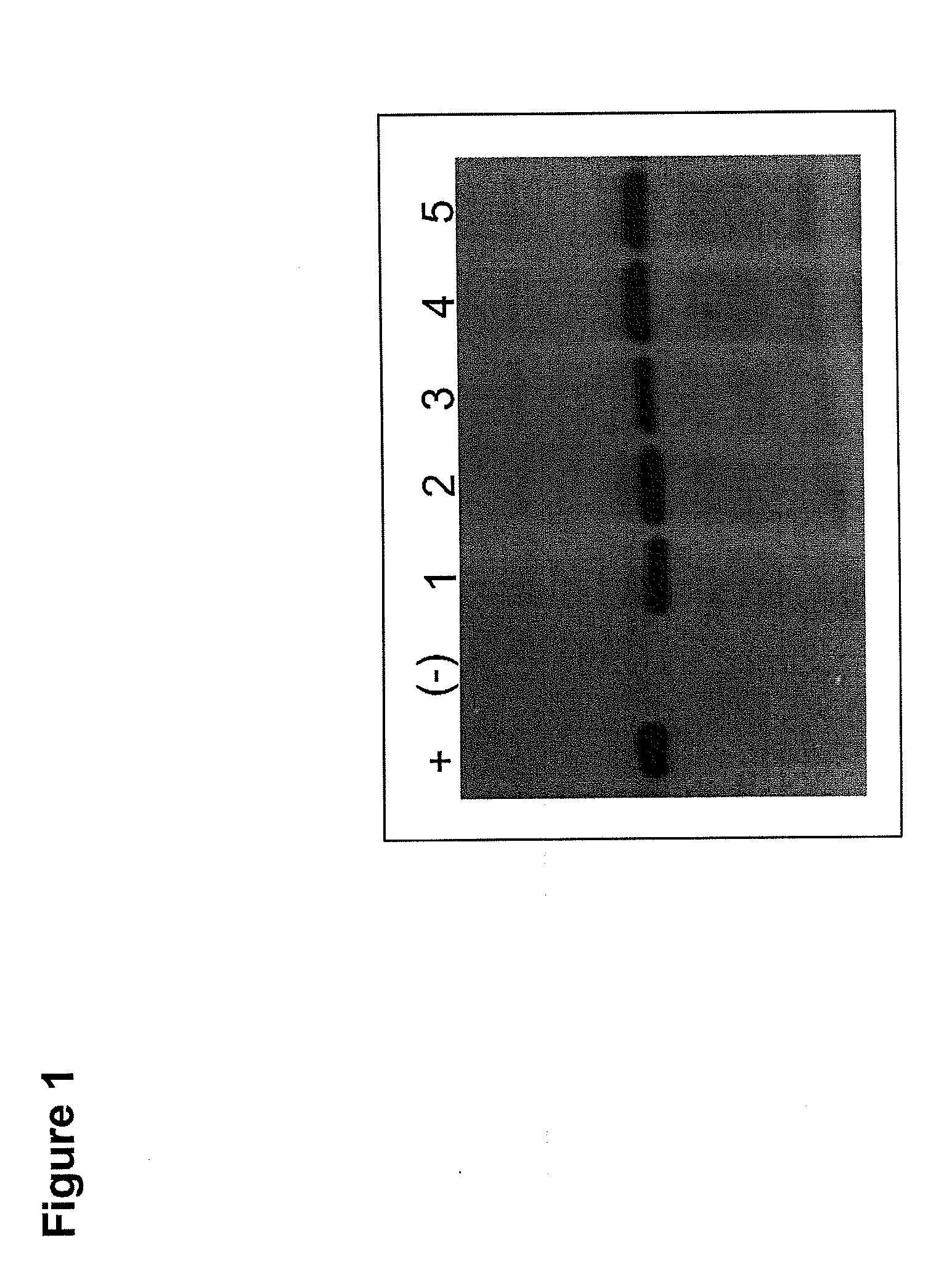
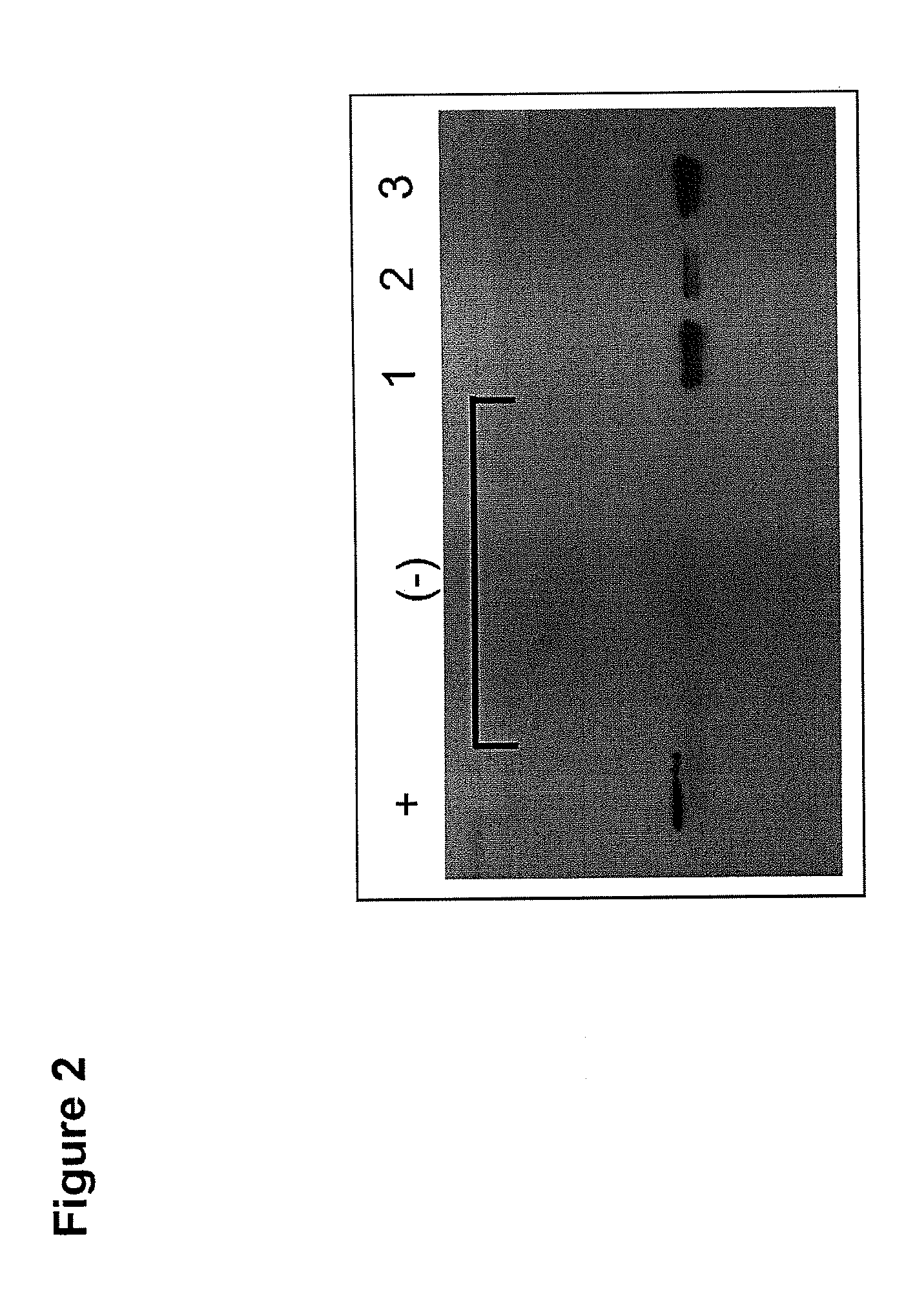
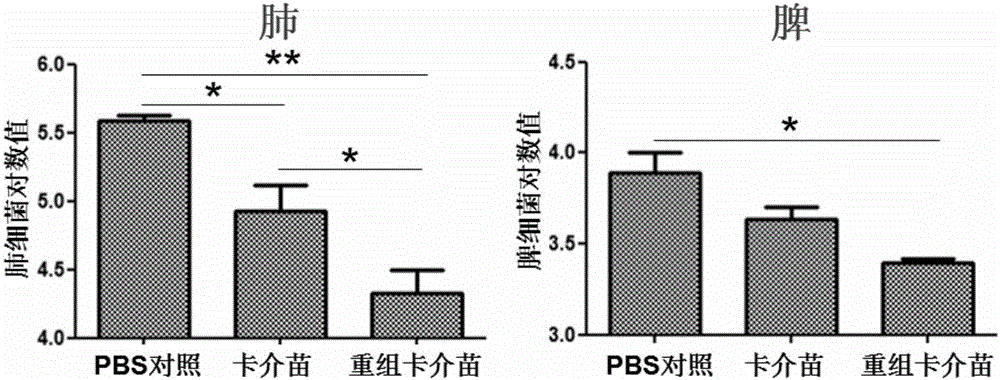
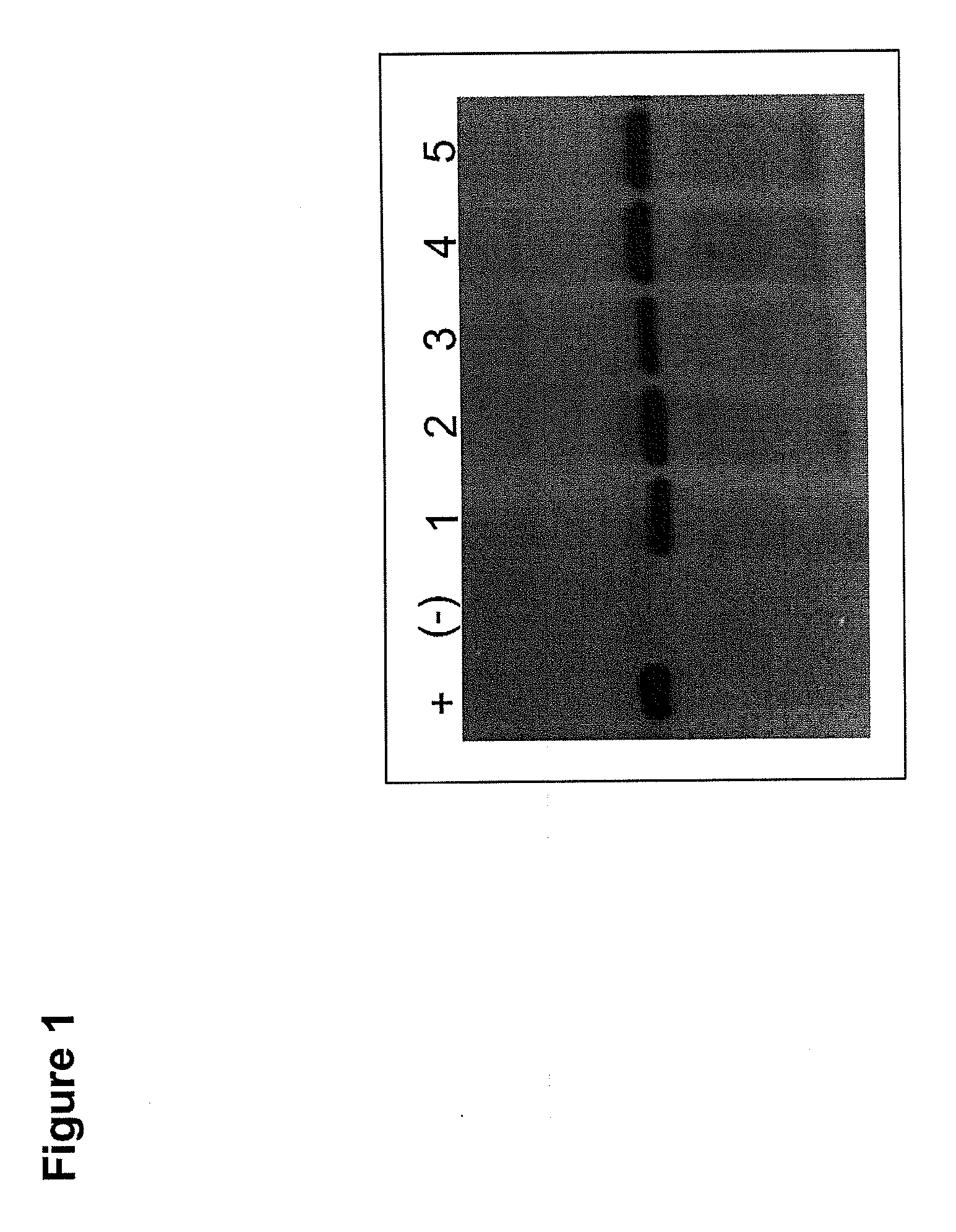
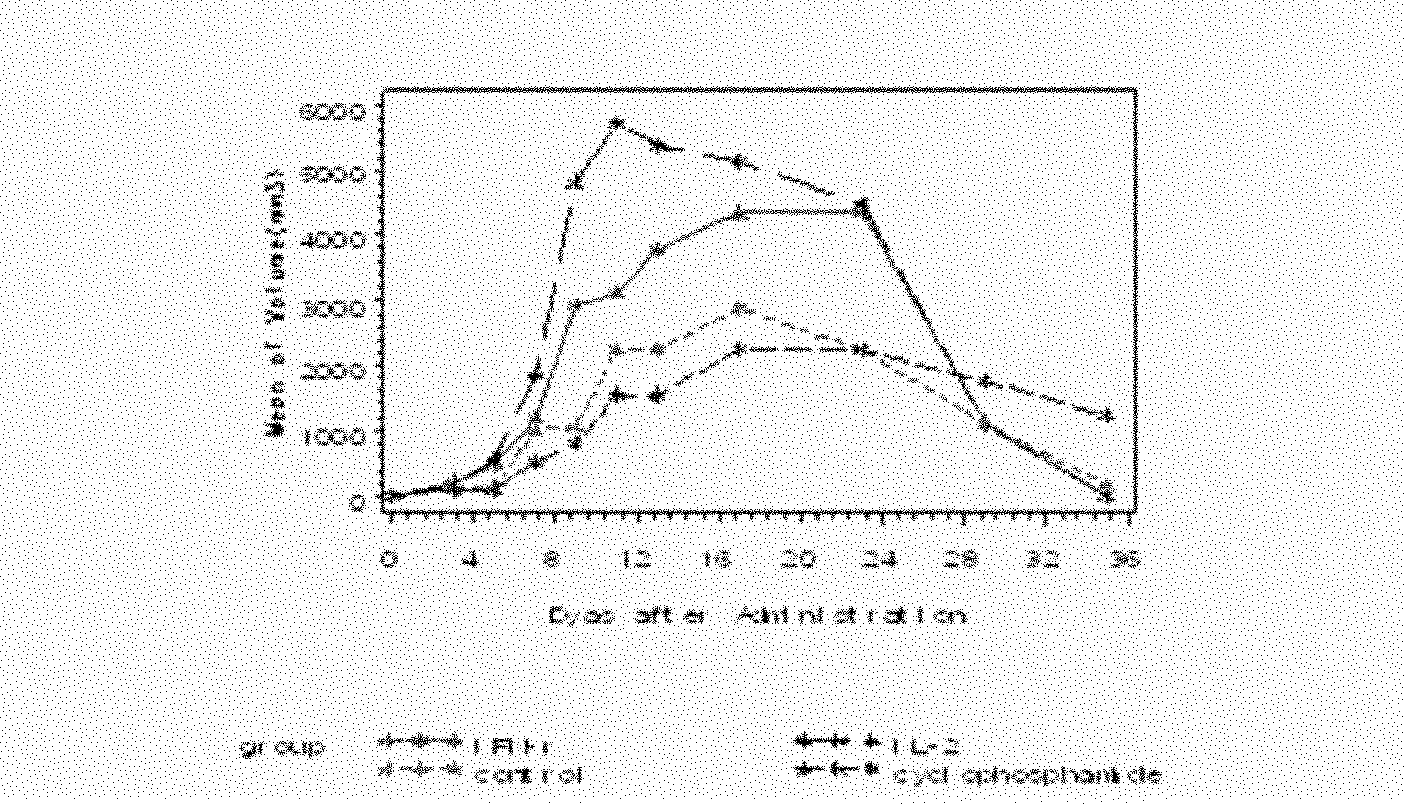
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
75 results about "Bacille Calmette Guerin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical Definition of Bacille Calmette Guerin. Bacille Calmette Guerin: An effective immunization against tuberculosis. Commonly abbreviated BCG, it is an attenuated (weakened) version of a bacterium called Mycobacterium bovis which is closely related to Mycobacterium tuberculosis, the agent responsible for tuberculosis.
