production line
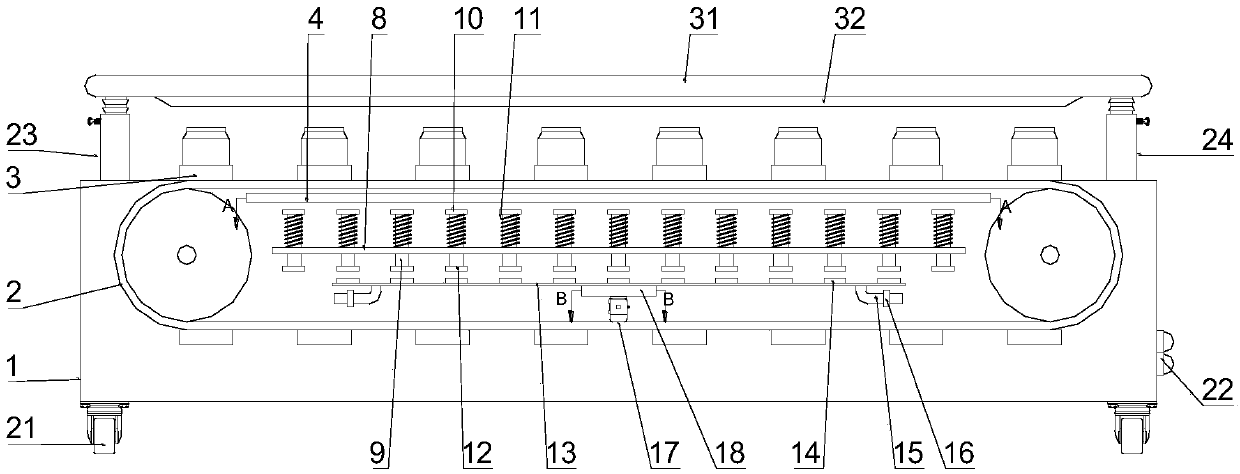
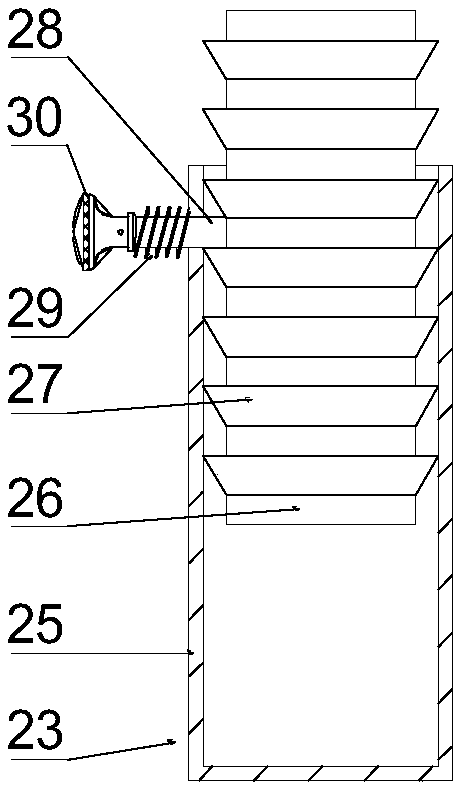
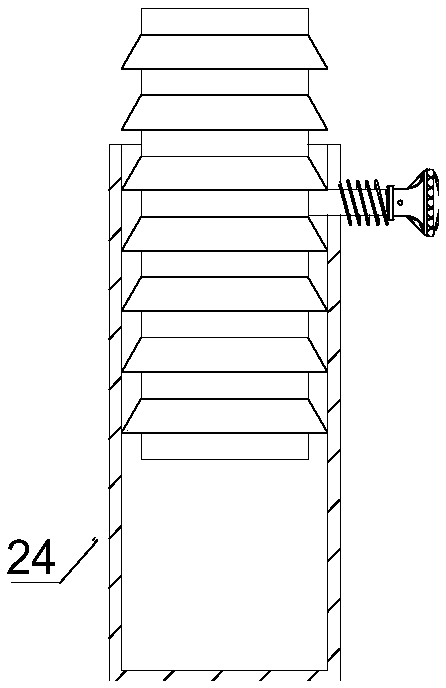
The invention discloses a medicine production line, which comprises a base, wherein a through groove is formed in the upper surface of the base along the length direction; a conveying mechanism is arranged in the through groove; a medicine placing mechanism, a hot-pressed encapsulating mechanism and a medicine collecting device are sequentially arranged at the upper surface of the base along the length direction; a control box is arranged at the side surface of the base, and is respectively and electrically connected with the conveying mechanism, the medicine placing mechanism and the hot-pressed encapsulating mechanism. The medicine production line has the beneficial effects that the structure is simple, and the practicality is high.
