An electrolyte promoting carbonate decomposition and a lithium-air battery
A lithium-air battery and electrolyte technology, applied in electrical components, secondary batteries, fuel cell-type half-cells, and secondary-battery-type half-cells, etc., can solve problems such as the inability of catalysts to catalyze
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Electrolyte No.1 and Li-air battery No.1
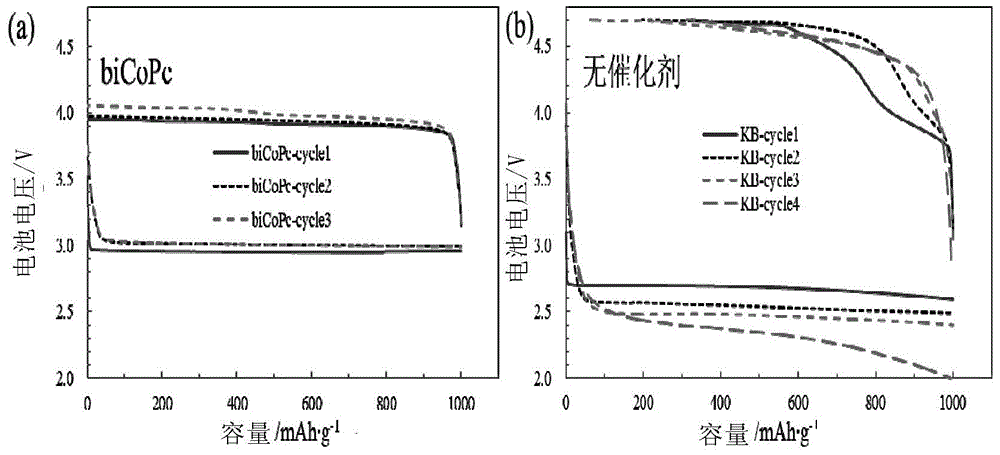
[0088] The method of dispersing the catalyst is as follows: 0.005mol / L dinuclear cobalt phthalocyanine (biCoPc) and 1mol / LLiTFSI are dissolved in tetraethylene glycol dimethyl ether (TEGDME) to prepare an electrolyte solution. As a comparison, 0.005 mol / L mononuclear cobalt phthalocyanine (CoPc) and 1 mol / L LiTFSI were dissolved in tetraethylene glycol dimethyl ether (TEGDME) to prepare an electrolyte solution. At the same time, a catalyst-free 1mol / L LiTFSI / TEGDME electrolyte was prepared.
[0089] The method for preparing lithium-lithium carbonate battery is as follows: 0.15g Li 2 CO 3 Add 5mL N-methylpyrrolidone (NMP) with 0.15g Ketjen Black EC600JD high-conductivity carbon black (KB), add 0.026g polyimide (PI) as a binder, and obtain electrode material slurry after magnetic stirring for 2 hours. The material is evenly coated on the surface of the nickel foam, and then the nickel foam coated with the slurry is moved into ...
Embodiment 2
[0093] Electrolyte No.2 and lithium-air battery No.2
[0094] The method of dispersing the catalyst is as follows: 0.005mol / L dinuclear cobalt phthalocyanine (biCoPc) and 1mol / LLiTFSI are dissolved in tetraethylene glycol dimethyl ether (TEGDME) to prepare an electrolyte solution. As a comparison, 0.005 mol / L mononuclear cobalt phthalocyanine (CoPc) and 1 mol / L LiTFSI were dissolved in tetraethylene glycol dimethyl ether (TEGDME) to prepare an electrolyte solution. At the same time, a catalyst-free 1mol / L LiTFSI / TEGDME electrolyte was prepared.
[0095] The method for preparing lithium-lithium carbonate battery is as follows: 1.30~1.50mg isotope-labeled Li 2 13 CO 3 Add 1mL of N-methylpyrrolidone (NMP) to Ketjen Black EC600JD high-conductivity carbon black (KB) at a mass ratio of 1:1, add 5mg of polyimide (PI) as a binder, and obtain electrode material slurry after ultrasonic dispersion for 2 hours The slurry is evenly applied to the surface of nickel foam, and then the ni...
Embodiment 3
[0103] Electrolyte No.3 and lithium-air battery No.3
[0104] The method of dispersing the catalyst is as follows: 0.005mol / L biCoPc and 1mol / L LiClO 4 Dissolved in DMSO, prepared as electrolyte. At the same time, prepare 1mol / L LiClO without catalyst 4 / DMSO electrolyte.
[0105] The method for preparing lithium-carbon dioxide / oxygen battery is as follows: take KB 0.15g, add 0.45mL 6wt% polytetrafluoroethylene (PTFE) emulsion as binder, and 5mL deionized water as dispersant, obtain electrode material after magnetic stirring for 2h Slurry, evenly apply the slurry to the surface of nickel foam, then move the nickel foam coated with slurry into an oven, dry it at 80°C and then move it into a vacuum oven, dry it in vacuum at 120°C for 12 hours, and cut it into pieces with a slicer A disc with a diameter of 14mm is used as the positive pole piece of the lithium-air battery, and the KB load is controlled to be 1±0.2mg / cm 2 . Spread the positive electrode sheet on the pre-drill...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com