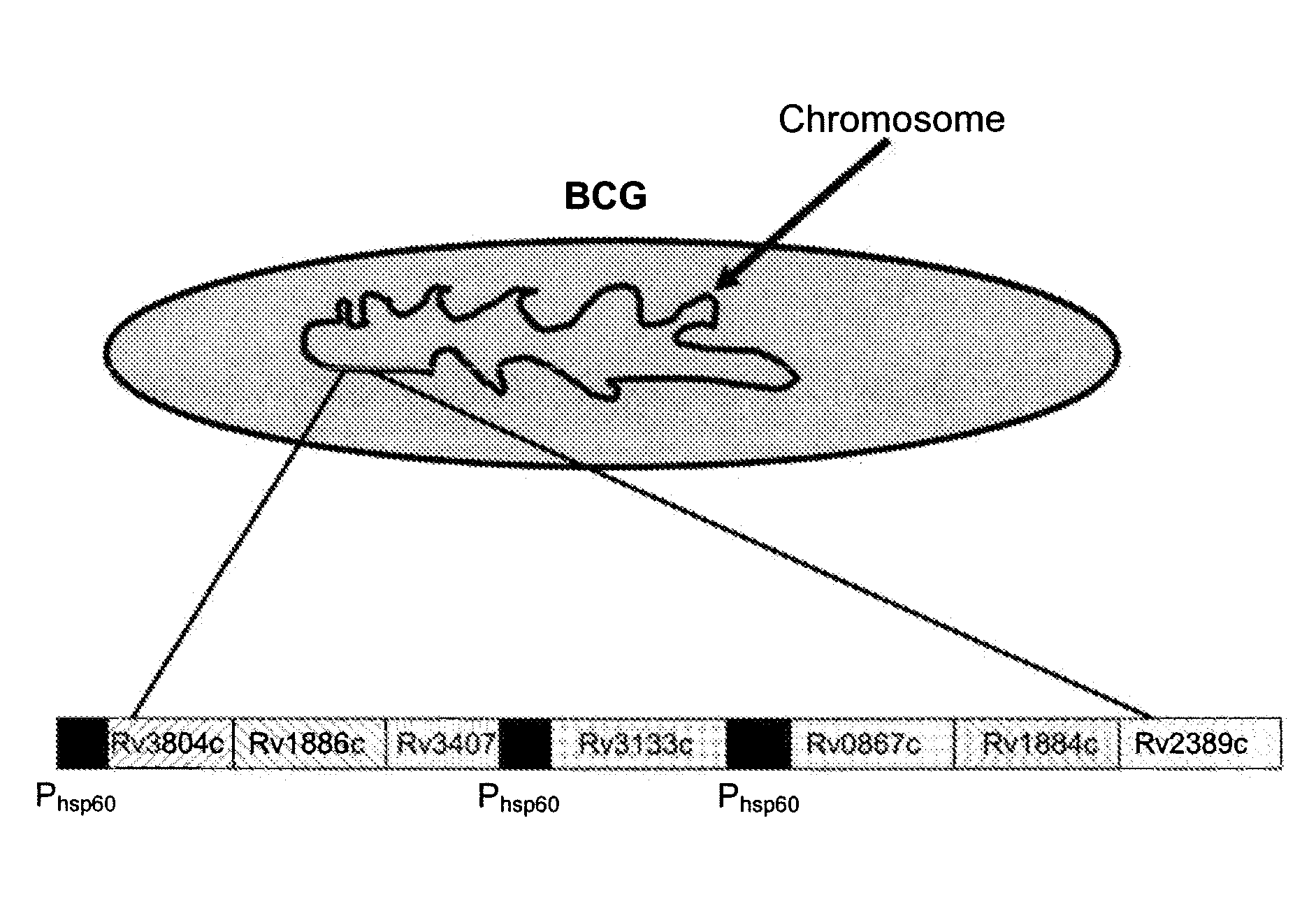
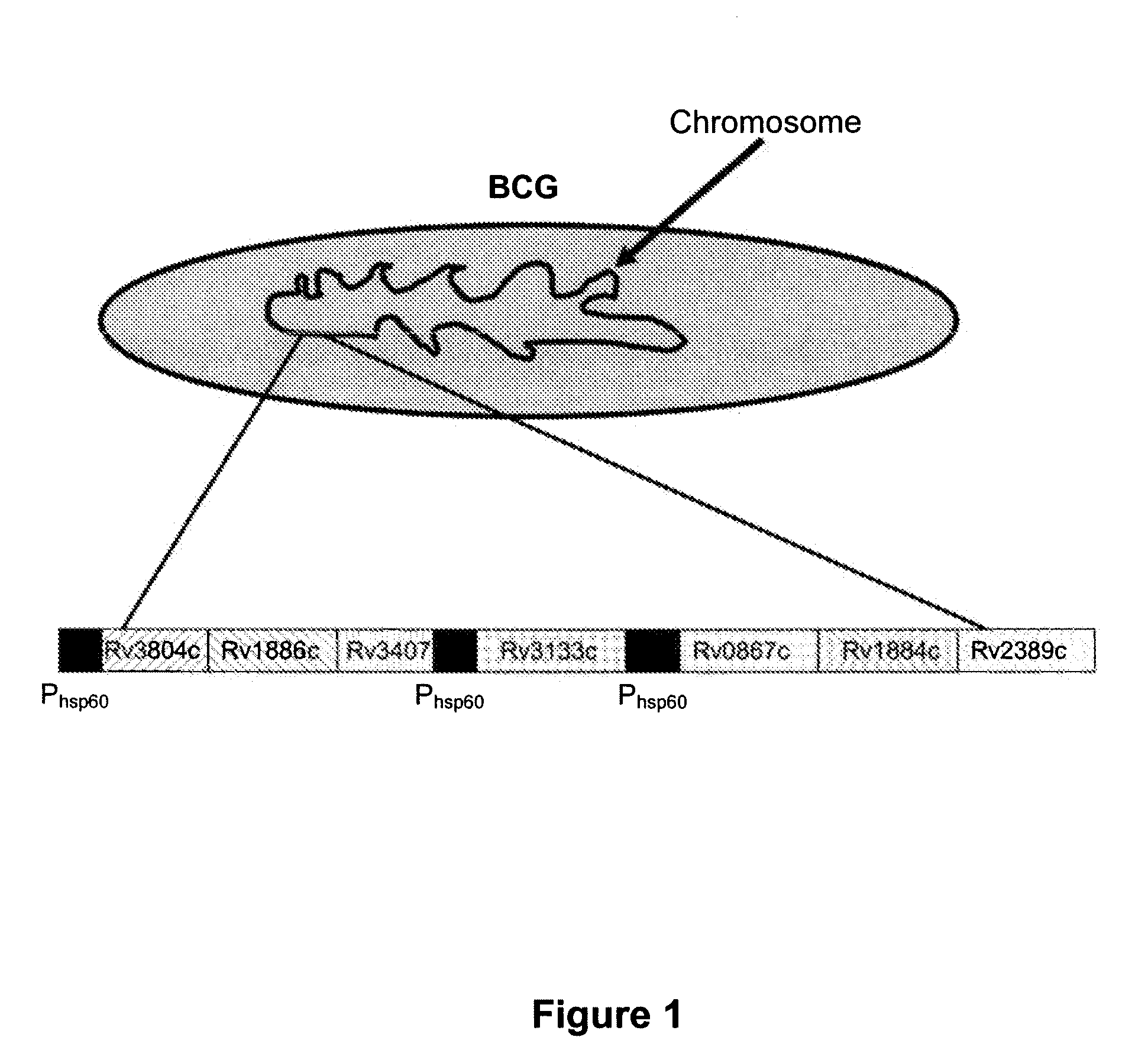
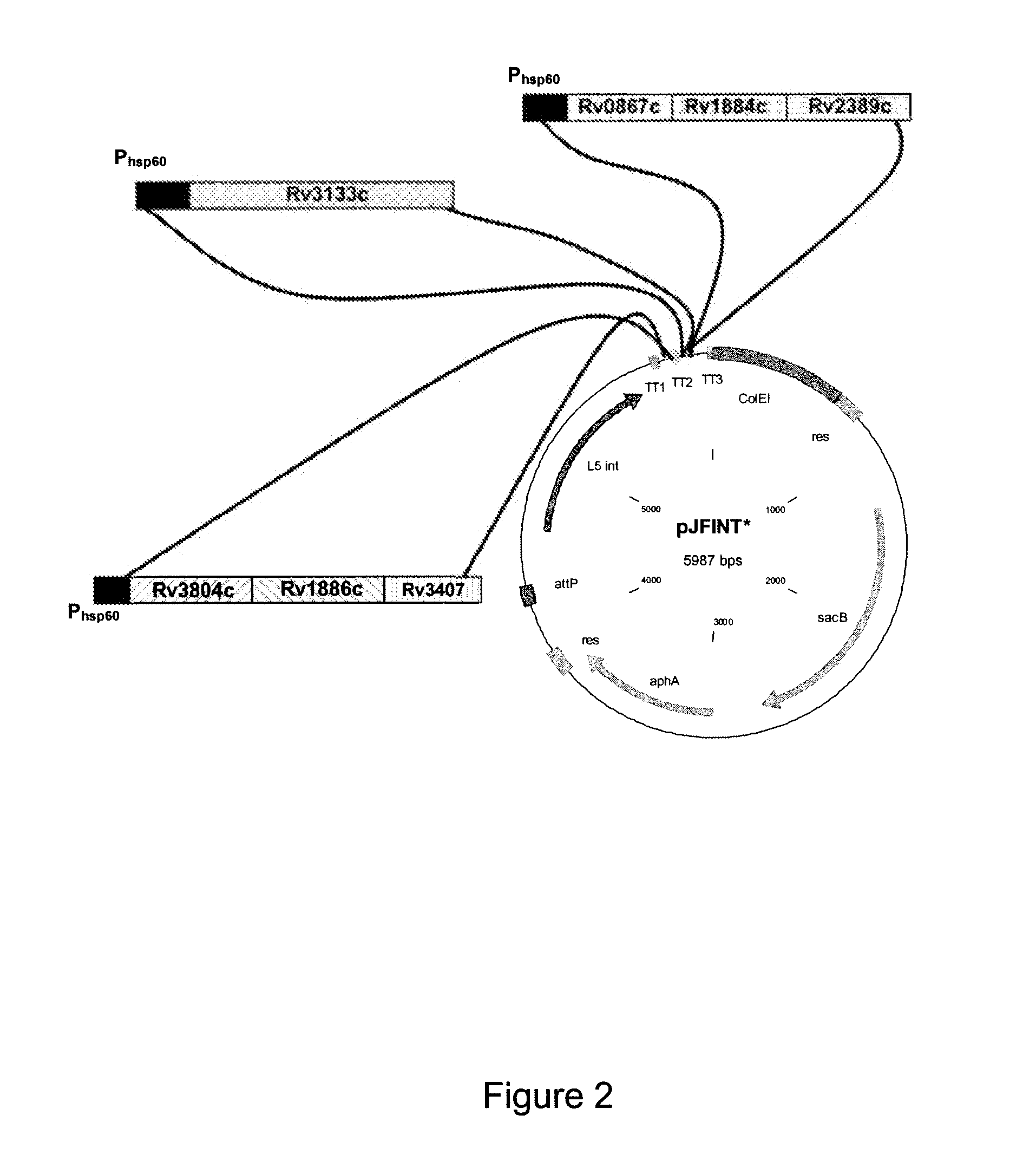
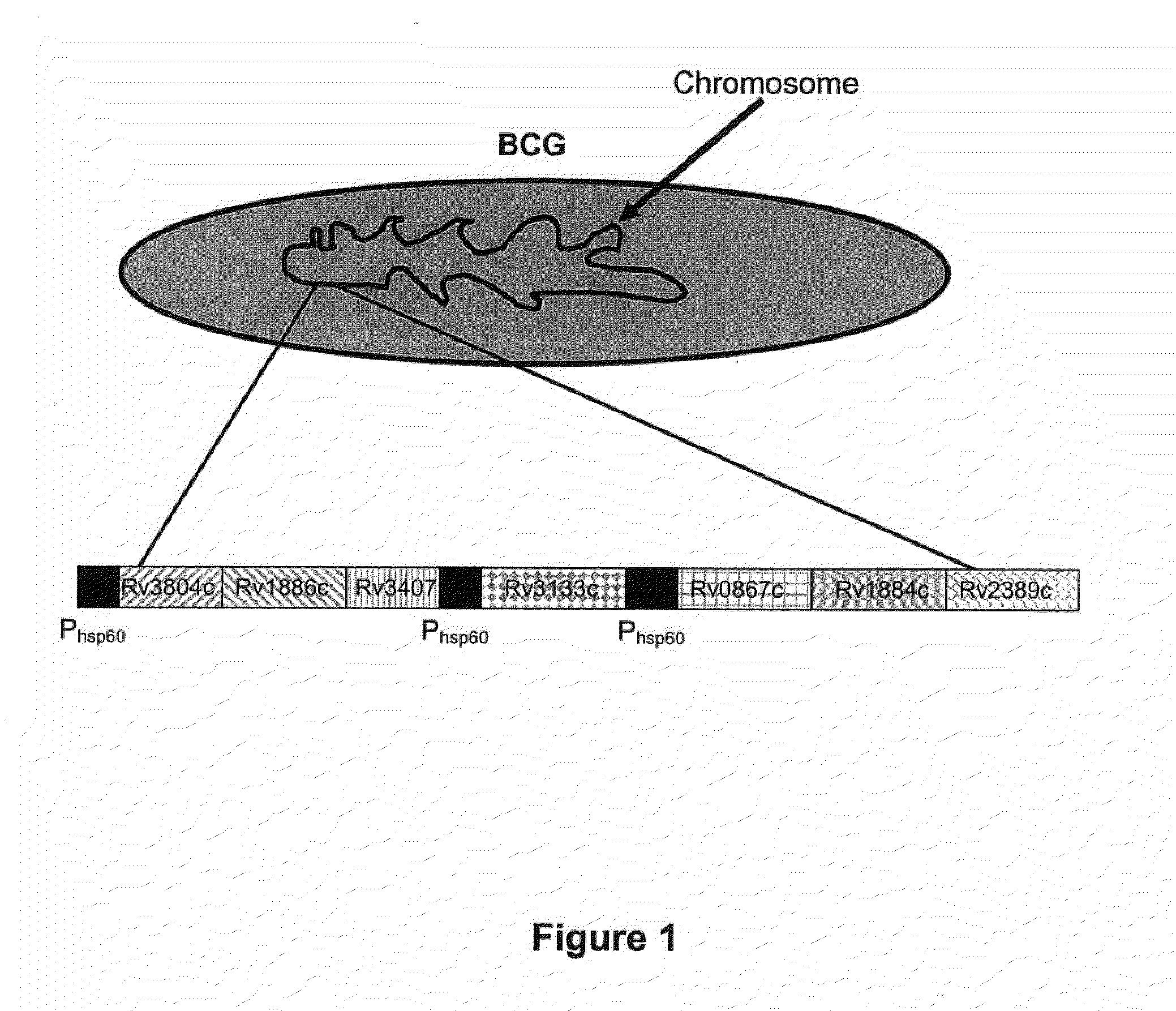
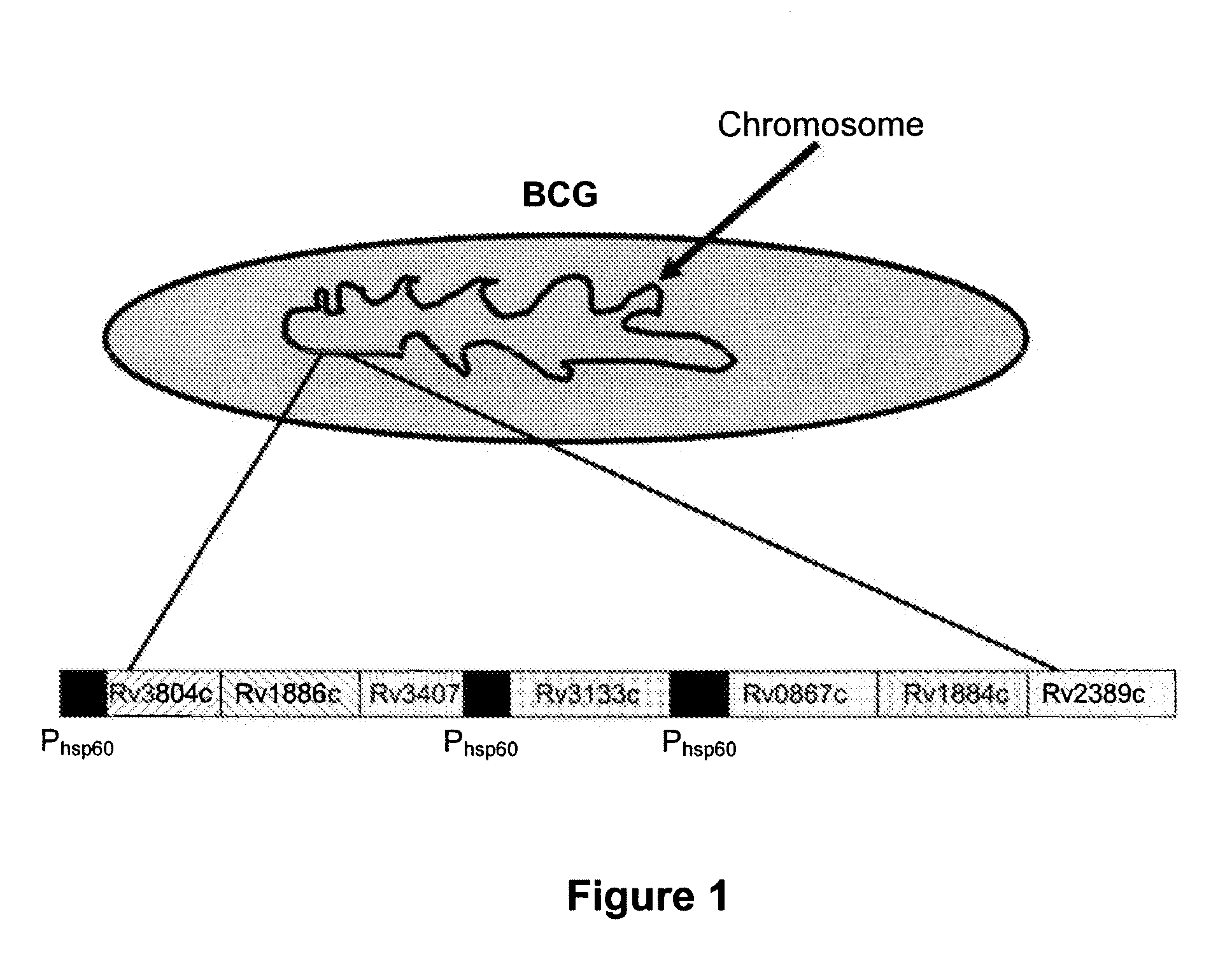
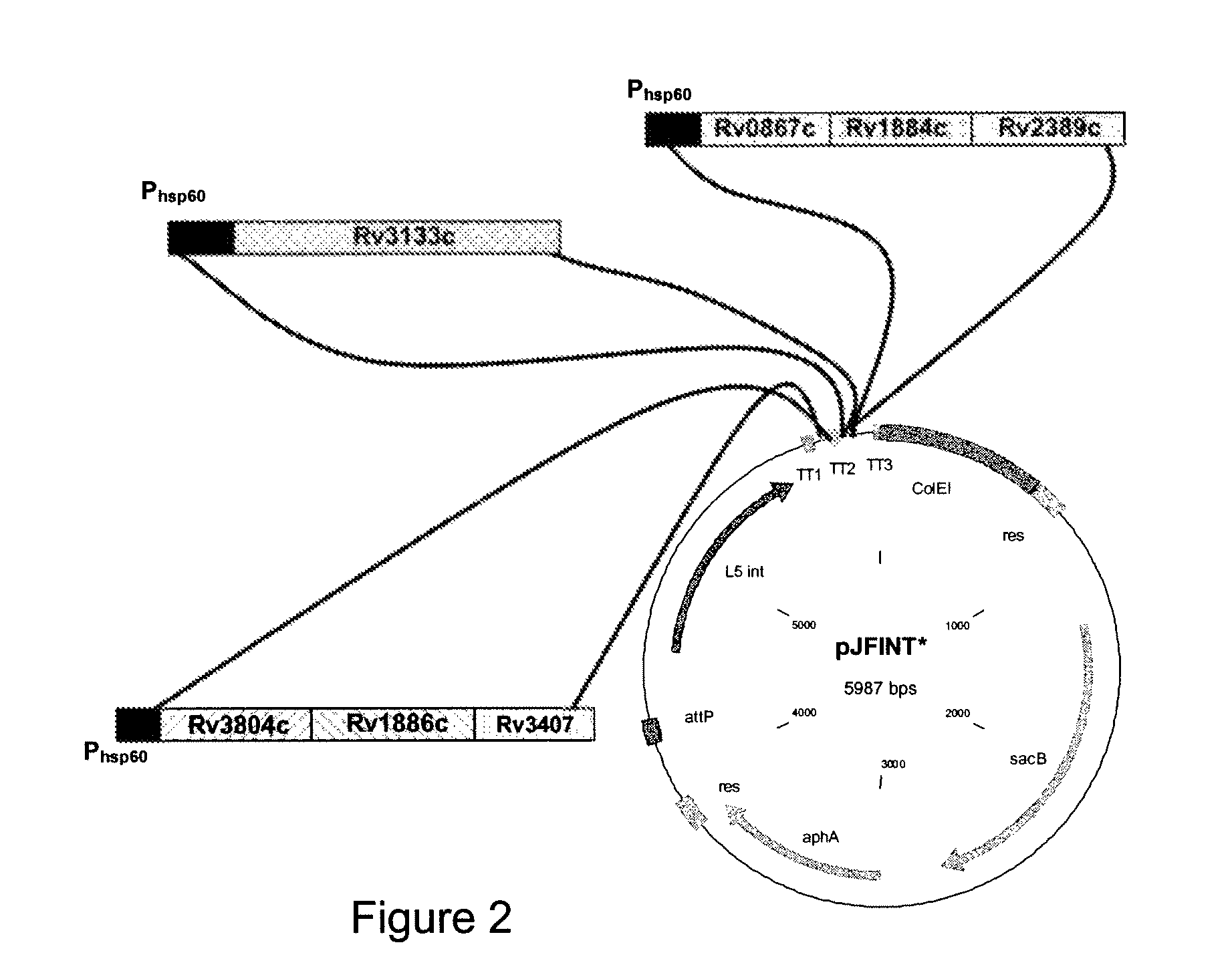
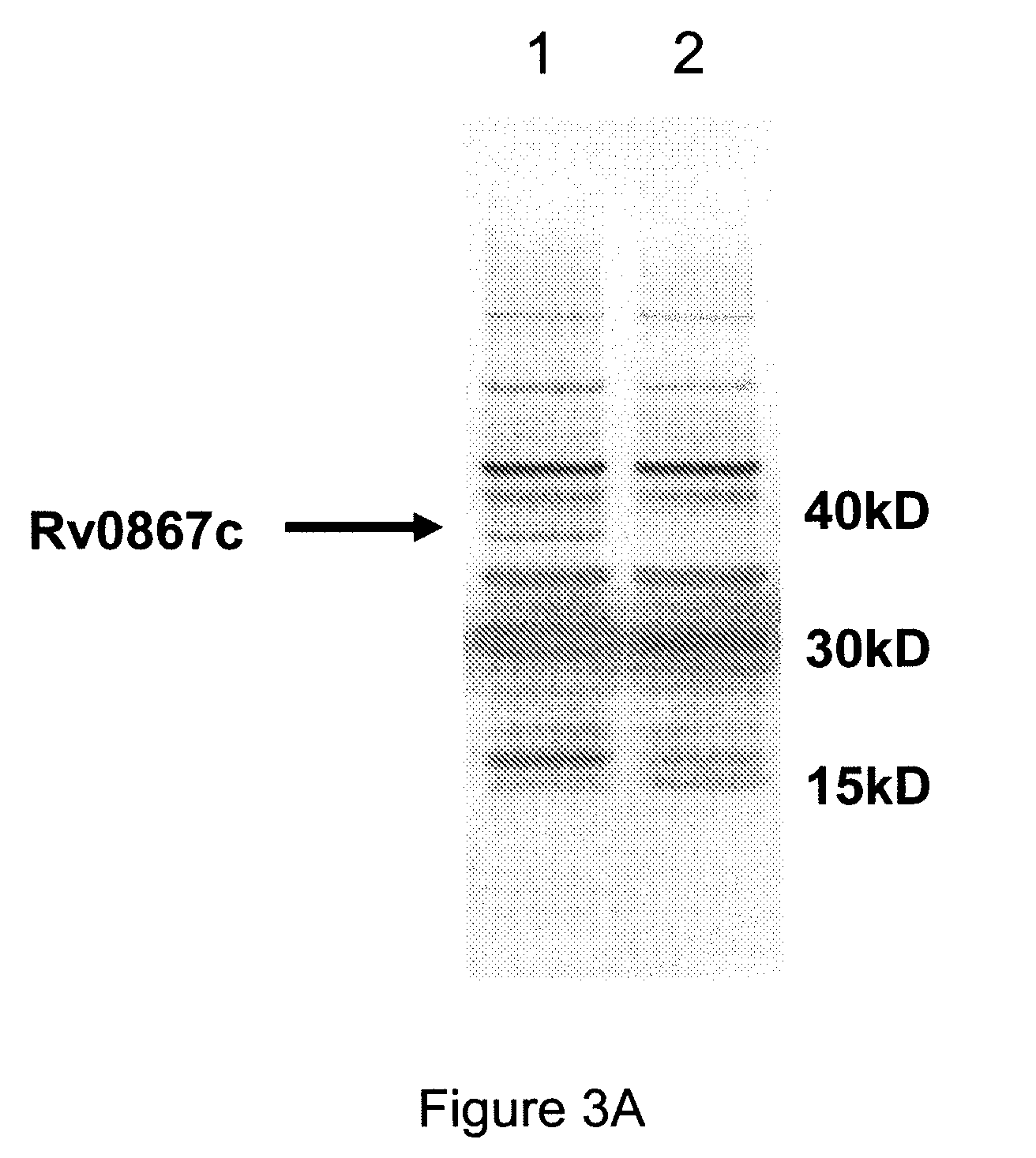
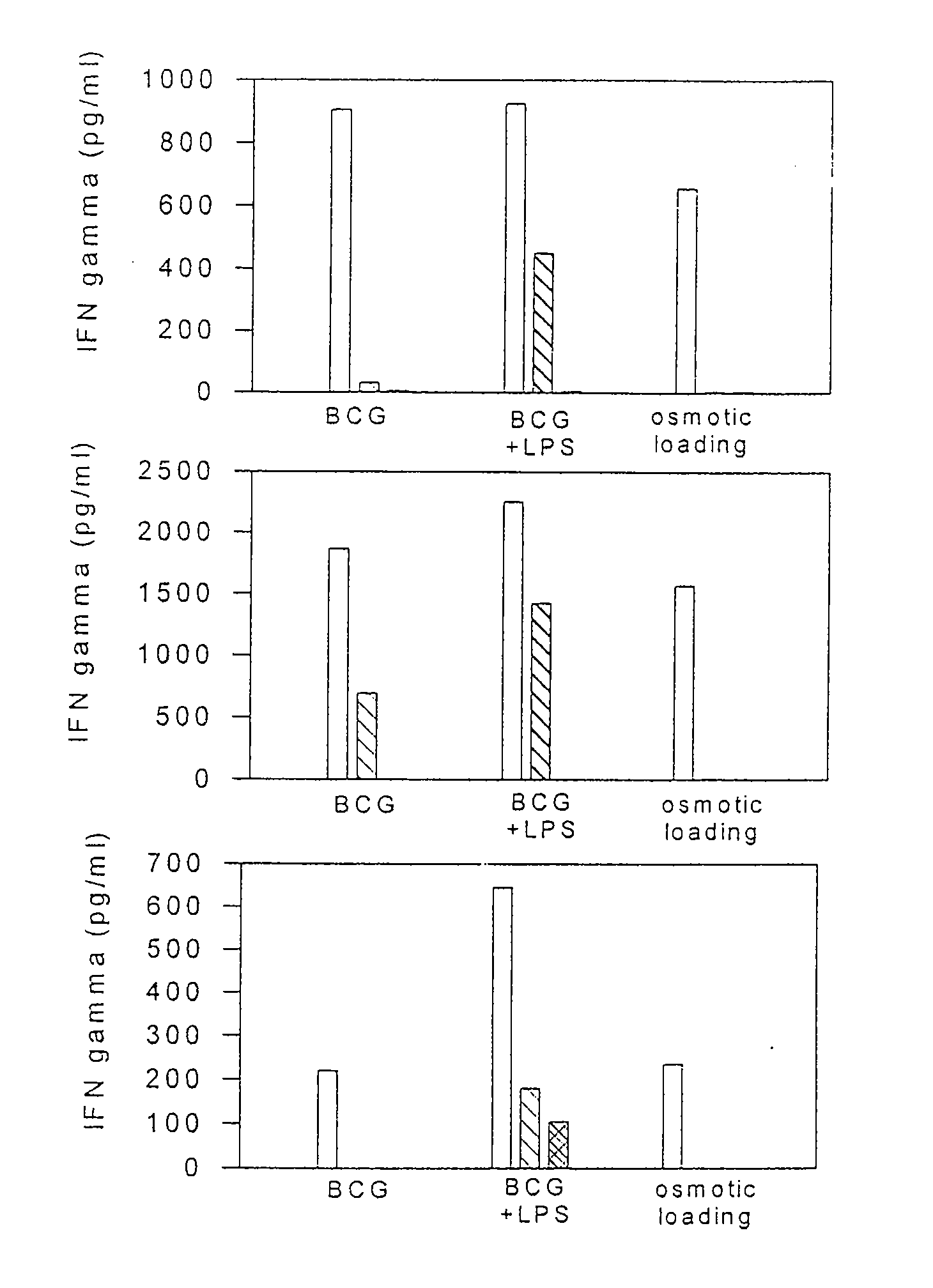
Novel recombinant bcg tuberculosis vaccine designed to elicit immune responses to mycobacterium tuberculosis in all physiological stages of infection and disease
A vaccine against Mycobacteria tuberculosis (Mtb) is provided. The vaccine comprises a recombinant Bacille Calmette-Guerin (BCG) subunit-based vaccine in which one or more Mtb antigens and one or more Mtb resuscitation or reactivation antigens are overexpressed, and in which at least a portion of the DosR regulon is up-regulated. The vaccine is protective against active Mtb infection both pre- and post-exposure to Mtb, and thus prevents disease symptoms due to the recurrence of a latent Mtb infection.
Owner:INT AIDS VACCINE INITIATIVE INC
Novel Recombinant BCG Tuberculosis Vaccine Designed to Elicit Immune Responses to Mycobacterium Tuberculosis in all Physiological Stages of Infection and Disease
A vaccine against Mycobacteria tuberculosis (Mtb) is provided. The vaccine comprises a recombinant Bacille Calmette-Guerin (BCG) subunit-based vaccine in which one or more Mtb antigens and one or more Mtb resuscitation or reactivation antigens are overexpressed, and in which at least a portion of the DosR regulon is up-regulated. The vaccine is protective against active Mtb infection both pre- and post-exposure to Mtb, and thus prevents disease symptoms due to the recurrence of a latent Mtb infection.
Owner:INT AIDS VACCINE INITIATIVE INC
Recombinant BCG tuberculosis vaccine designed to elicit immune responses to Mycobacterium tuberculosis in all physiological stages of infection and disease
A vaccine against Mycobacteria tuberculosis (Mtb) is provided. The vaccine comprises a recombinant Bacille Calmette-Guerin (BCG) subunit-based vaccine in which one or more Mtb antigens and one or more Mtb resuscitation or reactivation antigens are overexpressed, and in which at least a portion of the DosR regulon is up-regulated. The vaccine is protective against active Mtb infection both pre- and post-exposure to Mtb, and thus prevents disease symptoms due to the recurrence of a latent Mtb infection.
