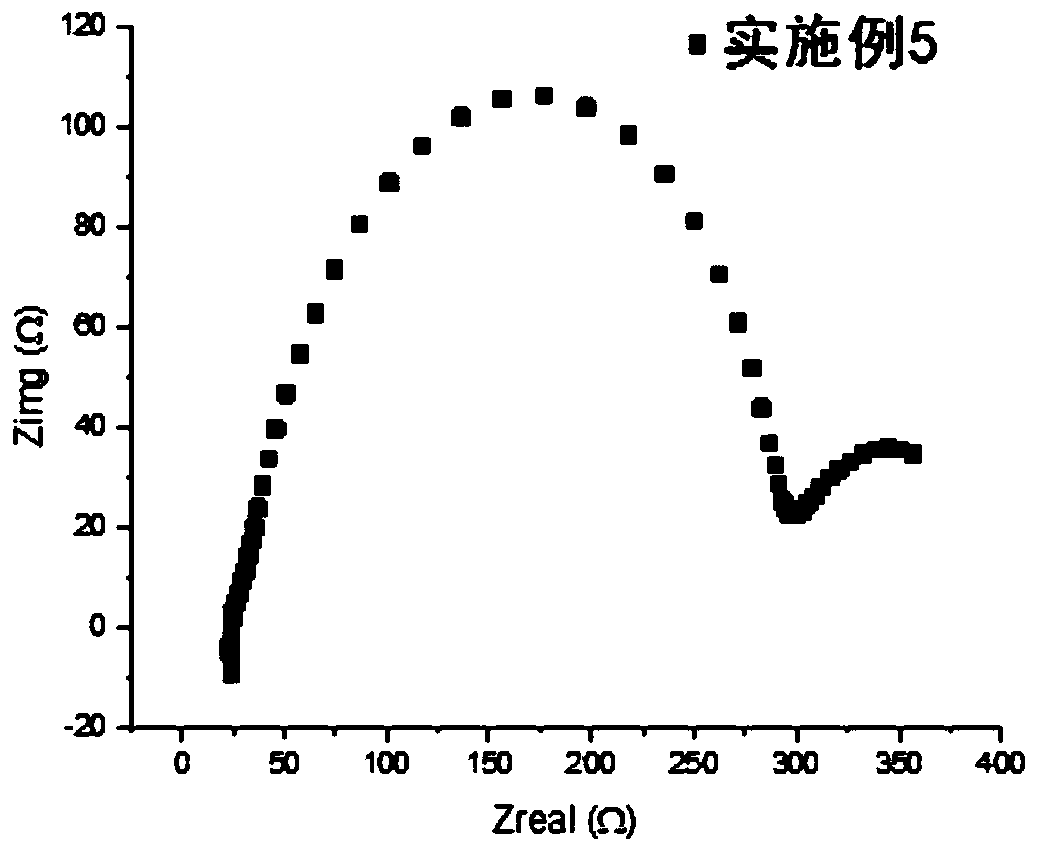
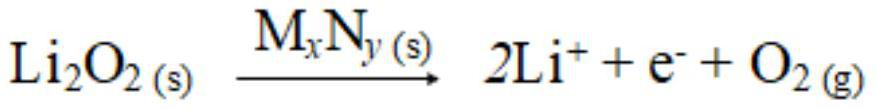Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Decomposition potential" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Decomposition potential or Decomposition voltage, in electrochemistry, refers to the minimum voltage (difference in electrode potential) between anode and cathode of an electrolytic cell that is needed for electrolysis to occur.
Removal of substances from metal and semi-metal compounds
InactiveUS6712952B1Polycrystalline material growthFrom normal temperature solutionsMelting temperatureMetal
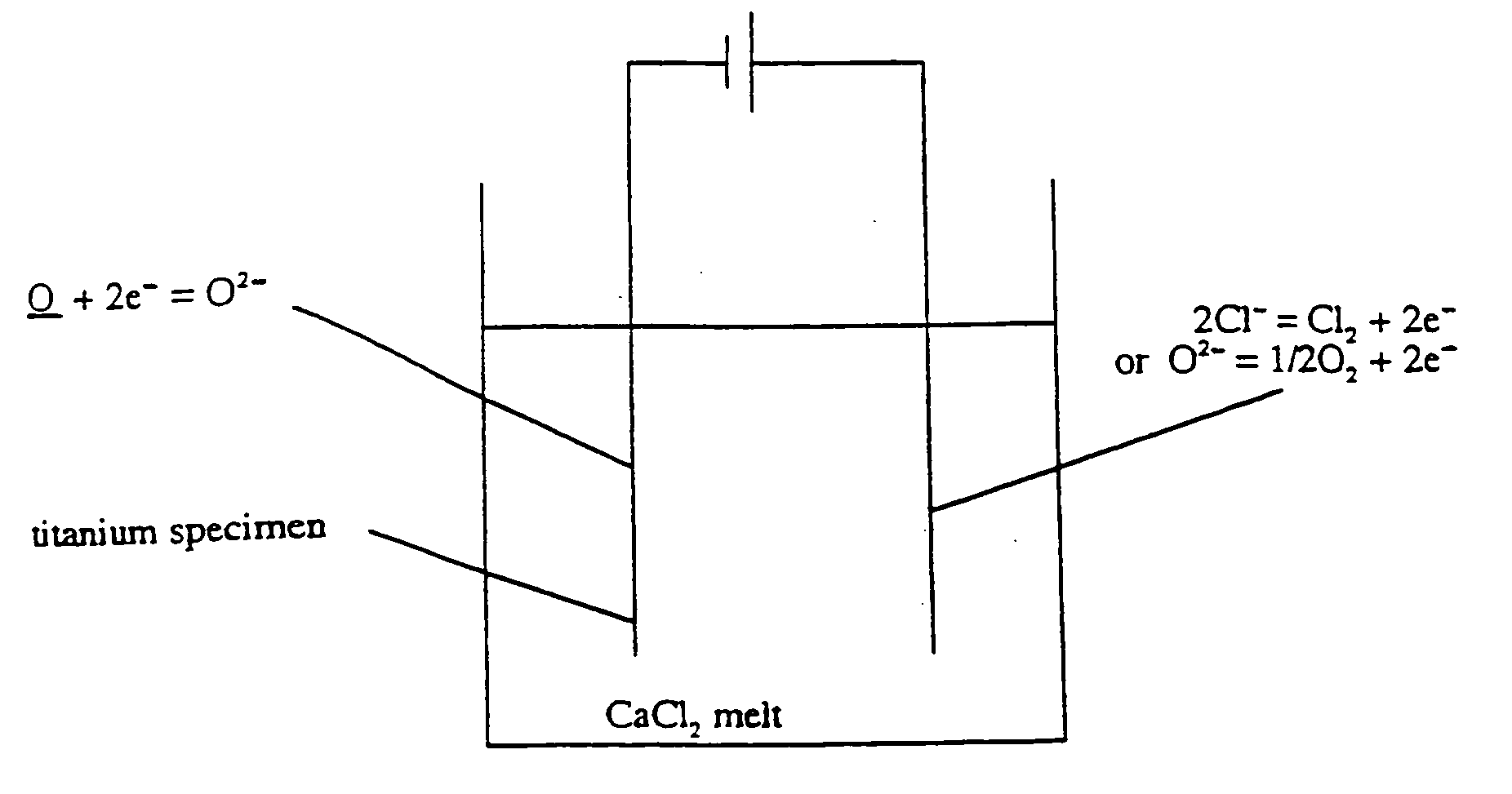
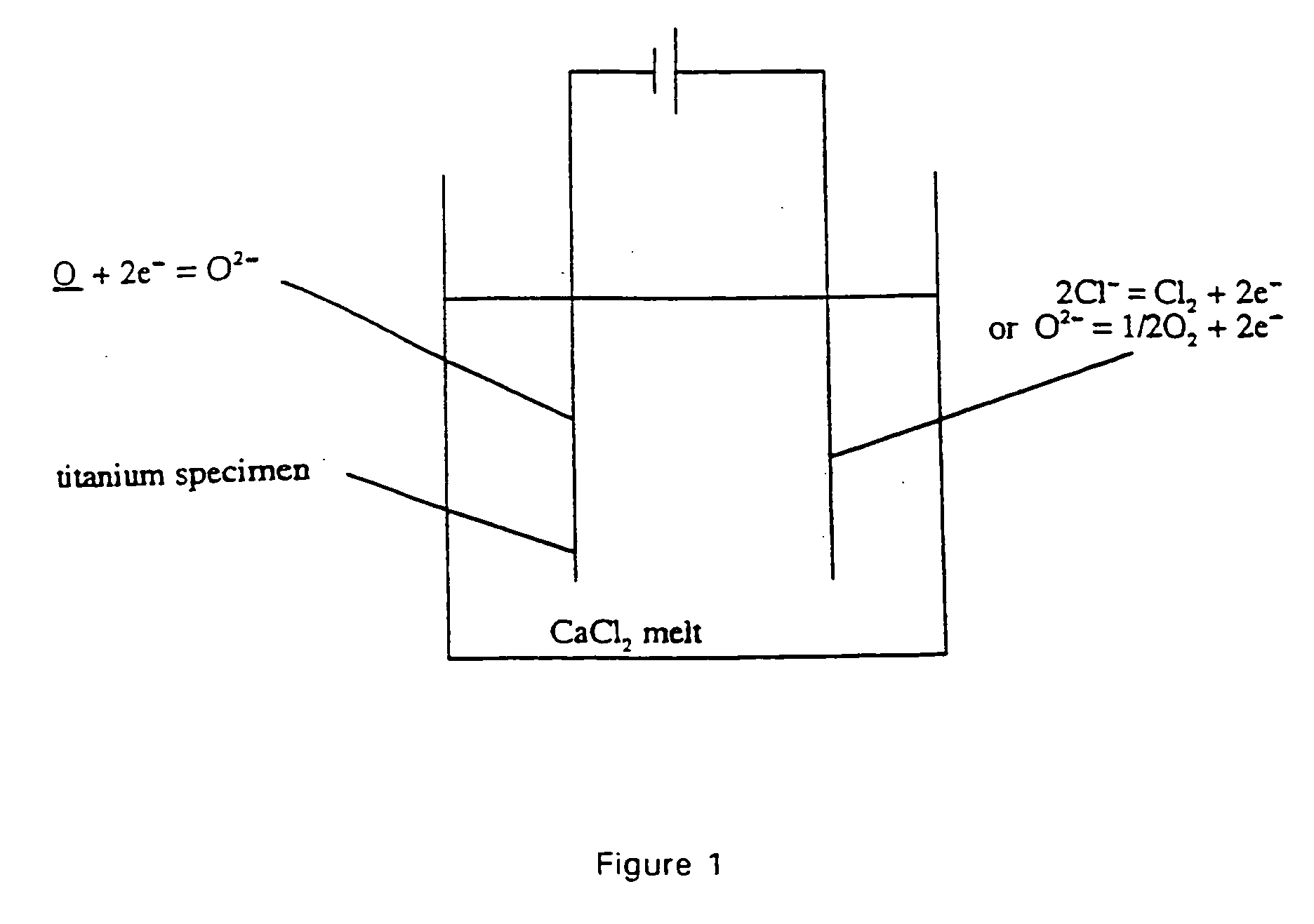
The present invention pertains to a method for removing a substance (X) from a solid metal or semi-metal compound (M<1>X) by electrolysis in a melt of M<2>Y, which comprises conducting the electrolysis under conditions such that reaction of X rather than M<2 >deposition occurs at a electrode surface, and that X dissolves in the electrolyte M<2>Y. The substance X is either removed from the surface (i.e., M<1>X) or by means of diffusion extracted from the case material. The temperature of the fused salt is chosen below the melting temperature of the metal M<1>. The potential is chosen below the decomposition potential of the electrolyte.