Owner:陈吉美
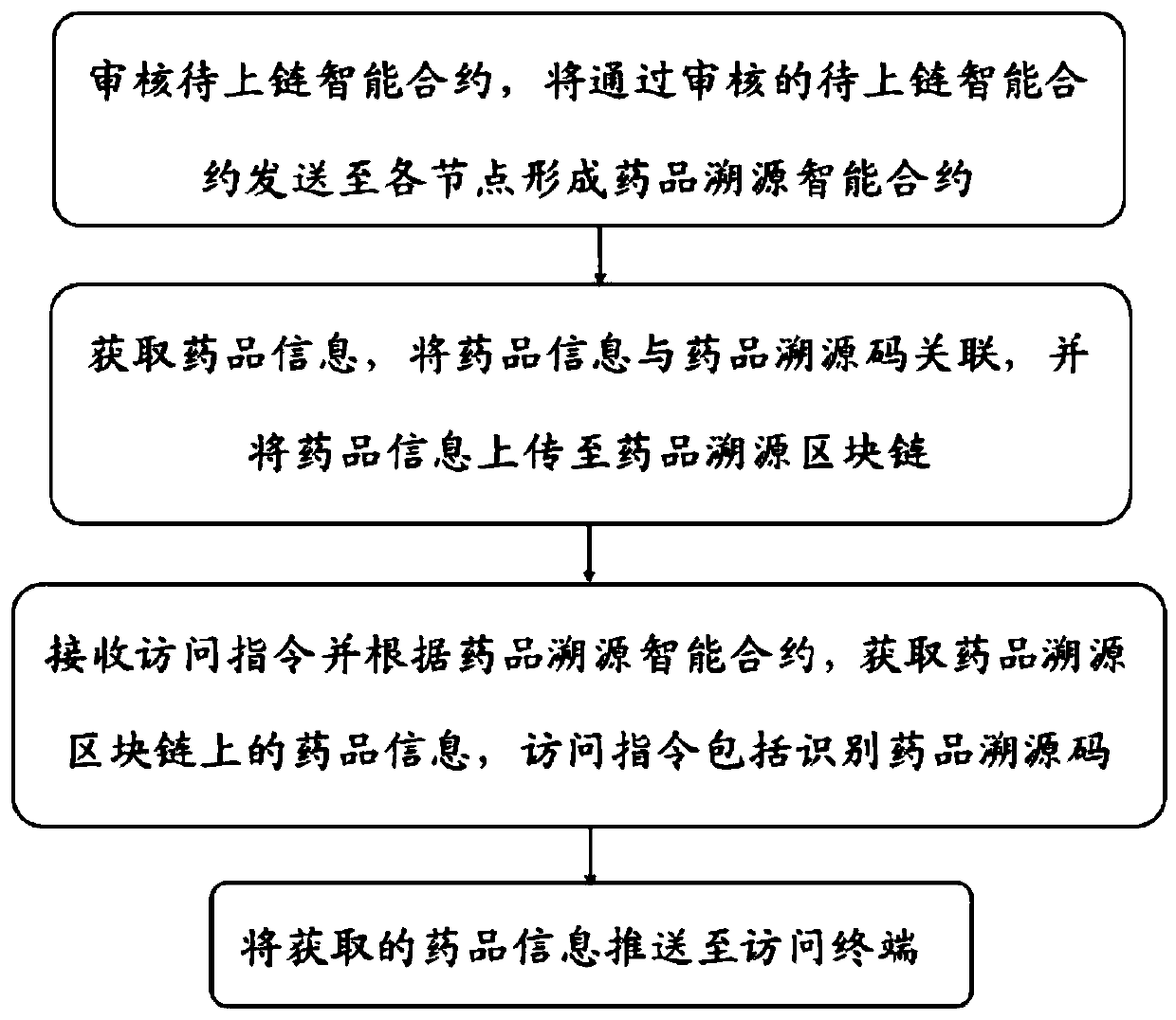
Block chain-based drug traceability method and device and medium
PendingCN111008844AIncreased sense of securityGuaranteed credibilityDatabase distribution/replicationDigital data protectionDrug productComputer science
The invention discloses a block chain-based drug traceability method and device and a medium. The block chain-based drug traceability method comprises the steps: auditing a to-be-uploaded intelligentcontract, and sending the to-be-uploaded intelligent contract passing the auditing to each node to form a drug traceability intelligent contract; obtaining drug information, associating the drug information with the drug traceability code, and uploading the drug information to the drug traceability block chain; receiving an access instruction and obtaining the drug information on the drug traceability block chain according to the drug traceability intelligent contract, wherein the access instruction comprises identifying the drug traceability code; and pushing the acquired drug information toan access terminal. The drug traceability block chain related to the drug traceability method is is tamper-proof and can retain the uniqueness of the drug information and the detection certificates and can accurately and quickly provide data such as materials in different states in the drug production, circulation and detection process to consumers in real time so as to realize the tracing of thewhole drug production process.
Owner:山东浪潮质量链科技有限公司
Homogeneous vibration device used for bottled powder medicine production
InactiveCN107628285AAvoid disengagement from the limit ringThe effect of homogeneous oscillation is goodSolid materialJigging conveyorsVibration amplitudeCoil spring
The invention discloses a homogeneous vibration device used for bottled powder medicine production. The homogeneous vibration device comprises a rack, a conveying belt, a fixing plate, vibration pieces, elastic ropes, a connecting plate, movable pillars, spiral springs, colliding blocks, a guiding device, an intermittent driving device, a first height adjusting device, a second height adjusting device and a limiting plate. A second magnet and a first magnet in the moving state are alternately repelled by each other and attracted to each other, and therefore the first magnet can move verticallyin a reciprocating manner, and the colliding blocks collide with the vibration pieces inconstantly. Due to the effect of the elastic ropes, the vibration pieces vibrate vertically in installation holes, and therefore the upper side belt face of the conveying belt is impacted, a medicine bottle is vibrated, and a powder medicine loaded in the medicine bottle is homogeneously vibrated. Due to the limiting plate on the upper side, the phenomenon that the vibration amplitude of the medicine bottle is large, and the medicine bottle disengages from a limiting ring is avoided. By means of the homogeneous vibration device used for bottled powder medicine production, a good homogeneous vibration effect is maintained on the powder medicine, meanwhile, production is continuous, and a very good practical effect is achieved.