Owner:INT AIDS VACCINE INITIATIVE INC
Method to increase class i presentation of exogenous antigens by human dendritic cells
InactiveUS20080171023A1Enhance MHC-class I processingEnhance immune responseBiocideArtificial cell constructsMHC class IDendritic cell
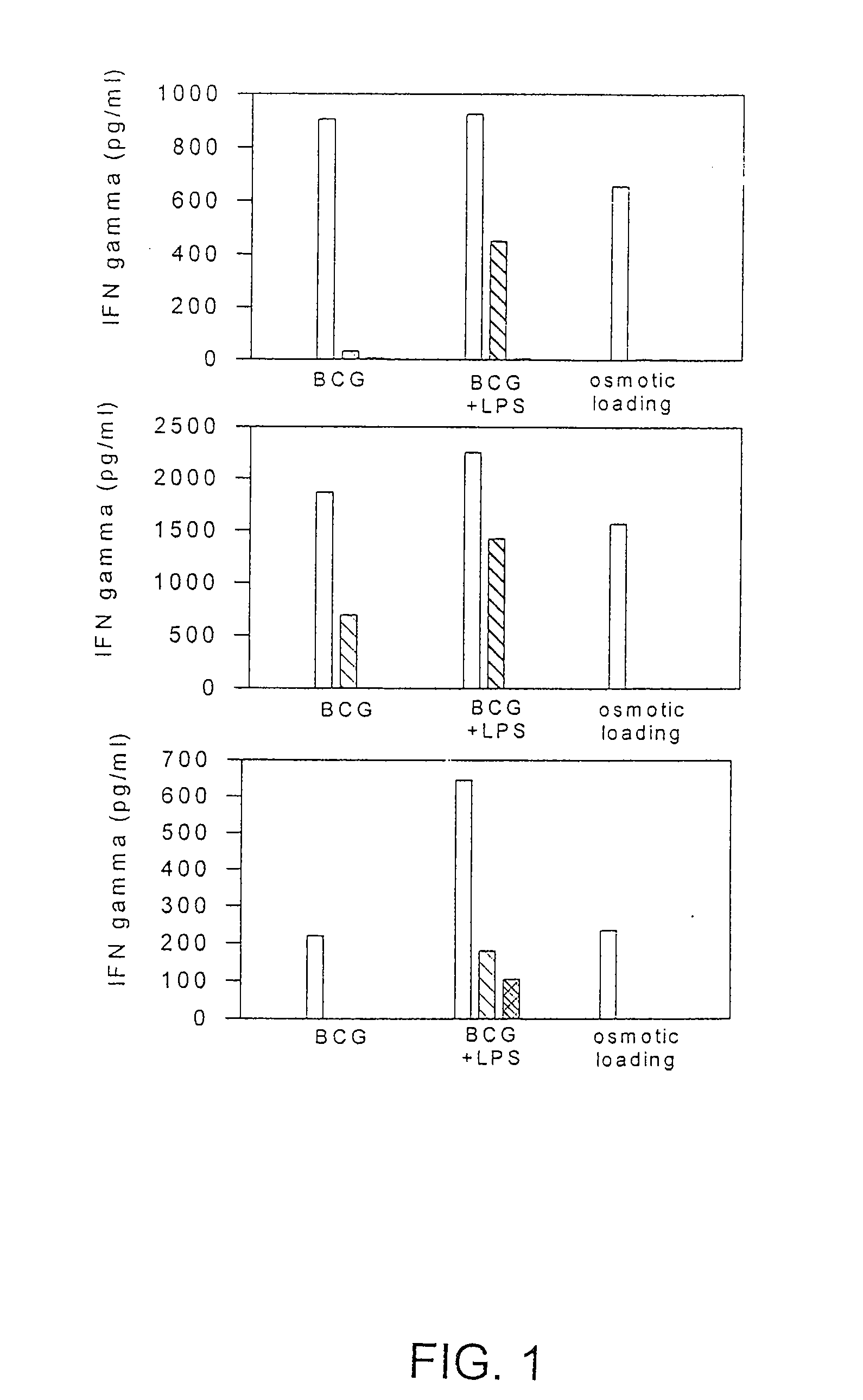
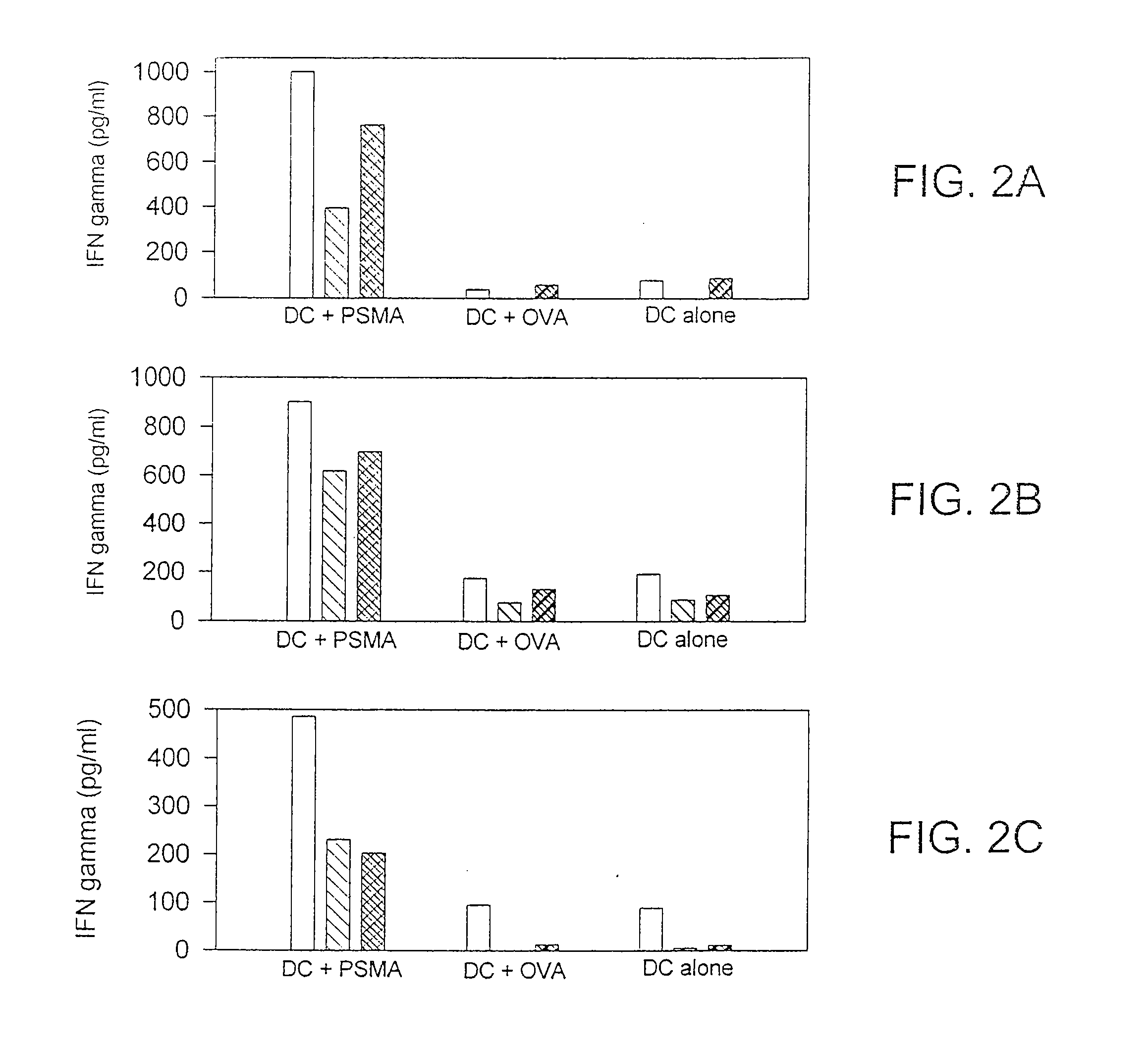
Methods and compositions for use of human dendritic cells to activate T cells for immunotherapeutic responses against primary and metastatic cancer are disclosed. In one embodiment, human dendritic cells exposed to a tumor associated antigen, or an antigenic fragment thereof in combination with bacillus Calmette-Guerin (BCG), are administered to a cancer patient to activate a predominantly CD8+T cell response in vivo. In an alternate embodiment, human dendritic cells are exposed to a tumor associated antigen or a specific antigenic peptide in combination with BCG in vitro and incubated or cultured with primed or unprimed T cells to activate a predominantly CD8+T cell response in vitro. The activated T cells are then administered to a cancer patient. Antigen in combination with BCG is processed by dendritic cells through the MHC-CLASS I compartment which provides for a predominantly CD8+T cell response. The addition of LPS provides for a greater number of mature dendritic cells enhancing the T cell response to antigen. Methods and compositions for human dendritic cells with extended life span and cryopreserved dendritic cells are disclosed.
Owner:NORTHWEST BIOTHERAPEUTICS INC
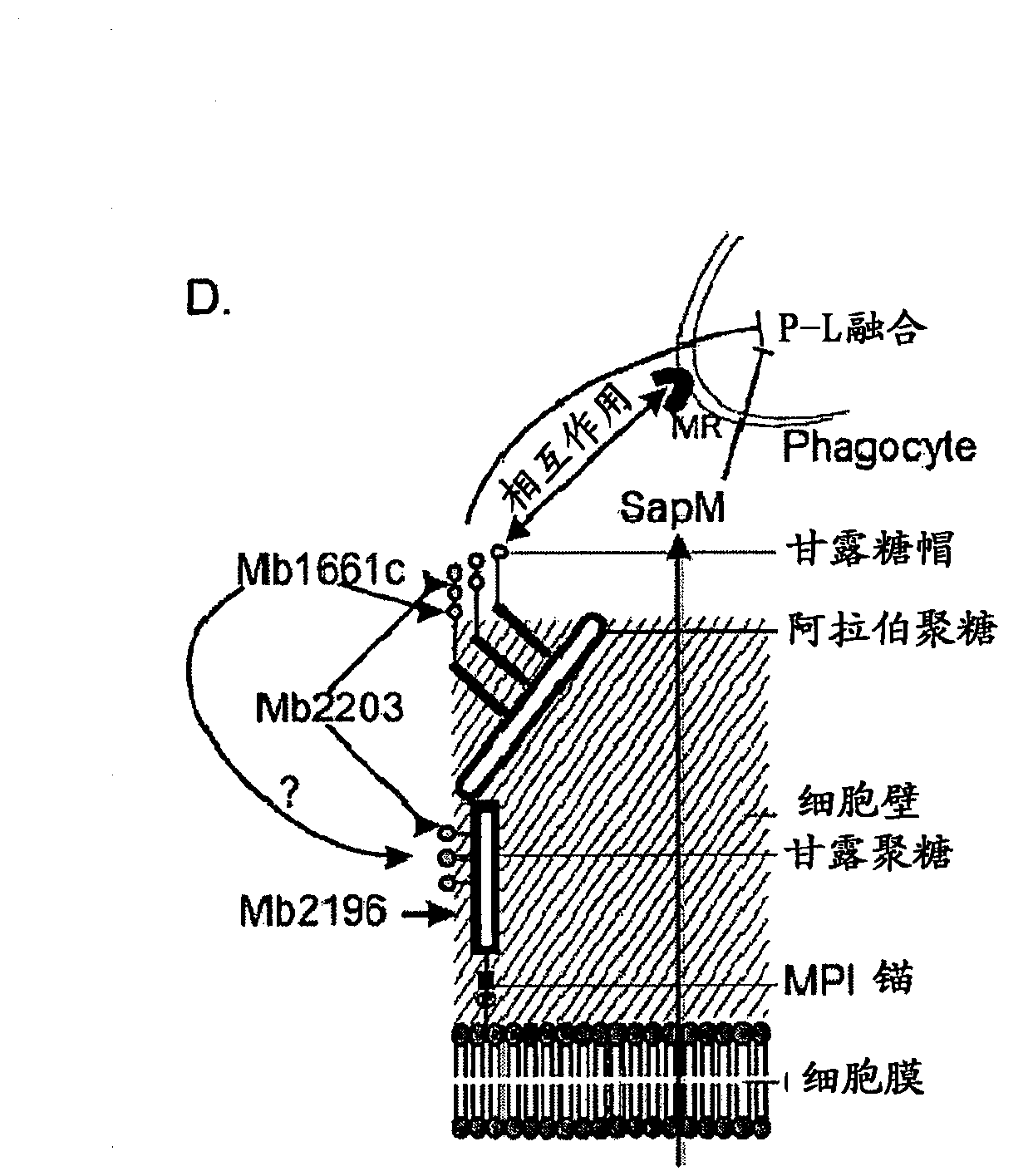
Mycobacterium mutants for vaccines with improved protective efficacy
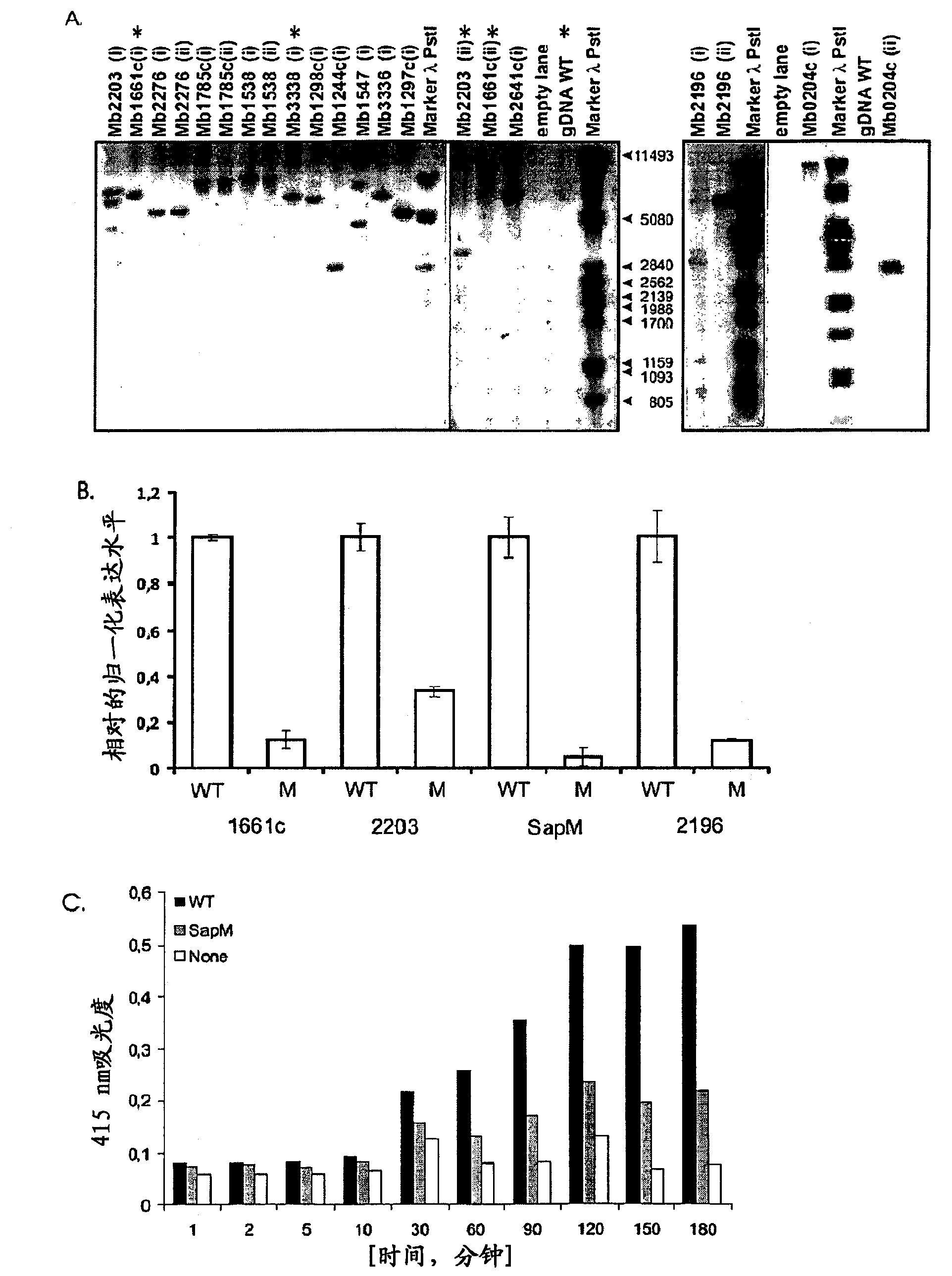
Tuberculosis (TB) is a major health problem and currently, the only licensed TB vaccine is Mycobacterium bovis Bacille Calmette-Guerin (M. bovis BCG). In the present invention, mutation of mycobacterial components reportedly involved in phagosome maturation inhibition was evaluated for vaccine purposes, as such mutations should result in better vaccine antigen processing and presentation. Thus, BCG mutants in genes coding for ManLAM capping a-1,2-mannosyltransferases and the PI3P phosphatase SapM were evaluated as TB vaccines in a stringent mouse model. Vaccination with both ManLAM capping mutants and the SapM mutant resulted in significantly longer survival as compared to non-vaccinated mice, whereas vaccination with the parental BCG did not. Moreover, mice vaccinated with the SapM mutant survived significantly longer than mice vaccinated with the parental BCG.; The mutant BCG strains showed unaltered phagocytosis, replication, lysosome colocalization and oxidant activity in macrophages and similarly induced autophagy in the latter. Additionally, replication and granuloma formation in mice was unaffected, indicating BCG-equivalent safety of these vaccines.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +2
Construction method of mycobacterium tuberculosis fusion protein Mtb 10.4-Hsp16.3, expression method thereof, purification method thereof and application thereof
ActiveCN102154324AEfficient purificationImproves and prolongs protective effectAntibacterial agentsBacterial antigen ingredientsCloning SiteTGE VACCINE
The invention discloses a construction method of mycobacterium tuberculosis fusion protein Mtb 10.4-Hsp16.3, an expression method thereof, a purification method thereof and application thereof. The construction method comprises the following steps: performing the PCR (polymerase chain reaction) amplification to Mtb 10.4 gene and Hsp16.3 gene, and sequentially inserting the amplified genes into a multi-clone site of a cloning vector to construct a recombinant vector; then expressing in the colibacillus; and finally purifying to obtain the fusion protein Mtb 10.4-Hsp16.3, wherein the fusion protein can be applied in a tuberculosis subunit vaccine. The invention has the advantages that the fusion protein vaccine can induce the higher cell of the animal to react with body fluid immunoreaction; the animal challenge test shows that the vaccine plays a part in immunity enhancing on the basis of the BCG (bacille calmette guerin) immunity, so that the protective effect of the BCG can be improved and prolonged; and the vaccine has no obvious side effects.
Owner:LANZHOU UNIVERSITY
Inactivated Poliomyelitis Vaccine Derived From Sabin Strain Of Polio Virus
InactiveUS20080193478A1Effective immunizationEffective and stableSsRNA viruses positive-senseViral antigen ingredientsAntigenHuman immunodeficiency
An inactivated Polio Vaccine derived from Sabin strain for safe and effective immunization against Poliomyelitis is provided. A process of preparation for such vaccine and formulations thereof are also provided. Administration of the vaccine of the present invention along with other antigens provides immunization not only against polio infection but also against other pathogens causing Hepatitis C. Hepatitis D. Hepatitis E. Meningitis A. Meningitis B. Meningitis C. Meningitis W. Meningitis Y. Pnemococcal (23 valent or more). Smallpox, Typhoid, Bacille Calmette Guerin, Tuberculosis. Human Immunodeficiency Virus. Anthrax or the like, to which children or adults not immunized earlier are susceptible, particularly to which children are susceptible.