Owner:METALYSIS
Lithium-ion battery
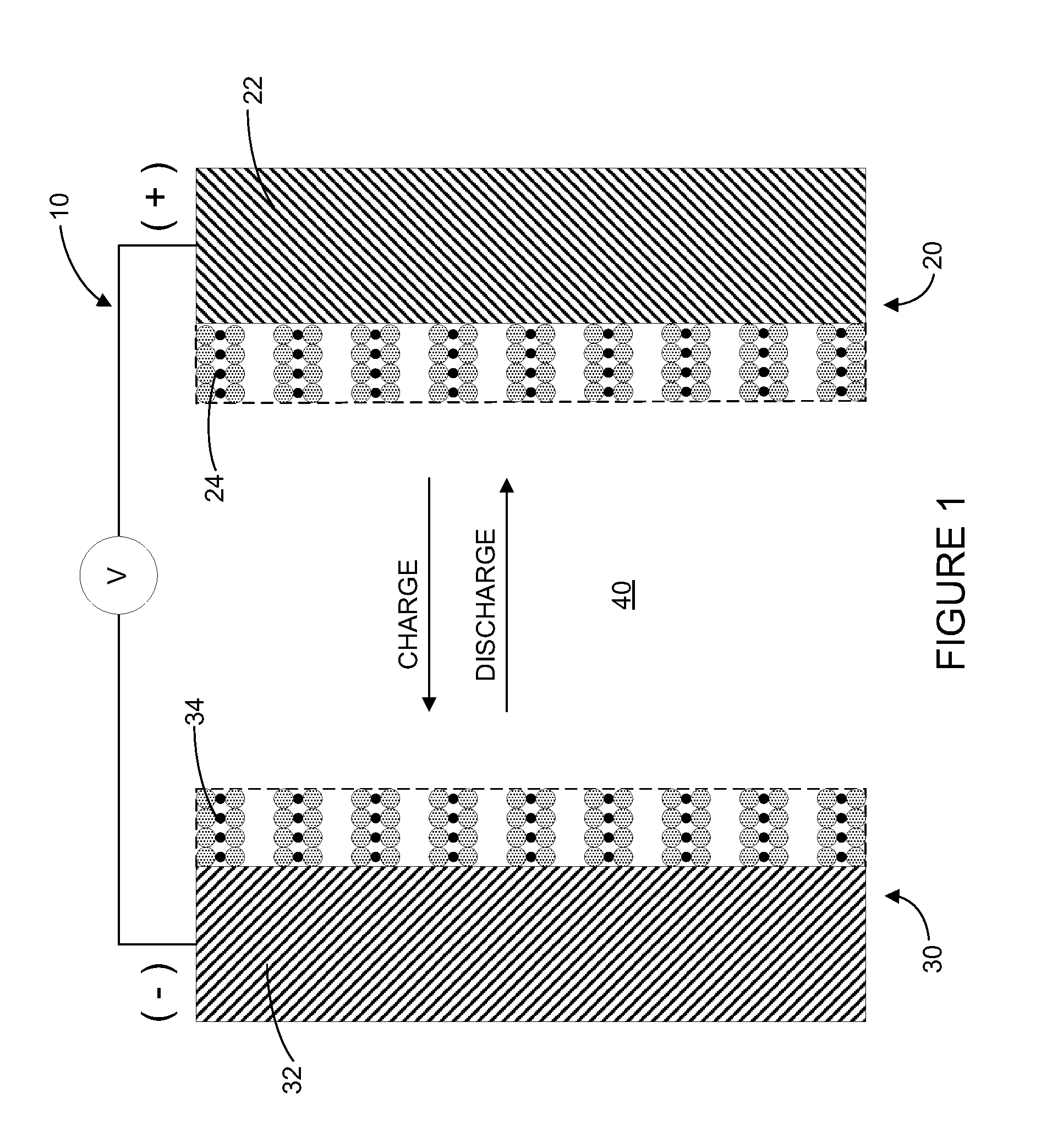
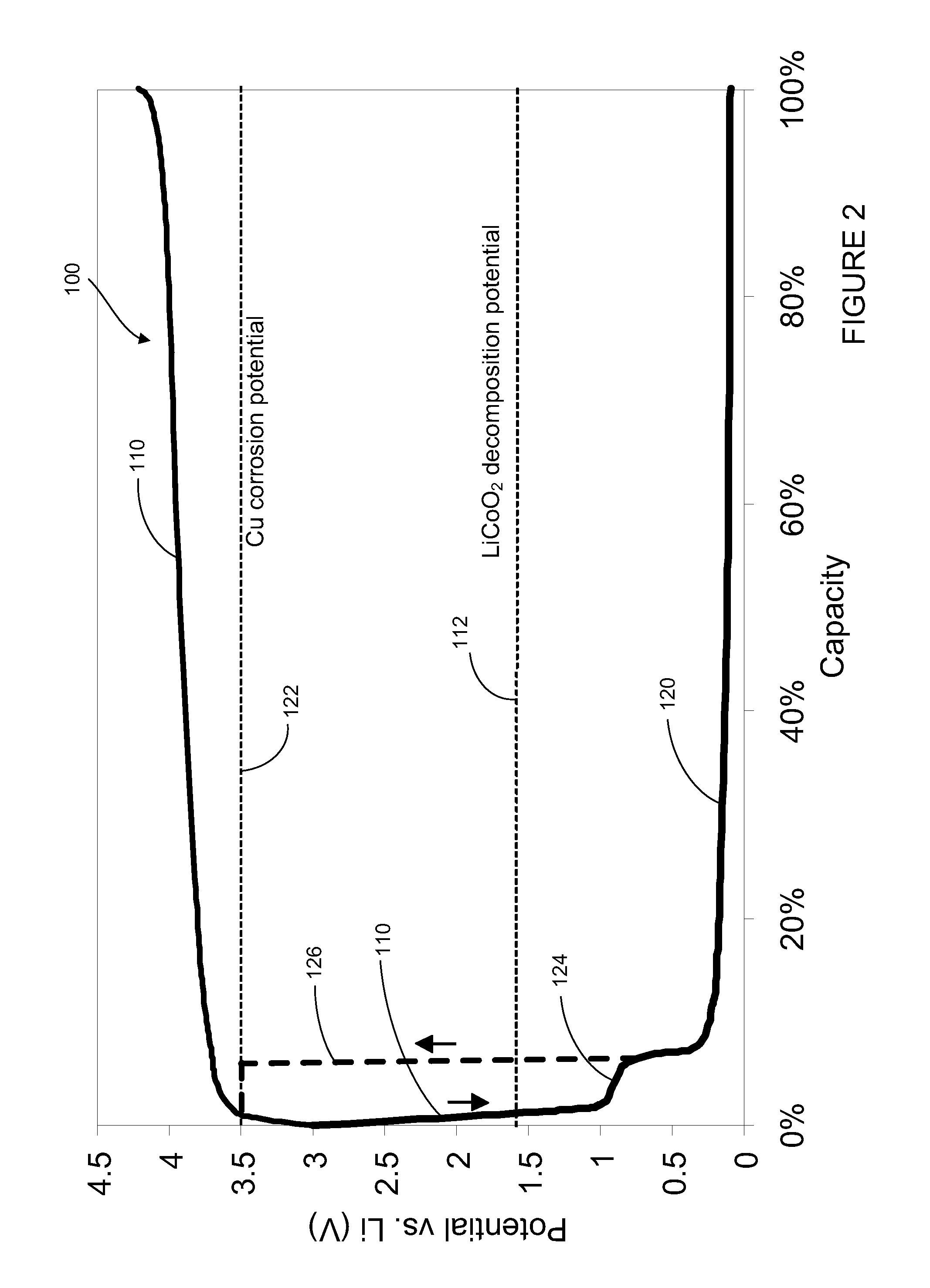
ActiveUS7794869B2Active material electrodesNon-aqueous electrolyte accumulator electrodesElectrical connectionEngineering
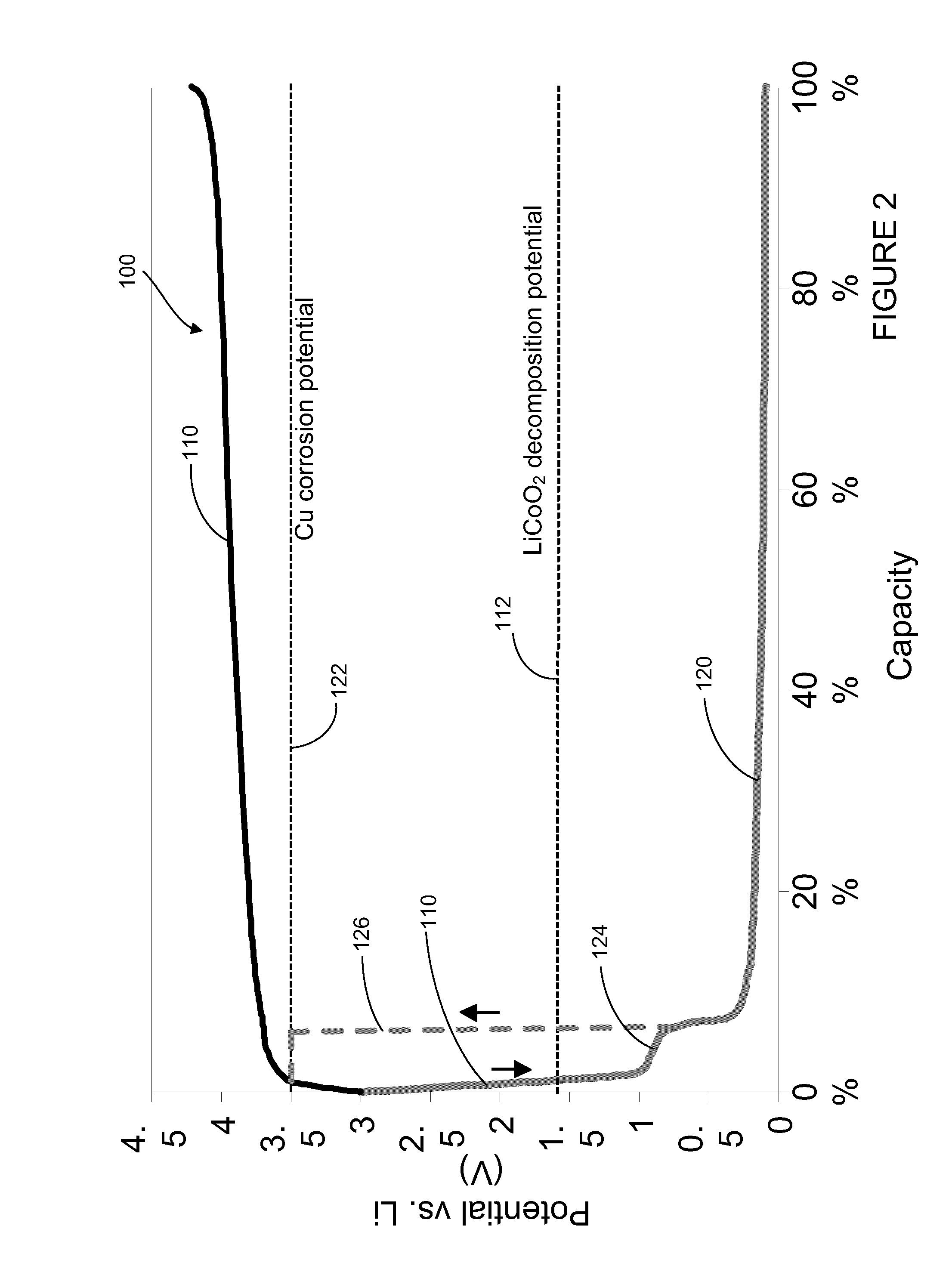
A battery includes a positive electrode having a current collector and a first active material and a negative electrode having a current collector and a second active material. The battery also includes an auxiliary electrode having a current collector and a third active material. The auxiliary electrode is configured for selective electrical connection to one of the positive electrode and the negative electrode. The first active material, second active material, and third active material are configured to allow doping and undoping of lithium ions. The third active material exhibits charging and discharging capacity below a corrosion potential of the current collector of the negative electrode and above a decomposition potential of the first active material.
Owner:MEDTRONIC INC
Removal of oxygen from metal oxides and solid solutions by electrolysis in a fused salt
InactiveUS20040159559A1Polycrystalline material growthFrom normal temperature solutionsMolten saltOxygen
The present invention pertains to a method for removing a substance (X) from a solid metal or semi-metal compound (M<1>X) by electrolysis in a melt of M<2>Y, which comprises conducting the electrolysis under conditions such that reaction of X rather than M<2 >deposition occurs at a electrode surface, and that X dissolves in the electrolyte M<2>Y. The substance X is either removed from the surface (i.e., M<1>X) or by means of diffusion extracted from the case material. The temperature of the fused salt is chosen below the melting temperature of the metal M<1>. The potential is chosen below the decomposition potential of the electrolyte.
Owner:METALYSIS
Medical device having lithium-ion battery
A medical device includes a rechargeable lithium-ion battery for providing power to the medical device. The lithium-ion battery includes a positive electrode comprising a current collector and a first active material and a negative electrode comprising a current collector, a second active material, and a third active material. The first active material, second active material, and third active material are configured to allow doping and undoping of lithium ions. The third active material exhibits charging and discharging capacity below a corrosion potential of the current collector of the negative electrode and above a decomposition potential of the first active material.
Owner:MEDTRONIC INC
Method for preparing rare earth alloy by molten salt electrolysis
ActiveCN103572329AInhibition formationImprove deoxidation efficiencyAlkaline earth metalPresent method
Provided in the present invention are a rare earth metal and a rare earth metal alloy and a method for the preparation of these by molten salt electrolysis. In the method for the preparation of the rare earth metal alloy by molten salt electrolysis, the electrolyte is an alkali metal or the chloride-fused salt of an alkaline earth metal, the positive electrode is an inert electrode or graphite, the negative electrode is composed of a rare-earth metal oxide and the oxides of other alloy components and / or metal powders, and electrolysis is induced by the passage of a direct current. During the electrolytic process, the temperature of electrolysis is higher than the melting point of the rare earth metal alloy produced and lower than the melting point of the negative electrode; the surface layer of the positive electrode is in the first stage electrolyzed to a liquid metal film which accumulates to a certain volume before falling to the crucible at the bottom. The current density of the negative electrode is sufficient to separate out from said negative electrode the components of the rare earth metal alloy. The electrolysis voltage is lower than the decomposition potential of the electrolyte and higher than the decomposition potential of the oxides corresponding to each component of the rare earth metal alloy. A crucible is used to collect the rare earth metal and the alloy obtained through the present method. The present method is technically simple and environmentally-friendly, while featuring low energy consumption, high current efficiency, and low costs.
Owner:LESHAN YOUYAN RARE EARTH NEW MATERIAL CO LTD
A crosslinked polymer electrolyte suitable for lithium secondary battery and a preparation method thereof
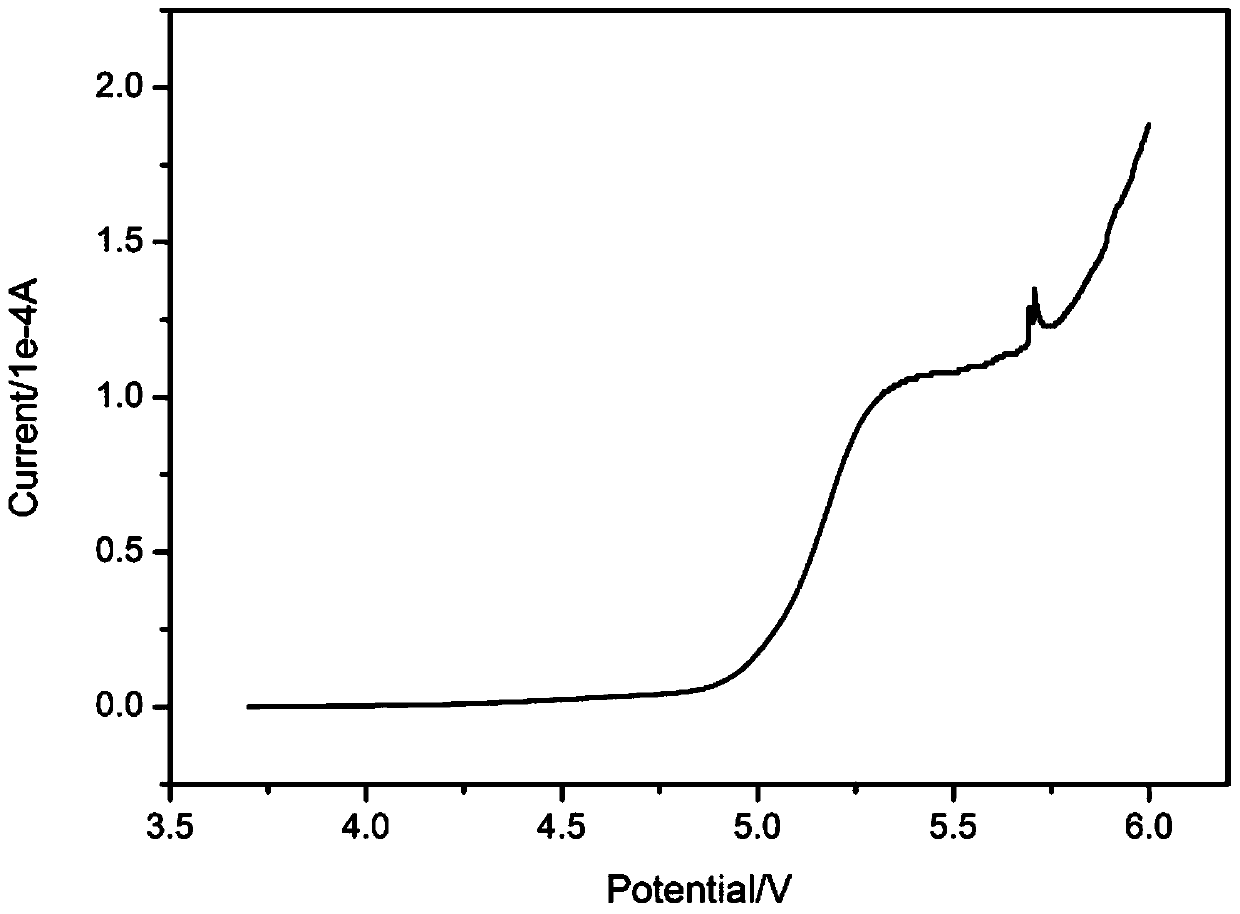
The invention discloses a crosslinked polymer electrolyte suitable for a lithium secondary battery and a preparation method thereof. The electrolyte is crosslinked by a low molecular weight polyetherderivative under the action of a crosslinking agent. The electrolyte has the advantages of simple preparation, high room temperature conductivity, good flexibility and oxidation decomposition potential of higher than 4.5 V.