Owner:ANHUI HUARUI PHARMA TECH DEV CO LTD
Preprocessing system for near-infrared online detection and application thereof
InactiveCN102200507ARealize automatic positive and negative flushingEasy to cleanPreparing sample for investigationColor/spectral properties measurementsInfraredSolid particle
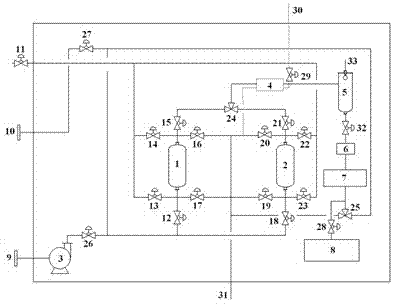
The invention provides a preprocessing system for near-infrared online detection, which is composed of a filter, a back-up filter, a variable-frequency centrifugal pump, a temperature control device, a buffering device, a flowmeter, a near-infrared spectrum acquisition device, an automatic sampling device, a liquid inlet and a liquid outlet. The preprocessing system provided by the invention can run automatically, continuously and reliably, and can be switched for use under the condition of not affecting the function of the whole system, therefore, the automatic positive and negative washing can be realized simultaneously, the samples of acquired spectrums can be acquired in a targeted mode, and the cleaning and beauty of the whole system can be maintained. By using the preprocessing system provided by the invention, the problem of difficulty in detection caused by more insoluble solid particles, unstable flow, easily-produced bubbles and large temperature fluctuation in the process of near-infrared online detection is solved, so that the acquired near-infrared spectrum is stable, reliable, and good in repeatability, thereby improving the accuracy of results of near-infrared on-line detection, promoting the development and application of the near-infrared spectral analysis technology in pharmaceutical production processes, and then truly realizing on-line quality control.
Owner:ZHEJIANG UNIV
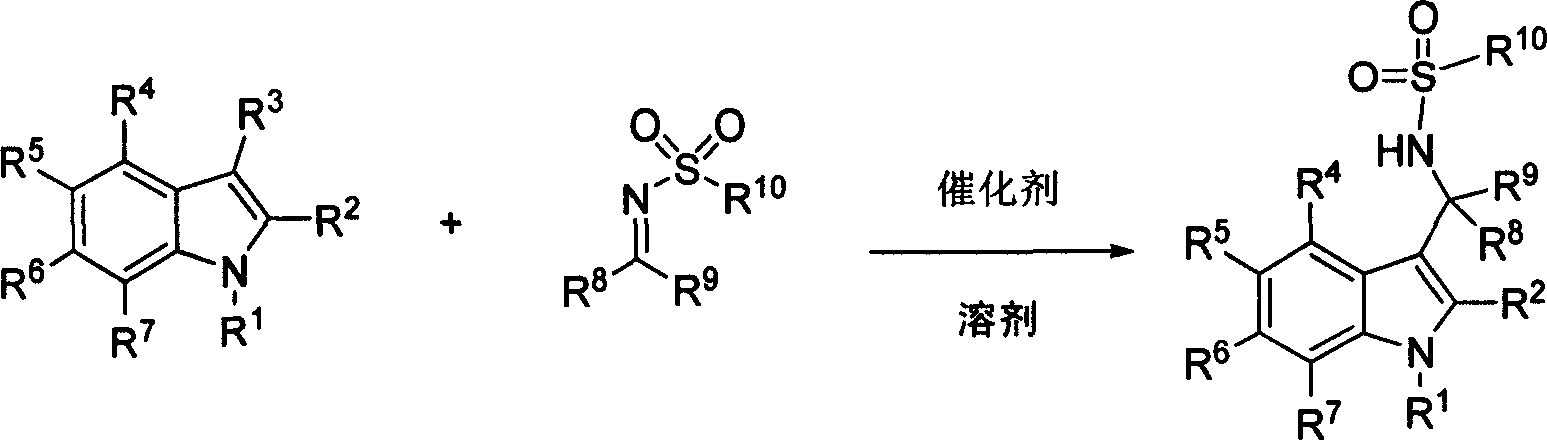
Process of synthesizing 3-methyl amino indole compound
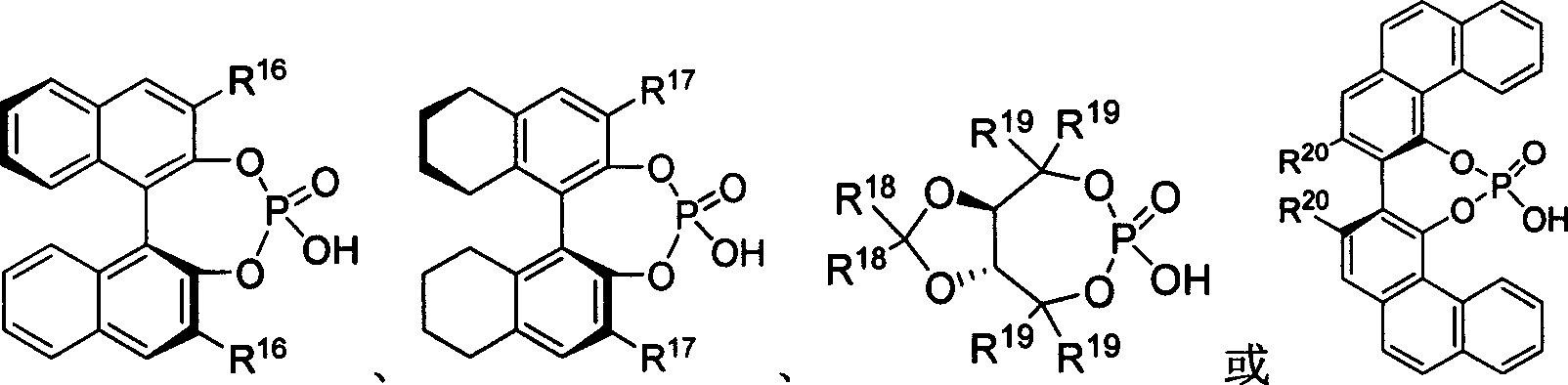
InactiveCN100999490AImprove efficiencyHigh enantioselectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsImidePhosphate
The present invention takes chiral phosphate as catalyst, uses sulfonyl imide and indole compounds synthesize 3-methylamino-indole compound of high-efficiency and high enantioselectivity. Compared with the existing method, it can be applied to many different types of indole and sulfonyl imide compounds, mild reaction conditions, simple operation. In addition, the reaction without joining any metal salts compounds, so useful for drug production and processing. And the reaction yields are better, high enantioselectivity.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Rapid grinding and crushing machine for powder pharmaceutical production
InactiveCN106513149AImprove crushing efficiencyFast crushingGrain treatmentsCooling chamberEngineering
