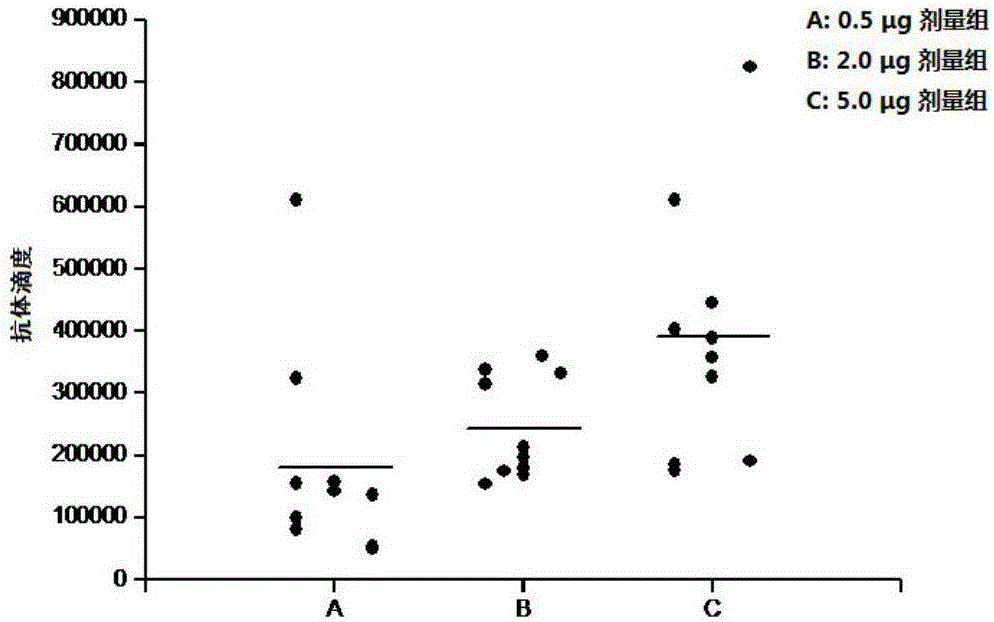
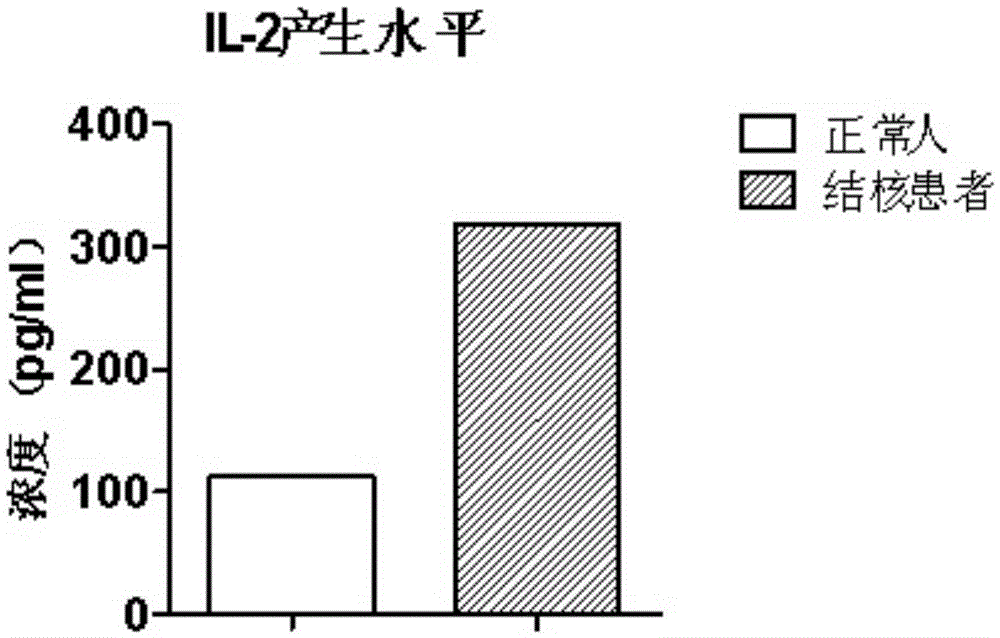
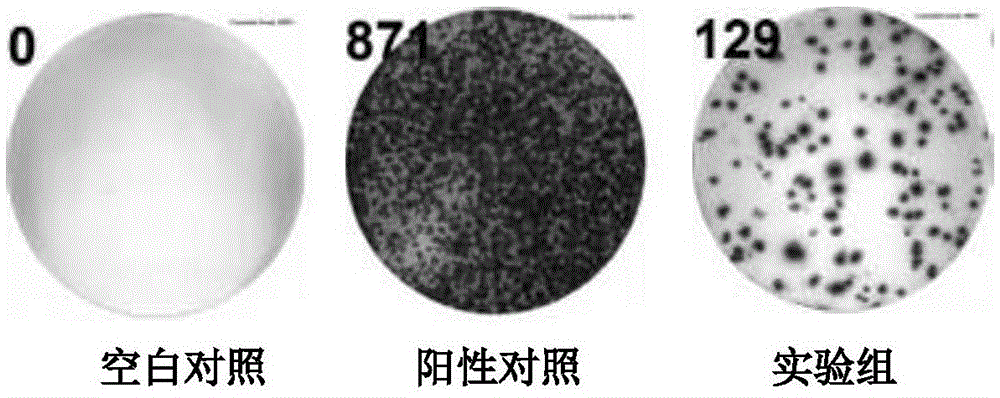
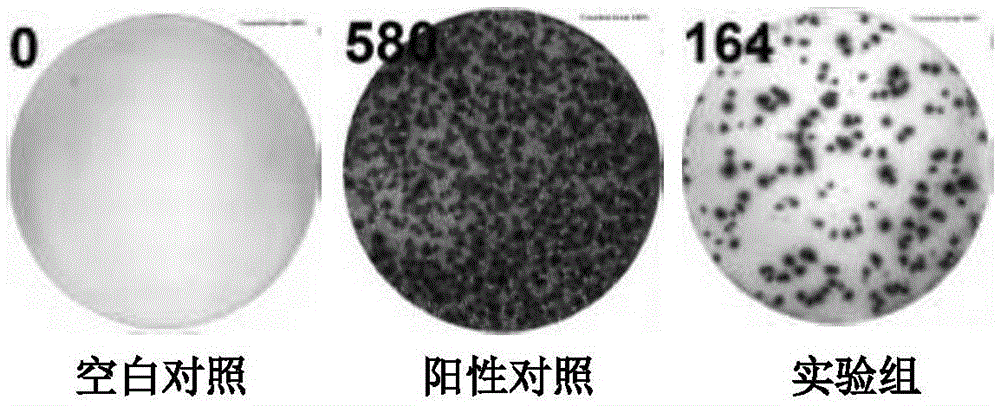
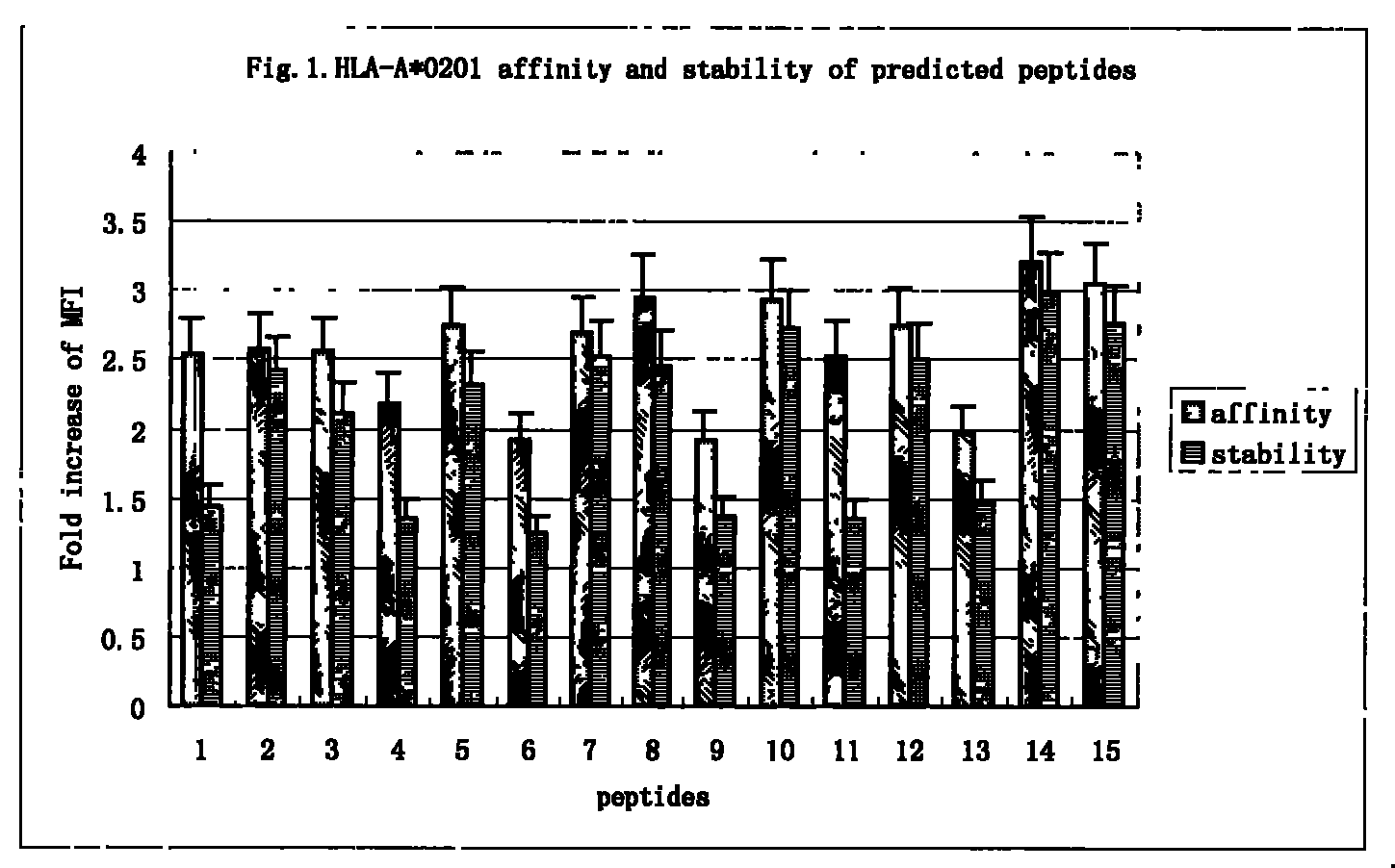
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
84 results about "Bcg bladder instillation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
BCG is used in the treatment of superficial forms of bladder cancer. Since the late 1970s, evidence has become available that instillation of BCG into the bladder is an effective form of immunotherapy in this disease. While the mechanism is unclear, it appears a local immune reaction is mounted against the tumor.