Owner:HARBIN INST OF TECH WUXI RES INST OF NEW MATERIALS +1
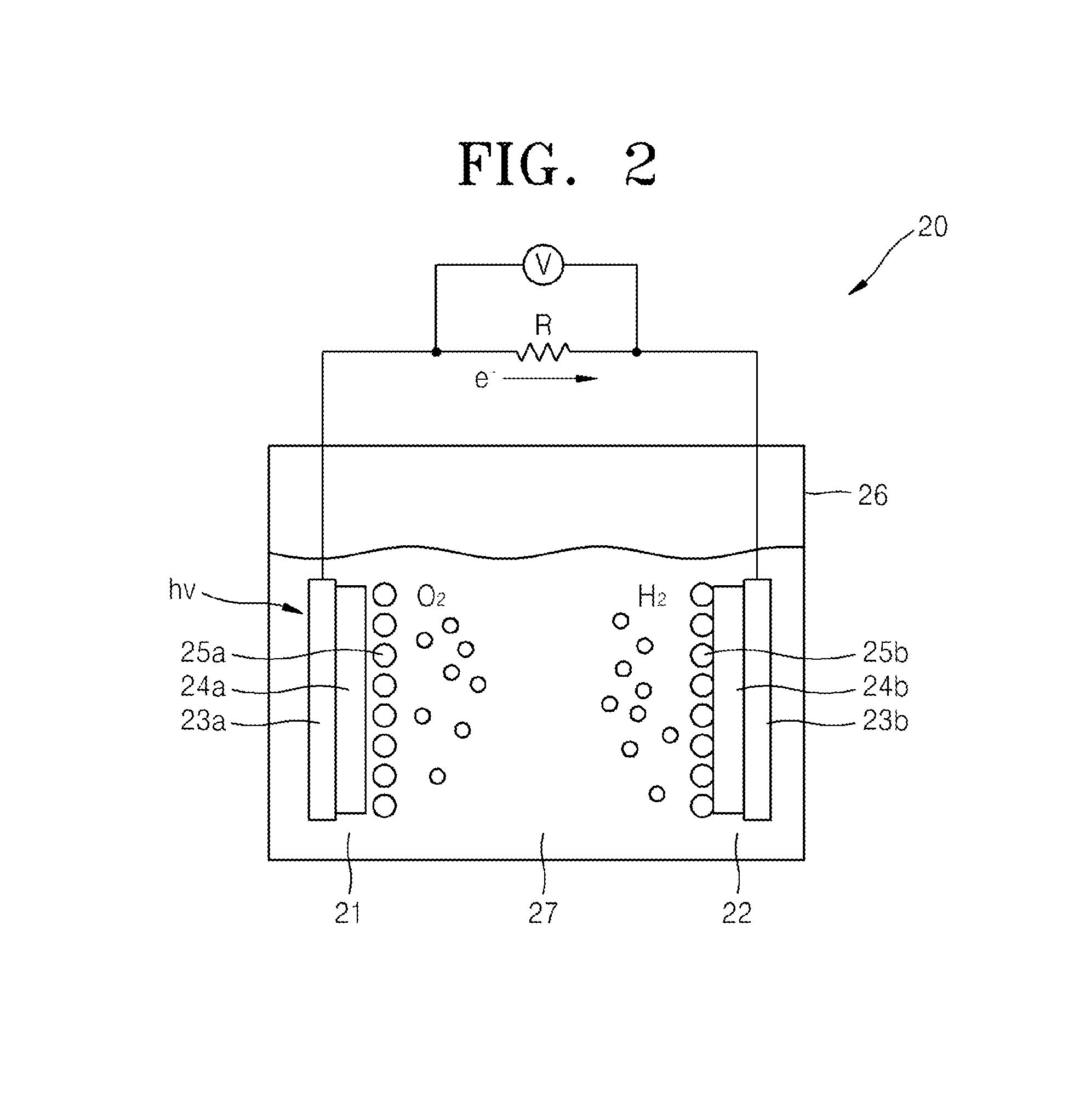
Composite protective layer for photoelectrode structure, photoelectrode structure including the composite protective layer, and photoelectrochemical cell including photoelectrode structure
A composite protective layer for a photoelectrode, the composite protective layer including a chemical protective layer; and a physical protective layer, wherein the chemical protective layer has corrosion rate of 0.1 Coulombs per square centimeter per 10 hours or less when evaluated at a water decomposition potential, and the physical protective layer has a moisture transmittance rate of 0.001 grams per square meter per day or less and has an electrical conductivity.
Owner:SAMSUNG ELECTRONICS CO LTD
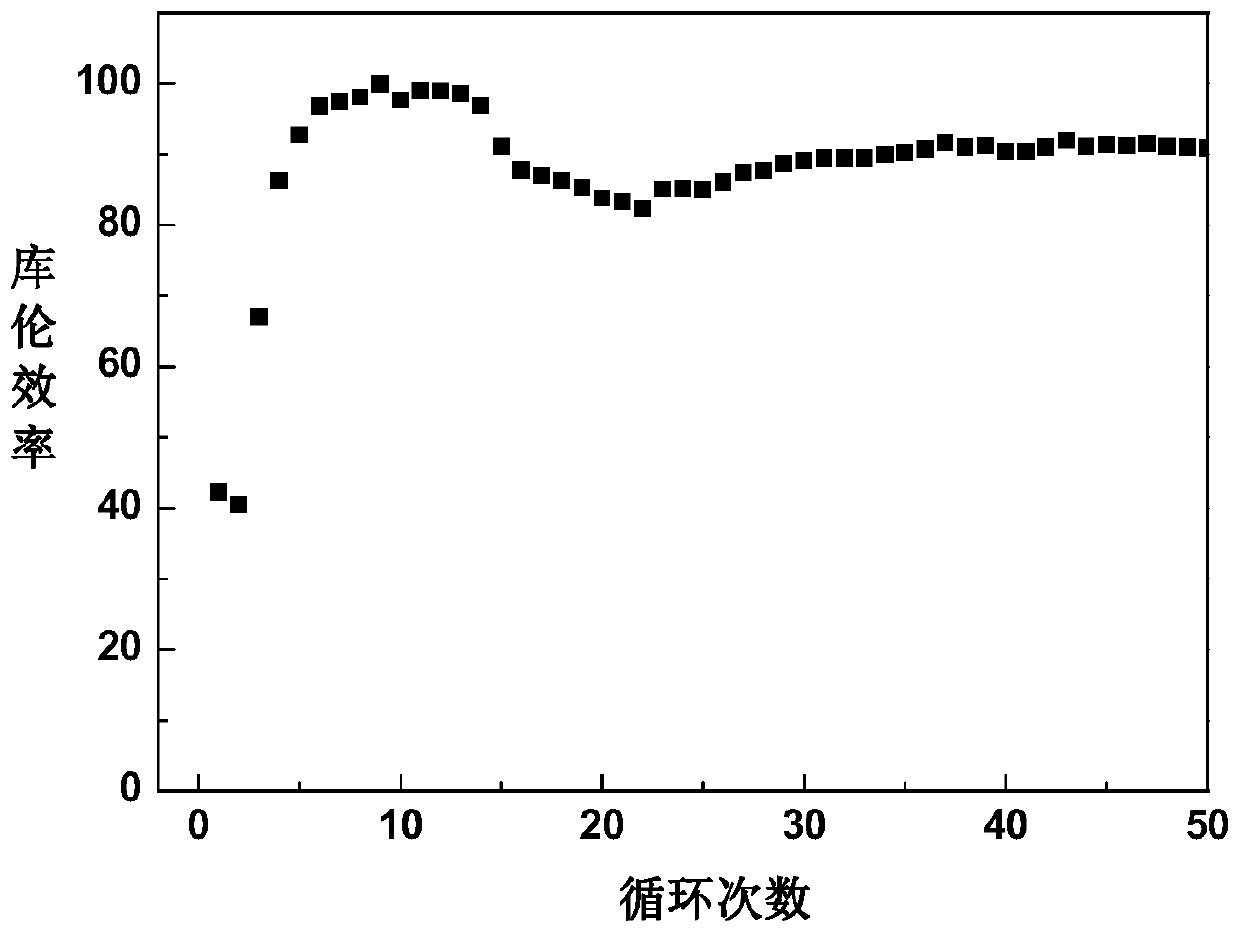
Electrolytic solution of rechargeable magnesium cell and application method thereof
InactiveCN103794815AHigh anodic oxidation decomposition potentialHigh deposition-dissolution efficiencySecondary cellsCyclic processSolvent
The invention discloses an electrolytic solution of a rechargeable magnesium cell and an application method thereof. The electrolytic solution comprises the following constituents: a solute is a phenyl amines compound and alkyl magnesium halide or a phenyl amines compound and alkyl magnesium and also can contain aluminium trichloride, and a solvent is ether; the concentration of the electrolytic solution is 0.1-3 mol / L. The electrolytic solution provided by the invention is applied to the rechargeable magnesium cell, a positive electrode oxidative decomposition potential can achieve more than 2.3V vs.Mg, and the efficiency of a stable circulation process can keep more than 90%; the electrolytic solution provided by the invention has the advantages that the preparation is simple, the raw material is cheap, the magnesium sedimentation-digestion efficiency is high, and the positive electrode oxidative decomposition potential is high.
Owner:SHANGHAI JIAO TONG UNIV
Electrolyte adopting propylene carbonate as main solvent
InactiveCN103985905AImprove initial discharge capacityImprove cycle lifeSecondary cellsOrganic solventDecomposition
The invention relates to an electrolyte adopting propylene carbonate as a main solvent, which relates to the electrolyte of a lithium ion battery. The electrolyte contains lithium salt, organic solvent and additive; the organic solvent contains propylene carbonate and chain-type carbonic ester; the additive contains a film-forming additive A and an additive B for inhibiting the embedding of the propylene carbonate; the electrolyte contains the following components by mass percent: 10 to 18 percent of lithium salt, 25 to 60 percent of propylene carbonate, 15 to 60 percent of chain-type carbonic ester, 0.5 to 5 percent of additive A and 0.1 to 10 percent of the additive B. Sulfite is used as the additive of the lithium ion battery electrolyte; since the sulfite is high in reduction potential which is higher than the decomposition potential of the propylene carbonate, the sulfite forms a layer of stable and compact SEI film on the surface of graphite before the decomposition of the propylene carbonate, the propylene carbonate solvent can be effectively prevented from being collectively embedded into the graphite layer along with the lithium ion, the initial discharging capacity of the battery is improved, and the cycle life of the battery is prolonged.
Owner:XIAMEN UNIV +1
Lithium-ion battery
ActiveUS20100239908A1Electrode carriers/collectorsOrganic electrolyte cellsEngineeringLithium electrode
A lithium-ion battery includes a positive electrode comprising a current collector and a first active material and a negative electrode comprising a current collector, a second active material, and a third active material. The second active material comprises a lithium titanate material and the third active material is selected from the group consisting of LixVO2 where x is between 0.05 and 0.4, LiMxMn(2−x)O4 where M is a metal and x is less than or equal to 1, V6O13, V2O5, V3O8, MoO3, TiS2, WO2, MoO2, RuO2, and combinations thereof. The third active material exhibits charging and discharging capacity below a corrosion potential of the current collector of the negative electrode and above a decomposition potential of the first active material.
Owner:MEDTRONIC INC
High voltage electrolyte for lithium ion battery
The invention belongs to the field of lithium ion battery electrolyte technology, and provides high voltage electrolyte for a lithium ion battery. The high voltage electrolyte comprises lithium salt, an organic solvent and an additive; and the high voltage electrolyte is characterized in that the organic solvent comprises fluoroester and a common carbonic ester organic solvent, wherein the organic solvent comprises 5-30% of the fluoroester by mass; the additive comprises ionic liquid and an film forming additive, and the mass percentage content is 0.01-8%. The high voltage electrolyte can enhance oxidative decomposition potential of the electrolyte, prolong cycle life of the lithium ion battery, and solve gas expansion and other problems of the lithium ion battery.