The invention discloses a rapid grinding and crushing machine for powder pharmaceutical production. The rapid grinding and crushing machine comprises a machine case. An electric motor and a feed port are arranged at the upper end of the machine case. The lower end of the electric motor is provided with a grinding tank and a stirrer. The stirrer comprises a stirring shaft, crushing blades and a plurality of grinding blocks. A grinding medium is arranged in the grinding tank. The lower end of the grinding tank is provided with a filter screen. A circulating chamber is arranged under the filter screen. A separating screen is arranged at the lower end of the circulating chamber. The lower end of the separating screen is provided with a storage tank. A discharge port is arranged on the lower portion of the storage tank. The grinding tank is also further provided with a delivery pump, a cooling chamber, cooling fluid and a temperature sensor, and the cooling fluid and the temperature sensor are arranged in the cooling chamber. A cooling device is internally connected to the cooling chamber. The front end face of the machine case is provided with a control panel and a control circuit module. The rapid grinding and crushing machine has the advantages of being reasonable in structural design, high in speed, sufficient in grinding and easy and convenient to operate.
Owner:张桂菊
Preparation method of positively-charged ceramic micro-nano fiber membrane
InactiveCN103451850AHigh porosityLarge specific surface areaHeating/cooling textile fabricsNon-woven fabricsPorosityFiber
The invention discloses a preparation method of a positively-charged ceramic micro-nano fiber membrane. The preparation method comprises the steps of adding spinnable polymer in a solvent, then adding a ceramic precursor in the solvent, and obtaining spinning solution after stirring and aging; pouring the spinning solution in a micro pump, adopting an injection needle head which is scraped smoothly as a spray nozzle, and obtaining composite fiber through electrostatic spinning; firstly roasting the composite fiber in anoxic atmosphere at 200-500 DEG C, then roasting the composite fiber in inertia or reducing atmosphere at 400-400 DEG C for 1-5 hours, and obtaining the positively-charged ceramic micro-nano fiber membrane. According to the preparation method of the positively-charged ceramic micro-nano fiber membrane, disclosed by the invention, the positively-charged ceramic micro-nano fiber membrane which is prepared by combining the electrostatic spinning and a step-by-step roasting technology has high porosity, high specific surface area, high surface electrical performance and good high-temperature-resisting and corrosion-resisting performances, has great advantages of separating, shielding and removing bacteria and virus and has a wide application prospect in environment cleaning, pharmaceuticals production, bioengineering, foundation medicine and the like.