Kit for detecting mycobacterium tuberculosis infection and monitoring clinical treatment effect and application of kit
ActiveCN104020297AIncreased sensitivityImprove featuresDisease diagnosisBiological testingTherapeutic effectSpecific antibody
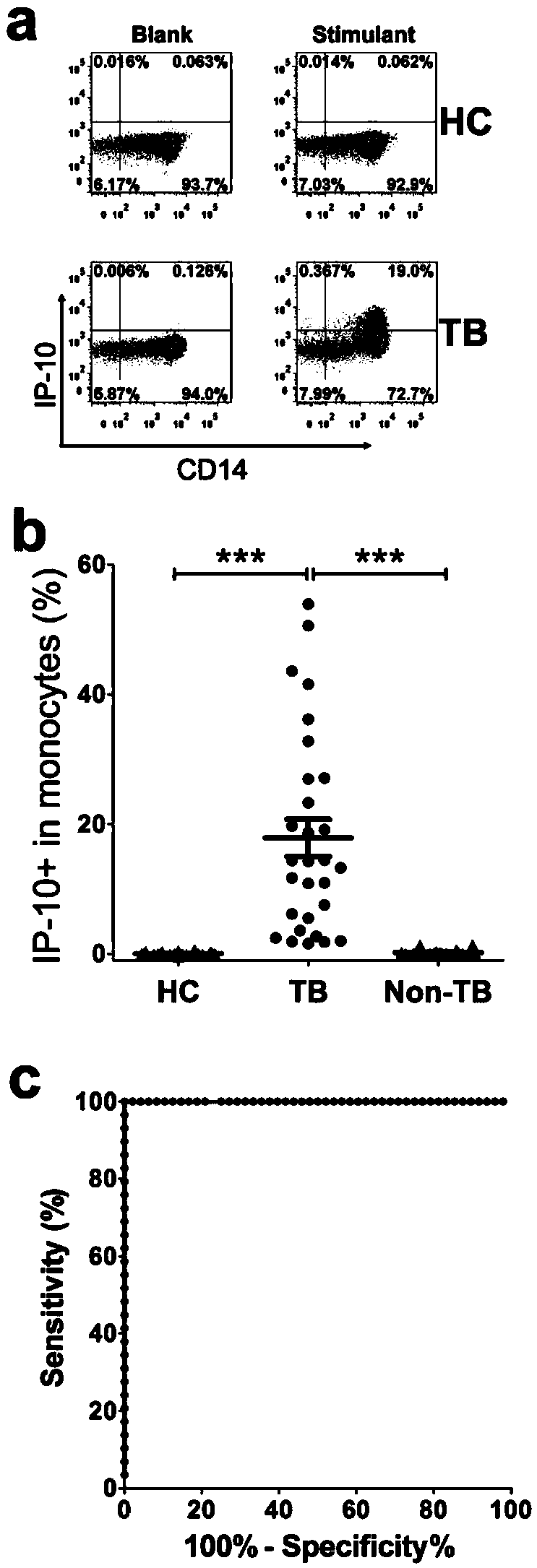
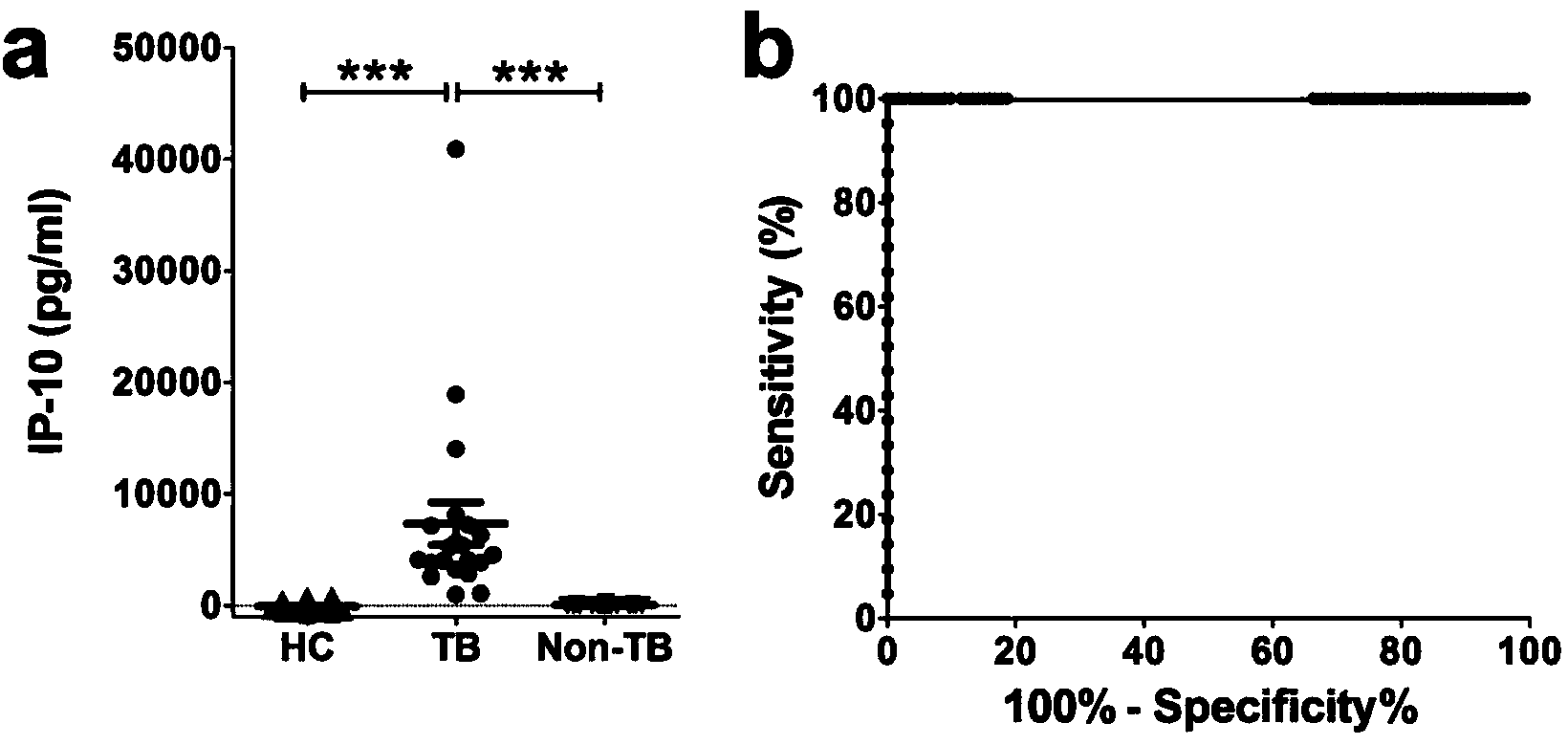
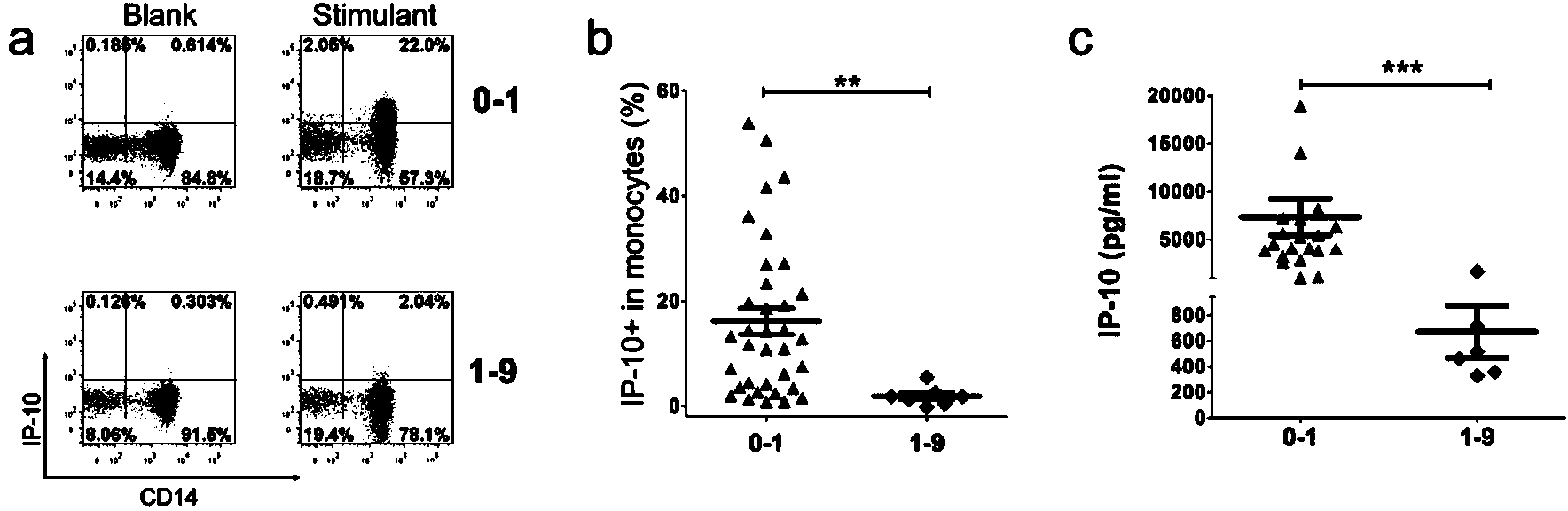
The invention discloses a kit for detecting the mycobacterium tuberculosis infection and monitoring a clinical treatment effect. The kit provided by the invention comprises a specific antibody, namely an IP-10 antibody and / or CD14 antibody, an antigen irritant and a positive contrast irritant. The kit disclosed by the invention can be used for diagnosing an active tuberculosis patient or a tuberculosis latent infection patient and is not affected by BCG (bacillus calmette-guerin) inoculation. The sensitivity and the specificity of the kit for diagnosing the active tuberculosis patient are higher than those of a commercial T-SPOT.TB kit and can be up to 100 percent. After the antituberculosis therapy is performed for 1 month, the detection rates of over 80 percent of clinical tuberculosis patients are converted to be negative, so that the kit can be used for detecting the clinical antituberculosis treatment effect.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Antigen epitope for exciting human anti-tubercle bacillus protective immunoreaction and its use
InactiveCN1858059AHelp preventAids in healingAntibacterial agentsBacterial antigen ingredientsMolecular ImmunologyBCG vaccine
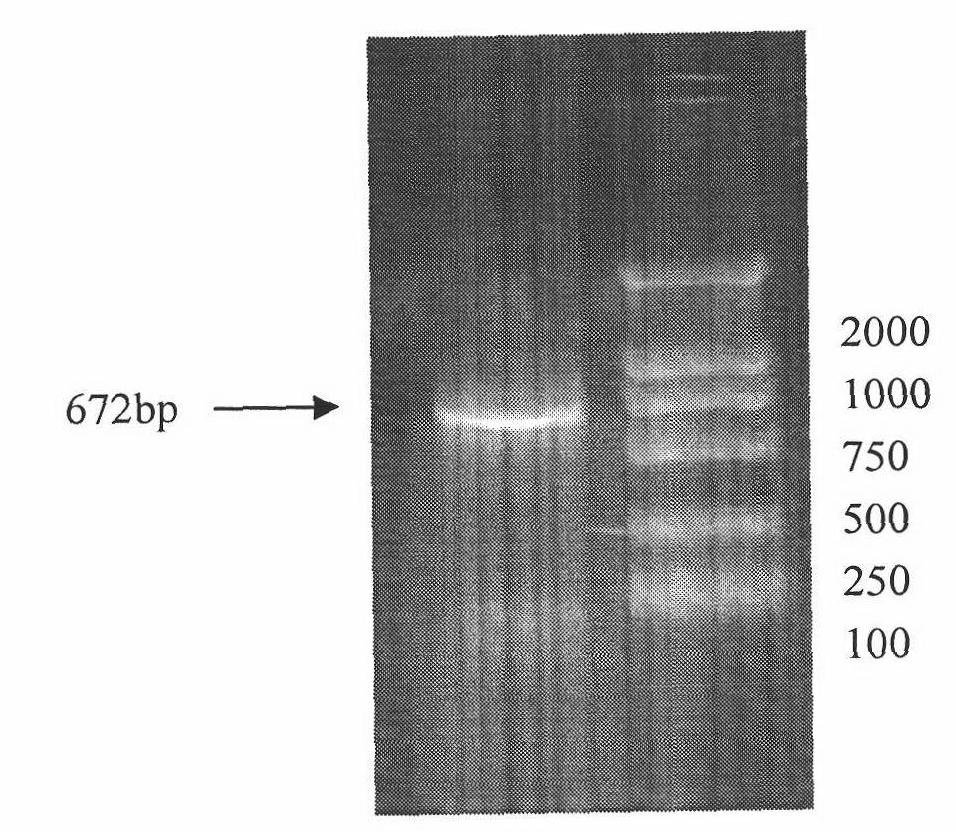
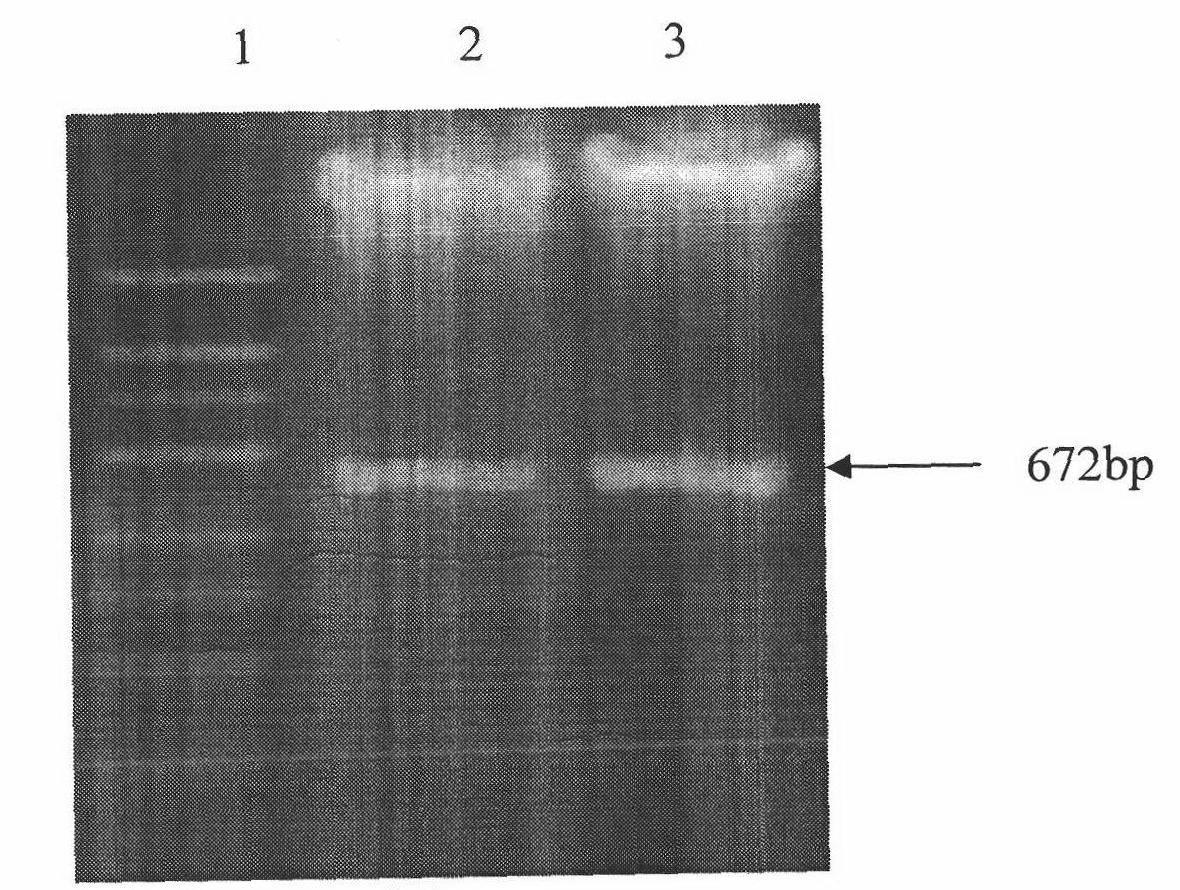
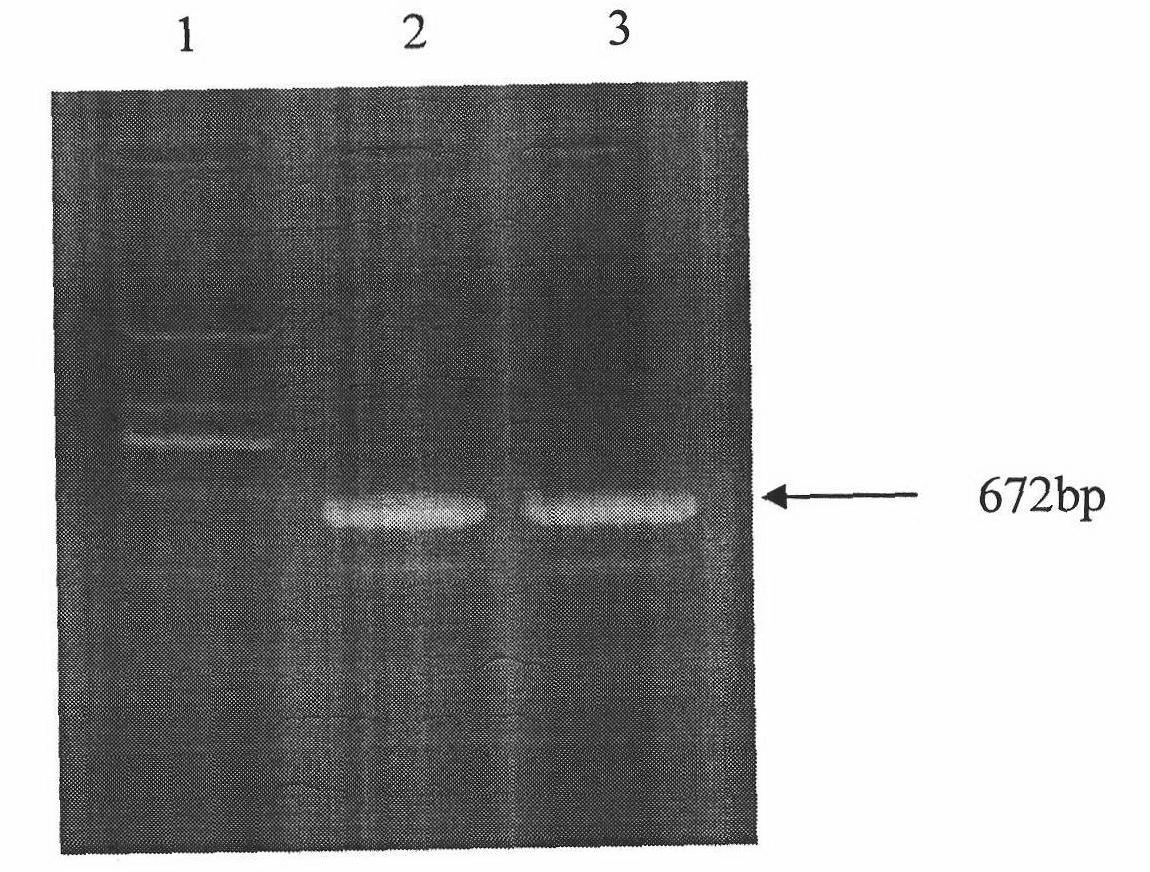
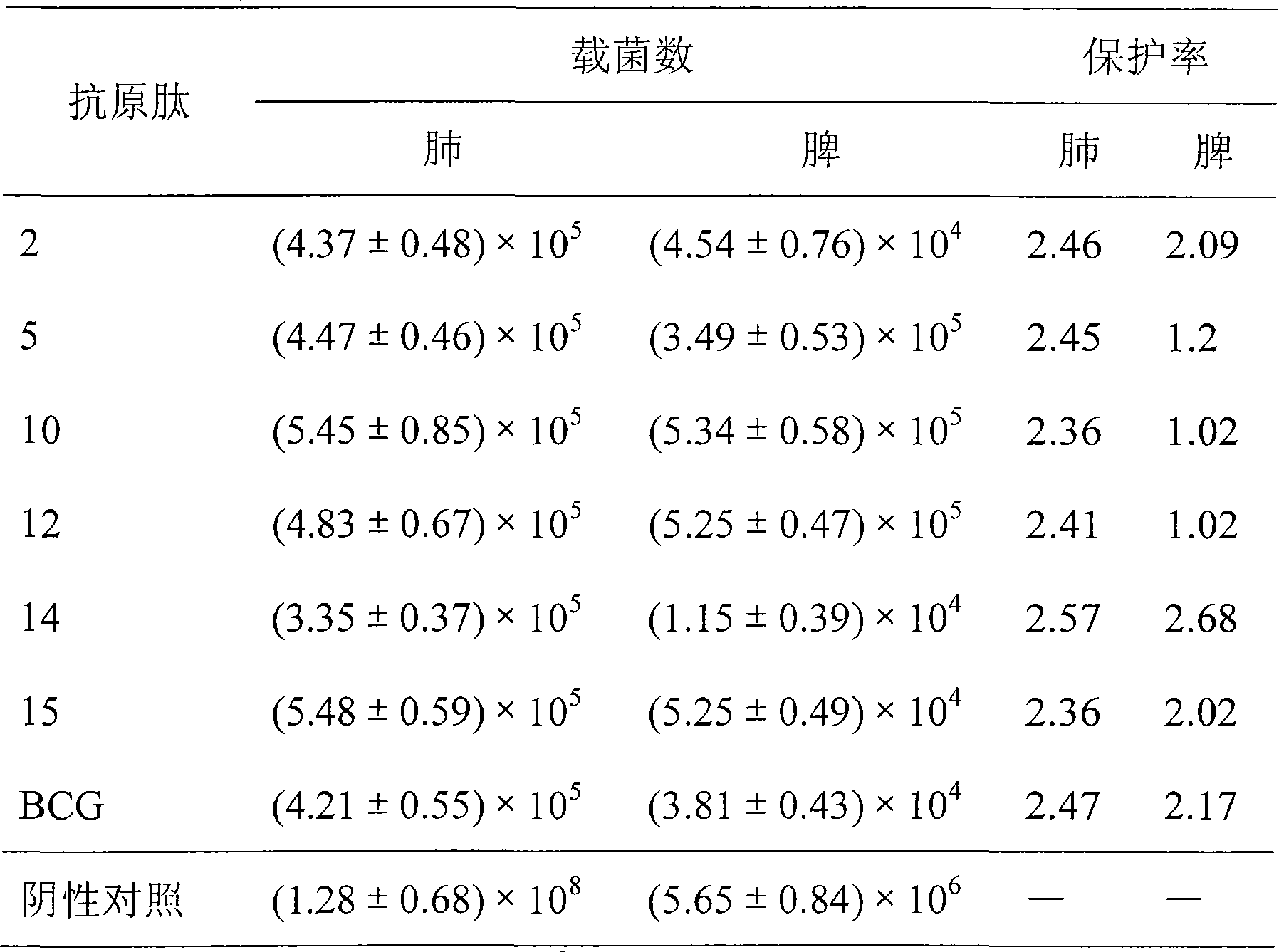
The present invention relates to molecular immunology technology, and aims at screening out antigen epitope molecular simulation peptide capable of exciting human body's protective immunoreaction against tubercle bacillus, researching protective immunoreaction mechanism against tuberculosis, and further developing new type of concatenate polyepitope tuberculosis vaccine. The present invention provides one kind of antigen epitope molecular simulation peptide capable of exciting human body's protective immunoreaction against tubercle bacillus, and the peptide contains the amino acid sequence selected from SEQ ID Nos. 2, 5, 10, 12, 14 and 15. The present invention also provides the screening process and use of the peptide. The present invention may be used in preventing and controlling tuberculosis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method to increase class i presentation of exogenous antigens by human dendritic cells