Owner:SHANGHAI SINOPOLY JIAHUA BATTERY TECH
Electrode active materialand lithium secondary battery
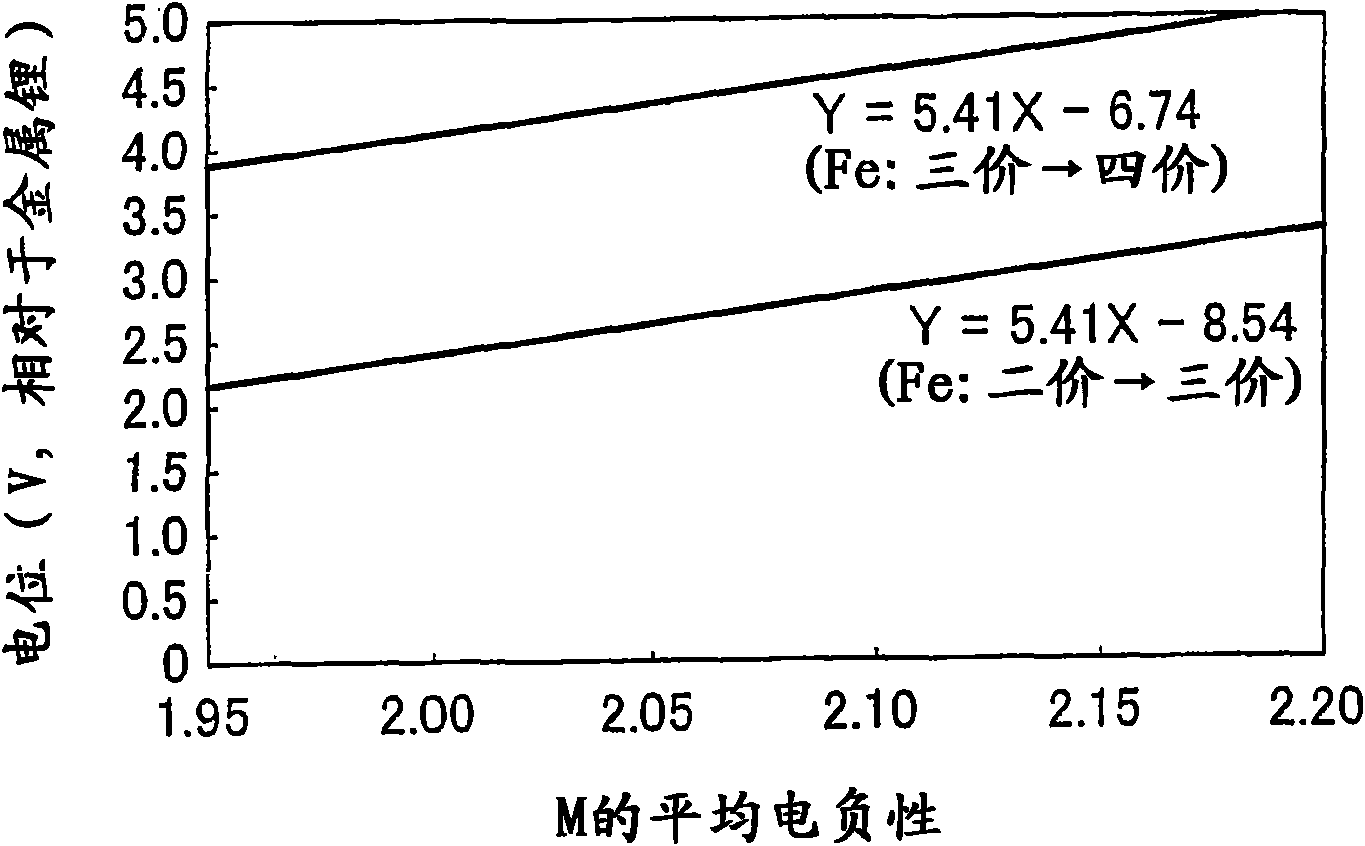
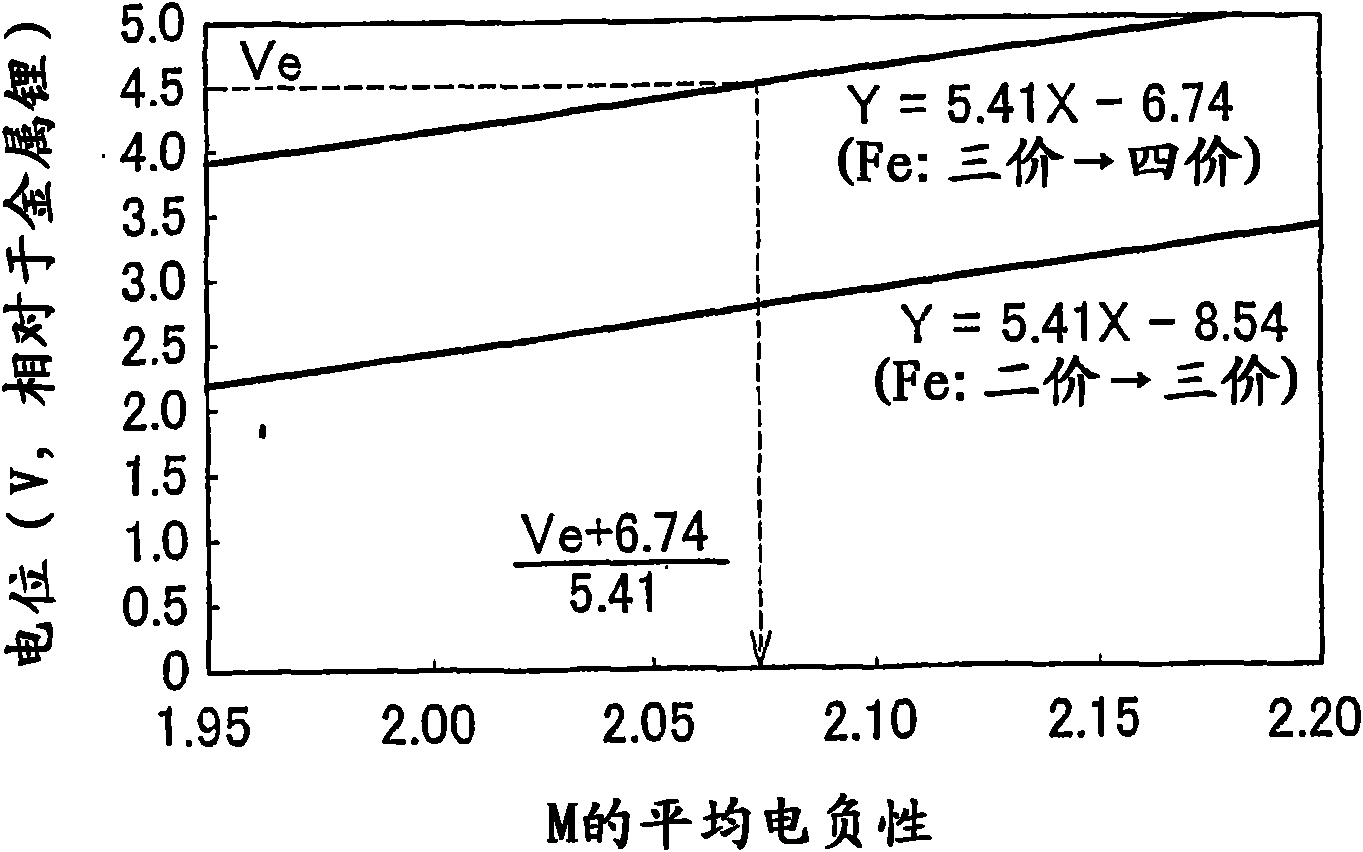
ActiveCN101657919AExcellent charge and discharge characteristicsIncrease capacityElectrode manufacturing processesSecondary cells charging/dischargingLithiumElectronegativity
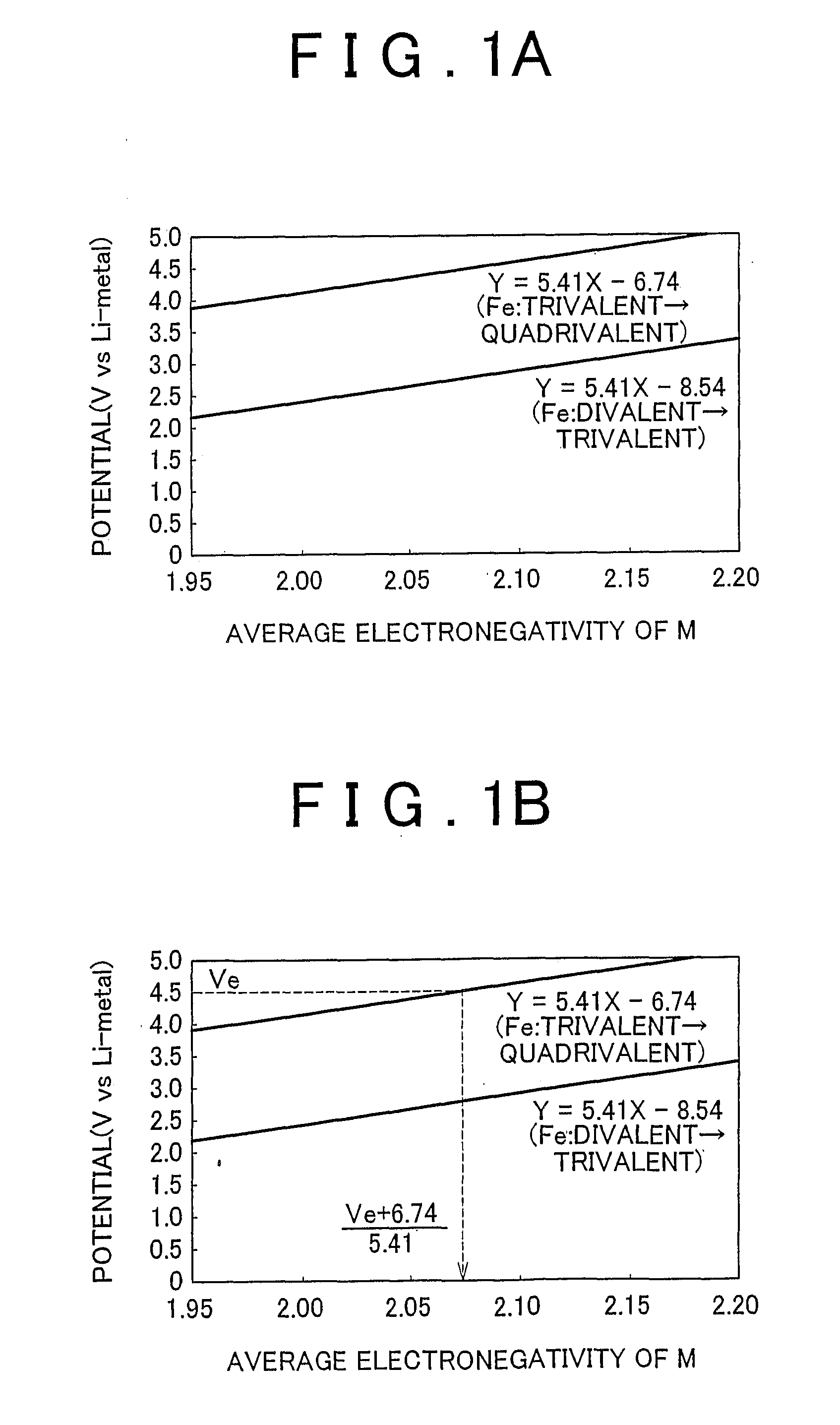
Electrode active material that is used together with an electrolyte solution having an electrolyte decomposition potential Ve is represented by the general expression LixFeMyO2 and is amorphous. In the expression, x and y are values which independently satisfy 1 < x = 2.5 and O < y = 3, respectively, and z = (x + (valence of Fe) + (valence of M) x y) / 2 to satisfy stoichiometry, and M representsone or two or more types of glass former element. The average electronegativity of M is less than (Ve + 6.74 / 5.41.
Owner:TOYOTA JIDOSHA KK +1
Silicon-containing organic compound and application thereof
InactiveCN109575067AImprove antioxidant capacityLow electronegativitySilicon organic compoundsSecondary cellsSilanesSolvent
The invention discloses a silicon-containing organic compound and application thereof. The silicon-containing organosilicon compound mainly comprises organic silicon carbonates, organic silicon carboxylates and ether modified silanes. The silicon-containing organic compound can be applied to lithium ion battery electrolyte, and serves as a solvent for the lithium ion battery electrolyte, the lithium ion battery electrolyte further comprises ethylene carbonate, propylene carbonate, methyl ethyl carbonate and diethyl carbonate which are used as basic solvents as well as lithium hexafluorophosphate, vinylene carbonate, 1,3-propanesulfonate and fluoroethylene carbonate which are taken as additives. The organosilicon compound electrolyte solvent has high oxidative decomposition potential, hightemperature resistance and high voltage resisting stability of a lithium ion battery can be improved quite well, wettability and low viscosity are good, the lithium ion conductivity can be improved quite well, the rate capability and the safety of the battery are improved, and the silicon-containing organic compound is a novel second-generation lithium ion battery solvent.