Owner:XIAN UNIV OF TECH
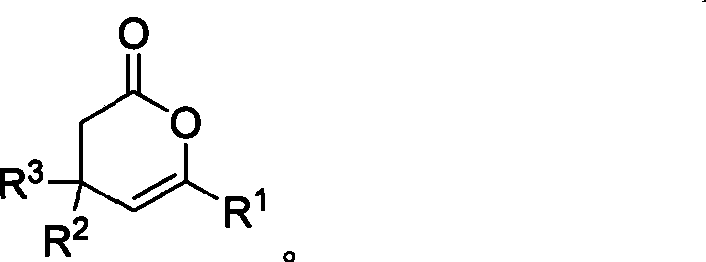
Method for synthesizing 4,6-substituted 3,4- dihydro-pyran-2-ketone derivative
InactiveCN101481369AIncrease production capacityEasy to handleOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsKetoneCarbene
The invention provides a method for effectively synthesizing a 4,6-substituted 3,-4-dihydro-pyran-2one derivative by efficiently synthesizing aldehyde-substituted cyclopropane compound with N-heterocyclic carbene as a catalyst. Compared with the existing method, the method has the advantages of wide suitable substrate range, convenient catalyst acquisition, mild reaction condition, simple operation, and high reaction efficiency. The method is performed without any metallic salt compounds, which is good for drug production and treatment.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
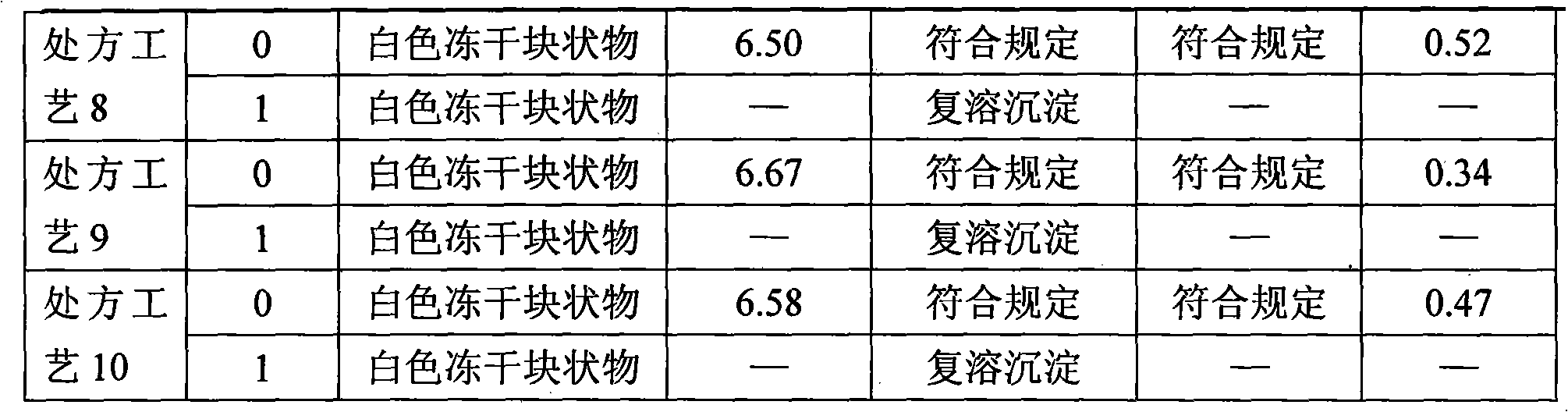
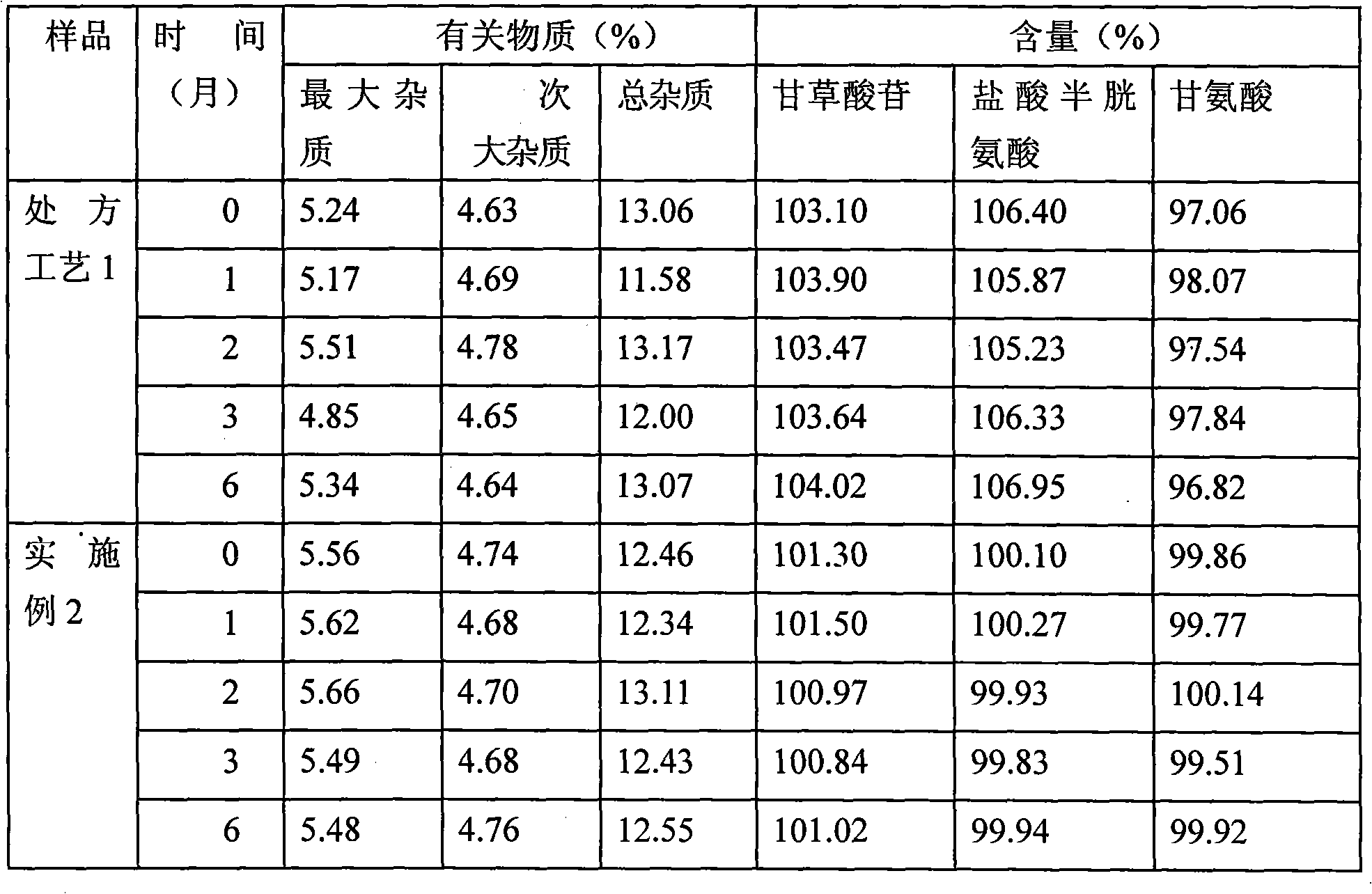
Powder injection of compound glycyrrhizic acid glycosides and preparation method thereof
The present invention relates to compound glycyrrhizin type powder injection and the preparation method thereof, in particular to compound glycyrrhizin for injection, compound glycyrrhizic acid mono-ammonium S powder injection and the preparation method thereof, which are characterized in that the powder injection consisting of glycyrrhizin (or mono-ammonium glycyrrhizinate), glycine and cysteine hydrochloride that are taken as the active ingredient and the bearer acceptable in medicine and the preparation method are included in the prescription; wherein, the bearer acceptable in medicine contains dextran. The compound glycyrrhizin type powder injection of the present invention can be preserved at room temperature, thus remarkably improving the stability of the medicine, better guaranteeing safety and significance of the medicine and effectively decreasing the storage and transportation cost in production and transportation processes of the medicine.
Owner:TIBET WEIXINKANG MEDICINE CO LTD
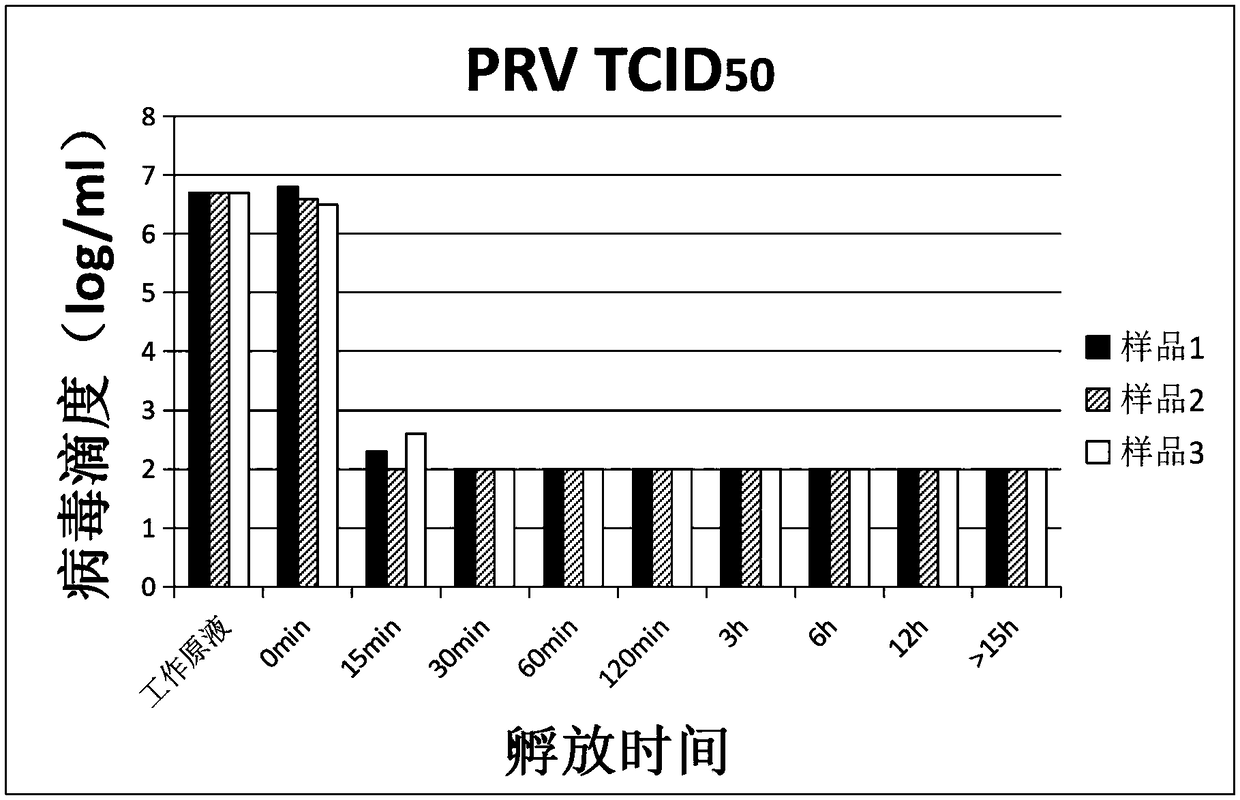
Verification method of low pH incubation virus inactivation
InactiveCN108342368AImprove scalabilitySimple purification processSsRNA viruses negative-senseMicrobiological testing/measurementVirus inactivationValidation methods