InactiveUS20080171023A1Enhance MHC-class I processingEnhance immune responseBiocideArtificial cell constructsMHC class IDendritic cell
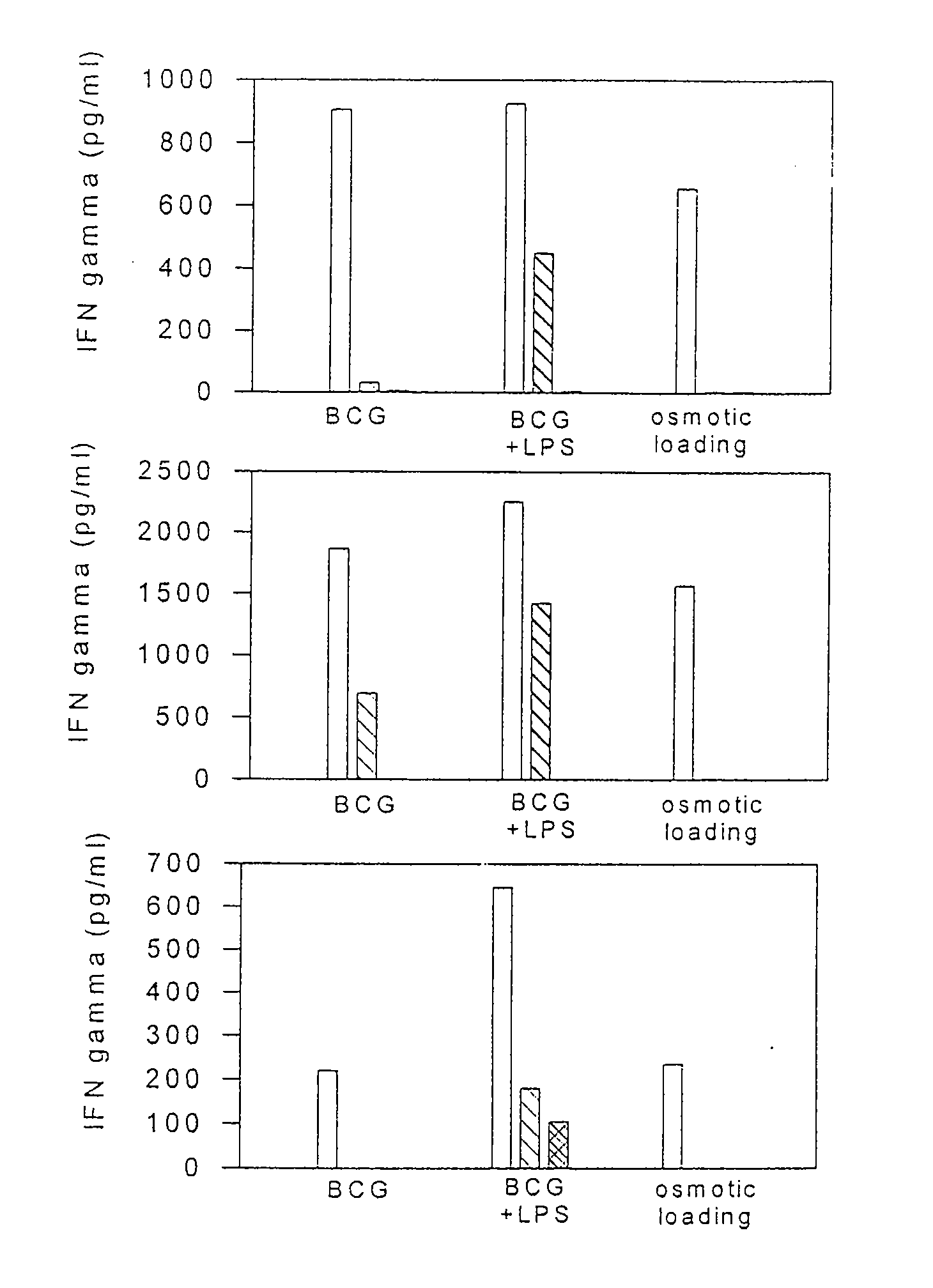
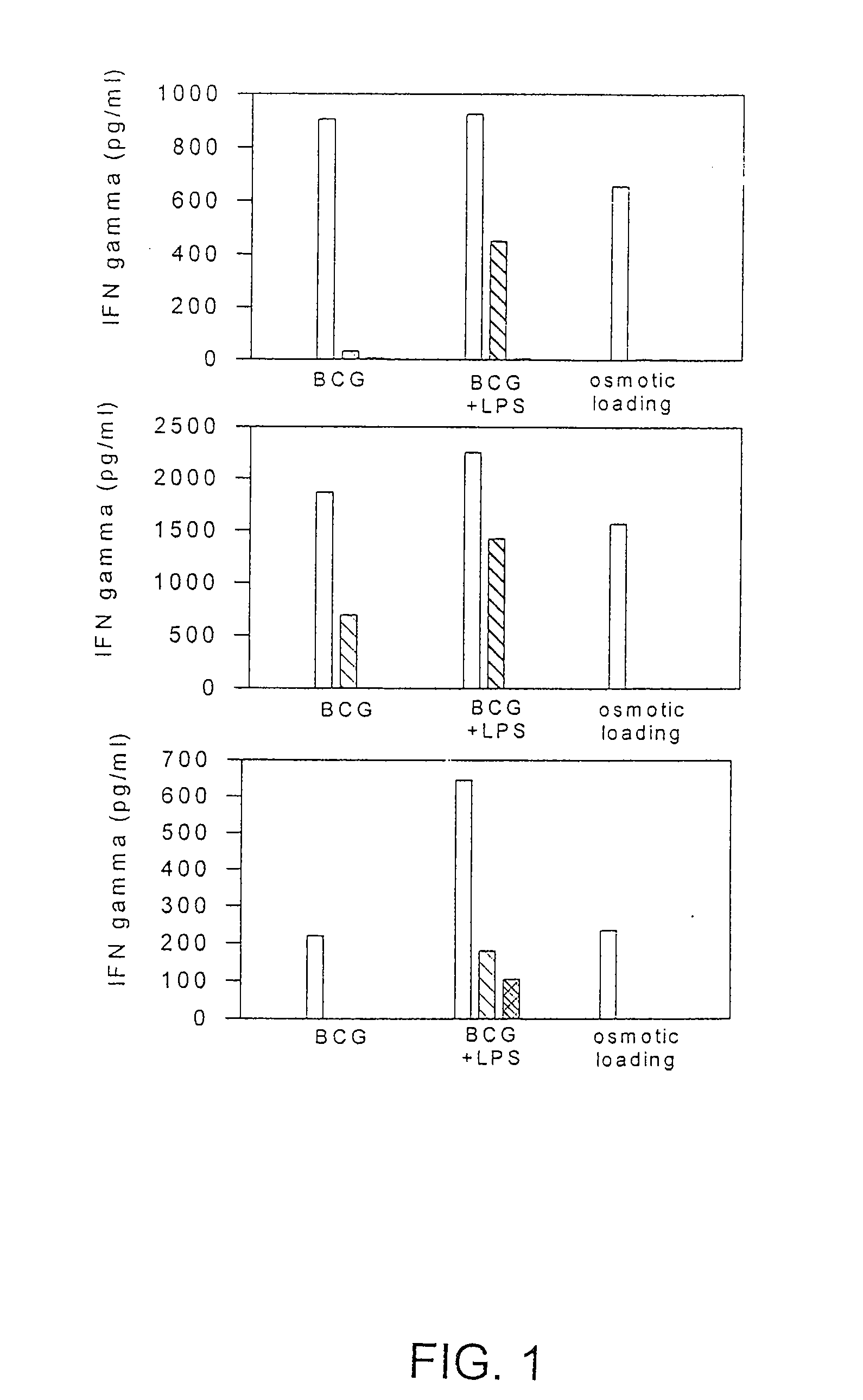
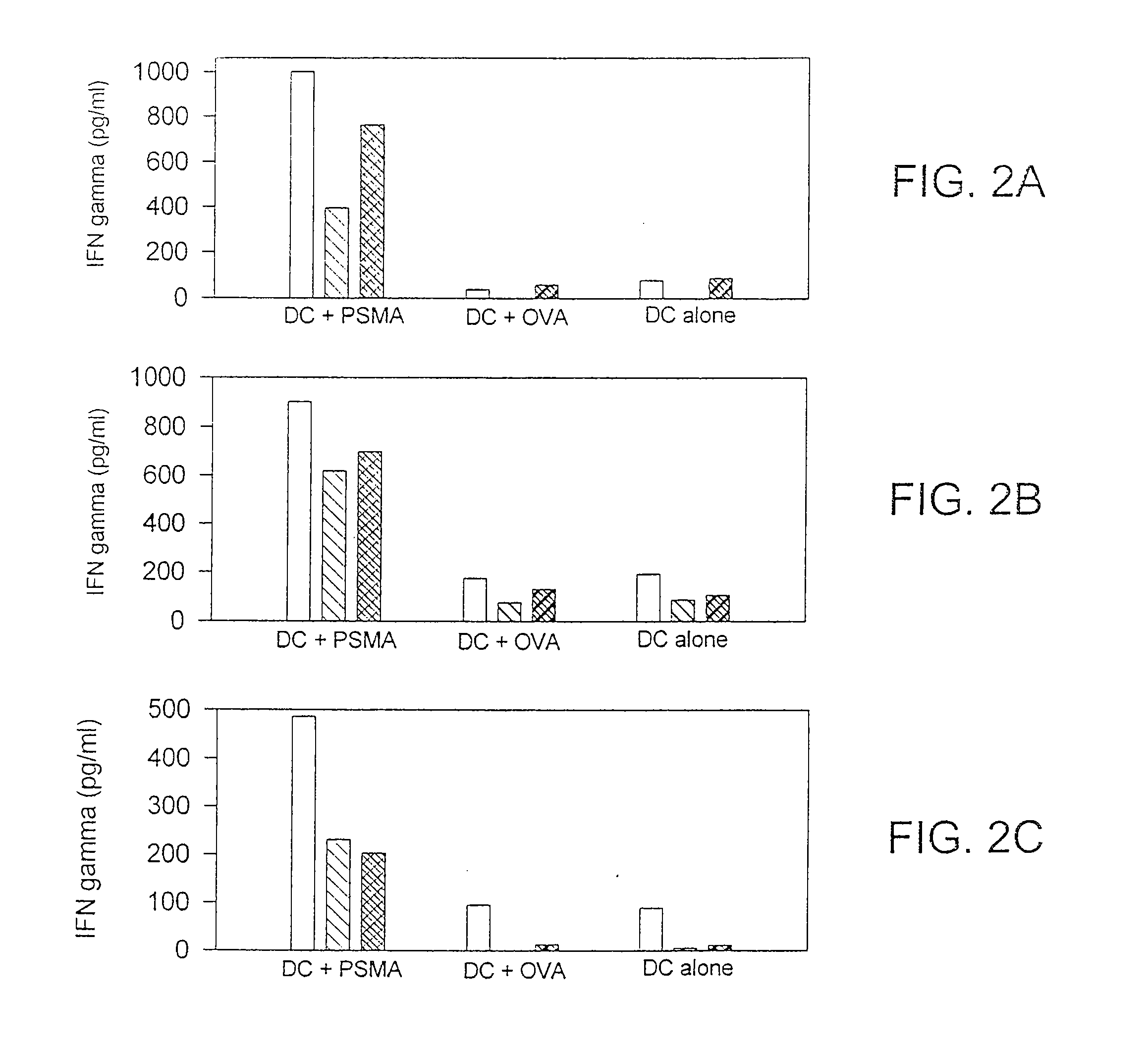
Methods and compositions for use of human dendritic cells to activate T cells for immunotherapeutic responses against primary and metastatic cancer are disclosed. In one embodiment, human dendritic cells exposed to a tumor associated antigen, or an antigenic fragment thereof in combination with bacillus Calmette-Guerin (BCG), are administered to a cancer patient to activate a predominantly CD8+T cell response in vivo. In an alternate embodiment, human dendritic cells are exposed to a tumor associated antigen or a specific antigenic peptide in combination with BCG in vitro and incubated or cultured with primed or unprimed T cells to activate a predominantly CD8+T cell response in vitro. The activated T cells are then administered to a cancer patient. Antigen in combination with BCG is processed by dendritic cells through the MHC-CLASS I compartment which provides for a predominantly CD8+T cell response. The addition of LPS provides for a greater number of mature dendritic cells enhancing the T cell response to antigen. Methods and compositions for human dendritic cells with extended life span and cryopreserved dendritic cells are disclosed.
Owner:NORTHWEST BIOTHERAPEUTICS INC
Bovine tuberculosis antibody identifying and detecting test strip prepared by applying Rv3872 novel fusion protein
InactiveCN101900727AWith identificationFunctionalBacteriaMicroorganism based processesAntigenNitrocellulose
The invention belongs to the technical field of animal infectious disease gene engineering and discloses a bovine tuberculosis antibody detecting immune colloidal gold test strip prepared by utilizing RV3872, ESAT6 and CFP10 fusion protein, and a preparation method and application. A colloidal gold immunochromatographic test strip is established by using the fusion protein as a colloidal gold labeled antigen and a capture antigen in a detection region of a nitrocellulose membrane. The detection of the bovine tuberculosis antibody by using test strip has prominent advantages of strong specificity and high sensitivity, and simultaneously bacillus calmette-guerin immunity and nontuberculosis mycobacteria infection can be identified and detected. The test strip comprises recombinant Escherichia coli BL21 / pET28a-MPBrce, and the strain expresses mycobacterium bovis RCE proteins and is preserved in the China Center for Type Culture Collection with the collection number of CCTCC No:M208244.
Owner:HUAZHONG AGRI UNIV
Immune cell cryopreservation liquid and cryopreservation method