Owner:SHANSHAN ADVANCED MATERIALS QUZHOU CO LTD
Sulfide solid electrolyte
InactiveUS20150214572A1Reduce decompositionSolid electrolytesSolid electrolyte cellsOctahedronTetrahedron
A main object of the present invention is to provide a sulfide solid electrolyte whose reduction decomposition potential can be decreased more than a conventional LGPS-based sulfide solid electrolyte. The present invention is a sulfide solid electrolyte comprising Li, Al, Ge, P, and S, wherein M0=M2 / M1 is 0<M0<0.323 where M1 is a mole fraction of contained P, and M2 is a mole fraction of contained Al, and in a case where each of X1 and X2 is an element selected from the group consisting of P, Ge, and Al, a crystal structure thereof includes an octahedron O formed by Li and S, a tetrahedron T1 formed by S and X1, and a tetrahedron T2 formed by S and X2, wherein the octahedron O and the tetrahedron T2 share a ridge, and the octahedron O and the tetrahedron T2 share an apex.
Owner:TOYOTA JIDOSHA KK +1
Nonaqueous electrolyte secondary battery
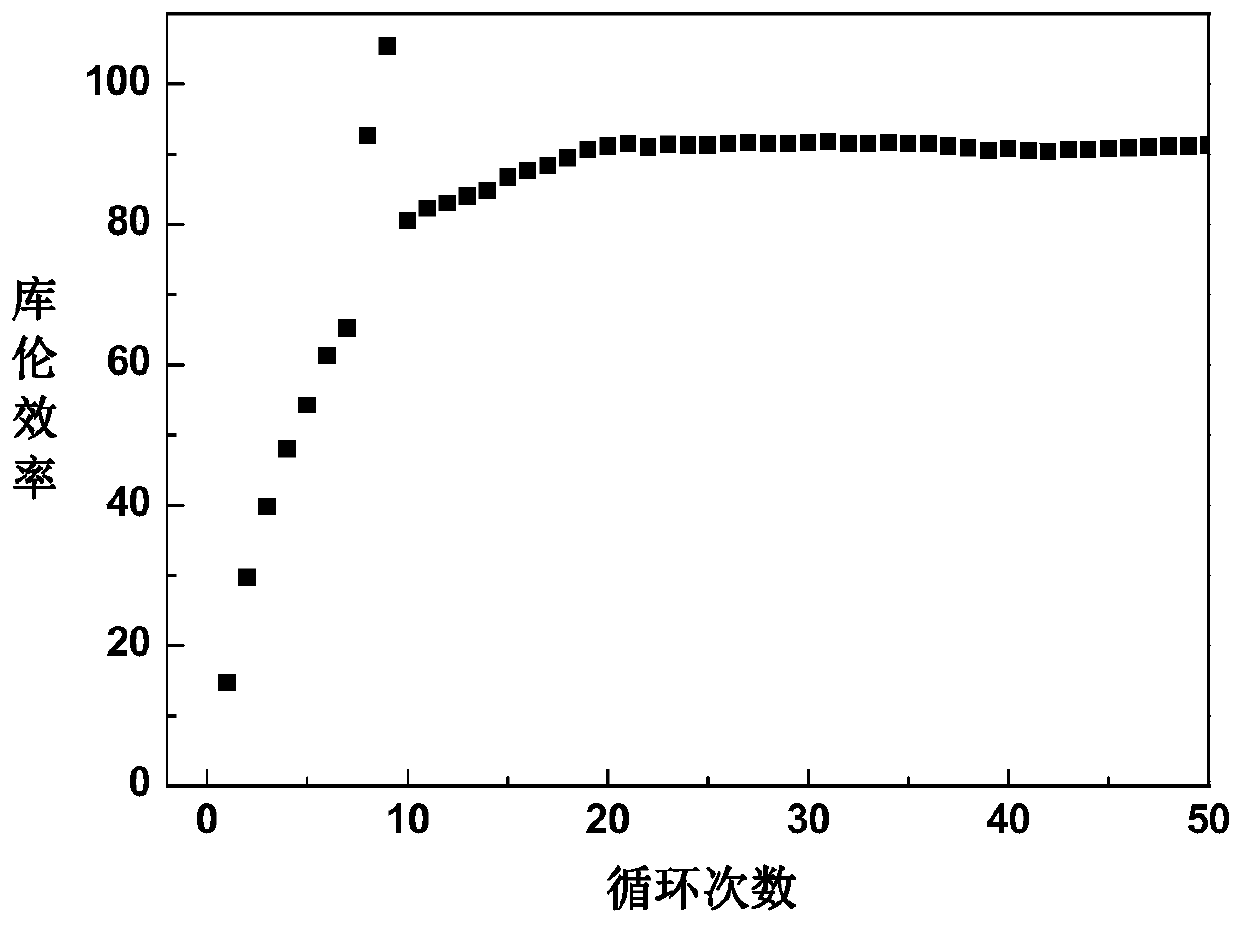
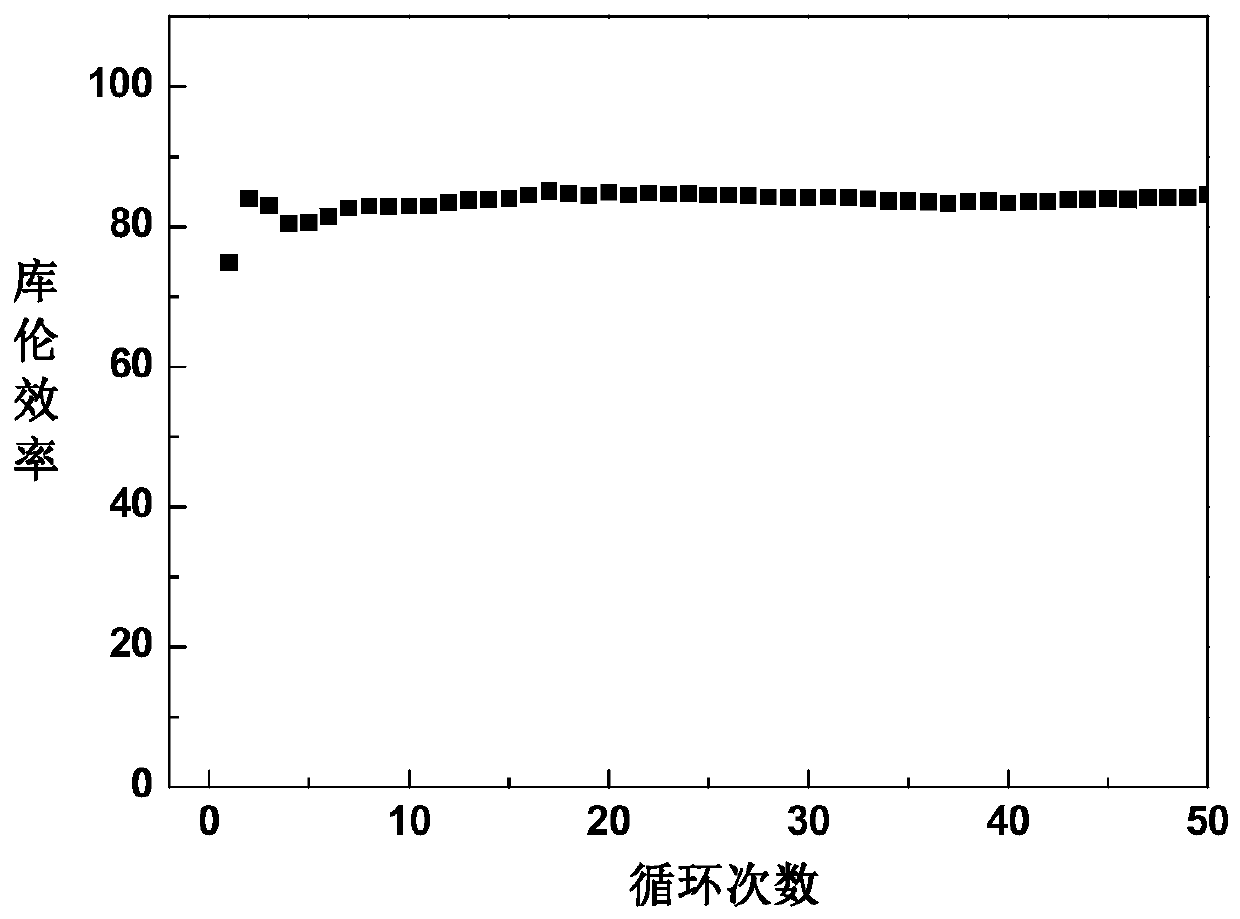
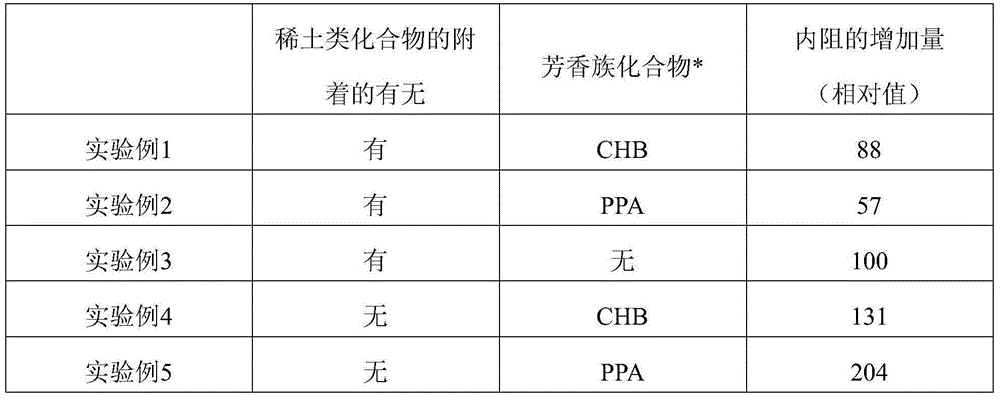
InactiveUS20160064738A1Increase internal resistanceIncrease resistanceLi-accumulatorsNon-aqueous electrolyte accumulator electrodesLithiumRare earth
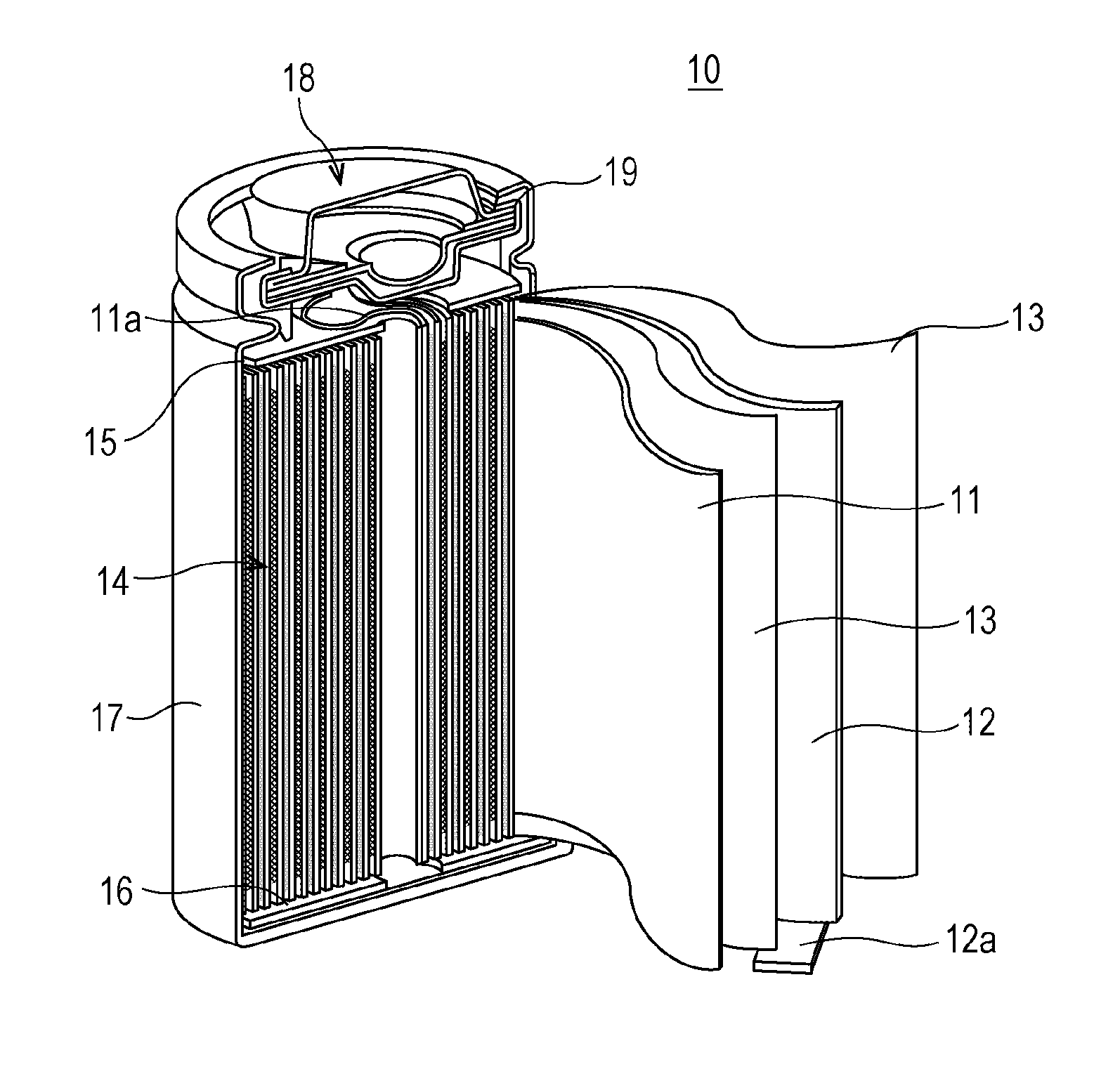
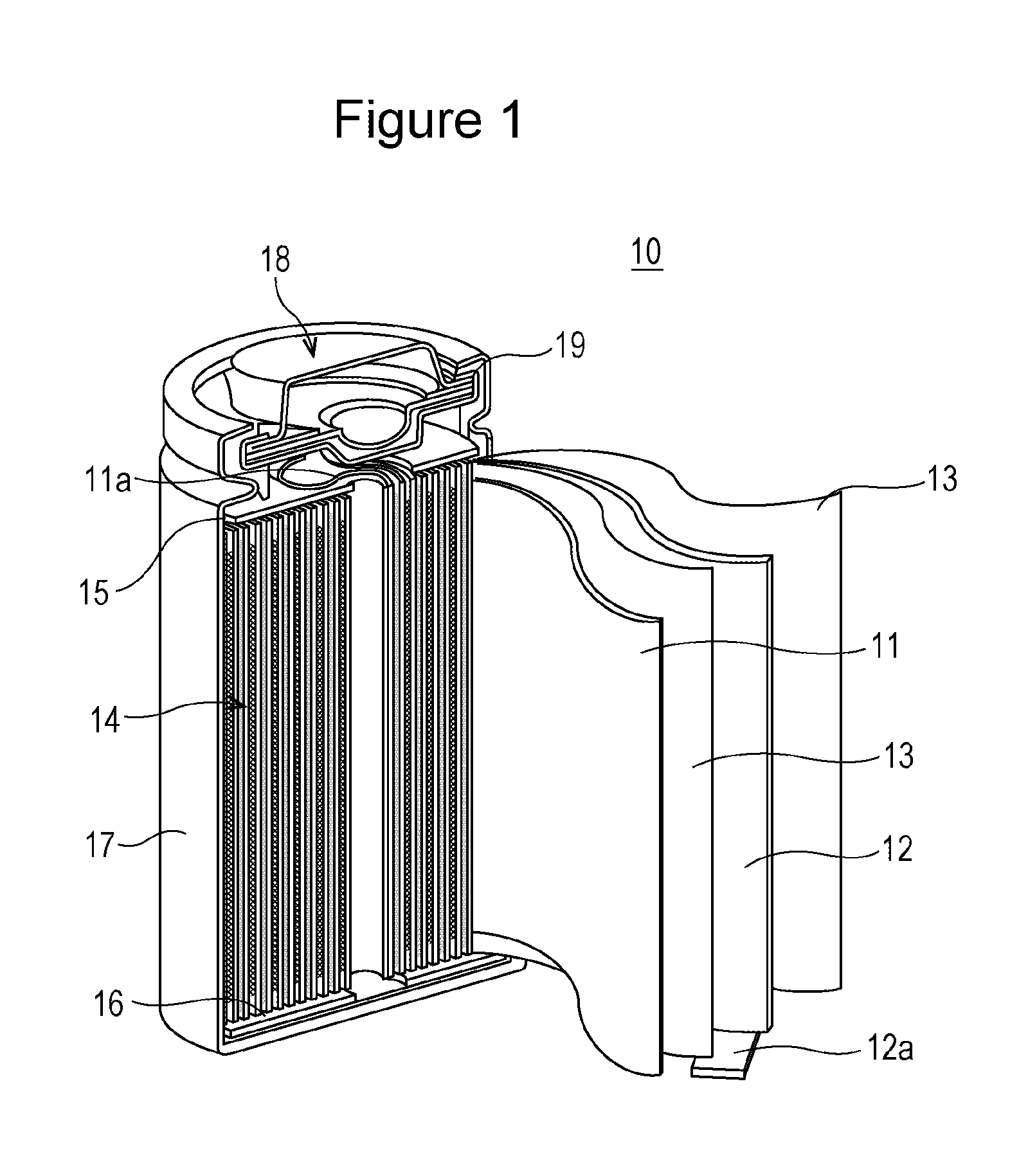
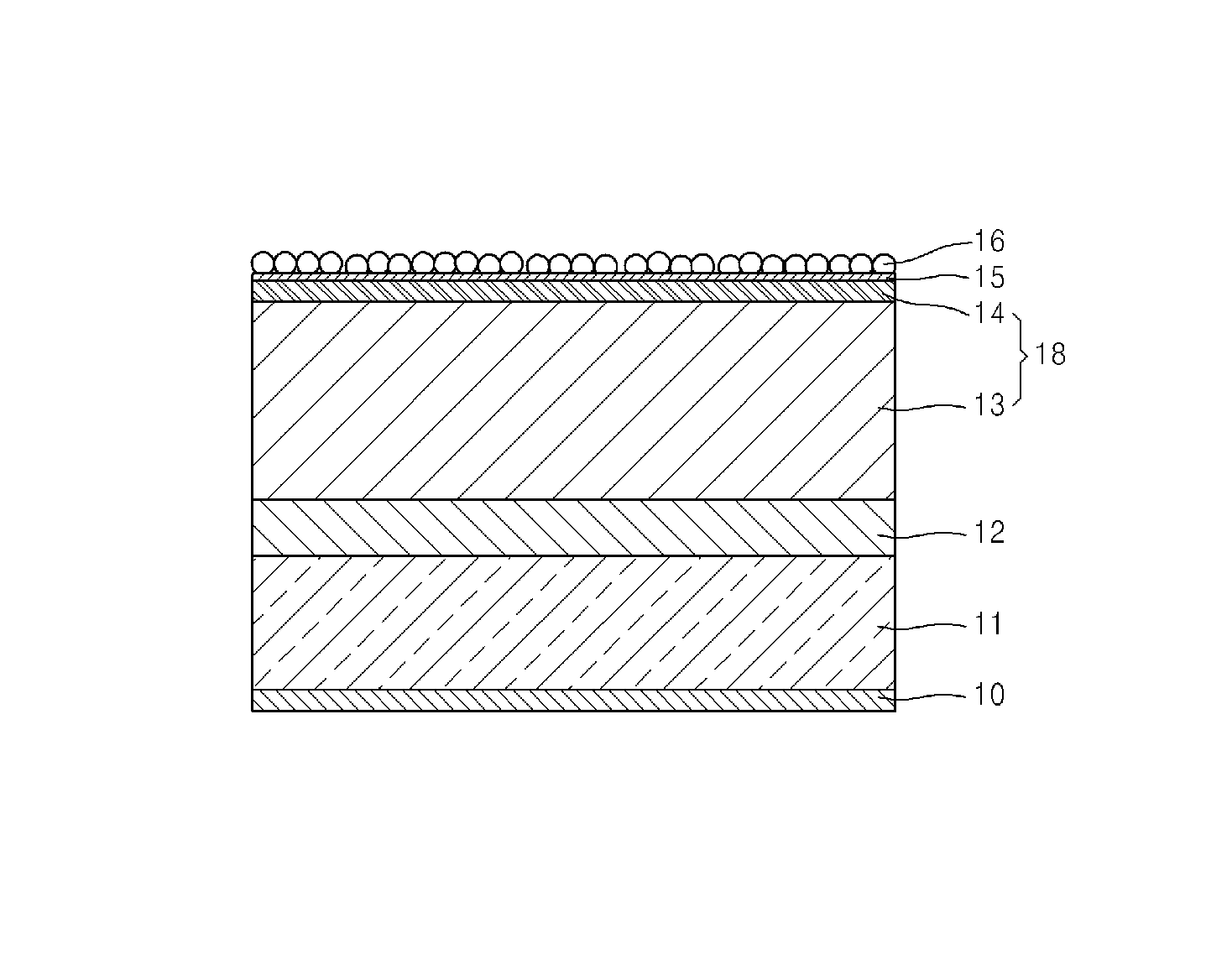
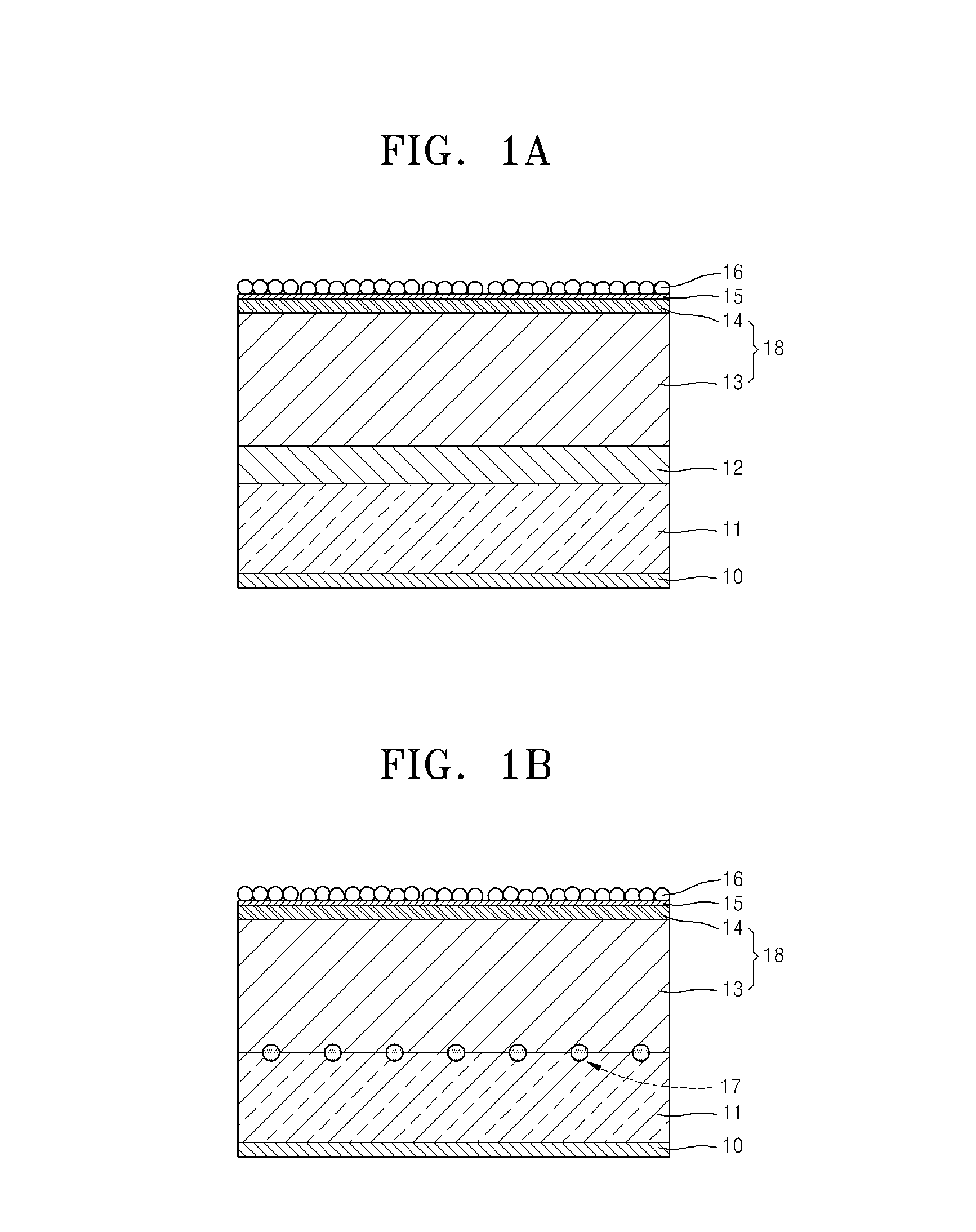
An aspect of the invention resides in a nonaqueous electrolyte secondary battery (10) including a positive electrode (11), a negative electrode (12) and a nonaqueous electrolytic solution, the positive electrode including a positive electrode active material containing a lithium transition metal oxide having a rare earth compound attached on the surface, the nonaqueous electrolytic solution including an aromatic compound having an oxidative decomposition potential in the range of 4.2 to 5.0 V vs. Li / Li+. The rare earth compound is preferably a rare earth hydroxide, a rare earth oxyhydroxide or a rare earth oxide.