Owner:PANACEA BIOTEC
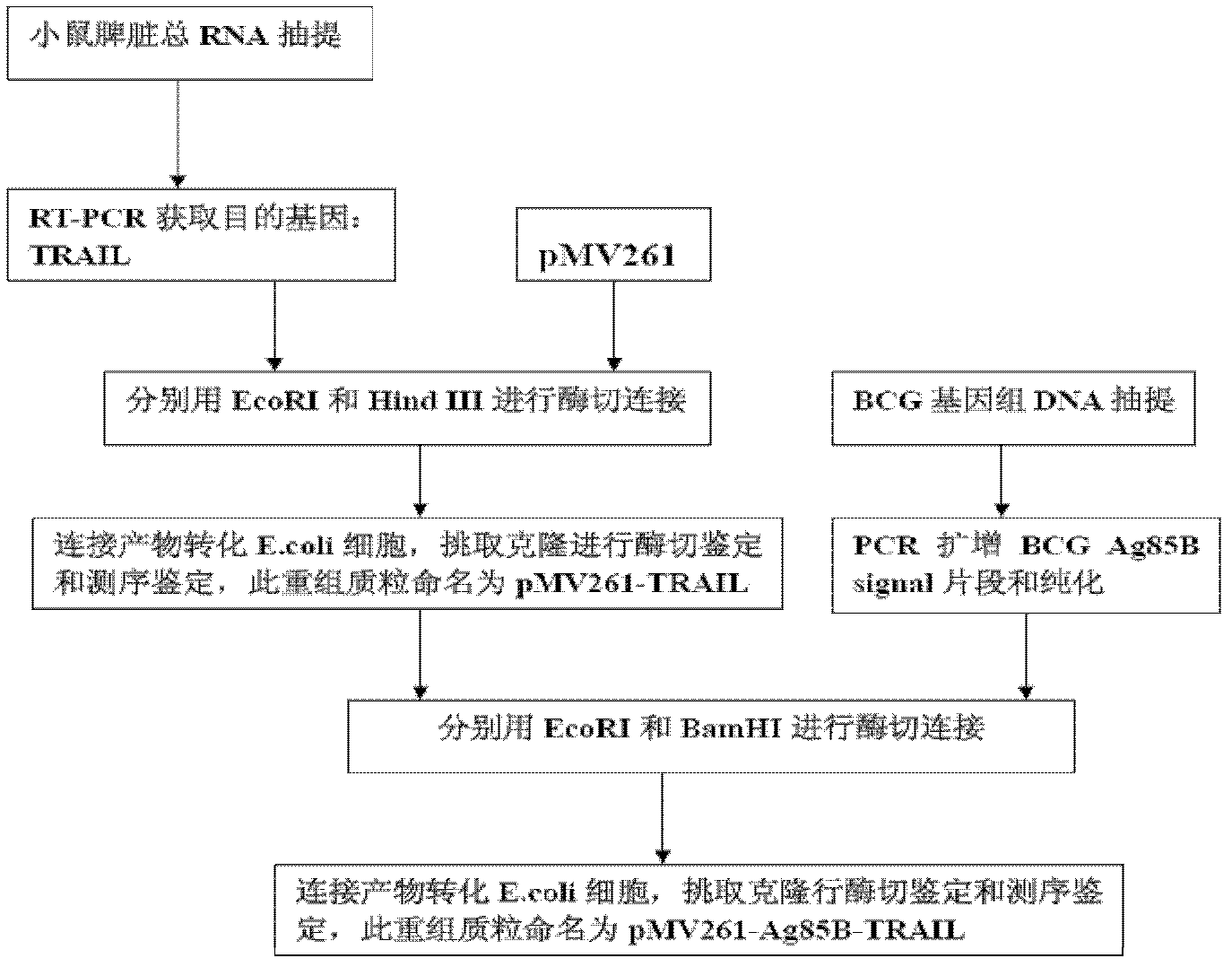
Construction and application of TRAIL (Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand) recombinant bacille calmette guerin (rBCG)
InactiveCN102327604AConnection direction is correctMeet the design requirementsAntibacterial agentsBacterial antigen ingredientsAntigenSide effect
The invention provides construction of TRAIL (Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand) recombinant bacille calmette guerin (rBCG), and relates to a shuttle expression vector comprising a signal peptide fragment of a major secretory antigen Ag85B of BCG and a gene fragment of a TRAIL and a construction method thereof. The obtained shuttle expression vector pMV261-Ag85B-TRAIL is used for constructing rBCGTRAIL, and can be applied to preparation of TRAIL rBCG for treating superficial bladder tumors, preventing postoperative recurrence thereof and preventing tuberculosis. The rBCG has dual functions of TRAIL and BCG, so that cooperative and synergistic actions of the TRAIL and BCG can be better brought into play; rBCG-TRAIL can secrete TRAIL, and the using amount of the rBCG-TRAIL can be lower than that of the BCG under the condition that the same or better immune effect is achieved, so that the toxic or side effect is reduced; and the rBCG-TRAIL can directly secrete TRAIL efficiently on a certain part, so that tumor cells can be killed in cooperation with the rBCG-TRAIL, and high cost caused by the use of a foreign cell factor is avoided.
Owner:沈周俊
Immunogenic formulation
ActiveUS20110200634A1SsRNA viruses negative-senseBacterial antigen ingredientsHeterologousFreeze-drying
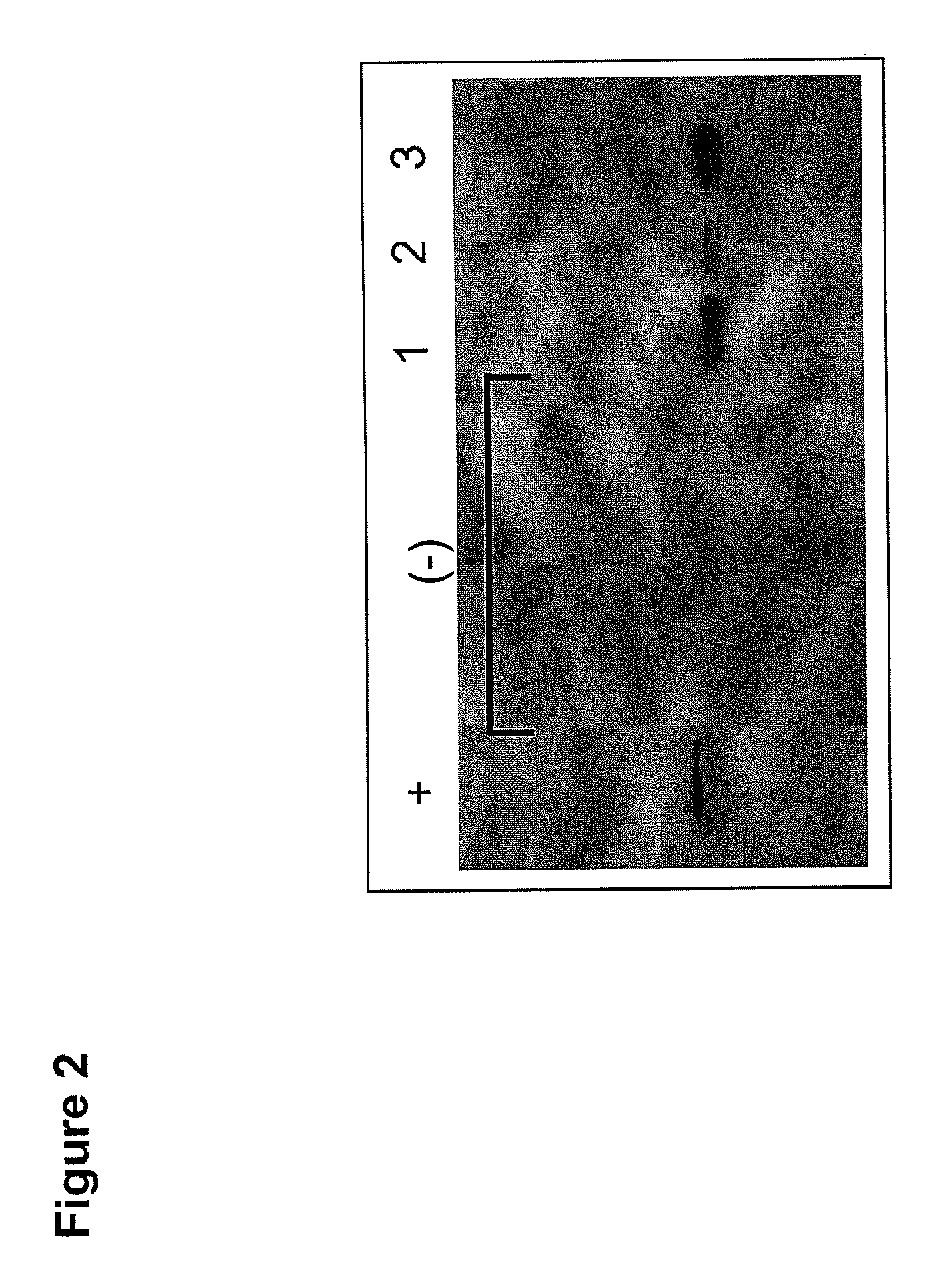
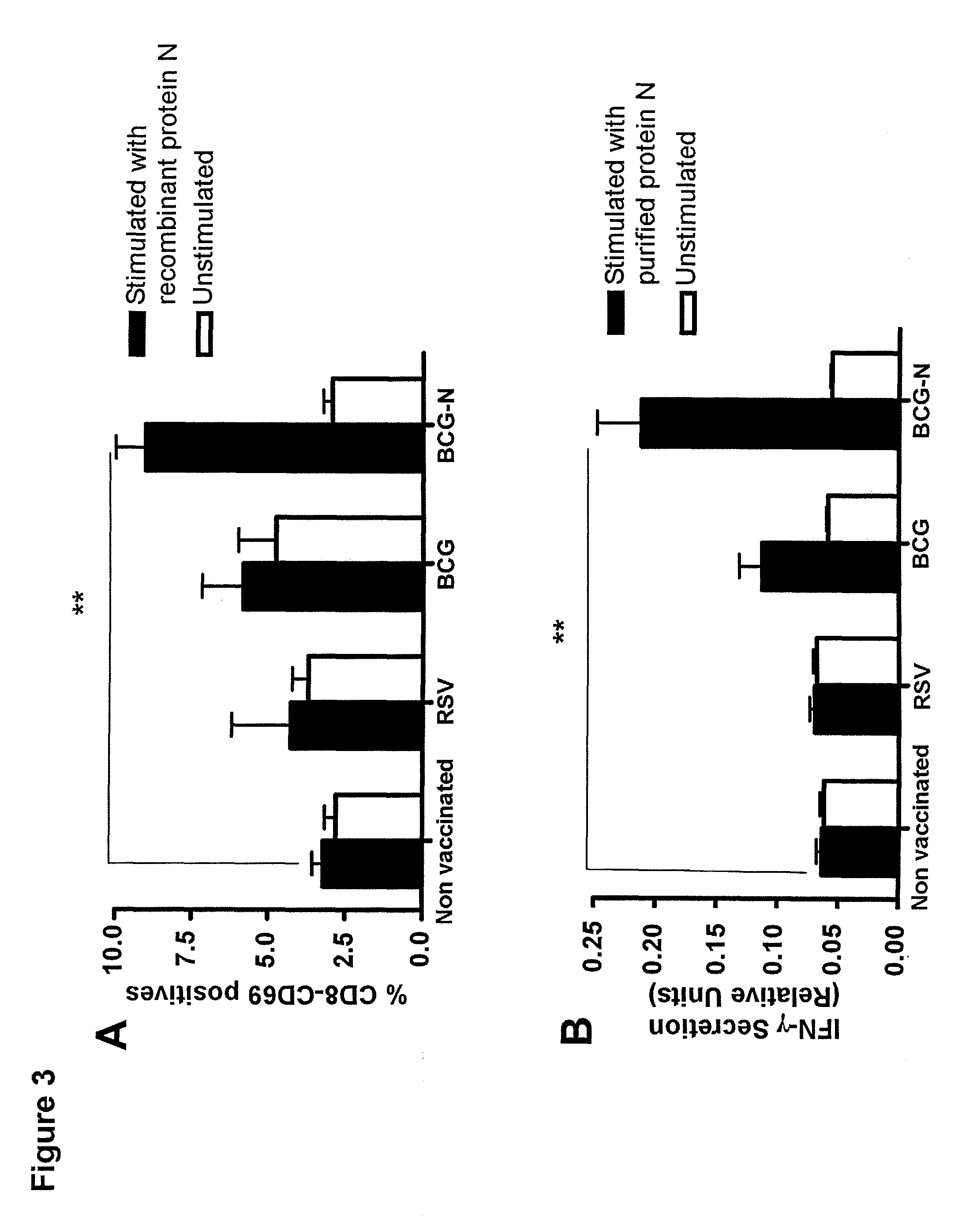
The present invention describes an immunogenic formulation to be used in mammals against the respiratory syncytial virus (RSV), consisting in the Calmette-Guérin bacillus (BCG) strain or other attenuated Mycobacterium strain that expresses heterologously at least one protein or immunogenic fragment of the RSV subtype A or RSV subtype B strains, originated from proteins NS1, NS2, N, P, M, SH, M2 (ORF1), M2 (ORF2), L, F or G. The genetic material that encodes for these proteins or immunogenic fragments is inserted into the BCG genome or extrachromosomally in one or several copies, which expression is regulated by endogenous or exogenous BCG promoters, either constitutive or inducible. The viral proteins or immunogenic fragments can be expressed by BCG as cytoplasmic-soluble, extracellularly-secreted or cell membrane-bound proteins. The preparation can further contain combinations of previously described formulations. The formulation can be stabilized by freeze-drying (conservation range from 4° C. and 25° C.) or through low temperatures (−80° C.) in a buffered saline solution to be conserved prior to its use.
Owner:PONTIFISIA UNIVERSIDAD KATOLIKA DE CHILE
Diphasic quick differential medium of mycobacterium tuberculosis and application of medium
InactiveCN103993065APromote growthHigh speedMicrobiological testing/measurementMicroorganism based processesLiquid mediumAntituberculosis drug
The invention relates to a preparation method of a diphasic quick differential medium of mycobacterium tuberculosis, a method for quickly differentiating the mycobacterium tuberculosis by using the medium, a method for testing the drug resistance on antituberculous drugs caused by the mycobacterium tuberculosis and a method for quickly culturing a bacillus Calmette and Guerin vaccine by using a liquid medium in the medium. The diphasic medium prepared by adopting the method can be used for quickly culturing the mycobacterium tuberculosis, the diphasic quick culture medium is capable of differentiating the mycobacterium tuberculosis within 2-5 days, the differentiated mycobacterium tuberculosis can be subjected to drug resistance detection of the antituberculous drugs such as rifampicin, isoniazide and streptomycin by virtue of a reverse transcription PCR (polymerase chain reaction) method, and the liquid medium prepared by adopting the method is capable of finishing the culture of the bacillus Calmette and Guerin vaccine within 6-9 days.
Owner:JINING MEDICAL UNIV
Antituberculous recombinant bacillus calmette-guerin with mazG gene deleted
The invention provides antituberculous recombinant bacillus calmette-guerin (BCG delta mazG) with a mazG gene deleted. The mazG gene in a genome of the antituberculous recombinant bacillus calmette-guerin with the mazG gene deleted is replaced with a hygromycin resistant gene. The invention further provides a preparation method of the BCG delta mazG and application thereof in preparing a recombinant vaccine for preventing tuberculosis. After the BCG delta mazG is inoculated subcutaneously into a mouse, a T cell immune response is obviously improved, after the immunized mouse is infected with a mycobacterium tuberculosis standard strain H37Rv, the bacterial load of the infected animal lung and spleen is significantly decreased, the pathological degree is relieved, and after the immunized mouse is infected for five weeks, a T cell secondary immune response is significantly improved. It is found through bacterial in-vivo survival tests that after high-dose intravenous inoculation is conducted, the BCG delta mazG can survive on viscera of the spleen, the lung, the liver and the kidney of the mouse continuously for 20 w or above, and the amount of bacteria of a BCG delta mazG recombinant strain is obviously increased compared with a wild strain. It is proved through experiments that the BCG delta mazG recombinant strain has antituberculous immunogenicity, protective efficacy and in-vivo persistence activity which are significantly improved, and the antituberculous recombinant bacillus calmette-guerin (BCG delta mazG) with the mazG gene deleted is expected to become one of the novel candidate vaccines for preventing tuberculosis infection.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
Immunogenic formulation
ActiveUS8398993B2Easy to eliminateAvoid and attenuate damageSsRNA viruses negative-senseBacterial antigen ingredientsHeterologousFreeze-drying
The present invention describes an immunogenic formulation to be used in mammals against the respiratory syncytial virus (RSV), consisting in the Calmette-Guérin bacillus (BCG) strain or other attenuated Mycobacterium strain that expresses heterologously at least one protein or immunogenic fragment of the RSV subtype A or RSV subtype B strains, originated from proteins NS1, NS2, N, P, M, SH, M2 (ORF1), M2 (ORF2), L, F or G. The genetic material that encodes for these proteins or immunogenic fragments is inserted into the BCG genome or extrachromosomally in one or several copies, which expression is regulated by endogenous or exogenous BCG promoters, either constitutive or inducible. The viral proteins or immunogenic fragments can be expressed by BCG as cytoplasmic-soluble, extracellularly-secreted or cell membrane-bound proteins. The preparation can further contain combinations of previously described formulations. The formulation can be stabilized by freeze-drying (conservation range from 4° C. and 25° C.) or through low temperatures (−80° C.) in a buffered saline solution to be conserved prior to its use.
Owner:PONTIFISIA UNIVERSIDAD KATOLIKA DE CHILE
Recombinant BCG tuberculosis vaccine designed to elicit immune responses to mycobacterium tuberculosis in all physiological stages of infection and disease
A vaccine against Mycobacteria tuberculosis (Mtb) is provided. The vaccine comprises a recombinant Bacille Calmette-Guerin (BCG) subunit-based vaccine in which one or more Mtb antigens and one or more Mtb resuscitation or reactivation antigens are overexpressed, and in which at least a portion of the DosR regulon is up-regulated. The vaccine is protective against active Mtb infection both pre- and post-exposure to Mtb, and thus prevents disease symptoms due to the recurrence of a latent Mtb infection.