ActiveCN108142412AAvoid pollutionReduce riskDead animal preservationHydroxyethyl starchProliferation activity
The invention discloses an immune cell cryopreservation liquid and a cryopreservation method. The cryopreservation liquid comprises a basic culture medium and additives. The additives include, by final concentration, 1-5 g / mL of trehalose, 3-6 v / v% of propylene glycol, 3-5 v / v% of acetamide, 5-10 v / v% of dextran, 1-3 g / mL of hydroxyethyl starch, 0.5-1.5 g / mL of glucose, 50-150 U / mL of heparin sodium, 5-15 mg / mL of herba phyllanthi urinariae extract and 0.5-1.5 mg / mL of bacillus Calmette-Guerin composite polysaccharides. The cryopreservation liquid is simple in formulation, interaction and synergistic effects of the components are achieved, endothelial progenitor cell cryopreservation effects can be evidently improved by the adoption of the cryopreservation method, insusceptibility of post-reviving cell proliferation activity can be guaranteed, and it is guaranteed that intracellular moisture of cells close to a freezing point is free of crystallization.
Owner:重庆斯德姆生物技术有限公司
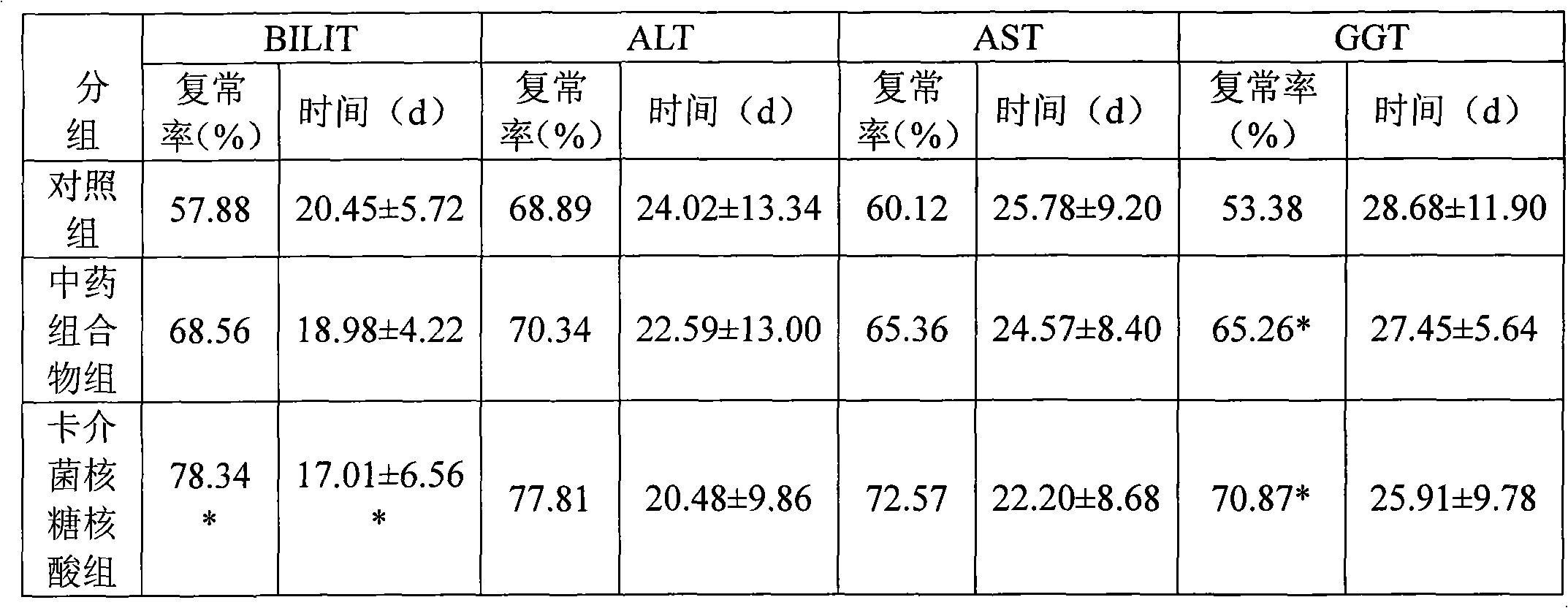
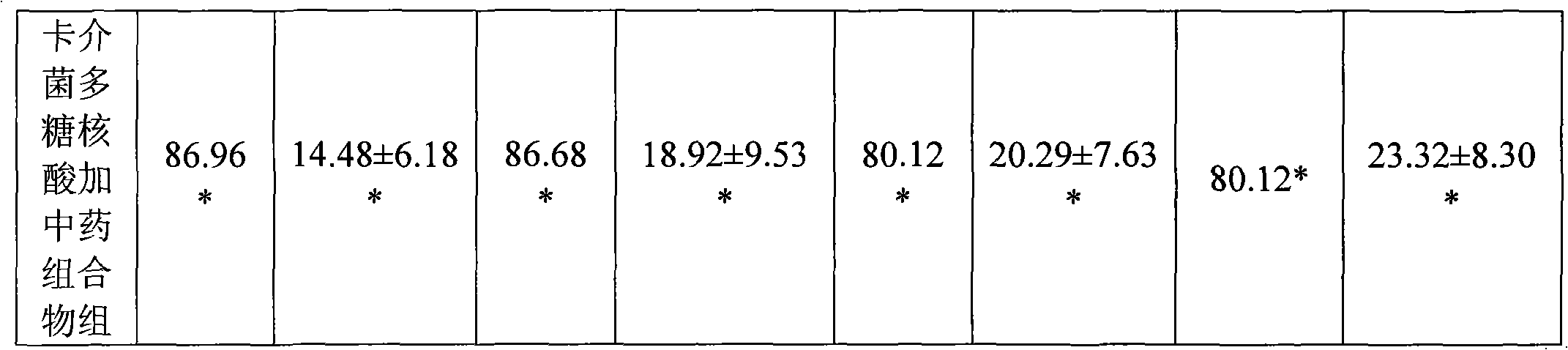
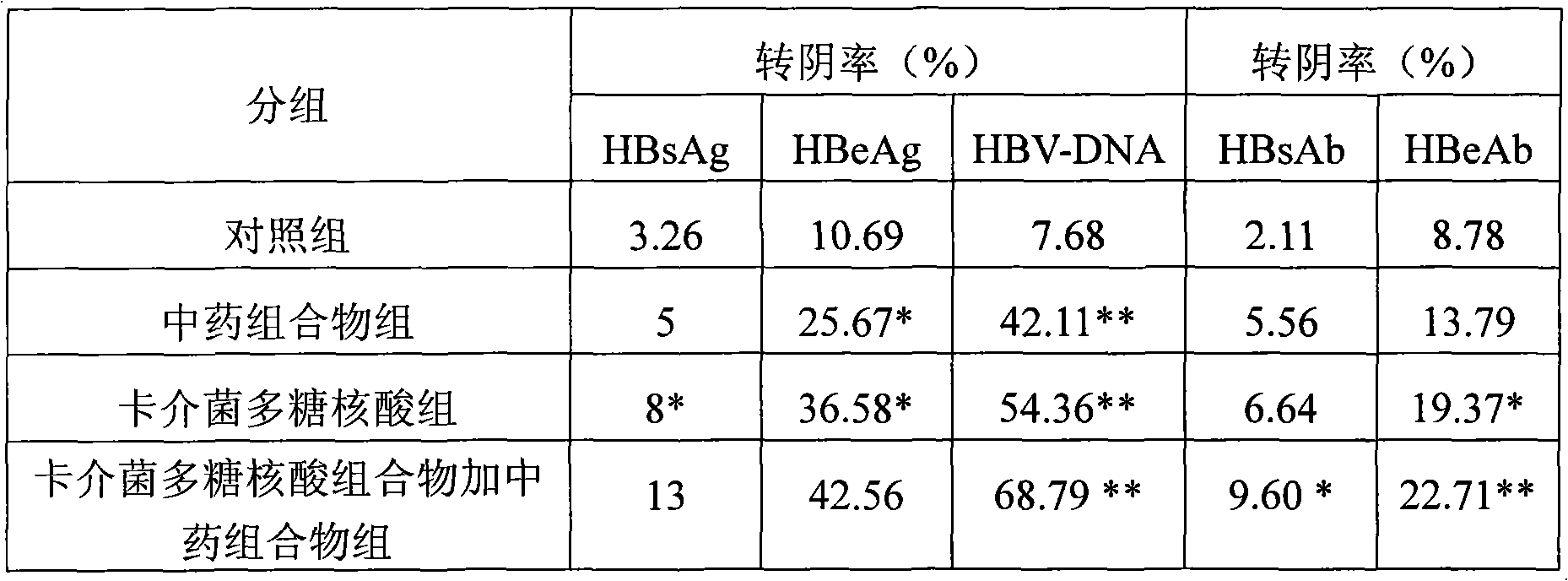
Medicine combination and application thereof in preparing preparations for treating chronic hepatitis B
ActiveCN101628046ARegulate immune functionImprove immunityBacteria material medical ingredientsDigestive systemToxic materialSpleen
The invention relates to a medicine combination and the application thereof in preparing preparations for treating chronic hepatitis B. The combination comprises a medicine combination with polysaccharide nucleic acid of bacillus calmette guerin as the active component and a Chinese traditional medicine combination, wherein the Chinese traditional medicine combination is prepared from astragalus, herba artemisiae scopariae, oldenlandia diffusa, herba lysimachiae, codonopsis pilosula, processed polygonum capitatum, salvia, radix paeoniae alba, chinaberry fruit, taraxacum, cortex moutan, poria and atractylodes; the polysaccharide nucleic acid of bacillus calmette Guerin is nonspecific immunity active reinforcer, and can effectively restrain the reproduction of hepatitis virus and promote the rapid negative turning of HBV-DNA and HbeAg; and the Chinese traditional medicine combination has the efficacy of invigorating qi and spleen, promoting blood circulation by removing blood stasis and clearing away heat and toxic material. The medicine combination can reduce the incidence rate of variation and drug resistance and effectively promote the improvement under the condition of improving the healing efficacy, and provides a novel, safe and effective drug choice for clinics.