The invention relates to a verification method of low pH incubation virus inactivation. The method comprises the steps of selecting indicator viruses and corresponding host cells; amplifying and purifying the indicator viruses; titering virus working primary liquid and samples; optimizing the pH value of virus inactivation verification experiments; estimating the low pH incubation virus inactivation effect. By means of the method, the full process of genetically engineered drug production technology virus inactivation can be simulated in a laboratory, and the amplifying and purifying technology of the indicator viruses is optimized, so that the titer of the working primary liquid of the indicator viruses is increased, the pH value is unified to 7.0, the quality is stable, and subsequent operation is facilitated; meanwhile, the specific technological details like the determination of the optimal incubation pH value and the optimization of the pH adjusting mode of low pH incubation are optimized, and the efficiency and the effectiveness of the inactivation of common DNA viruses, RNA viruses, enveloped viruses and non-enveloped viruses are improved.
Owner:CANVEST WUHAN BIOTECH
Process for preparing mefenamic acid
ActiveCN101475505AImprove productivityLow yieldOrganic chemistryOrganic compound preparationDimethylaniline N-oxideAnti-inflammatory analgesics
The invention belongs to the technical field of anti-inflammatory drug production, and relates to a method for preparing mefenamic acid. The method comprises: adding o-chlorobenzoic acid and 2,3-dimethylaniline into a system which is formed by a non-protonic polar solvent and a dehydrant, performing condensation reaction in the presence of an acid-binding agent, a catalyst and a phase-transfer catalyst, and obtaining mefenamic sodium; acidifying the mefenamic sodium and obtaining coarse mefenamic acid products; and refining the coarse mefenamic acid products in an organic solvent and water and obtaining finished mefenamic acid products. The method improves the production efficiency of the mefenamic acid and reduces the production cost of the mefenamic acid.
Owner:BAOJI TIANXIN PHARM CO LTD
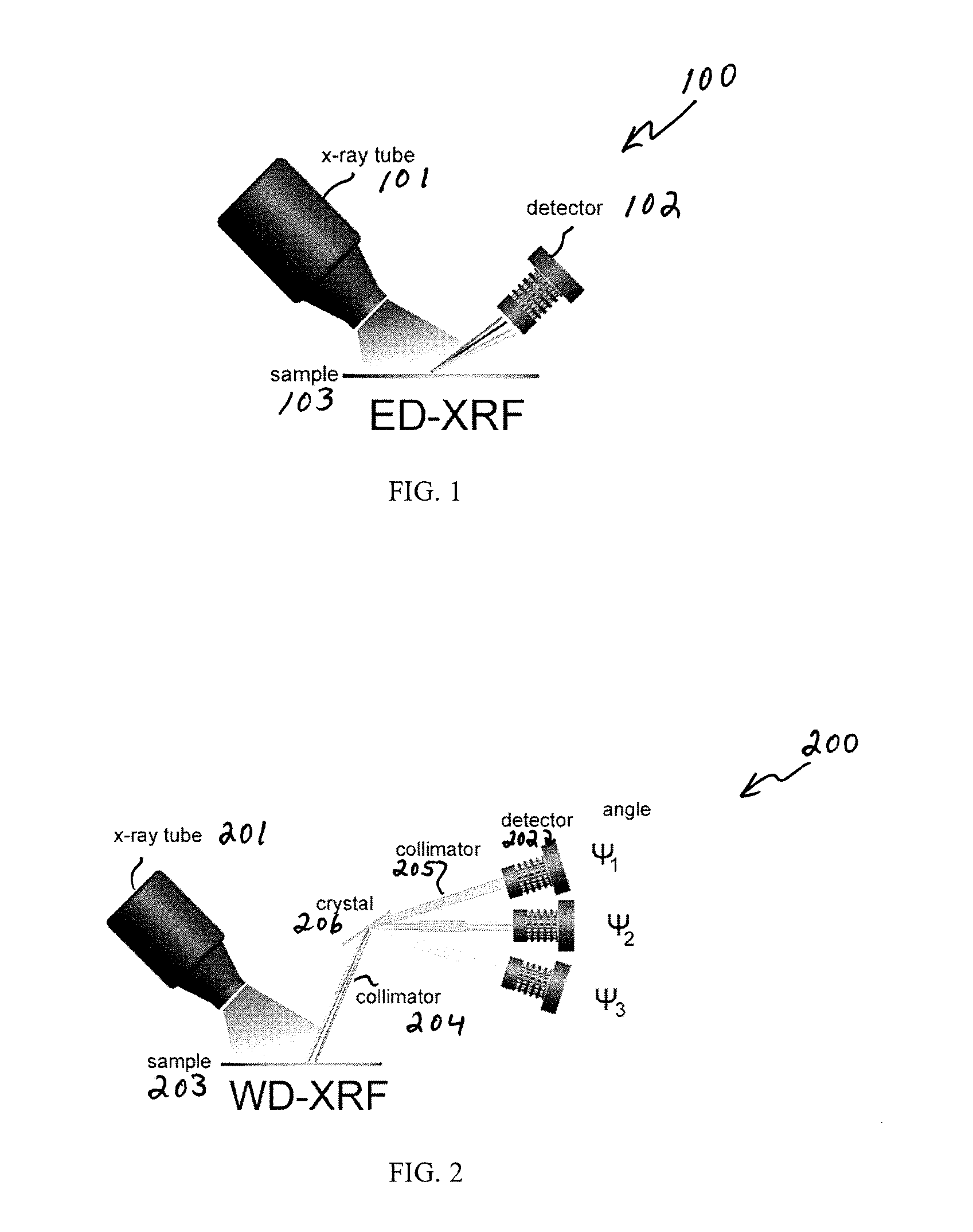
Metal analysis during pharmaceutical manufacturing
ActiveUS20170038319A1Material analysis using wave/particle radiationTesting medicinal preparationsMetal impuritiesPharmaceutical drug