Owner:INT AIDS VACCINE INITIATIVE INC
Construction method of drug evaluation model for dermal pathology of tuberculosis rabbit
InactiveCN102430119AObvious symptomsOrganic active ingredientsBacterial antigen ingredientsBCG immunizationTreating tuberculosis
The invention discloses a construction method of a drug evaluation model for dermal pathology of a tuberculosis rabbit. The method comprises: selecting a rabbit, injecting an immune drug into rabbit, conducting BCG (Bacillus Calmette Guerin) vaccine immune injection on the 15th to 20th day of drug intervention, and carrying out bacterial and pathological examination so as to construct a pathological model. The method of the invention establishes a pathological model of drug intervention on drug cutaneous tuberculosis with obvious symptoms so as to conduct visual research on tuberculosis pathology and bacterial pathogenicity, thus providing a visual animal model for vaccines and screening of drugs treating a tuberculosis necrotic liquefied cavity. And the model is stable and repeatable. The invention establishes a drug intervention procedure and observation indexes for research of drug intervention on a tuberculosis granuloma liquefaction process, and also provides a research basis for probing an immune mechanism about the formation of a tuberculosis liquefied cavity.
Owner:LANZHOU UNIVERSITY
Modified new coronavirus S gene, recombinant plasmid and recombinant bacillus calmette guerin vaccine constructed by same and application of recombinant bacillus calmette guerin vaccine
PendingCN113403330AEvoke an immune responsePrevent intrusionAntibacterial agentsSsRNA viruses positive-senseGene terminatorVirus Protein
The invention discloses a modified new coronavirus SARS-CoV-2S protein, a recombinant plasmid and a recombinant bacillus calmette guerin vaccine constructed by the modified new coronavirus SARS-CoV-2S protein, and application of the recombinant bacillus calmette guerin vaccine, and belongs to the technical field of biological agents. Through modifying a partial sequence of gene promoter region and a partial sequence of gene terminator region, the modified new coronavirus S gene complete sequence can be smoothly expressed in an escherichia coli-mycobacterium tuberculosis shuttle plasmid pMV261 to obtain a recombinant plasmid pMVS; the recombinant plasmid pMVS is introduced into BCG through electric transformation, and recombinant BCG (rBCG) is obtained; the rBCG can successfully express S protein, arouse human immune response and induce antibody generation to prevent virus invasion, and the constructed rBCG is a subunit vaccine, has a better protection effect than parent BCG, can prevent or treat new coronavirus and mycobacterium tuberculosis, and has huge social benefits.
Owner:JINING MEDICAL UNIV
Method for preparing pure protein derivative of tubercle bacillus by using mycobacterium tuberculosis attenuated strain H37Ra
ActiveCN112813121AHarm reductionLower security level requirementsBacteriaMicroorganism based processesEpidemiological MonitoringImmunity response
The invention discloses a method for preparing a pure protein derivative of tubercle bacillus by using a mycobacterium tuberculosis attenuated strain H37Ra, and belongs to the technical field of biological protein preparation. The mycobacterium tuberculosis attenuated strain CMCC93020 (H37Ra) is used as a strain, and a finished product of the pure protein derivative of the tubercle bacillus is obtained through amplification, inactivation, salting-out precipitation, degerming filtration, inspection, dilution and subpackaging. The defects in the prior art that strains have great harm to operators and the environment and have high requirements for production conditions (P3 production workshops) are overcome, and finally the protein which can be used for clinical diagnosis of tuberculosis, screening of bacillus calmette-guerin vaccine inoculation objects, monitoring of organism immune responses after bacillus calmette-guerin vaccine inoculation and epidemiological monitoring is obtained.
Owner:成都可恩生物科技有限公司
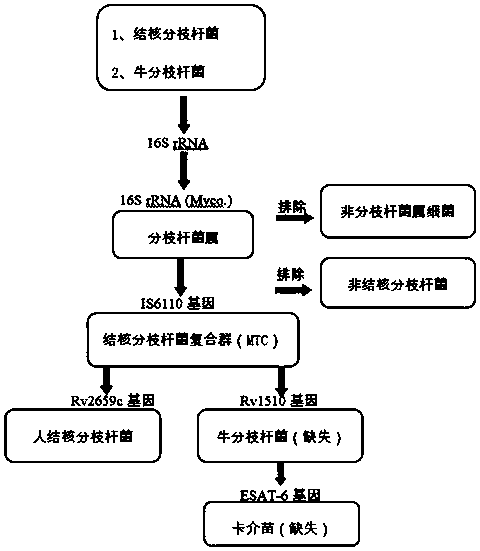
Technical method for identifying human mycobacterium tuberculosis, mycobacterium bovis and bacillus calmette-guerin (BCG)
PendingCN111154898AMicrobiological testing/measurementMicroorganism based processesTuberculosis mycobacteriumBacille Calmette Guerin
The invention provides a technical method for identifying human mycobacterium tuberculosis, mycobacterium bovis and bacillus calmette-guerin (BCG). Five target genes, i.e., 16S rRNA, IS6110, Rv2659c,ESAT6 and Rv1510 are selected for multiple PCR amplification, the mycobacterium tuberculosis can be rapidly and accurately identified, and interference caused by the mycobacterium bovis and BCG low-virulent strains (BCG) is effectively removed.
Owner:NANJING UNIV
Specific skin regent for diagnozing tuberculomyces infection and active tuberculosis
InactiveCN1683009ABacterial antigen ingredientsDrug compositionsMycobacterium InfectionsCalmette-Guerin Bacillus
The present invention relates to specific skin reagent for diagnosing tuberculous infection and active tuberculosis, and belongs to the field of medical immunological diagnosis technology. The specific skin reagent consists of diluent liquid and dissolved tuberculous allergen, and the tuberculous allergen is tuberculous mycobacterium ESAT6 protein. The present invention also proposes applying tuberculous mycobacterium ESAT6 protein for human skin test as tuberculous allergen to detect tuberculous infecting person and tuberculosis patient and identify the specific reaction of inoculated Calmette-Guerin bacillus vaccine. The present invention can induce the immune response of tuberculous infecting person. The reagent can induce delayed allergic reaction of tuberculous infecting person and can identify BCG inoculation from the allergic reaction of non-tuberculous mycobacterium and tuberculous mycobacterium infection.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY
Gold-labeled diagnosis reagent based on combined protein for tubercle bacillus
InactiveCN1844929AIncreased sensitivityImprove featuresSugar derivativesBiological testingAntigenSpecific test
The invention discloses a medical immunity diagnose technique, especially providing a bacillus tubercle mycobacterium gold mark diagnose agent based on combined protein. Wherein, it uses B cell antigen determinants of bacillus tubercle mycobacterium main excrete proteins as ESAT6, MPT64, PstS-1, Ag85B to combine a new group of protein as antigen, as the specific test antibody in the clinic serology diagnosis of phthisis. The invention combines the B cell antigen determinants of bacillus tubercle mycobacterium main excrete proteins to be displayed by gene project, and purified to attain one new protein, and via gold mark filter method to test the serum of tuberculosis patient. The invention has high sensitivity, simple operation, and high speed, which can identify the sensitized condition caused by contacting the non- bacillus tubercle mycobacterium or inoculating beg vaccine, and the real bacillus tubercle mycobacterium. It can be used in the clinic serology diagnosis of clinic tuberculosis.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY
Methods and systems for determining m. tuberculosis infection
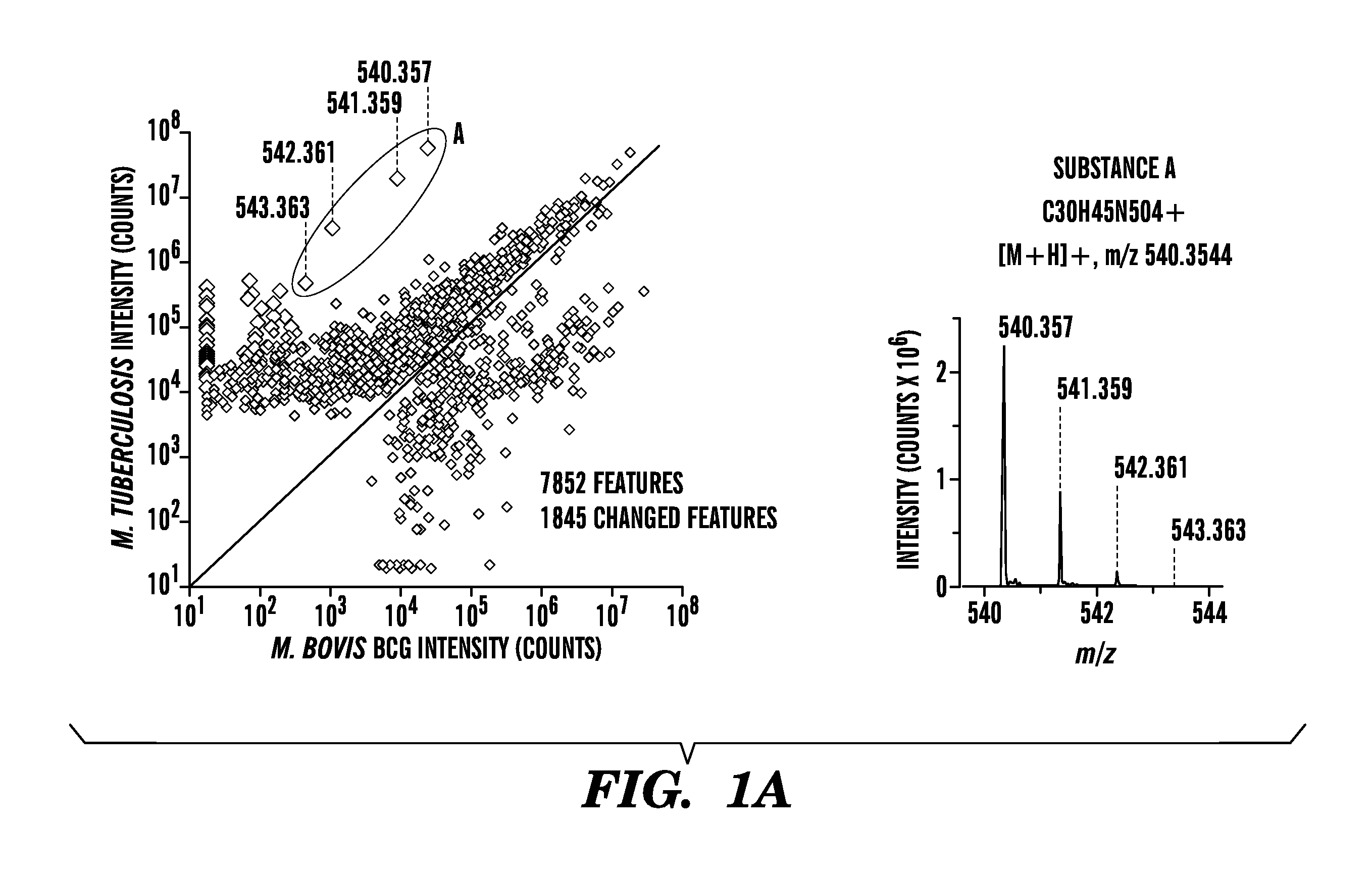
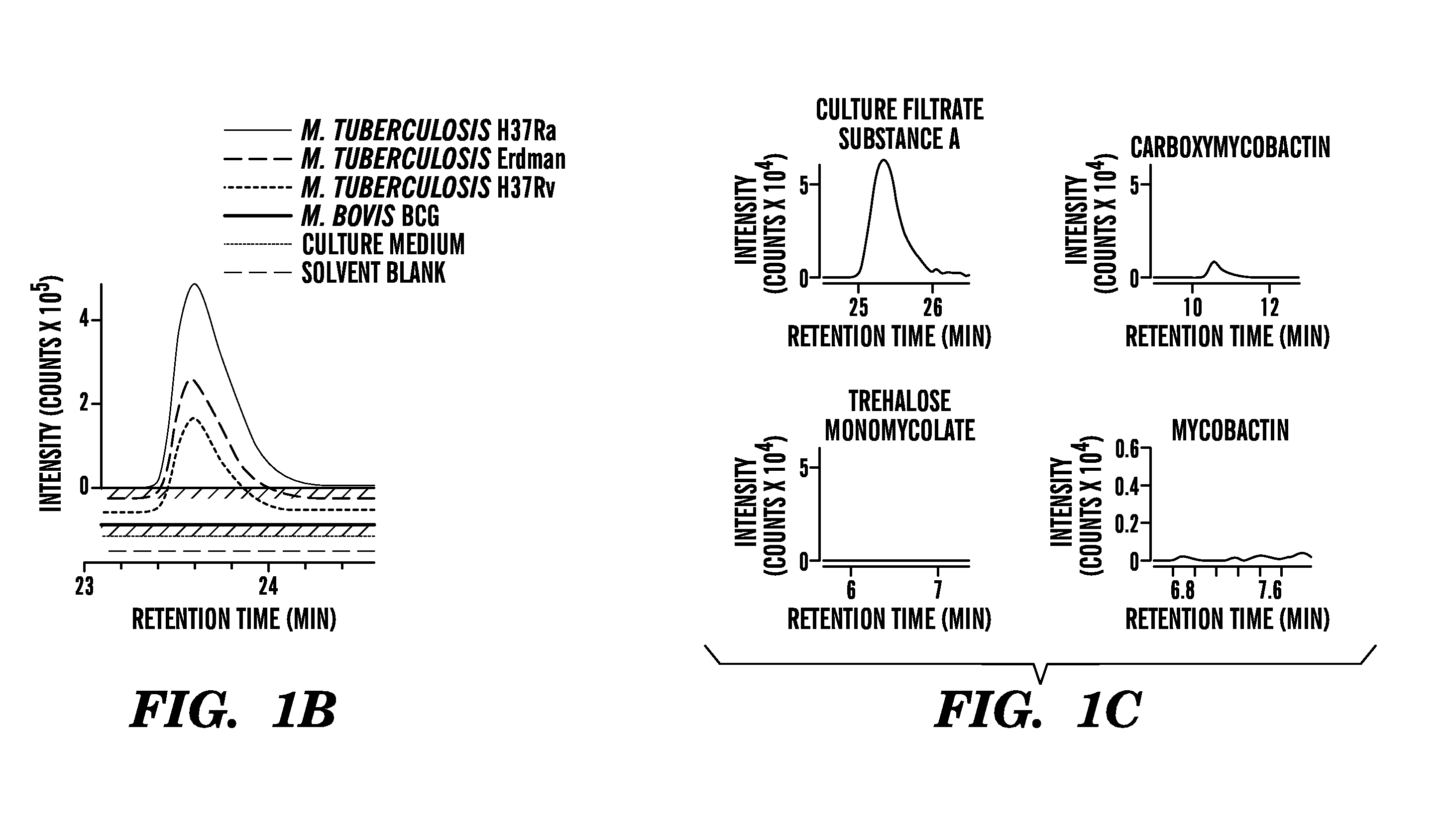
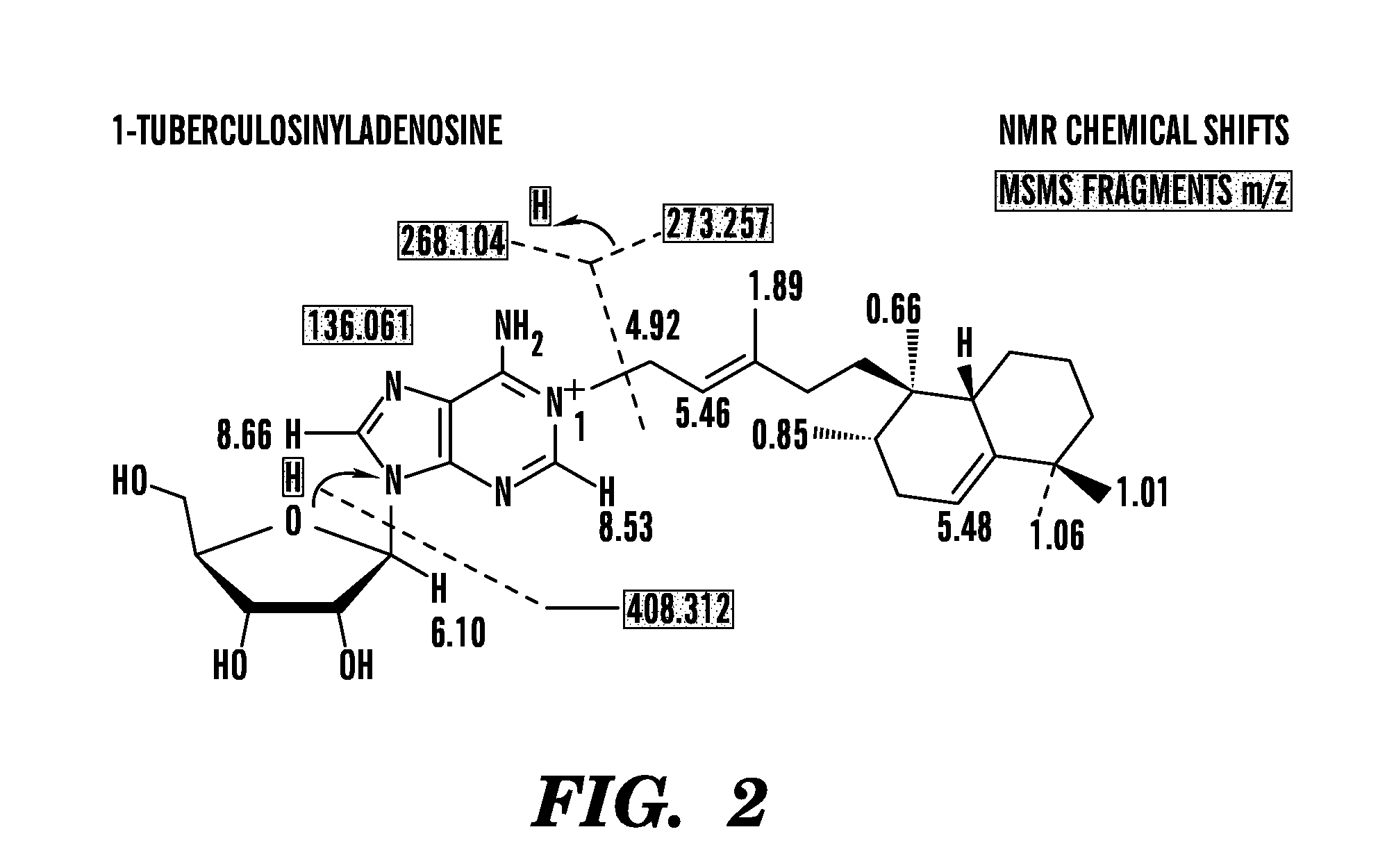
Embodiments of the invention relate to methods and systems for the detection of Mycobacterium tuberculosis. Mycobacterium tuberculosis kills more than one million people each year. To better understand why M. tuberculosis is virulent and to discover chemical markers of this pathogen, we compared its lipid profile to that of the attenuated but related mycobacterium, Mycobacterium bovis Bacille Calmette Guerin (BCG). This strategy identified previously unknown compounds that are specific to M. tuberculosis, e.g. 1-tuberculosinyladenosine, N6-tuberculosinyladenosine, and various tuberculosinyladenosines having mycolic acids, produced by the Rv3378c enzyme.