Owner:JIUZHITANG
Bacillus calmette-guerin compound polysaccharide, and preparation method and use thereof
InactiveCN103305567AGood clinical efficacyWide range of indicationsOrganic active ingredientsMicroorganism based processesDiseaseSide effect
The invention discloses bacillus calmette-guerin compound polysaccharide, and a preparation method and use thereof. The preparation method comprises the steps of extracting bacillus calmette-guerin compound polysaccharide from a bacillus calmette-guerin cell wall by using a biochemical method; firstly, continuing to inoculate subculture containing a bacillus calmette-guerin standard strain on Sutong culture medium to carry out extended cultivation until mycoderm from milk white to dark orange grows; carrying out compound polysaccharide crude extraction and compound polysaccharide purification. The prepared bacillus calmette-guerin compound polysaccharide comprises arabinose, mannose, glucoside and galactose; the molecular weight is 2000-36000 Daltons. The bacillus calmette-guerin compound polysaccharide can simulate an immune system of people; activation of a plurality of immune cells and generation of cell factors are facilitated, so as to play the immunomodulatory effect. The preparation has no toxic and side effect on application to a human body, and can be used for preventing influenza, asthma, an anaphylactic disease and intracellular infectious diseases, and assisting auxiliary anti-tumor treatment.
Owner:CENT SOUTH UNIV
PCR(Polymerase Chain Reaction) primers and method for identifying mycobacterium bovis
InactiveCN102409102ASimple and precise identificationEasy to operateMicrobiological testing/measurementMicroorganism based processesBacteroidesNucleotide
The invention provides five pairs of primers and an identification method for PCR(Polymerase Chain Reaction) identification of mycobacterium bovis. The five pairs of primers respectively amplify aiming at a 16SrRNA conserved region of mycobacterium bacteria, a mycobacterium tuberculosis complex(MTBC)Rv0577 gene, Rv1970 of a mycobacterium tuberculosis RD7 region, a mycobacterium bovis pncA gene and an RD1 region gene to generate specific amplified fragments, and the nucleotide sequences of the specific amplified fragments are as shown in SEQ ID No.1-5. According to the identification method provided by the invention, the total DNA of a sample is taken as a template, PCR amplification is respectively performed by the five pairs of primers, and the result is judged according to the size of an amplified band. The primers provided by the invention can specifically identify mycobacteria, MTBC, mycobacterium tuberculosis, mycobacterium bovis and mycobacterium bovis BCG(Bacillus Calmette-Guerin), and the detection method has good sensitivity and simplicity of method and operation, and can realize quick large-flux detection of mycobacterium bovis.
Owner:CHINA AGRI UNIV
Mycobacterium tuberculosis PGL-tb1 oligosaccharide conjugate as well as preparation method and application thereof
ActiveCN106390113ASolve protection problemsFix stability issuesAntibacterial agentsBacterial antigen ingredientsAntigenChemical structure
The invention relates to a mycobacterium tuberculosis PGL-tb1 oligosaccharide conjugate as well as a preparation method and an application thereof. In the mycobacterium tuberculosis PGL-tb1 oligosaccharide conjugate provided by the invention, oligosaccharide, which is clear and simple in chemical structure, instead of a mixture, can be massively synthesized by virtue of a chemical method, and by coupling a designed linker to a vector, an antigen can be prepared; the problem of low and unstable BCG (Bacillus Calmette Guerin) vaccine immune protection force can be solved; and the mycobacterium tuberculosis PGL-tb1 oligosaccharide conjugate, for some special people low in immunity, can also achieve a relatively immune effect.
Owner:CANSINO BIOLOGICS INC
Mycobacterium tuberculosis fusion protein and application thereof in induction of peripheral blood mononuclear cells to generate cytokines
ActiveCN105601747AIncreased sensitivityStrong specificityAntibacterial agentsBacterial antigen ingredientsAntigenPeripheral blood mononuclear cell
The invention discloses a mycobacterium tuberculosis fusion protein and an application thereof in induction of peripheral blood mononuclear cells (PBMCs) to generate cytokines. The fusion protein includes three proteins PPE41, ESAT-6 and PE25, and the proteins are connected through connecting peptides. Compared with present stimulants, the fusion protein provided by the invention has the advantages of efficient effect, strong sensitivity, high specificity and good stimulation effect. The fusion protein stimulates the PBMCs to generate a large amount of mycobacterium tuberculosis antigen specific IFN-gamma, IL-2, TNF-alpha and other tuberculosis related factors, and the above stimulation induction reaction is free from BCG vaccine interference. The fusion protein can effectively improve the tuberculosis detection rate and is of positive significance to control the tuberculosis. The fusion protein can be applied in researches of the tuberculosis pathopoiesis and immunoprophylaxis mechanisms and control of the tuberculosis as a stimulant.
Owner:SUN YAT SEN UNIV
Novel recombinant vaccine used for preventing tuberculosis
InactiveCN101850112AStable expressionImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsEscherichia coliImmunogenicity Study
The invention relates to the construction of a human GM-CSF gene and Mycobacterium tuberculosis ESAT6 gene chimerically expressed GMCSF-ESAT6 protein recombinant Bacillus Calmette Guerin (BCG) vaccine and immunogenicity research thereof, namely the sequence of the human GM-CFS gene and the sequence of the Mycobacterium tuberculosis ESAT6 gene are inserted into the sequence of the same escherichiacoli-Mycobacterium tuberculosis shuttle plasmid pMV361 by gene engineering technology so as to construct a recombinant shuttle plasmid rpMV361GMCSF-ESAT6; and a vector is introduced into a BCG vaccine by an electroporation method so as to construct a recombinant BCG vaccine rBCG:GMCSF-ESAT6. The recombinant BCG vaccine can express the human GM-CSF and Mycobacterium tuberculosis ESAT6 gene chimeric protein GMCSF-ESAT6 stably, and has an immunogenicity superior to that of the conventional BCG vaccine. The invention provides a process for preparing the recombinant BCG vaccine, researches the immunity thereof, and belongs to the field of gene engineering and the field of tuberculosis vaccine. The novel recombinant vaccine prevents the generation and the propagration of tuberculosis more effectively.
Owner:SICHUAN UNIV
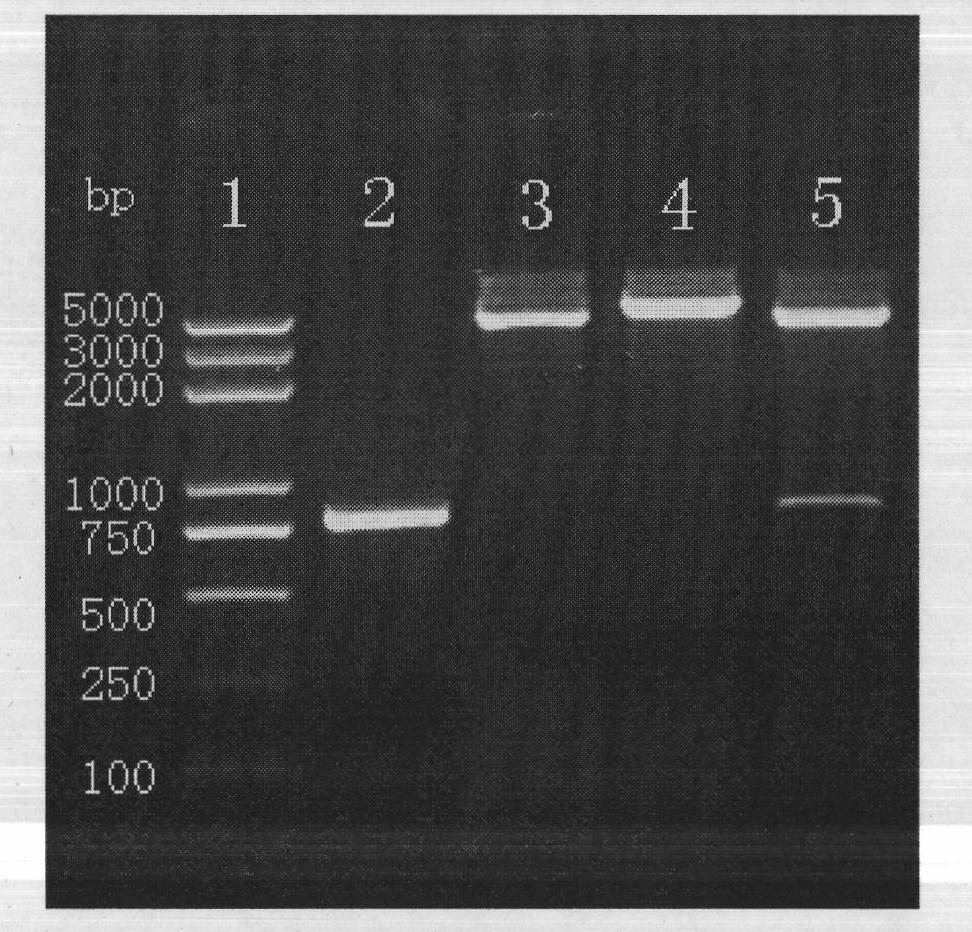
Construction and application of TRAIL (Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand) recombinant bacille calmette guerin (rBCG)
InactiveCN102327604AConnection direction is correctMeet the design requirementsAntibacterial agentsBacterial antigen ingredientsAntigenSide effect
The invention provides construction of TRAIL (Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand) recombinant bacille calmette guerin (rBCG), and relates to a shuttle expression vector comprising a signal peptide fragment of a major secretory antigen Ag85B of BCG and a gene fragment of a TRAIL and a construction method thereof. The obtained shuttle expression vector pMV261-Ag85B-TRAIL is used for constructing rBCGTRAIL, and can be applied to preparation of TRAIL rBCG for treating superficial bladder tumors, preventing postoperative recurrence thereof and preventing tuberculosis. The rBCG has dual functions of TRAIL and BCG, so that cooperative and synergistic actions of the TRAIL and BCG can be better brought into play; rBCG-TRAIL can secrete TRAIL, and the using amount of the rBCG-TRAIL can be lower than that of the BCG under the condition that the same or better immune effect is achieved, so that the toxic or side effect is reduced; and the rBCG-TRAIL can directly secrete TRAIL efficiently on a certain part, so that tumor cells can be killed in cooperation with the rBCG-TRAIL, and high cost caused by the use of a foreign cell factor is avoided.
