Process of synthesizing 3-methyl amino indole compound
A methylaminoindole compound and a technology for the indole compound are applied in the field of synthesizing optically active 3-methylaminoindole compounds, and the effects of mild reaction conditions and easy operation are achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
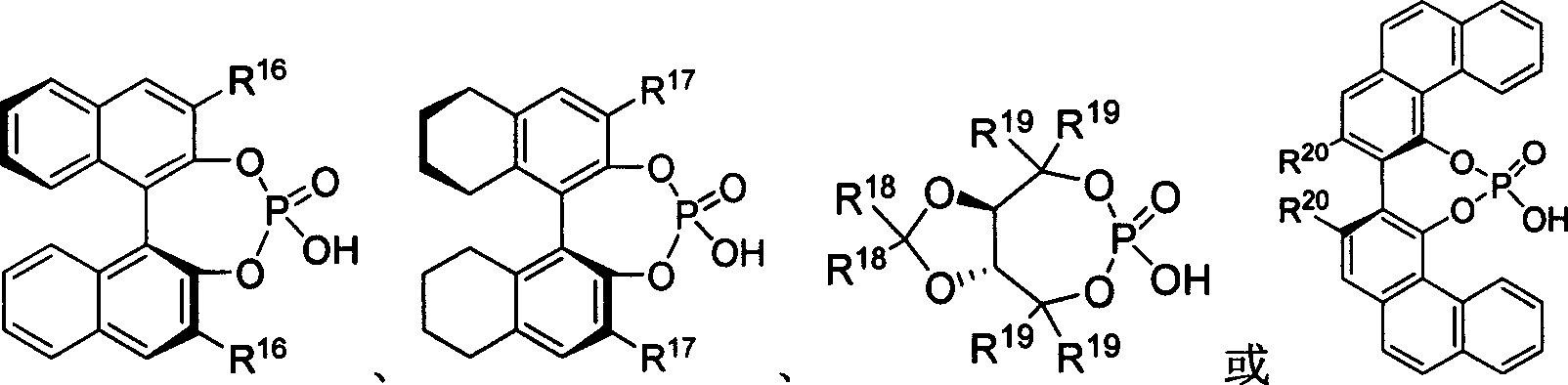
[0020] Embodiment 1: the preparation of chiral phosphoric acid
[0021] Under the protection of argon at room temperature, dissolve diphenol or dihydroxy derivatives (0.5mmol) in 1mL dry pyridine in a dry reaction tube, and dissolve (1.0mmol) phosphorus oxychloride Slowly added dropwise to the system, stirred at room temperature for 3 hours. 1 mL of water was slowly added dropwise to the system, and stirred at room temperature for 30 minutes. Dichloromethane was added to dissolve, washed with 1N hydrochloric acid aqueous solution (10mL×3), the organic layer was dried over anhydrous sodium sulfate, the solvent was spun off under reduced pressure, and the residue was separated by column chromatography to obtain the product.
[0022] C1: (S)-3,3'-[3,5-bis(trifluoromethyl)phenyl]2-1,1'-binaphthol phosphate
[0023] (S)-3,3′-[3,5-Bis(trifluoromethyl)phenyl]2-1,1′-binaphthyl phosphate
[0024] (or its enantiomers)
[0025] Solid, 89% yield. IR (CHCl 3 ) 1620, 1501, 1474, 137...