Owner:THE BRIGHAM & WOMENS HOSPITAL INC
Preparation method of muramyl dipeptide-anti-CD20 immune conjugate and application thereof
InactiveCN103087196AMaintain immunogenicityReduce dosageDipeptide ingredientsAntipyreticCD20Malignant lymphoma
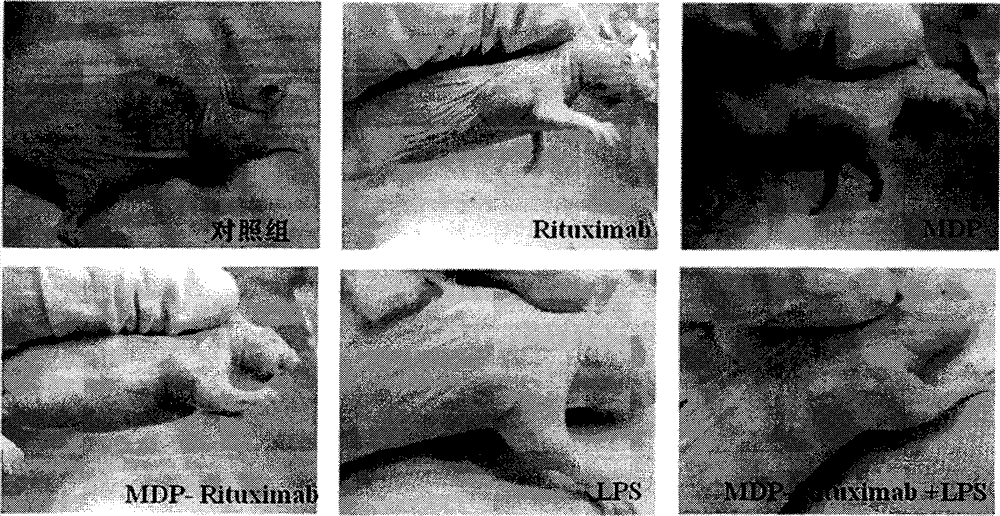
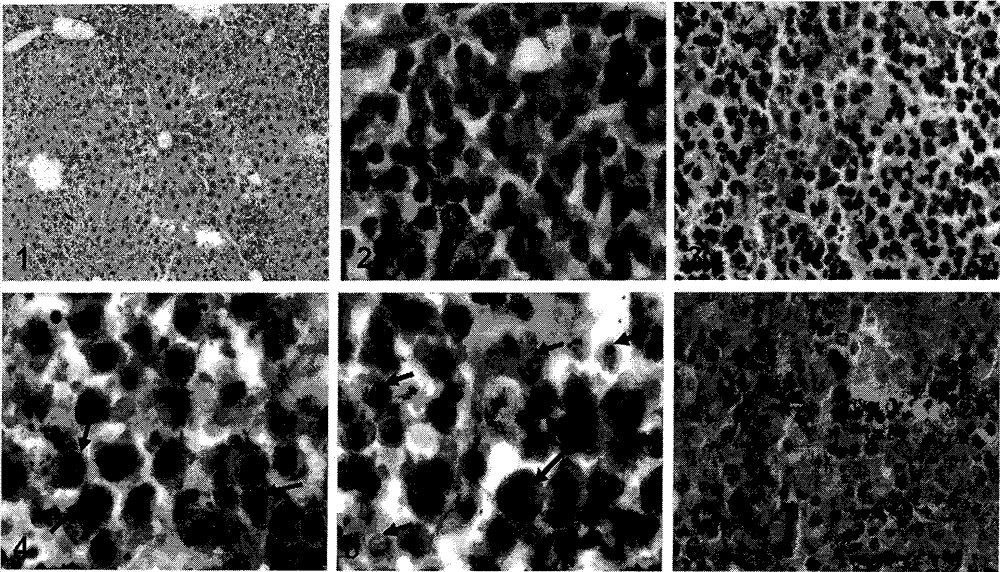
By taking guide functions of specific cellular immunity and monoclonal antibody immunity generated in vaccine inoculation as theoretical basis, the effective component of bacillus calmette Guerin (BCG), namely muramyl dipeptide (MDP), and a monoclonal antibody Rituximab of lymphoma cell membrane resistant antigen CD20 are combined to prepare a muramyl dipeptide-anti-CD20 immune conjugate, namely MDP-Rituximab, wherein in-vivo and in-vitro anti-tumour effects of immunocompetent cells subjected to BCG immunity under mediation of MDP-Rituximab are known. The conjugate disclosed by the invention keeps the immunogenicity of MDP, stimulates organisms to generate specific T cell immune response, and brings MDP to be around residual lymphoma cells with the help of immune guide function that a Rituximab antibody is combined with lymphoma cell membrane CD20 molecules; the final purpose of eliminating residual tumours is achieved through immune response mediated by MDP induced T cells; therefore, due to the method, the drug resistance caused by exclusive use of Rituximab is overcome; furthermore, the use amount of the antibody is reduced; and the application prospect of the muramyl dipeptide-anti-CD20 immune conjugate in malignant lymphoma is predicted.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Recombinant calmette-Guerin bacillus vaccine for secretion of human interferon-alphaza and its constructing method
InactiveCN1710071AImprove drug efficacyReduce the risk of tumor recurrenceRecombinant DNA-technologySignal peptideInterferon
This invention involves reorganization BCG vaccine for secretion of people interferon- alpha2a, and structuring method. Utilize gene engineering to clone BCG vaccine Ag85B signal peptide part and the genes of people IFN - alpha 2a playing a secreting role to pMV261 to receive the BCG vaccine shuttling expression carrier pMSIFN - alpha 2a. Adopt electric boring technology to lead pMSIFN - alpha 2a into BCG to recombinate rBGGIFN - alpha2a. Depending on rBGGIFN - alpha 2a duplicating in BCG and the secretion function of the signal peptide, can secrete IFN - alpha2a efficiently.
Owner:SHANGHAI JIAO TONG UNIV
Construction and application of PhoPR gene overexpressed BCG strain
The invention relates to a construction and an application of a novel PhoPR gene overexpressed BCG (Bacillus Calmette-Guerin) mutant strain. The construction comprises the following steps: firstly, converting a carrier containing a BCG overexpressed PhoPR gene into BCG, screening, detecting and confirming a successfully converted strain as the novel PhoPR gene overexpressed BCG mutant strain. Theinvention relates to the novel PhoPR gene overexpressed BCG mutant strain, namely, BCG: PhoPR strain. Such a strain is mainly used for researching the functions of BCG PhoPR gene overexpressed associated protein PhoPR at the aspects of growth of mycobacterium tuberculosis in host, drug tolerance, permeability regulation, acid resistance, pathogenicity, and the like, and is used for developing andresearching novel vaccines for interfering and treating tuberculosis.
Owner:SHIHEZI UNIVERSITY
A kind of recombinant BCG and its application
ActiveCN108949783BImprove defectsConsistent structureAntibacterial agentsBacterial antigen ingredientsEscherichia coliBacterial strain
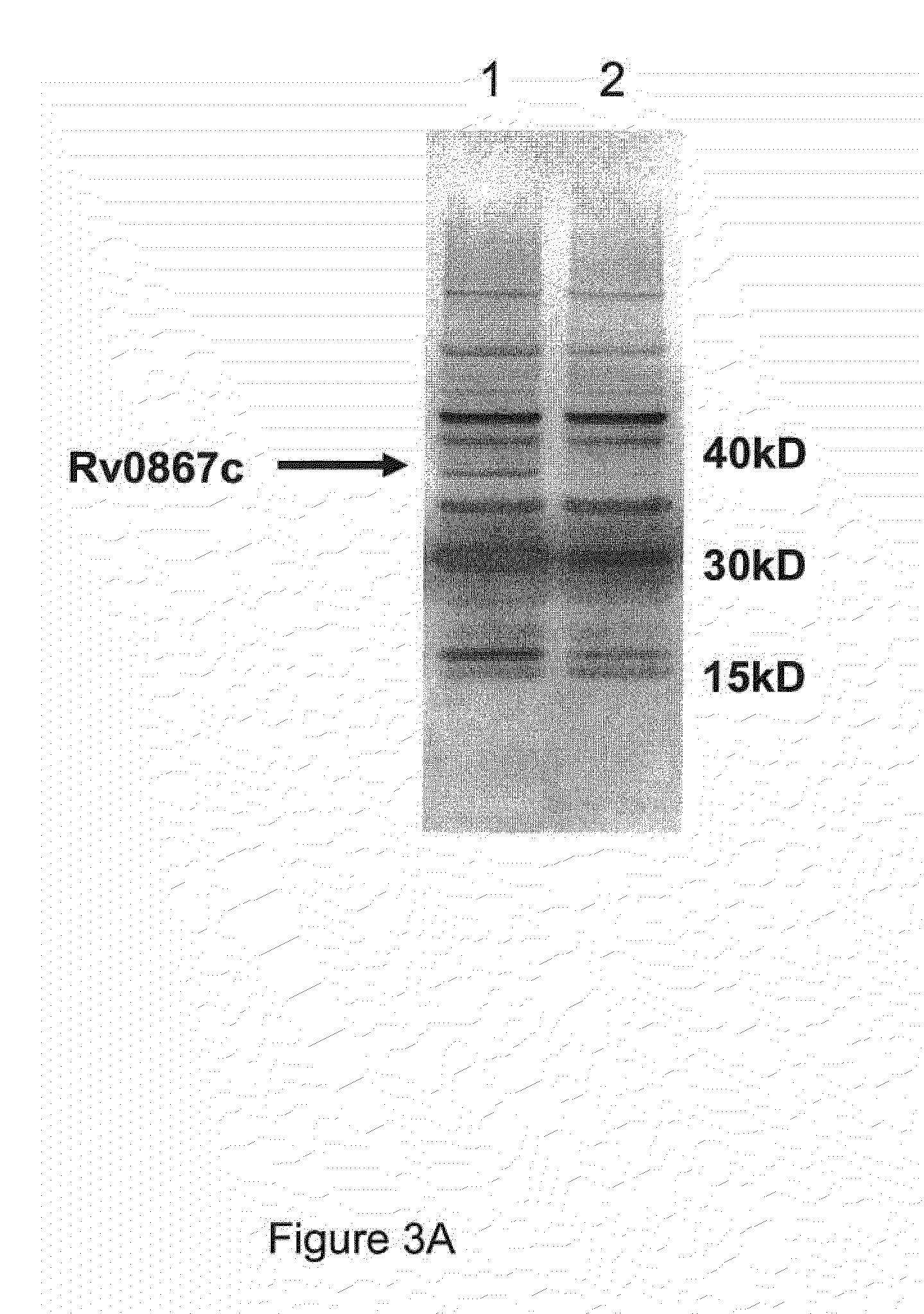
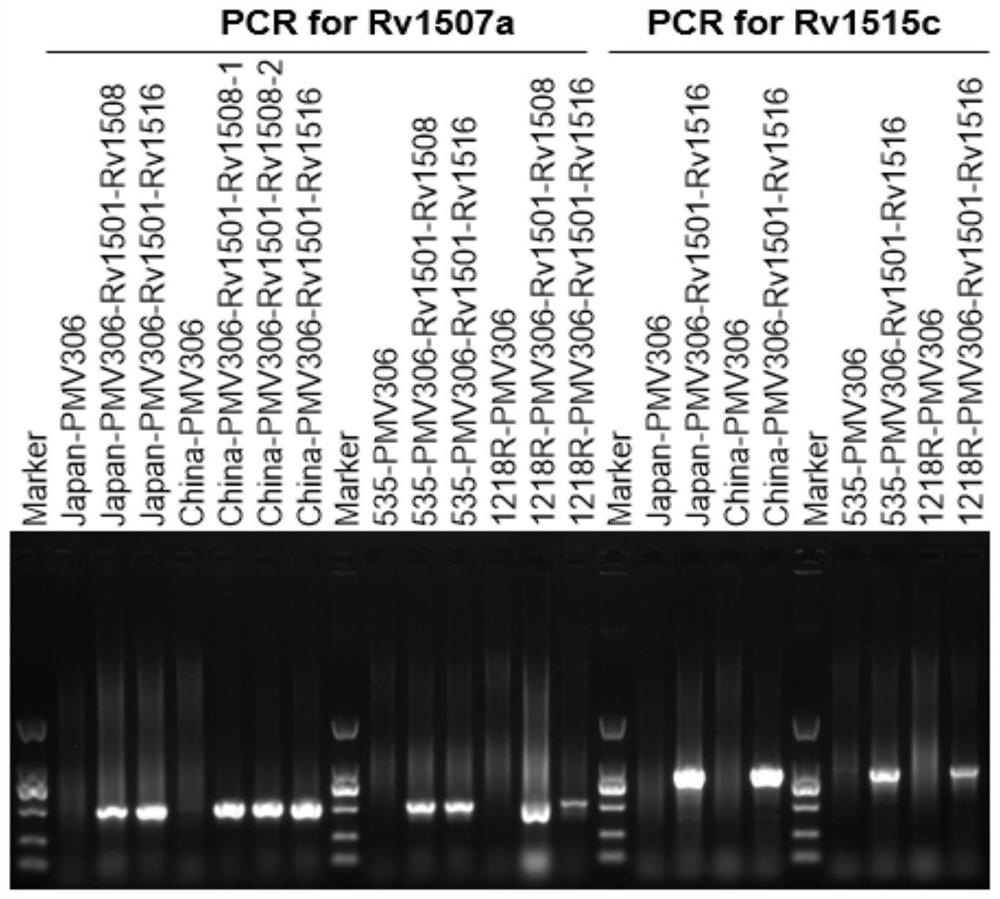
The invention belongs to the technical field of gene engineering and tuberculosis vaccine, and provides a recombinant bacillus calmette guerin (BCG) vaccine containing mycobacterium tuberculosis genome RD4 zone related coding genes. According a preparation method, the recombinant bacillus calmette guerin vaccine is produced through transformation of recombinant Escherichia coli-mycobacterium shuttle plasmids containing genes used for coding of mycobacterium tuberculosis genome RD4 zone proteins into bacillus calmette guerin vaccine. The recombinant bacillus calmette guerin vaccine is capable of realizing RD4 zone gene and protein expression. After immunization of animals with the recombinant bacillus calmette guerin vaccine, the safety of recombinant BCG bacterial strain containing complete RD4 zone is not reduced, the safety of recombinant BCG bacterial strain containing a part of RD4 zone (Rv1501-Rv1508c) is increased obviously. The recombinant BCG bacteria strain containing the complete or a part of RD4 zone genes possess better anti-infection protection effects. The recombinant bacillus calmette guerin vaccine can be used in prevention or treatment of tuberculosis.