A system and method for detecting, measuring, and analyzing for metallic impurities in pharmaceutical drugs and compounds utilizes an x-ray fluorescence system. The system and method may be co-located with a pharmaceutical manufacturing process for in-line continuous monitoring of metal impurities. The pharmaceutical products may be in a form selected from a powder, slurry, pill, tablet, and gel.
Owner:UHV TECH INC
Closure container for single dose disposable pharmaceutical delivery system
The disposable unit dose pharmaceutical delivery system may be used by trained medical personnel for intradermal or subcutaneous injections in applications currently being serviced by conventional pre-filled syringes. The delivery system may also be used by personnel with little or no medical training for mass immunizations in underdeveloped parts of the world, insulin injections, and / or emergency epinephrine injections. The delivery system includes an envelope with a tear-away section and a dispensing section. When the tear-away section has been removed, a needle is exposed and is ready for insertion in the patient. The dispensing portion is squeezed between the thumb and forefinger to inject the pharmaceutical into the patient. The exterior surface of the envelope may be used for advertising by a pharmaceutical producer, a supplier or otherwise.
Owner:SERATOUCH
Ginkgo snail-killing micro emulsion and preparation method thereof
The invention discloses a gingko snail killing microemulsion agent and a preparing method, which relates to the technical field of plant source agricultural drug production. The invention takes the seed shell extract of the gingko containing a ginkgoic acid as the source drug, and the invention is made by configuring auxiliary surface active agents, solution agent and antifreeze agent as well as water. The producing method comprises the following operating steps: the gingko external seed shell extract, the microemulsion agent and the solution agent are blended to be an even and transparent oil phase; the steamed water or the deionized water are added slowly to form an oil enclosed water type emulsion during the agitating process; through agitating and heating, the emulsion is rapidly converted into a water enclosed oil type; a steady O or W type emulsion after being