Owner:SANYO ELECTRIC CO LTD
Ultraviolet polymerized glyceryl carbonate (meth) acrylate-based polymer electrolyte suitable for lithium secondary battery and preparation method thereof
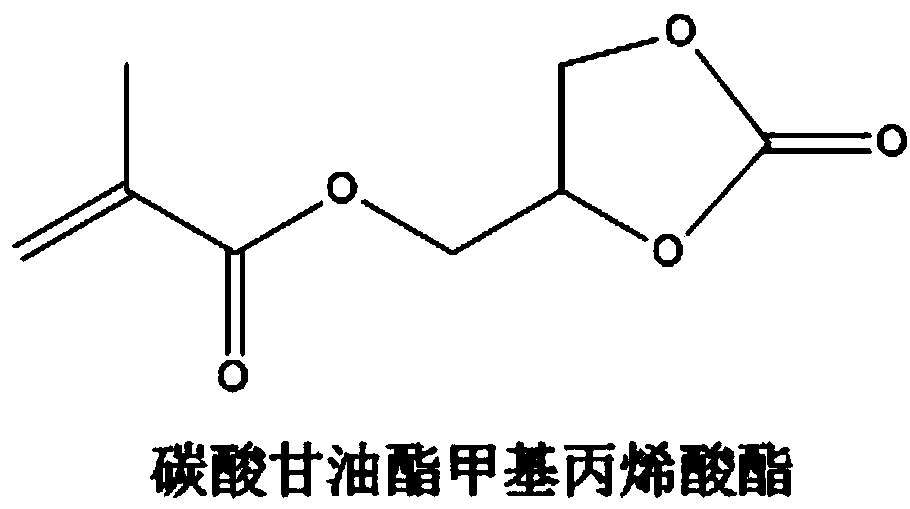
ActiveCN109216762AEasy to prepareHigh conductivity at room temperatureSolid electrolytesSecondary cellsPolymer sciencePolyethylene glycol
The invention discloses an ultraviolet polymerized glyceryl carbonate (meth) acrylate-based polymer electrolyte suitable for lithium secondary battery and a preparation method thereof, mainly comprising a lithium salt and a solid polymer matrix, wherein the lithium salt is dispersed in the solid polymer matrix, wherein the content of the lithium salt is 5-50 wt%. The solid polymer matrix is a copolymer of glycerol carbonate (meth) acrylate, polyethylene glycol (meth) acrylate and regulating monomer. The solid polymer electrolyte of the invention has the advantages of simple preparation, high room temperature conductivity, good flexibility and oxidation decomposition potential (4.8 V), and can be used as the electrolyte of a lithium ion battery.
Owner:HARBIN INST OF TECH WUXI RES INST OF NEW MATERIALS +1
Composite cathode material and preparation method and application thereof, and lithium ion battery
ActiveCN112271281APositive electrodesSecondary cells servicing/maintenanceLithium oxideElectrolytic agent
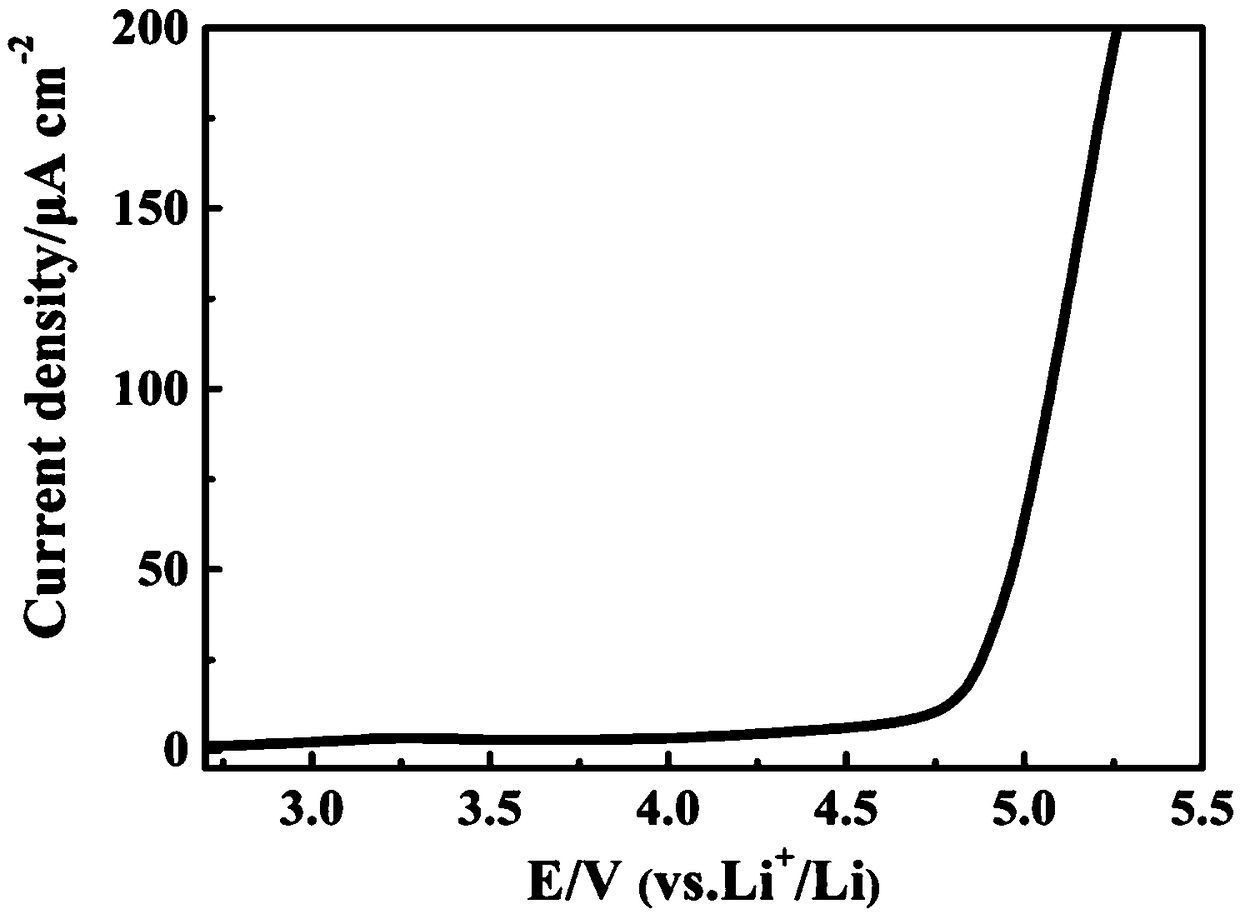
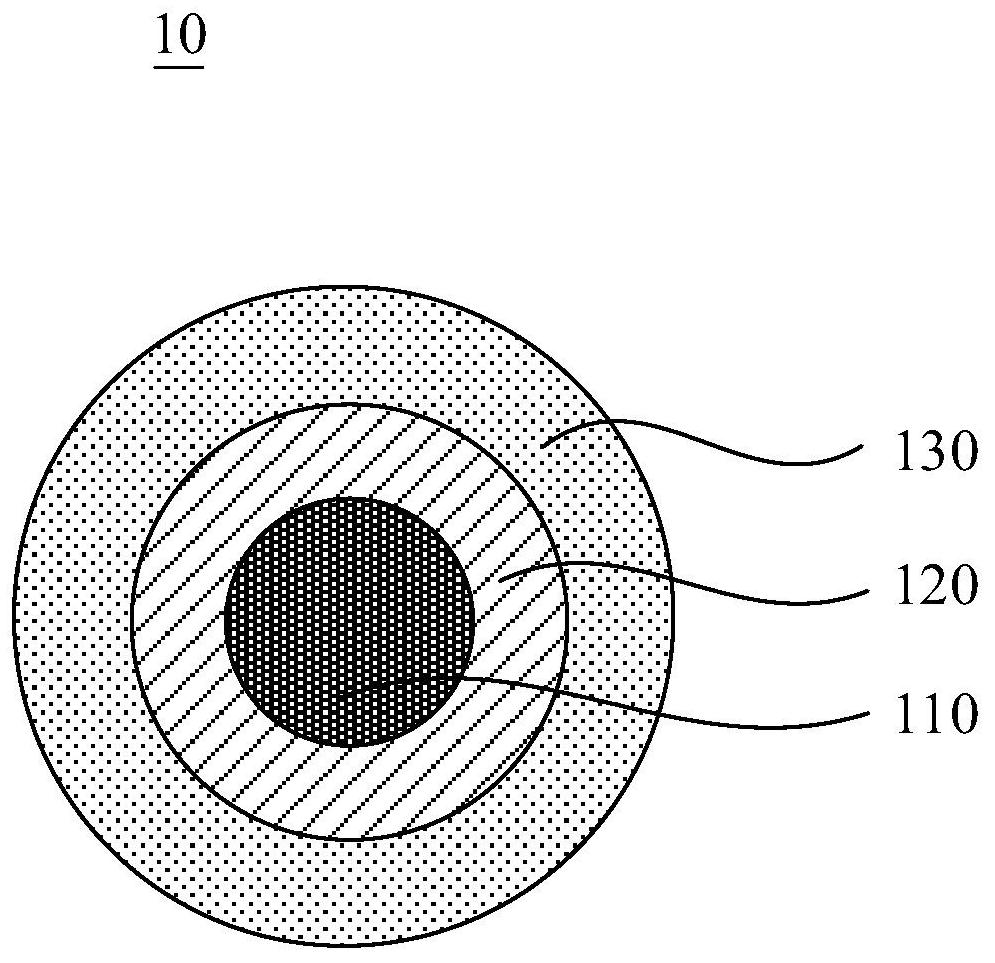
The invention relates to a composite cathode material and a preparation method thereof and a lithium ion battery, and belongs to the technical field of batteries. The composite cathode material comprises a cathode material, a metal compound layer and a lithium peroxide layer, the surface of the cathode material is coated with the metal compound layer, and the surface of the side, away from the cathode material, of the metal compound layer is coated with the lithium peroxide layer. The metal compound layer of the composite cathode material can be used as a catalyst to reduce the decomposition potential of lithium peroxide, and in the first charging process, the metal compound can catalyze efficient decomposition of lithium peroxide and release Li+ for efficient lithium supplementation; andmeanwhile, the metal compound layer is remained on the surface of the cathode material, so that the effect of a physical barrier layer can be achieved, the cathode material is prevented from being directly contacted with the electrolyte, the interface side reaction of the cathode material is reduced, the interface stability of the cathode material and the electrolyte is improved, and the cycle performance of the battery is improved.