Owner:沈周俊
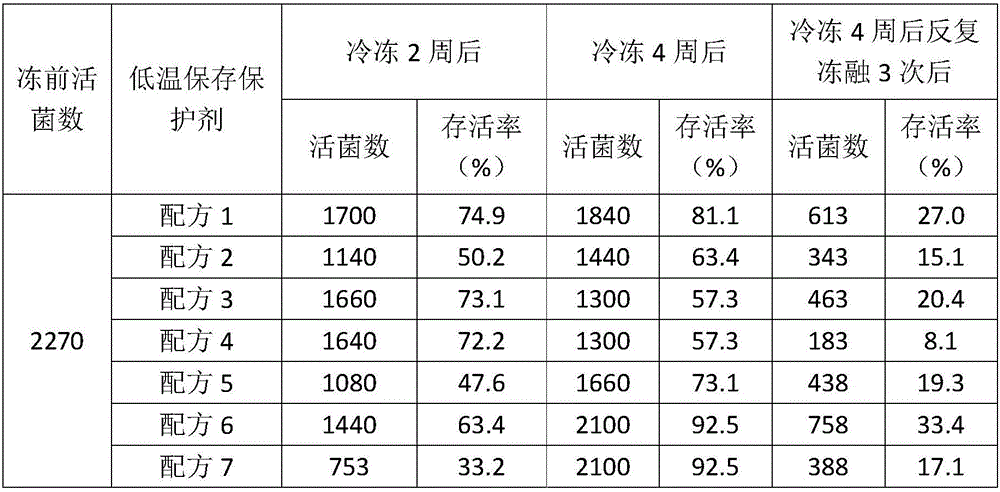
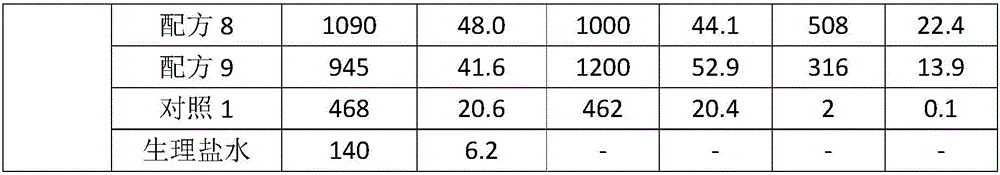
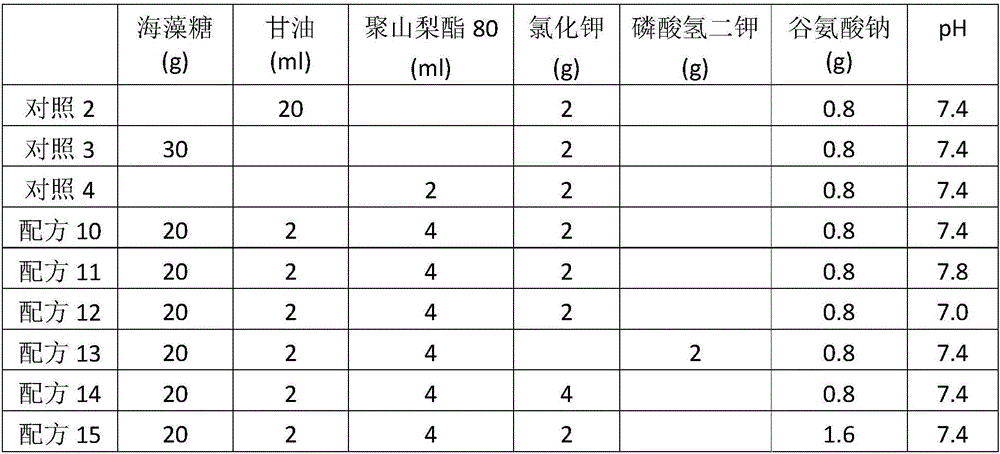
Low-temperature microorganism preservation protectant
The invention relates to a low-temperature microorganism preservation protectant, as well as a preparation method and use thereof. The low-temperature microorganism preservation protectant is prepared from the following components: non-reducing saccharide, glycerin, a nonionic surfactant, a potassium salt, glutamate and water. The invention also discloses a technical method for preserving bacillus calmette-guerin at low temperature for long. A technical support is provided for deep development of related researches on the bacillus calmette-guerin.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Method for treating bladder cancer through promoting pharmorubicin by bacillus calmette guerin vaccine
PendingCN109675023ASmall toxicityImprove treatment efficiencyCompound screeningOrganic active ingredientsSide effectRetention time
The invention discloses a method for treating bladder cancer through promoting pharmorubicin by a bacillus calmette guerin vaccine (BCG), and specifically relates to the field of combined treatment ofthe bladder cancer in clinical medicine. The method comprises a method for researching synergism of the BCG to the pharmorubicin in a cellular level and a method for synergism of the in-vivo BCG to the pharmorubicin. According to the invention, through using the method for researching the synergism of the BCG to the pharmorubicin in the cellular level and the method for the synergism of the in-vivo BCG to the pharmorubicin, a combined medication scheme is provided to improve treatment efficiency and reduce toxic and side effects during drug perfusion. The method disclosed by the invention isnot high in prefused drug concentration, but long in intra-bladder retention time, thereby being better in the anti-tumor effect without generating an adverse reaction.
Owner:GUANGDONG PHARMA UNIV
Mycobacterium tuberculosis fusion protein (EAMMH) and constructing, expressing and purifying method and application thereof
ActiveCN104098700AImprove shortcomingsStrong immunityAntibacterial agentsBacterial antigen ingredientsAntigenInclusion bodies
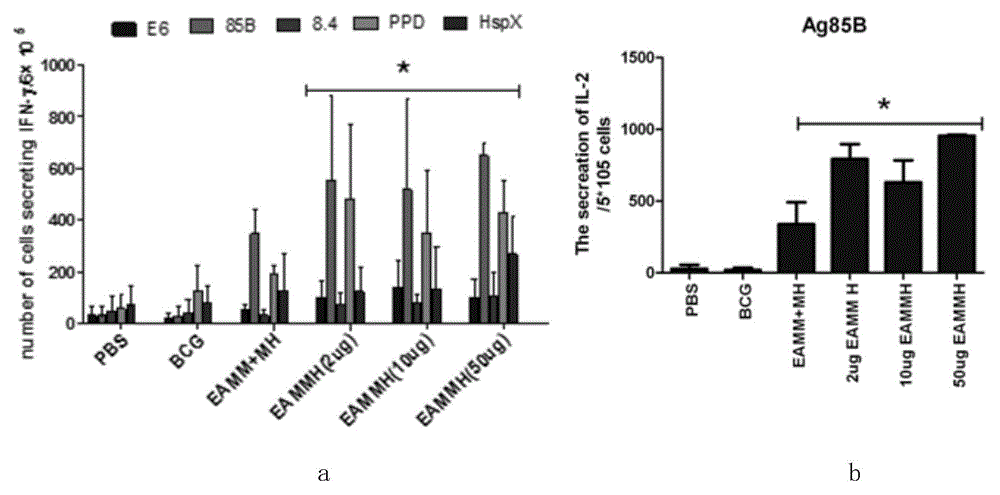
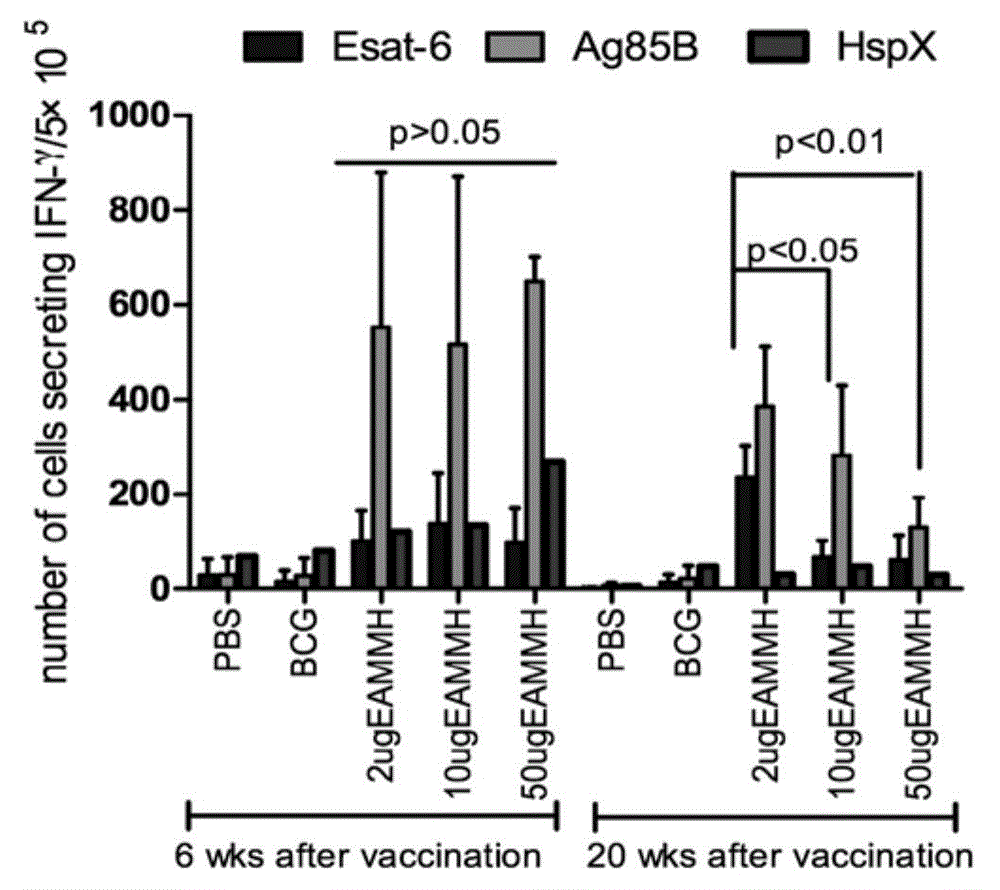
The invention discloses mycobacterium tuberculosis fusion protein EAMMH and a constructing, expressing and purifying method and application thereof. The fusion protein is expressed in a soluble form, and greatly improves EAMM inclusion body form expression weakness. Tuberculosis subunit vaccine (LT69) constructed by combination of the fusion protein and an adjuvant has strong protective immunity, is superior to traditional BCG (Bacillus Calmette-Guerin) vaccine and EAMM+MH combined vaccine; the vaccine as an enhanced vaccine can significantly enhance the BCG initial immune immunity and protection effect of anti tuberculosis, and to a certain extent, reduces the pathological injury of the lung; in addition, the subunit vaccine contains a wide variety of antigens of growth period and latency period of mycobacterium tuberculosis, can induce strong specific cellular immune and humoral immune response aiming at each period of tubercle bacillus antigen, has protective effect on the tubercle bacillus in different metabolic states, is long in protection time, and is expected to become an effective vaccine for clinical tuberculosis prevention.
Owner:LANZHOU UNIVERSITY
Recombinant bacillus calmette guerin vaccine for toxoplamasis and preparation method thereof
InactiveCN102198267AImprove thermal stabilityEasy transportationProtozoa antigen ingredientsMicroorganism based processesEscherichia coliGondii toxoplasma
The invention provides a recombinant bacillus calmette guerin (BCG) vaccine for toxoplamasis and a preparation method thereof. The preparation method for the recombinant bacillus calmette guerin vaccine comprises the following steps of: performing target (TA) cloning on an obtained toxoplasma gondii cyclophilin gene; sequencing and identifying correctly, performing enzyme cutting and recovering target fragments; connecting the target fragments with an Escherichia coli-mycobacterium shuttle expression vector pMV261 and an integrated expression vector pMV361 which are subjected to enzyme cutting reaction respectively; and converting recombinant plasmids to BCG, and screening by resistance and polymerase chain reaction (PCR) to obtain the recombinant bacillus calmette guerin vaccine for the positive toxoplasma gondii. The vaccine is high in heat stability and easy to transport, store and produce, is not needed to be purified and can be directly used for immune protection tests, and a complex process of protein aftertreatment is avoided, so the cost is reduced greatly, and the recombinant bacillus calmette guerin vaccine is suitable for vast rural areas.
Owner:JILIN UNIV
Cell-free preparations of immunopotentiators, and preparation methods and uses thereof
InactiveCN103505476AUniform particle sizePromote absorptionAntibacterial agentsOrganic active ingredientsGranularityBrucella
The invention relates to a cell-free preparation of a pseudomonas preparation, a cell-free preparation of a Bacillus Calmette-Guerin polysaccharide and nucleic acid preparation, a cell-free preparation of a Nocardia rubra cell wall skeleton preparation, a cell-free preparation of a Group A Streptococcus preparation, a cell-free preparation of a Pseudomonas aeruginosa preparation, and a cell-free preparation of a Brucella preparation. The above cell-free preparations have a granularity of 10-1000nm, preferably 10-800nm, and more preferably 10-500nm. The pyrogens of Gram-positive bacteria are below 320EU / ml, and preferably below 120EU / ml. The preparation methods of the cell-free preparations comprise the following steps: heating the preparations for boiling for 15-60min to obtain inactivated bacterial liquids; washing aseptic-test-qualified bacterial liquids, and breaking thalli under an aseptic condition by using a breaker; centrifuging a suspension obtained after breaking the thalli, collecting the above obtained precipitate, and washing the precipitate to prepare a suspension; and packaging the suspension, and carrying out heating disinfection to obtain the cell-free preparations of the preparations. The invention also relates to applications of the cell-free preparations.
Owner:熊慧
Composition comprising bacillus calmette guerin polysaccharides and bacillus calmette guerin nucleic acids and use of preparing medicament thereof
ActiveCN102238959BRegulate immune functionAvoid infectionOrganic active ingredientsBacterial antigen ingredientsMicroorganismIon exchange
A composition comprising BCG polysaccharide and BCG nucleic acid is provided, wherein the polysaccharide accounts for 10% to 69% of the mass of the composition, and the nucleic acid accounts for 30% to 89% of the mass of the composition, the The composition is prepared by mixing said polysaccharide and said nucleic acid, wherein said polysaccharide and said nucleic acid are obtained from a BCG culture by extracting said polysaccharide and said a mixture of nucleic acids, and separating said polysaccharide and said nucleic acids from this mixture by ion exchange chromatography. Also provided is the use of the composition comprising the polysaccharide and the nucleic acid in the preparation of biological agents for treating or preventing microbial infections, tumors, and allergies.