cooled to reach the constant indoor temperature is obtained. The invention has fine environmental compatibility of the emulsion conformation, also completely realizes the advantages of the gingko snail killing agent with high efficiency to the snail and safety to people and animals. The microemulsion agent takes water as the main solvent with slight influence to environment. In addition, the invention has remarkable synergism; the LC 50 and LC 90 of the 24h respectively are 0.56mg per liter and 3.5mg per liter, which are obviously better than that of the 24h snail killing effect (LC50 is equal to 1.35mg per liter and the LC90 is equal to 3.85mg per liter) of the external seed shell extract of the original drug.
Owner:JIANGSU UNIV
Method for preparing prepared rehmannia roots
InactiveCN106177152APromote digestion and absorptionReduce churnSenses disorderAntipyreticRadix Rehmanniae PreparataRehmannia
The invention discloses a method for preparing prepared rehmannia roots. The method includes the following steps that 1, dried rehamnnia roots are cleaned with water and dried; 2, materials are prepared, wherein the dried rehamnnia roots and rice wine in the weight ratio of 10:12 are prepared; 3, 28% of the total amount of the rice wine is added into the dried rehamnnia roots obtained in the step 2, and soaking is carried out till the rice wine is completely absorbed; 4, the dried rehamnnia roots provided with the rice wine are placed into a ceramic tank, enclosed and steamed for 24 hours in a water isolation mode; 5, the steamed dried rehamnnia roots are taken out and dried in the sun in a sunlight room till medium-well drying is achieved; 6, 9% of the total amount of the rice wine is added, soaking is carried out till all the rice wine is completely absorbed, and the step 4 and the step 5 are repeated; 7, soaking, steaming and airing are repeated for nine times jointly, finally drying is carried out through sunlight, and the prepared rehmannia roots are obtained. By means of the prepared rehmannia roots prepared with the method and prepared rehmannia root medicinal slices thereof can meet the Chinese patent medicine preparing requirements of pharmaceutical producing enterprises, and can also meet personal decoration decocting requirements.
Owner:山西广誉远国药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com