Embodiment 2
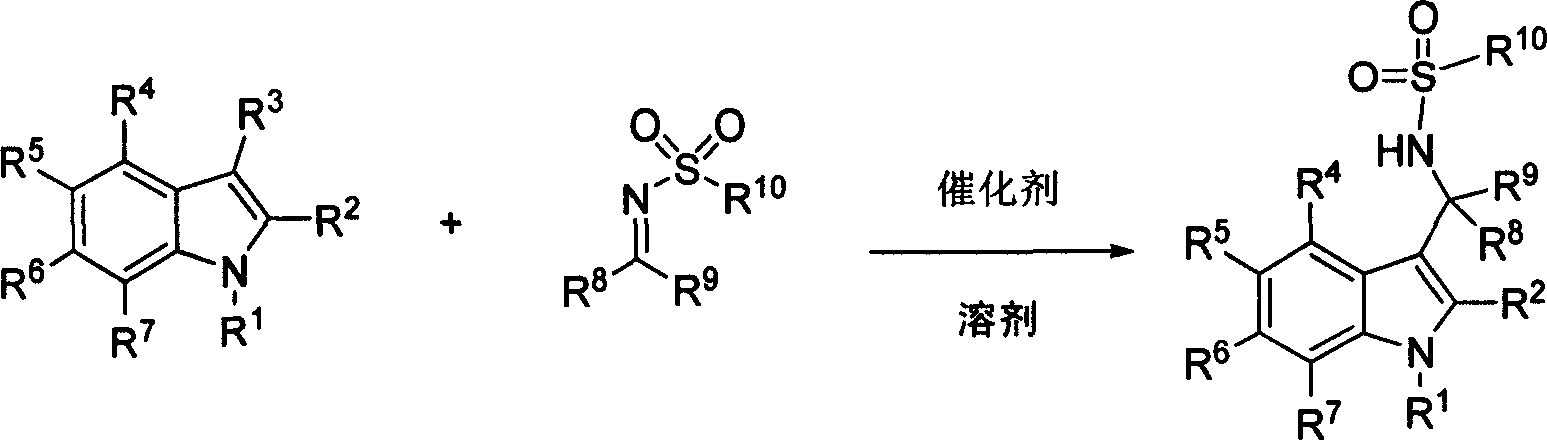
[0057] Example 2: Chiral phosphoric acid catalyzed addition reaction of indoles to sulfonimides
[0058] Add (chiral) phosphoric acid compound (0.025mmol), sulfonimide compound (0.25mmol), and toluene 1mL in sequence to a dry reaction tube, stir at room temperature for 10 minutes, then put it in a -60°C bath and stir for 5 minutes , add indole compound (0.75mmol), react under -60 ℃, the reaction finishes, add 10%NaHCO 3 aqueous solution (3mL), separated, the organic layer was washed once with 5mL water, once with saturated brine 5mL, and the organic layer was washed with anhydrous Na 2 SO 4 After drying, the solvent was removed under reduced pressure and the residue was separated by column chromatography to obtain the product.
[0059] P1: (R)-N-((3-(1H-indol-3-yl)(phenylmethyl)-4-methylphenylsulfonamide (R)-N-((1H-indol-3- yl)(phenyl)methyl)-4-methylbenzenesulfonamide
[0060] (or its enantiomers)
[0061] Catalyst is C7 (10mol%),-60 ℃; R f =0.40 (ethyl acetate / petrol...
Embodiment 3
[0142] Embodiment 3: removal and conversion (application example) of sulfonyl group in the product
[0143] P22: (R)-N-((3-(1H-indol)yl)(phenylmethyl)-benzylcarbonamide
[0144] (R)-Benzyl(1H-indol-3-yl)(phenyl)methylcarbamate
[0145]
[0146] In a dry reaction tube was added P2 (1.10 g, 2.5 mmol) followed by dry THF (10 mL). This solution was cooled to -78°C and liquid ammonia (50 mL) was added, then excess sodium metal (500 mg) was added in portions until the dark blue color was maintained for 5 minutes. Ammonium chloride solid (5 g) was added to quench at -78°C, the system slowly rose to room temperature, and the liquid ammonia volatilized. The resulting residue was partitioned with water (50 mL) and dichloromethane (50 mL), the organic phase was collected, and the aqueous phase was extracted with dichloromethane (4×50 mL). The organic layers were combined, dried and concentrated. The resulting white solid was dissolved in THF (30 mL), and water (15 mL) was added. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com