Owner:HUNAN SIQI BIOPHARM
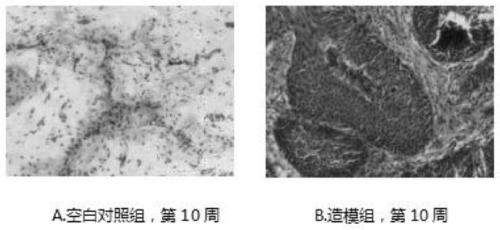
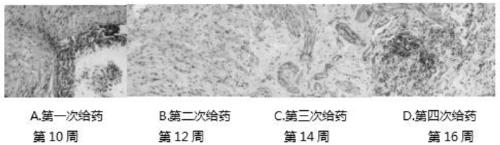
Construction method of drug evaluation model for dermal pathology of tuberculosis rabbit
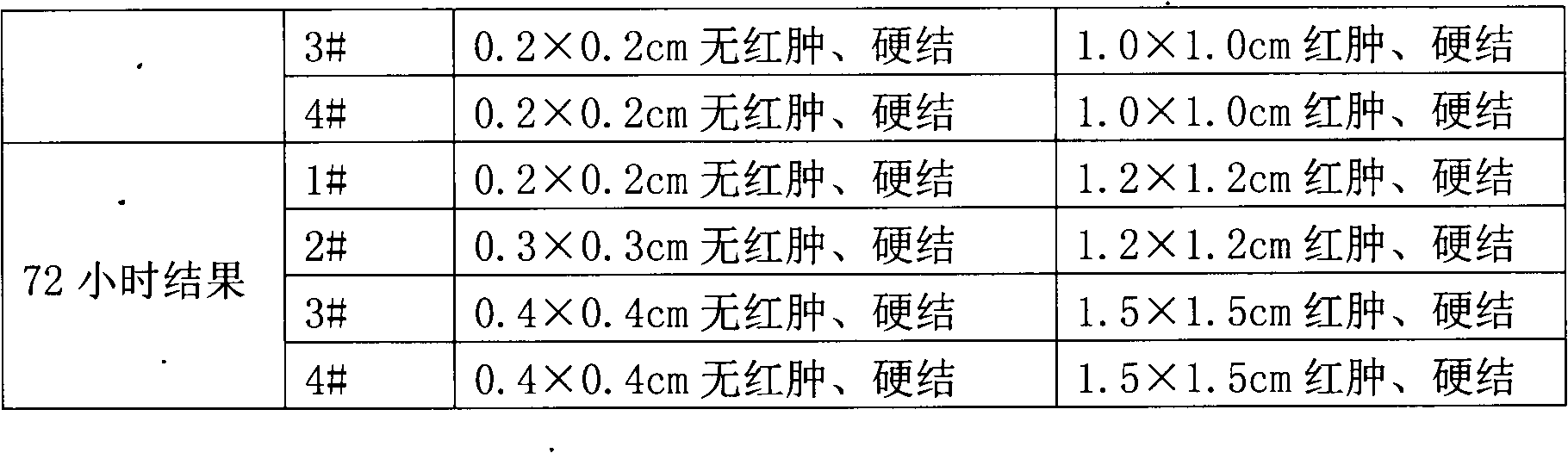
InactiveCN102430119AObvious symptomsOrganic active ingredientsBacterial antigen ingredientsBCG immunizationTreating tuberculosis
The invention discloses a construction method of a drug evaluation model for dermal pathology of a tuberculosis rabbit. The method comprises: selecting a rabbit, injecting an immune drug into rabbit, conducting BCG (Bacillus Calmette Guerin) vaccine immune injection on the 15th to 20th day of drug intervention, and carrying out bacterial and pathological examination so as to construct a pathological model. The method of the invention establishes a pathological model of drug intervention on drug cutaneous tuberculosis with obvious symptoms so as to conduct visual research on tuberculosis pathology and bacterial pathogenicity, thus providing a visual animal model for vaccines and screening of drugs treating a tuberculosis necrotic liquefied cavity. And the model is stable and repeatable. The invention establishes a drug intervention procedure and observation indexes for research of drug intervention on a tuberculosis granuloma liquefaction process, and also provides a research basis for probing an immune mechanism about the formation of a tuberculosis liquefied cavity.
Owner:LANZHOU UNIVERSITY
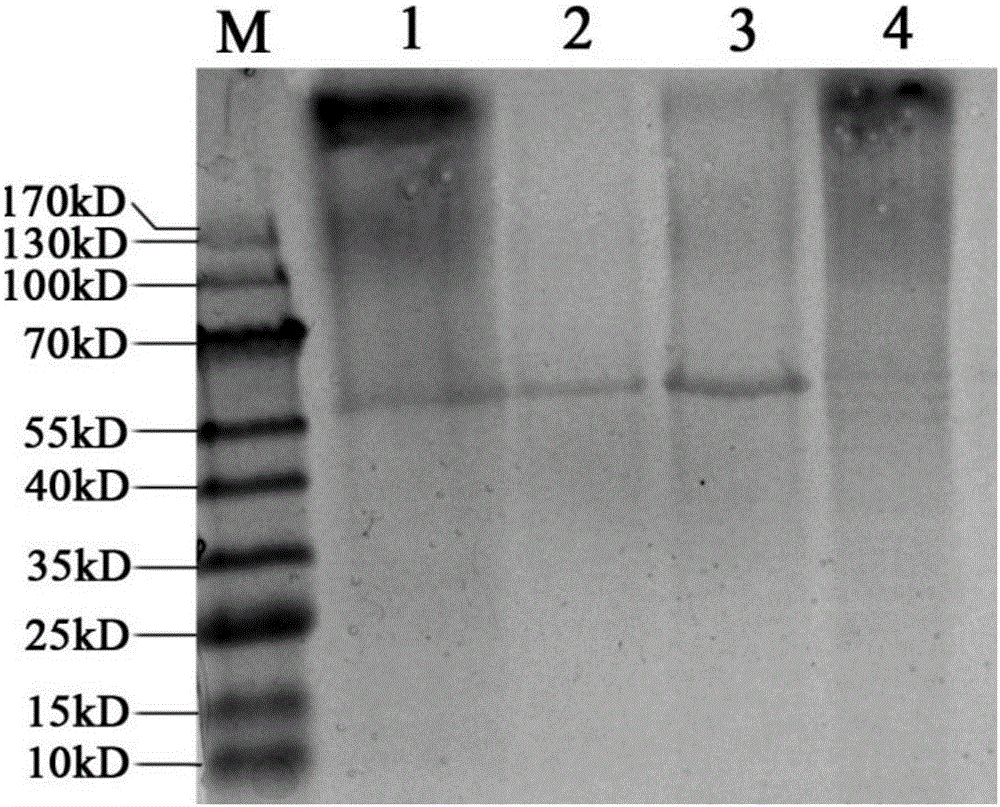
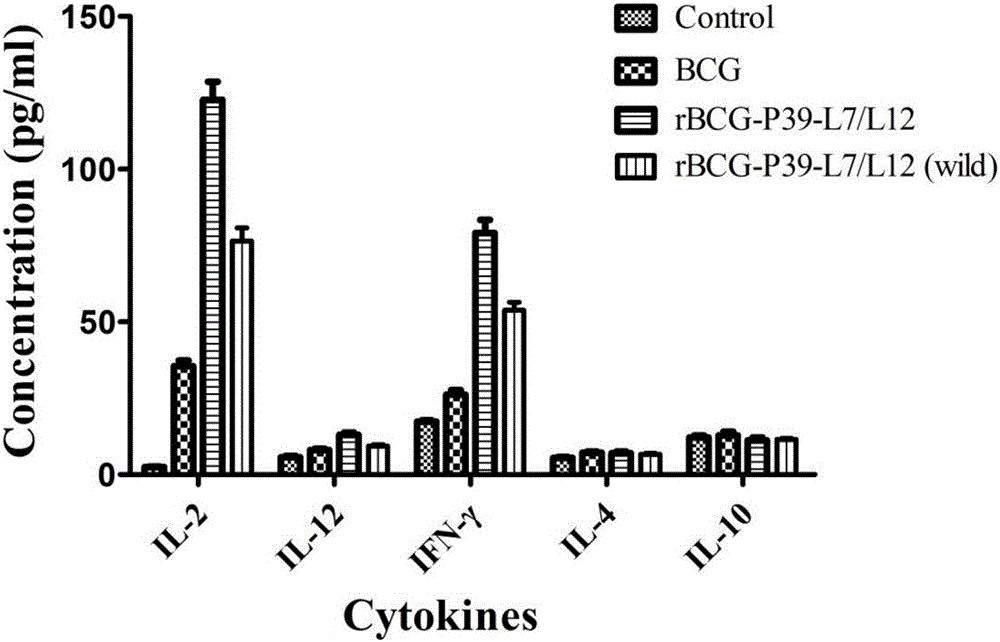
rBCG for expression of Br. Melitensis P39 and L7/L12 fusion gene and construction method thereof
ActiveCN106834331ASignificant immune adjuvant effectLow costAntibacterial agentsBacterial antigen ingredientsRibosomal proteinBCG vaccine
The invention provides rBCG for expression of Br. Melitensis P39 and L7 / L12 fusion gene. The rBCG is constructed by transferring an expression vector carrying codon-optimized Br. Melitensis P39 and L7 / L12 fusion gene into BCG. Brucellosis-generated cytoplasm binding protein PBP39 (coding gene is P39) and Brucellosis ribosomal protein L7 / L12 are both T-cell antigen. Bacillus Calmette-Guerin (BCG) vaccine is the only one commercial vaccine for preventing tuberculosis so far. The BCG vaccine has a remarkable immunologic adjuvant effect and is an exogenous gene expression host with good performance and high safety. By BCG expression of the codon-optimized Brucellosis P39 and L7 / L12 fusion gene, expression quantity of the target gene can be increased. The rBCG vaccine can simulate intracellur infection and parasitic characteristics of Brucellosis to more effectively induce body to generate immune response, can perform advantages of high safety, simple preparation, low cost, etc. of BCG as the expression host as well as the immunologic adjuvant effect of the BCG itself, and is expected to become a novel Brucellosis vaccine.
Owner:INNER MONGOLIA MEDICAL UNIV
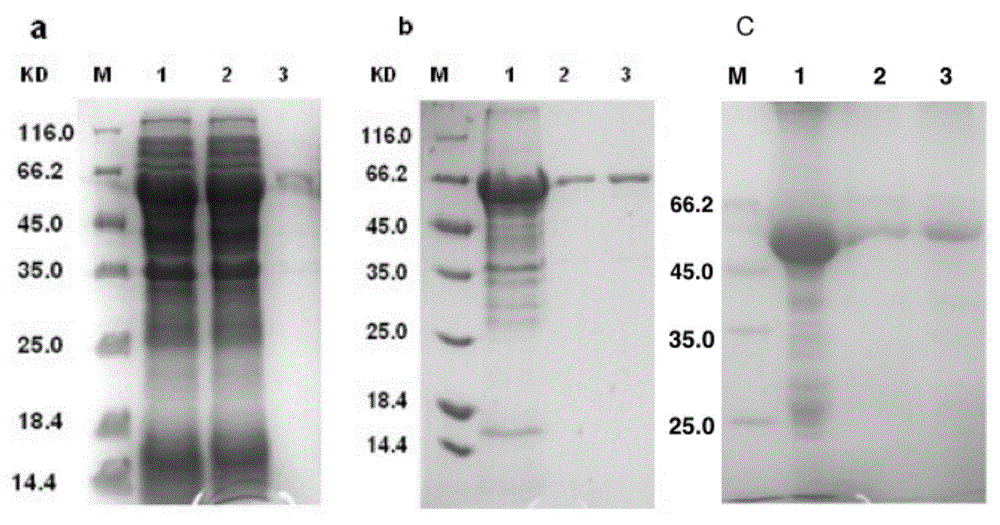
Recombinant human co-stimulatory molecule bacilli-calmette-guerin strain and process for making same
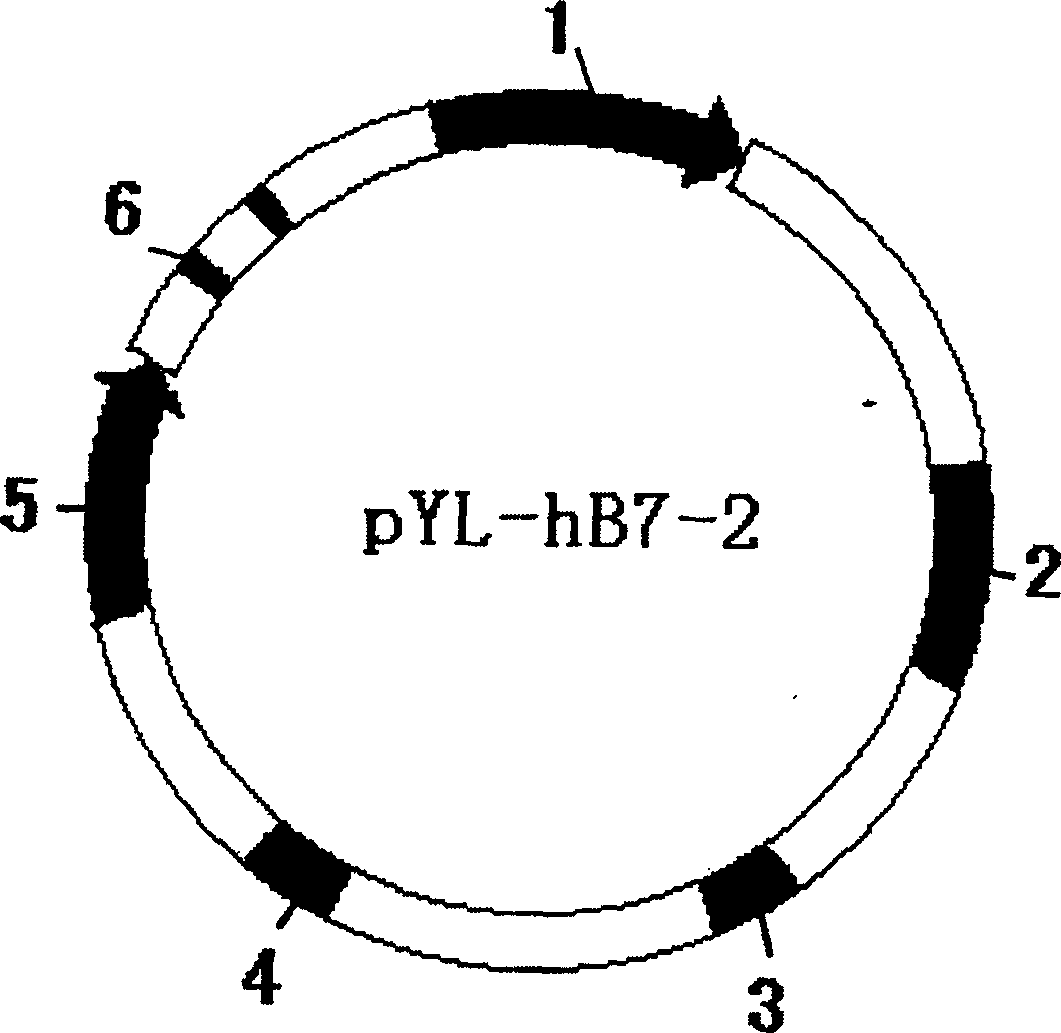
The invention discloses a recombinant human co-stimulatory molecule bacilli-calmette-guerin strain and process for preparation, wherein the cultrue preservation number is CGMCC No.1120. The preparing process consists of carrying out hB7-2(IgC+IgV) fragment polymerase chain reaction, establishing plasmid pYL-hB7-2 and transforming, carrying out E.Coli-pYL-hB7-2 monoclonal bacterial colony expansion, plasmid extraction and purification, electrophoresis, enzyme cutting and determination of pYL-hB7-2 plasmid through PCR reaction, bacillus Calmette-Guerin vaccine electrical transformation, rBCG-hB7-2 monoclonal bacterial colony selection, expansion, and determination.