Owner:FUDAN UNIV
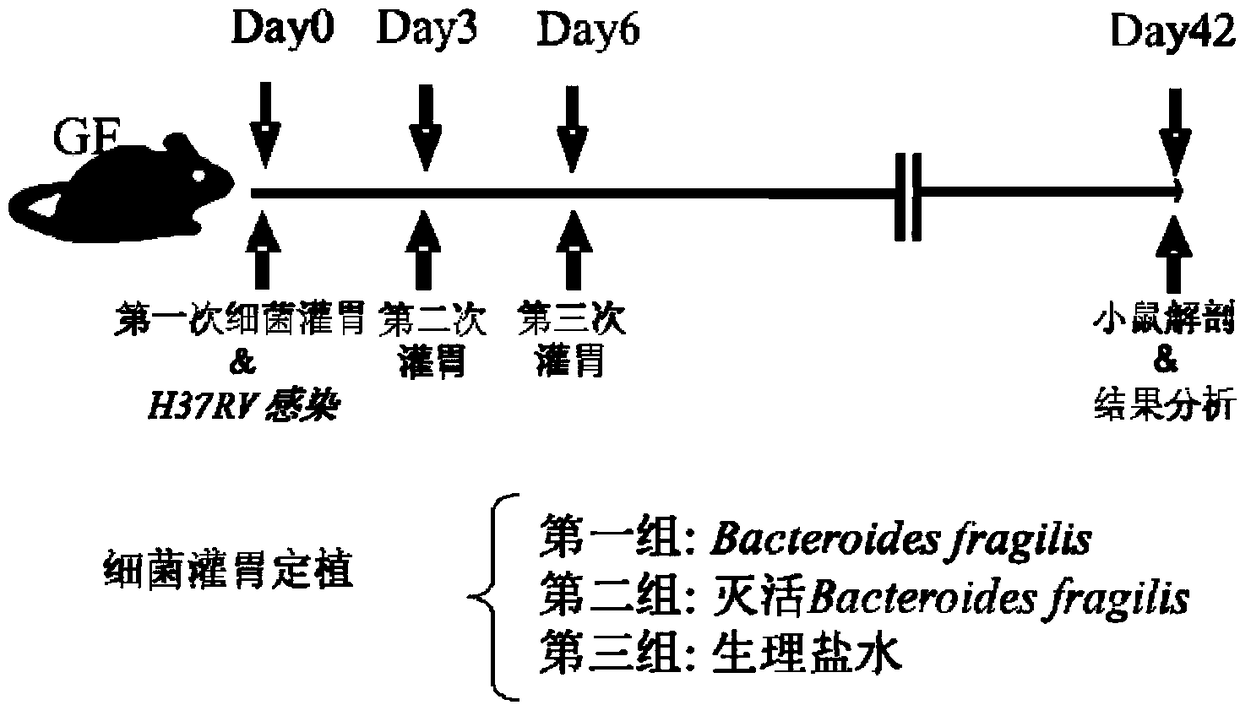
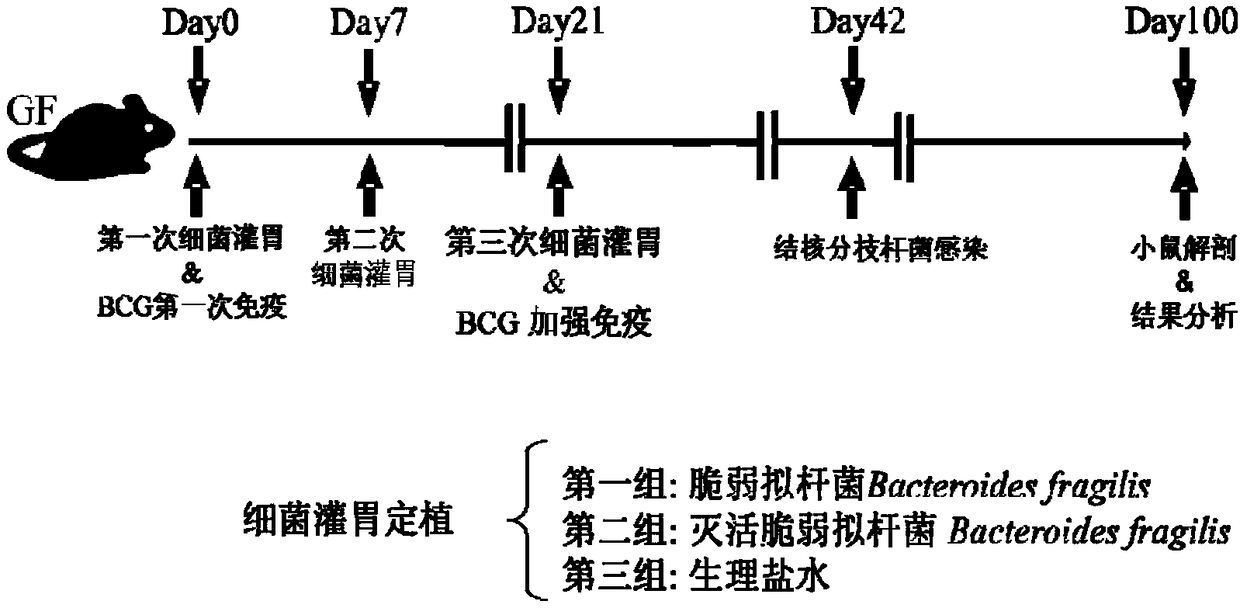
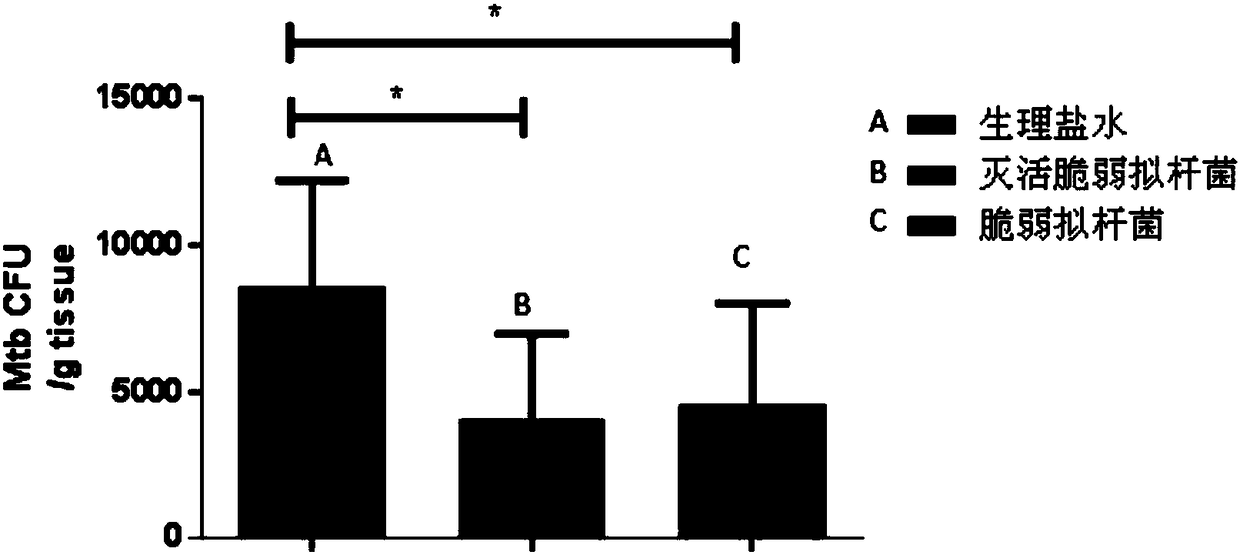
Use of Bacteroides fragilis in the preparation of drugs for the treatment and prevention of tuberculosis
InactiveCN109200063AGood immune protectionEnhanced inhibitory effectAntibacterial agentsUnknown materialsFood additiveMedicine
The invention belongs to the biomedical field, in particular to the use of Bacteroides fragilis in the preparation of drugs for the treatment and prevention of tuberculosis. The invention also provides the application of Bacteroides fragilis in the preparation of pharmaceutical compositions, foods, health products and food additives for treating and / or preventing tuberculosis. The Bacteroides fragilis provided by the invention can effectively treat and / or prevent tuberculosis and enhance the immune protection effect of tuberculosis bacillus Calmette-Guerin vaccine (BCG), and has wide application prospect.
Owner:REVAISSANT SHENZHEN BIOSCIENCES CO LTD
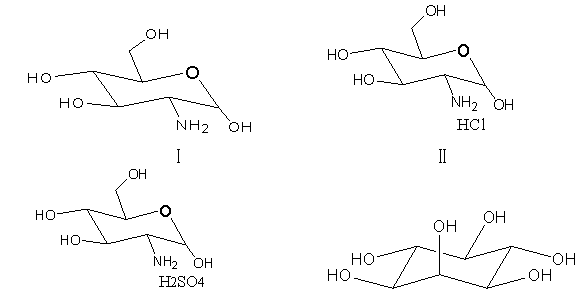
2-amino-2-deoxy-D-glucose and new application of hydrochloride, sulfate and myo-inositol thereof
InactiveCN103305586ARapid diagnosisBacteriaMicrobiological testing/measurementMycobacterium tuberculosis cultureInositol
The invention discloses 2-amino-2-deoxy-D-glucose and an application of hydrochloride, sulfate and myo-inositol thereof in preparing a mycobacterium tuberculosis culture medium or a bacillus calmette guerin vaccine mycobacterium culture medium. The 2-amino-2-deoxy-D-glucose and the hydrochloride, sulfate and myo-inositol thereof serving as a growth factor of mycobacterium tuberculosis have good application prospects in developing a novel mycobacterium tuberculosis rapid culture medium, and can be applied to the rapid diagnosis of tuberculosis, type identification and drug sensitivity detection; and by adding one of the four compounds or the mixture of several ones of the four compounds into the culture medium, the flora density in the liquid culture medium is far higher than that of a blank control group, the macroscopic bacterial colony appears one week earlier in the solid culture medium than in the blank control group, and the bacterial plaque is obviously larger than that of the blank control group.
Owner:GANSU AGRI UNIV
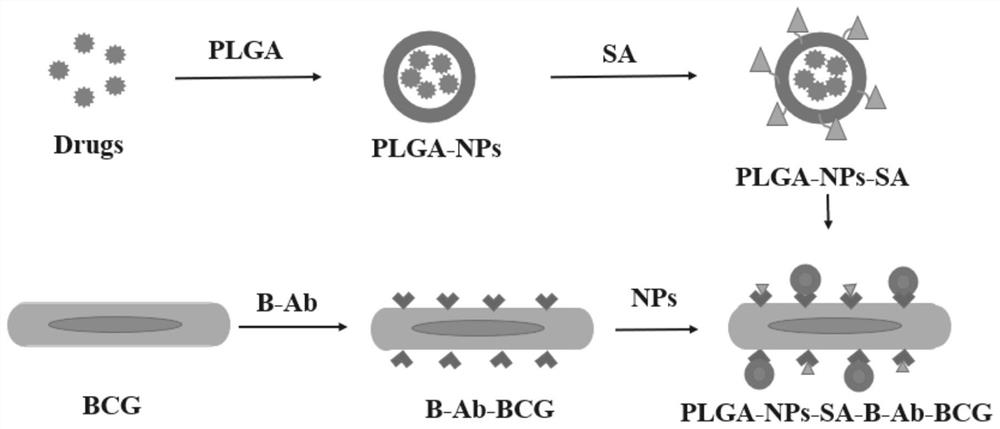
BCG (bacillus calmette-guerin) vaccine complex combined with nano drug carrier and preparation method of BCG vaccine complex
PendingCN112451679AImprove intratumoral transportPromote absorptionMacromolecular non-active ingredientsAntineoplastic agentsTumor targetingUrinary bladder epithelium
The invention discloses a BCG (bacillus calmette-guerin) vaccine complex combined with a nano drug carrier. The BCG vaccine complex is of a complex structure formed by combining the nano drug carrierand BCG vaccine thalli by virtue of an acting force between avidin and biotin protein; the nano drug carrier is nano particles which are coated with a polylactic acid-glycolic acid copolymer, subjected to surface modification by the avidin and have the particle size of 120nm to 180nm; and the surface of each BCG vaccine thallus is combined with one or more nano particles. The BCG vaccine complex disclosed by the invention has very good adhesion and absorption effects on the epithelial layer of the bladder, drug particles are loaded to the surfaces of the BCG thalli, and the BCG is used as thecarrier, so that the uptake of the nano drug carrier in the bladder and tumor targeting are enhanced, and the tumor killing effect of a drug can be remarkably improved. Meanwhile, the drug-loaded BCGcan stimulate strong immune response in part of the bladder and the whole body, so that a combined treatment mode combining immunotherapy and chemotherapy is realized.
Owner:THE SECOND HOSPITAL OF TIANJIN MEDICAL UNIV
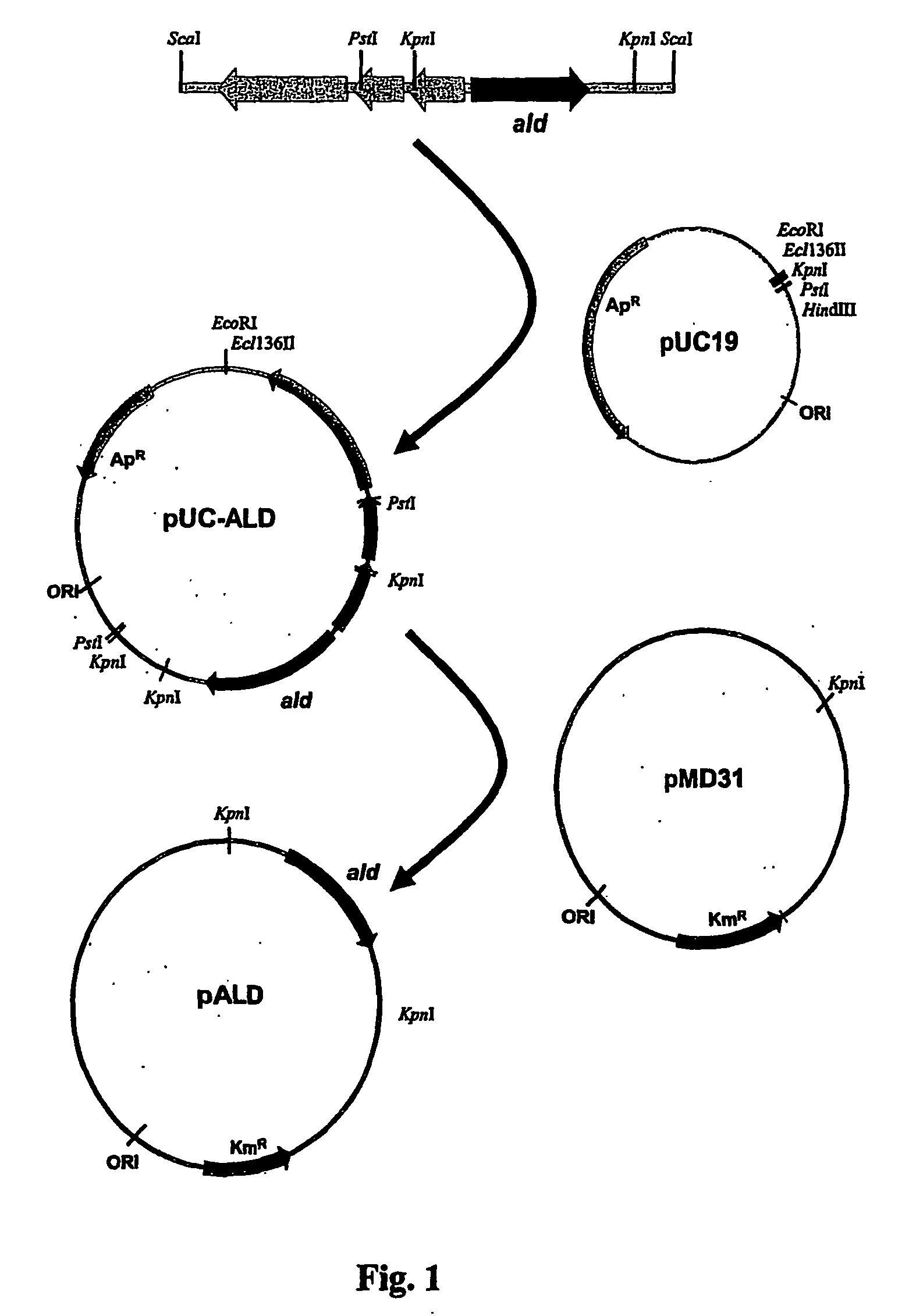
Tuberculosis Vaccines Including Recombinant BCG Strains Expressing Alanine Dehydrogenase, Serine Dehydratase and/or Glutamine Synthetase
InactiveUS20070264286A1Release growth inhibitionInduce long-term protective immunityAntibacterial agentsBacteriaSerine dehydrataseTGE VACCINE
The invention relates to a live recombinant Mycobacterium bovis-BCG strain comprising a nucleic acid capable of expression, the nucleic acid encoding at least one protein or polypeptide that exhibits alanine dehydrogenase activity, glutamine synthetase activity, or serine dehydratase activity.
Owner:LIU JUN
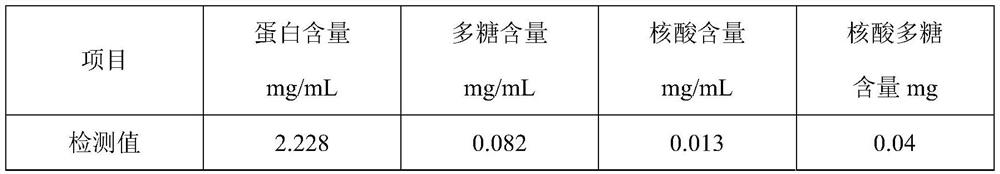
Use of BCG polysaccharide and Nucleic acid preparation in preparation of medicine for oral cavity local medication
InactiveCN1433770ATotal number of enhancementsEnhance immune functionOrganic active ingredientsRespiratory disorderDiseaseSide effect
Owner:CHENGDU INST OF BIOLOGICAL PROD
HBsAg-BCG combined vaccine for intracutaneous injection and preparation method thereof
InactiveUS7579003B2Improve securityEnhance humoral immunityAntibacterial agentsBacterial antigen ingredientsHeat stabilityBacille Calmette Guerin
The present invention provides a combined vaccine that includes hepatitis B vaccine (HeVac) and Bacille Calmette-Guerin (BCG) for intracutaneous injection and the method of preparation of such vaccine. The combined vaccine changes the now used liquid agent of the HeVac into a cryoprotectant, thus improving the heat stability of the HBsAg. Because of the cryoprotectant in the combined vaccine, the heat stability of the HBsAg is improved, and the efficacy of the vaccine is only slightly decreased after 30 days at 37° C., and the decrease is lower than with the liquid agent of the HeVac. The present invention changes the newborn's inoculation from two injections into one to simultaneously obtain prophylaxis of hepatitis B and tuberculosis.
Owner:CHANGCHUN INST OF BIOLOGICAL PRODS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com