Owner:SUNWODA ELECTRIC VEHICLE BATTERY CO LTD
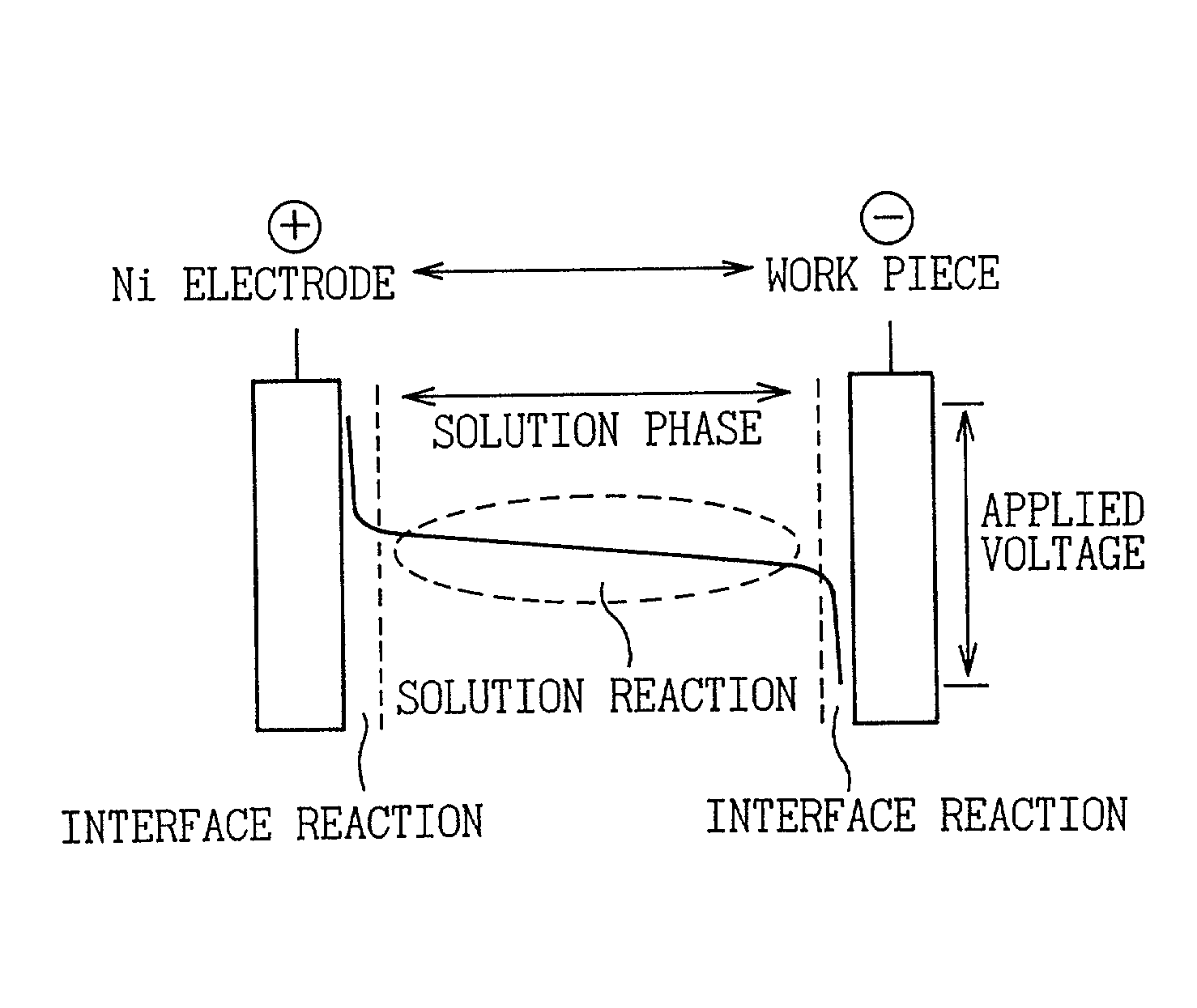
Electrolytic phosphate chemical treatment method
InactiveUS20020162752A1Increased level of controlImproving the reaction efficiency on a metal surfaceCellsPhosphatisationSolventDecomposition potential
The object of the present invention is to provide an electrolytic phosphate chemical treatment method capable of improving the reaction efficiency on a metal surface (interface) by preventing the reaction in the solution phase so as to reliably prevent sludge formation during continuous treatment. The present invention relates to a method of forming a film composed of a phosphate compound and a metal on the surface of an article to be treated by performing electrolytic treatment on a metal material article to be treated in a phosphate chemical treatment bath by contacting said metal material having electrical conductivity with said phosphate chemical treatment bath containing phosphate ions and phosphoric acid, nitrate ions, metal ions that form a complex with phosphate ions in said phosphate chemical treatment bath, and metal ions for which the dissolution-precipitation equilibrium potential at which ions dissolved in said phosphate chemical treatment bath are reduced and precipitate as metal is equal to or greater than -830 mV, which is the cathodic reaction decomposition potential of the solvent in the form of water when indicated as the hydrogen standard electrode potential, and is substantially free of metal ions other than those which are a component of the film; wherein the ORP (oxidation-reduction potential) of said phosphate chemical treatment bath (indicated as the potential relative to a standard hydrogen electrode) is maintained at equal to or greater than 700 mV.
Owner:DENSO CORP
An electrolyte promoting carbonate decomposition and a lithium-air battery
ActiveCN106654465APromote decompositionFuel and secondary cellsSecondary cellsElectricityOrganic solvent
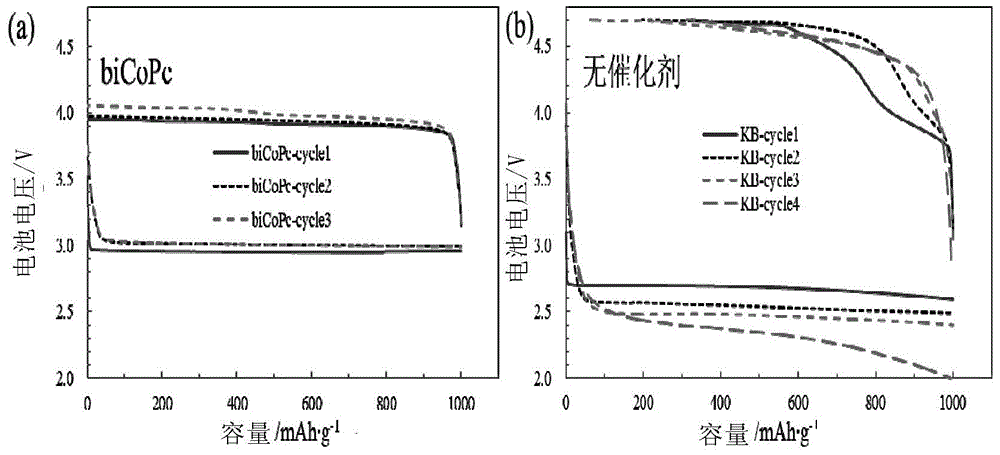
An electrolyte promoting carbonate decomposition and a lithium-air battery are provided. In particular, the electrolyte promoting carbonate decomposition is disclosed and includes a lithium salt, an organic solvent and a catalyst, wherein the catalyst is a double-core or multi-core phthalocyanine transition metal coordination compound. The corresponding lithium-air battery is also disclosed. A solution-phase carbonate decomposition catalytic system is adopted by the lithium-air battery to overcome the bad electric contact between carbonates and solid electrodes, thus effectively reducing decomposition potentials of the carbonates, effectively reducing lithium carbonate accumulation in the lithium-air battery and further improving cyclic performance of the battery.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Electrode Active Material and Lithium Secondary Battery
ActiveUS20100183923A1Improve discharge characteristicsAvoid problemsElectrode manufacturing processesActive material electrodesLithiumElectronegativity
Electrode active material that is used together with an electrolyte solution having an electrolyte decomposition potential Ve is represented by the general expression LixFeMyO2 and is amorphous. In the expression, x and y are values which independently satisfy 1<x≦2.5 and O<y≦3, respectively, and z=(x+(valence of Fe)+(valence of M)×y) / 2 to satisfy stoichiometry, and M represents one or two or more types of glass former element. The average electronegativity of M is less than (Ve+6.74 / 5.41.
Owner:KYUSHU UNIV +1
Lithium secondary battery
InactiveCN1485944APromote decompositionCell electrodesOrganic electrolyte cellsLithium-ion batteryElectrolyte
A lithium secondary battery including a positive electrode which is capable of occluding and releasing lithium, a negative electrode which is capable of occluding and releasing lithium, a separator between the positive electrode and the negative electrode, and a nonaqueous electrolyte comprising a nonaqueous solvent and a wettability improving agent. The nonaqueous solvent does not substantially wet the separator, and the wettability improving agent is dissolved in the nonaqueous solvent, improves the wettability of the nonaqueous solvent to the separator, and has an oxidative decomposition potential in a range of 4.5 V to 6.2 V.
Owner:SANYO ELECTRIC CO LTD
Charging method for nonaqueous electrolyte secondary battery
An initial charging operation is carried out by a charging step composed of two-stages or more to improve an initial charging and discharging efficiency, reduce the charge of wasteful materials and improve a high capacity and a high cyclic characteristic without deteriorating various kinds of battery properties. In order to realize the improvements, a nonaqueous solvent which is decomposed under a potential higher than the reduction and decomposition potential of a main solvent is included in electrolyte. This charging method is a method for achieving the addition effect of such a nonaqueous solvent as much as possible. As a specific means, the electrolyte to which vinylene carbonate is added is employed and a constant-current and constant-voltage charge under about 3.2 V is carried out for 1 to 2 hours before a battery is completely charged. Thus, a good coat can be formed on the surface of an anode while suppressing the quantity of electricity required for forming the coat.
Owner:MURATA MFG CO LTD
Polymer solid electrolyte, method of making same, and electrochemical cell
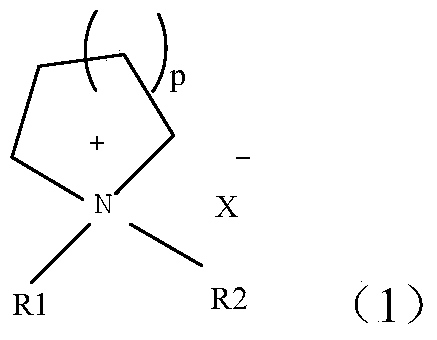
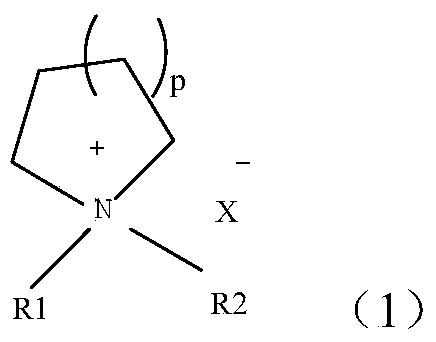
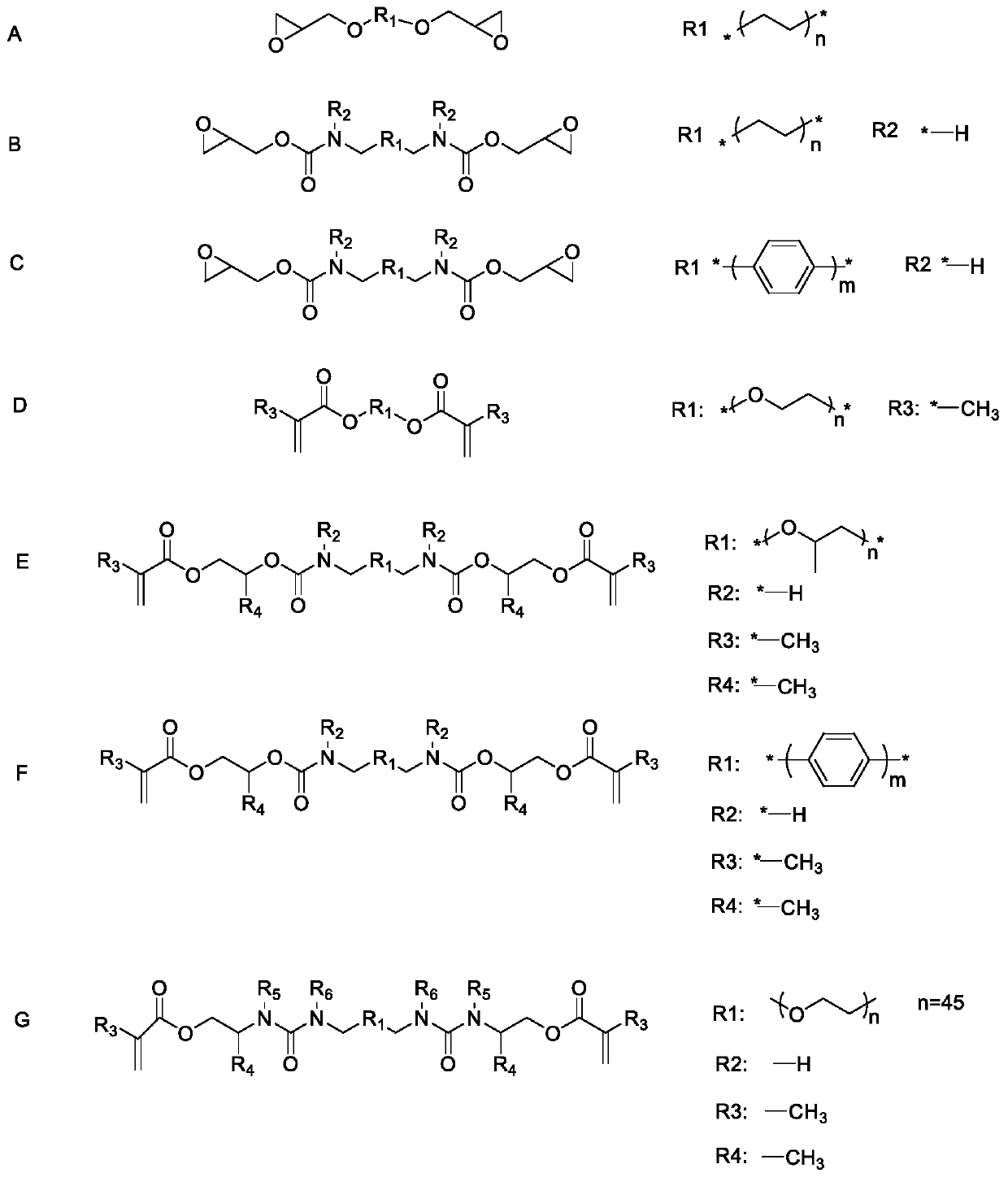
The invention relates to a polymer solid electrolyte and a method of making the same. The present invention generally relates to various polymer solid electrolyte materials suitable for various electrochemical devices. Certain embodiments of the invention are generally directed to solid electrolytes having relatively high ionic conductivity and other mechanical or electrical properties, e.g., tensile strength or decomposition potential. Certain aspects include a polymer, a plasticizer, and an electrolyte salt. In some cases, the polymer may exhibit certain structures (shown in the specification), where R1 can be one of the following groups (shown in the specification). In the structures, n is an integer between 1 and 10000, m is a integer between 1 and 5000, and R2 to R6 can each independently be one of the following structures shown the specification.
Owner:FACTORIAL INC
Composite protective layer for photoelectrode structure, photoelectrode structure including the composite protective layer, and photoelectrochemical cell including photoelectrode structure
InactiveUS9567680B2Extend your lifeElectrical apparatusMultiple component coatingsEvery HourPhotoelectrochemical cell
A composite protective layer for a photoelectrode, the composite protective layer including a chemical protective layer; and a physical protective layer, wherein the chemical protective layer has corrosion rate of 0.1 Coulombs per square centimeter per 10 hours or less when evaluated at a water decomposition potential, and the physical protective layer has a moisture transmittance rate of 0.001 grams per square meter per day or less and has an electrical conductivity.
Owner:SAMSUNG ELECTRONICS CO LTD
A kind of cross-linked polymer electrolyte suitable for lithium secondary battery and preparation method thereof
The invention discloses a crosslinked polymer electrolyte suitable for a lithium secondary battery and a preparation method thereof. The electrolyte is crosslinked by a low molecular weight polyetherderivative under the action of a crosslinking agent. The electrolyte has the advantages of simple preparation, high room temperature conductivity, good flexibility and oxidation decomposition potential of higher than 4.5 V.