Owner:天津市泌尿外科研究所 +4
A hemopoietic stem cell medium
InactiveCN107099504AHigh proliferation ratePromote proliferationCulture processBlood/immune system cellsVitamin CCell activity
The invention relates to the technical field of biology, and particularly relates to a hemopoietic stem cell medium. The medium includes a base medium and an additive added into the base medium. Based on final concentrations, the additive includes 100-200 mg / mL of FBS, 0.02-0.03 [mu]mol / mL of sodium selenite, 5-10 mg / mL of bacillus calmette-guerin polysaccharides and nucleic acids, 50-100 [mu]mol / mL of cepharanthine, 50-100 ng / mL of trehalose, 40-80 ng / mL of vitamin C, 1.5-2.5 mg / mL of a first cytokine slow-release microcapsule, and 25-50 mg / mL of a second cytokine slow-release microcapsule. The hemopoietic stem cell medium can significantly increase the propagation rate and cell activity of hemopoietic stem cells, allows the hemopoietic stem cells to be in a propagation undifferentiated state for long time, and maintains stem cell characteristics of the hemopoietic stem cells.
Owner:DONGGUAN BOALAI BIOLOGICAL TECH CO LTD
Bacillus calmette-guerin polysaccharide and nucleic acid preparation for treating respiratory diseases for livestock
InactiveCN101757041AHigh extraction rateShort timeOrganic active ingredientsBacteria material medical ingredientsDiseaseRespiratory tract disease
The invention relates to a bacillus calmette-guerin polysaccharide and nucleic acid preparation for treating respiratory diseases for livestock. The method for preparing the preparation comprises a new process of extracting polysaccharide and nucleic acid from bacillus calmette-guerin, particularly using a high-pressure homogenizing machine to crush the bacillus calmette-guerin, and crushing the bacterium by combining the high-pressure homogenizing machine with the conventional high-speed tissue stamping technique to extract the polysaccharide and nucleic acid from the bacillus calmette-guerin. The extract can be prepared into different preparations as required, and the preparations are used for preventing and treating the respiratory disease of the livestock.
Owner:TIANJIN RINGPU BIO TECH
Recombinant bacillus calmette-guerin vaccine strain with over-expression mycobacterium tuberculosis Rv3586 and application of recombinant bacillus calmette-guerin vaccine strain
ActiveCN106479946AGood immune protectionStrong cellular immune responseAntibacterial agentsBacterial antigen ingredientsMicrobiologyBcg bladder instillation
The invention relates to a recombinant bacillus calmette-guerin vaccine strain with over-expression mycobacterium tuberculosis Rv3586 and application of the recombinant bacillus calmette-guerin vaccine strain. Plasmids with encoding genes of the mycobacterium tuberculosis Rv3586 are transformed into bacillus calmette-guerin vaccine, and the recombinant bacillus calmette-guerin vaccine strain can be obtained by means of screening, is called as Bacillus calmette-Guerin rBCG-DisA and is called as rBCG-DisA for short, and a preservation number of the bacillus calmette-guerin vaccine strain is CCTCC M 2016335. The recombinant bacillus calmette-guerin vaccine strain and the application have the advantages that the recombinant bacillus calmette-guerin vaccine strain with the over-expression mycobacterium tuberculosis Rv3586 has merits of target antigens and bacillus calmette-guerin vaccine and can be used for individual immunization, or booster immunization can be carried out by the recombinant bacillus calmette-guerin vaccine strain and mycobacterium tuberculosis Ag85B-ESAT6 subunit vaccine, high immune response of normal and affected mice can be induced, the mycobacterium-resistant protective ability of organisms can be improved, and accordingly the recombinant bacillus calmette-guerin vaccine strain has an excellent application prospect.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Reagent and method for detecting Mycobacterium tuberculosis infection in vitro
InactiveCN102305855AValid in vitro assayOvercome the disadvantages of unsatisfactory effectImmunoglobulins against bacteriaFermentationAntigenParatuberculosis
The invention discloses a reagent and a method for detecting Mycobacterium tuberculosis infection in vitro. The reagent comprises a segment of specific T cell reactive polypeptide, namely M232 polypeptide shown as SEQ ID No.1 and screened from early secreting antigen target-6 (ESAT-6) polypeptide; and the M232 polypeptide is contacted with a T cell of a Mycobacterium tuberculosis host to detect cell factors released by the T cell and determine whether the T cell identifies the M232 polypeptide. The invention has the advantages of high sensitivity, no influence of Bacillus Calmette-Guerin (BCG) vaccine and nontuberculosis mycobacterial vaccine, high specificity, capacity of detecting patients with active pulmonary tuberculosis and patients with potential infection, and capacity of detecting healthy Mycobacterium tuberculosis contacts. The invention is particular suitable for detecting tuberculosis and / or potential infection of the tuberculosis for Chinese people. The invention also relates to a kit for detecting Mycobacterium tuberculosis infection in vitro, which comprises two mixed polypeptides, namely M232 and M233, and a tool for detecting the identification of the T cell on protein, or polypeptide or analogs of the polypeptide.
Owner:SUN YAT SEN UNIV
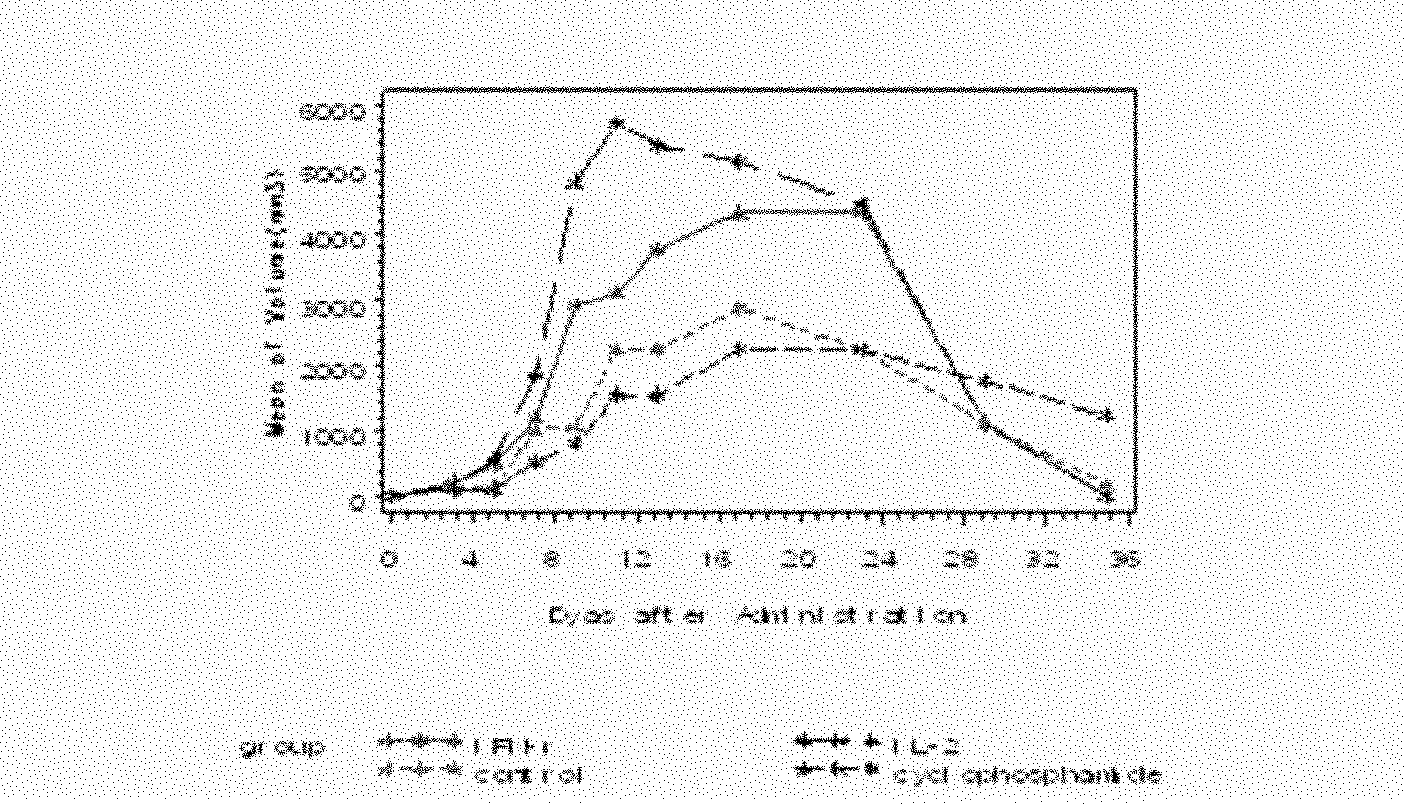
Recombinant bacillus calmette-guerin vaccine secreting bacteria resuscitation somatomedin and application thereof
InactiveCN101862450ASecretion persistsStable growth curveAntibacterial agentsBacterial antigen ingredientsBacteroidesGrowth Factor Gene
The invention discloses a recombinant bacillus calmette-guerin vaccine secreting bacteria resuscitation somatomedin and application thereof. The recombinant bacillus calmette-guerin vaccine uses the bacillus calmette-guerin vaccine as host cell. An exogenous expression vector carrying out transfection on the host cell is an expression vector comprising a bacteria resuscitation somatomedin genetic fragment. The recombinant bacillus calmette-guerin vaccine can continuously secrete Rpf factors with bioactivity and can induce a humoral immunity response level and a cellular immune response level. An antibody which is generated by aiming at the exogenous Rpf can interdict the stimulating effect of the Rpf to MTB. The level of inducing IFN-gamma of the recombinant bacillus calmette-guerin vaccine is superior to that of BCG. The recombinant bacillus calmette-guerin vaccine is applied to preparation of a preventive immunity vaccine for MTB infection and / or MTB inapparent infection.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Prediction method and prediction system of irresponsive gamma globulin Kawasaki disease
InactiveCN107480436AProlonged feverShort duration of feverHealth-index calculationCharacter and pattern recognitionSerum rashKawasaki disease
The invention provides a prediction method and prediction system of an irresponsive gamma globulin Kawasaki disease. The method comprises the following steps that 21 original parameters of SVM model building are collected; the modelling original parameters comprise gender, age, fever time during treatment, clinical classification, CRP detection value, WBC value, PLT value, Hb value, ALT value, AST value, ALB value, gamma globulin using time and clinical diagnosed symptom indicators; the clinical diagnosed symptom indicators comprise conjunctival injection, erythra, cracked lips, a strawberry-like tongue, lymphadenectasis of a neck, hard and swollen hands and feet, digit peeling, crissum peeling and a red and swollen bacillus calmette-guerin scar; discretization is conducted on the original parameters to obtain SVM characteristic values corresponding to the original parameters; the SVM characteristic values are regarded as base data, a SVM model is built, and through the SVM model, a complication with irresponsive gamma globulin of the Kawasaki disease is predicted. By means of the prediction method and prediction system, early intervening treatment can be conducted on a patient, recovery of damage to a coronary artery is promoted, and the prediction method and prediction system have important significance and value on diagnosis and treatment of the Kawasaki disease in the future.
Owner:SOOCHOW UNIV AFFILIATED CHILDRENS HOSPITAL +1
Antigen epitope for exciting protective immunity against tubercle bacillus of human body and uses thereof
InactiveCN101311189BHelp controlMulti-drug resistance problem solvedAntibacterial agentsBacterial antigen ingredientsMolecular ImmunologyScreening method
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Mycobacterium tuberculosis OS-tb oligosaccharide conjugate, and preparation method and application thereof
ActiveCN107184972ASolve protection problemsFix stability issuesAntibacterial agentsBacterial antigen ingredientsAntigenChemical structure
The invention provides a mycobacterium tuberculosis OS-tb oligosaccharide conjugate, and a preparation method and application thereof. In the mycobacterium tuberculosis OS-tb oligosaccharide conjugate, the chemical structure of oligosaccharide is clear and single, is not a mixture, and can be abundantly synthesized by a chemical method; through the design of a connecting body to be coupled with a carrier for preparing the antigen, the problems of instability and low immune protection force of BCG vaccine can be solved; a relatively good immune effect can also be generated on some special people groups with low immunity. Wide application values are realized in the preparation of the tuberculosis vaccine.
Owner:CANSINO BIOLOGICS INC
Human interferon alpha-2b recombinant bacillus calmette-guerin, construction method and identification method thereof
InactiveCN101381728AImprove anti-bladder cancer effectReduce the applied doseBacterial antigen ingredientsMicrobiological testing/measurementSide effectImmunocompetence
The invention relates to a recombinant BCG vaccine rBCG-IFN alpha-2b for secreting human IFN alpha-2b and a construction method and an identification method thereof, wherein a BCG vaccine Ag85B signal peptide fragment which has the function of secretion and genes of human IFN alpha-2b are cloned to pMV261 by the genetic engineering technology, and a BCG vaccine shuttle expression vector pMV261-Ag85B-IFN alpha-2b is obtained; and the vector is induced into BCG by the electrotransformation technology, and the recombinant BCG vaccine rBCG-IFN alpha-2b is established; and the human IFN alpha-2b can be highly efficiently secreted in virtue of the secretion function of the pMV261-Ag85B-IFN alpha-2b on BCG replication and signal peptide. The recombinant BCG vaccine rBCG-IFN alpha-2b obtained not only keeps the immunogenicity of the prior BCG but also can continuously secrete the cell factor-the IFN alpha-2b, thereby improving the immunocompetence of the BCG; the IFN alpha-2b can be directly acted on tumor cells to inhibit proliferation and induced differentiation of the tumor cells, has good antitumor action, and can reduce the application dosage and reduce the toxic and side effect caused by the BCG; and the IFN alpha-2b solves the problems of large application dosage, high incidence rate of side effects, short response time and expensive cost caused by exogenous IFN alpha-2b.
Owner:丁国庆
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com