Owner:HARBIN INST OF TECH WUXI RES INST OF NEW MATERIALS +1
Electrolyte for rechargeable magnesium battery
InactiveCN109713368AHigh deposition-dissolution efficiencyUnderstand the purposeSecondary cells servicing/maintenanceEtherDissolution
The invention discloses an electrolyte for a rechargeable magnesium battery using ether as a solvent and relates to the field of rechargeable magnesium batteries. The electrolyte comprises magnesium trifluoromethanesulfonate and aluminum trichloride in a molar ratio of 1: (1 to 5). The concentration of magnesium ions in the electrolyte is 0.1 to 2.0 mol.L-1. The electrolyte has a high anodic oxidation decomposition potential, high electrical conductivity, high reversible magnesium deposition-dissolution efficiency, excellent cycle performance and low electrolyte solution cost, and the anodic oxidation decomposition potential on stainless steel can reach over 2.0V vs.Mg / Mg2+. The stable magnesium deposition-dissolution efficiency of the system is higher than 90%, which promotes the development of a low-cost and high-performance rechargeable magnesium battery.
Owner:SHANGHAI JIAO TONG UNIV
Nonaqueous electrolyte secondary battery
InactiveCN105051964ALower internal resistanceCell electrodesLi-accumulatorsLithiumRare-earth element
A nonaqueous electrolyte secondary battery (10) according to one embodiment of the present invention is provided with: a positive electrode (11) comprising a positive electrode active material which contains a lithium-containing transition metal oxide, and to the surface of which a compound of a rare earth element adheres; a negative electrode (12); and a nonaqueous electrolyte solution. The nonaqueous electrolyte solution contains an aromatic compound that has an oxidative decomposition potential within the range of 4.2-5.0 V vs. Li / Li+. The compound of a rare earth element is preferably a hydroxide of a rare earth element, an oxyhydroxide of a rare earth element or an oxide of a rare earth element.
Owner:SANYO ELECTRIC CO LTD
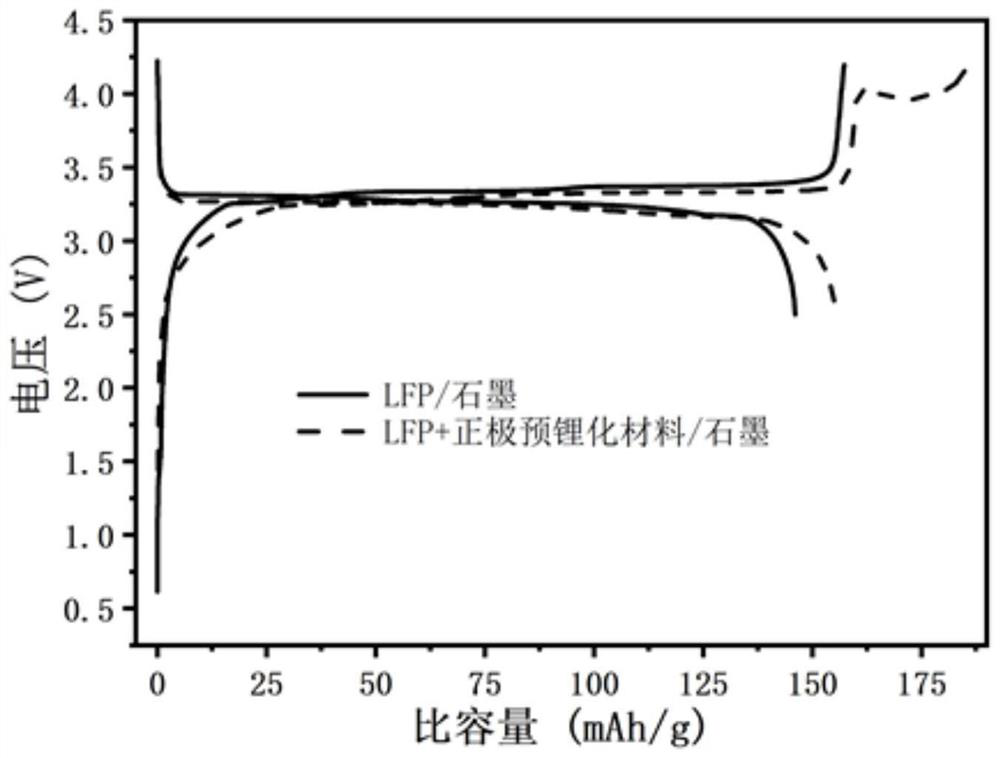
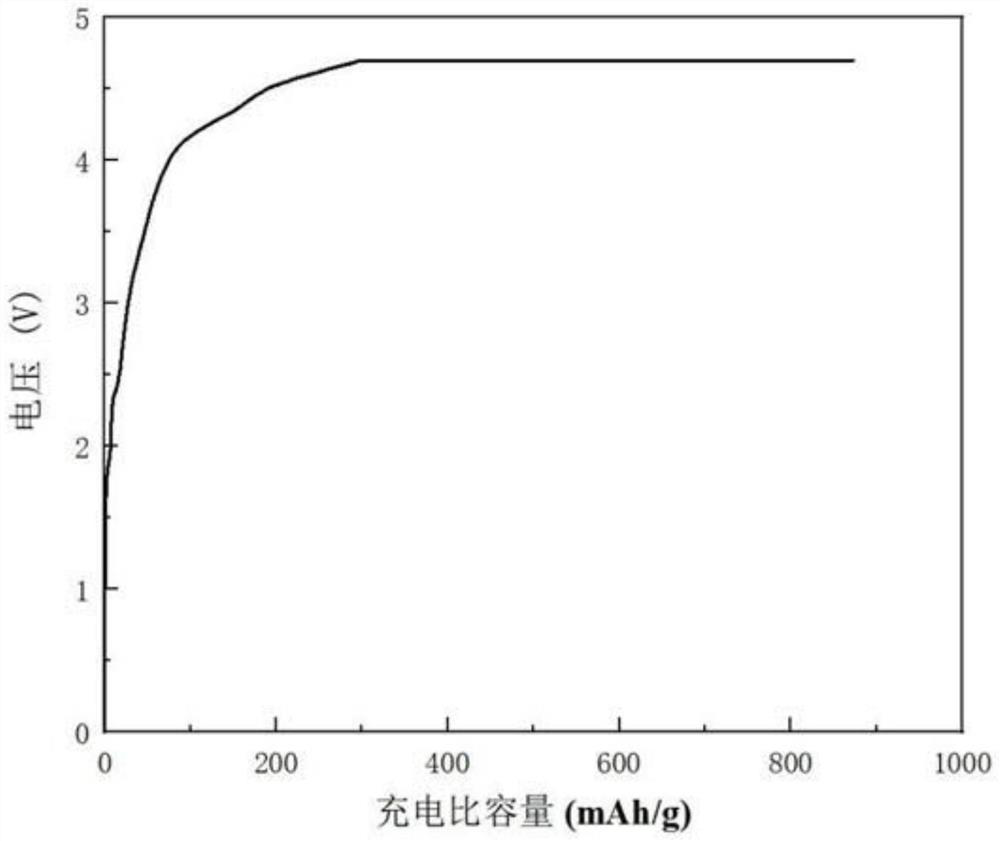
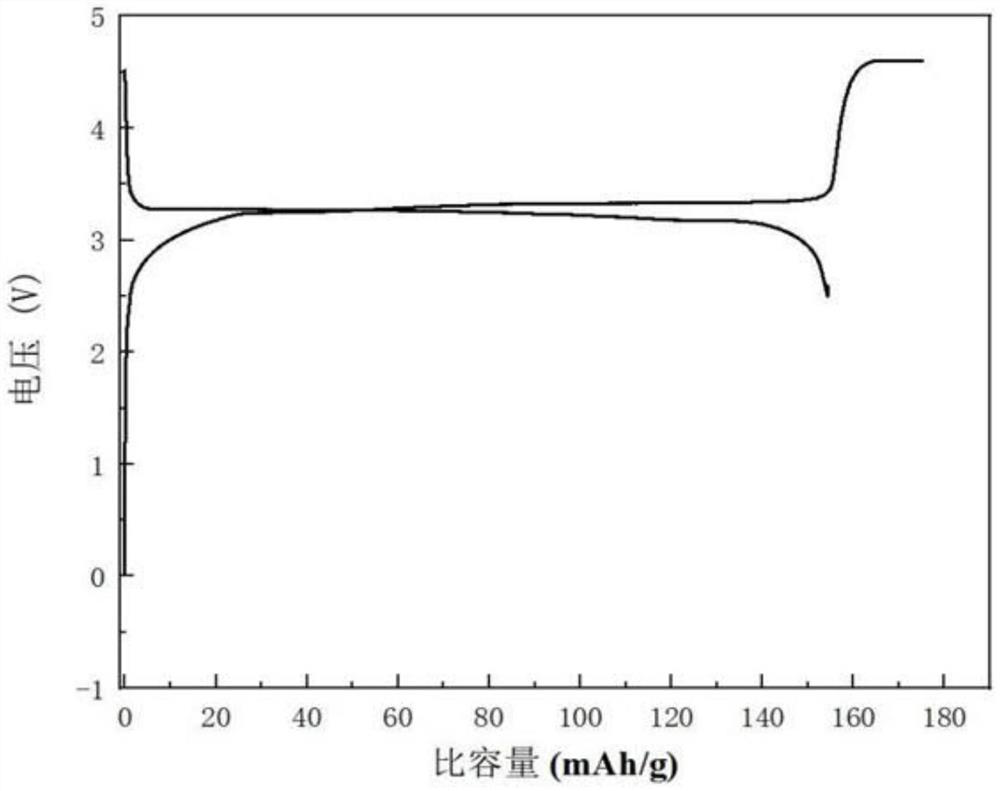
Compound for pre-lithiation, preparation method of compound, positive electrode pre-lithiation material, preparation method of positive electrode pre-lithiation material and lithium battery
The invention relates to the technical field of energy storage, and relates to a compound for pre-lithiation, a preparation method of the compound, a positive electrode pre-lithiation material, a preparation method of the positive electrode pre-lithiation material, and a lithium battery. The chemical formula of the compound is LixMyOz, wherein x is more than or equal to 3 and less than or equal to 12, y is more than or equal to 1 and less than or equal to 2, z is more than or equal to 4 and less than or equal to 11, and M is one or more selected from a group consisting of Nb, Ta, Zr, W, Sn, V, Ru, Ce or Bi. When the compound is used as a positive electrode pre-lithiation material, a decomposition potential is relatively low, charging specific capacity is high, a discharging process is irreversible, a relatively good lithium battery in-situ pre-lithiation effect can be realized, and the compound has good air stability, is compatible with an existing lithium battery production process and has a commercial application prospect. According to the lithium battery adopting the positive electrode pre-lithiation material, in the charging process of the battery, the compound is irreversibly decomposed to release active lithium ions, so active lithium loss caused by negative electrode SEI growth is supplemented, and the effects of improving the energy density of the lithium battery and prolonging the cycle life of the lithium battery can be achieved.
Owner:SONGSHAN LAKE MATERIALS LAB +1
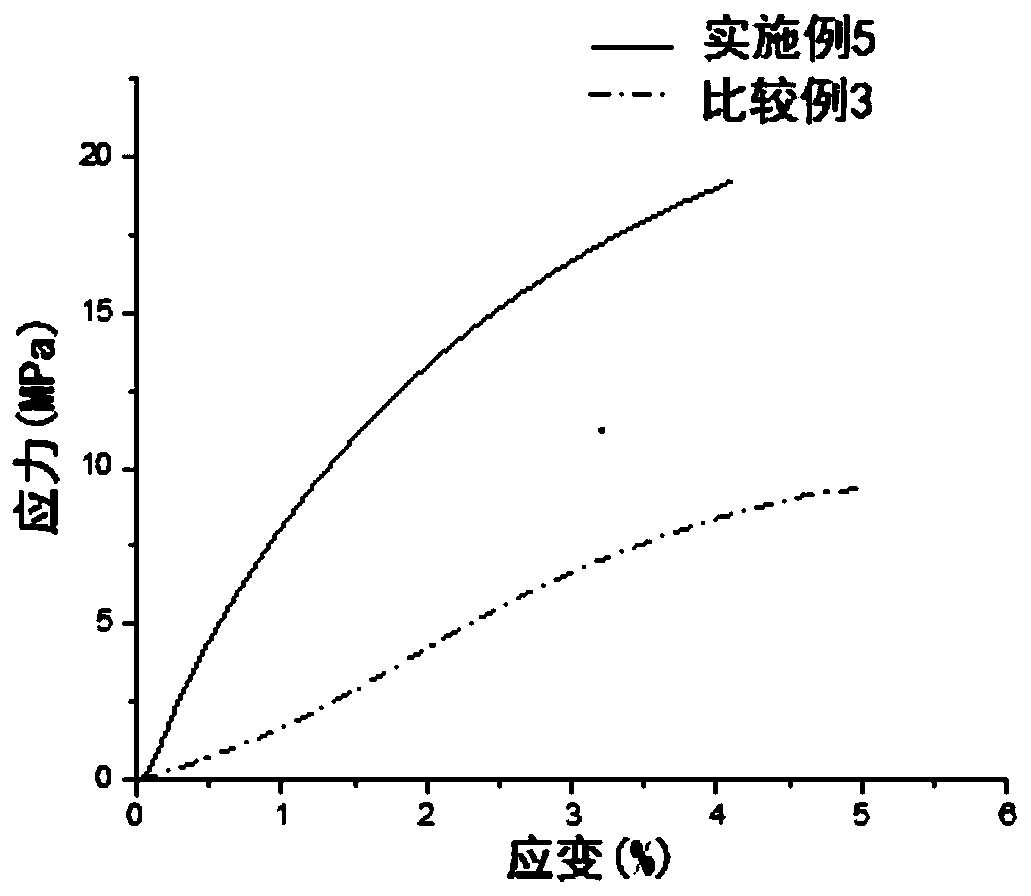
Positive electrode lithium supplementing material and preparation method and application thereof
ActiveCN114335433AEasy to operateSuitable for industrial productionElectrode manufacturing processesSecondary cellsLithium metasilicateLithium-ion battery
The invention discloses an anode lithium supplementing material and a preparation method and application thereof, and belongs to the field of energy storage. The positive electrode lithium supplementing material comprises a core material, the core material is at least one of a boron-doped lithium orthosilicate material and a boron-doped lithium metasilicate material, the chemical formula of the boron-doped lithium orthosilicate material is Li4Si1-xBxO4, the chemical formula of the boron-doped lithium metasilicate material is Li2Si1-yByO3, x is greater than or equal to 0.001 and less than or equal to 0.2, and y is greater than or equal to 0.001 and less than or equal to 0.2. According to the arrangement, lithium orthosilicate and / or lithium metasilicate are / is used as a main body, and the boron-doped lithium silicate material formed by non-metallic element boron doping is used as a lithium supplement agent, so that the ionic conductivity of the lithium silicate material can be improved, the decomposition potential of the lithium silicate material can be reduced, the active lithium release of the lithium silicate lithium supplement material can be promoted, and the energy density and the cycle life of the lithium ion battery can be remarkably improved.
Owner:SONGSHAN LAKE MATERIALS LAB +1
Polymer solid electrolyte, method of making the same, and electrochemical cell
InactiveCN111162311ASolid electrolytesFinal product manufactureSolid state electrolytePolymer science
The invention relates to a polymer solid electrolyte, a method of making the same, and an electrochemical cell. The present invention generally relates to various polymer solid electrolyte materials suitable for various electrochemical devices. Certain embodiments of the invention are generally directed to solid electrolytes having relatively high ionic conductivity and other mechanical or electrical properties, e.g., tensile strength or decomposition potential. Certain aspects include a polymer, a plasticizer, and an electrolyte salt. In some cases, the polymer may exhibit certain structuressuch as:,where R1 can be one of the following groups: where n is an integer between 1 and 10000, m is a integer between 1 and 5000, and R2 to R6 can each independently be one of the following structures: shown in the description.
Owner:林奈公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com